Abstract
Interleukin-2 is an important activation factor for NK cells but its effect on NK cell MMP production and matrix degradation is less well investigated. We have used freshly isolated human NK cells and the IL-2-independent NK cell line, YT, to investigate the effects of IL-2 stimulation on NK cell invasion of Matrigel as well as on MMP expression and production. In YT cells we found opposing early and late effects of IL-2 stimulation with an early (2h) increase in MMP-9 protein level and enhanced migration in the Matrigel invasion assay and by 30hrs a decreased mRNA expression of MMP-2, -9, -13, MT3- and MT6-MMP. We also found a pre-culture period of 48h with IL-2 to negatively affect YT cell migration. We furthermore found that freshly isolated human NK cells Matrigel invasion was MMP-dependent and it increased in response to IL-2. Importantly, in freshly isolated human NK cells we did not see a downregulation of MMPs after 24h IL-2 stimulation, but instead a significant upregulation of MT6-MMP mRNA. Because of the cellular localisation of MT6-MMP, which ensures a focalized proteolytic activity, and its high expression compared to the other MMPs in freshly isolated human NK cells makes it of interest to study further.
Keywords: NK cell, MMP, Interleukin 2, migration
Introduction
Natural killer (NK) cells need to be in close contact with tumour cells in order to trigger their cytolytic machinery (1). Previous studies on NK cell-infiltrated tumours have demonstrated that relatively few extravasated NK cells make direct contact with tumour cells and the preponderance of NK cells are often retained in the stroma surrounding the tumour and tumour islets (1–4).
In vitro studies using Interleukin-2 (IL-2)-activated NK cells isolated from mouse and rat have shown that their migration through Matrigel-covered filters is, in part, dependent on matrix metalloproteinases (MMPs) (5–7). MMP-2, -3, -7, -9, -10, -11, -13, MT1- and MT2-MMP have been detected in rodent NK cells (5, 6, 8). In human NK cells, however, the only MMPs described to date are MMP-1, -2, -9, MT1- and MT2-MMP (9–11).
Knowledge about the expression and regulation of MMPs and other matrix-degrading proteases in NK cells is limited. It has been shown that MMP-2 is upregulated in human YT NK cells following cross-linking of the activating receptor 2B4 (12). Stimulation of rat NK cells by the prostaglandin PGE2 enhanced the secretion of MMP-1 and -3, and facilitated migration in the Matrigel invasion model (7). Human NK cell invasion into type I collagen has further been shown to be enhanced by the chemokine CXCL12 in a MMP-dependent manner (10). Stimulation of human NK cells by IL-18 has in addition been shown to increase their invasiveness in a Matrigel-based assay as well as their secretion of MMP-2, -9 and MT1-MMP (11). A screening of MMP-2 and MMP-9 production using semi-quantitative gelatin zymography by Johnatty et al. showed that freshly isolated human NK cells cultured for 4 days produced lower amounts of these MMPs compared to other freshly isolated lymphocytes (13).
The role of IL-2 in NK cell invasion is mainly unclear and inherently difficult to study, as freshly isolated NK cells of rodent and human origin are dependent on exogenous IL-2 for survival and proliferation in culture. Long-term culture of human NK cells in IL-2-containing medium can substantially modify the amount of adhesion receptors (14). This could alter target recognition and negatively affect the migratory ability. On the other hand, an increased expression of matrix-degrading proteases, such as MMPs, may enhance NK cells’ ability to migrate through basal membranes and the underlying extracellular matrix, and ultimately result in greater quantities of NK cells getting in direct contact with tumour cells. It is therefore important to gain more knowledge about how MMP expression is regulated in NK cells. In particular, improved migratory capabilities of NK cells may enhance the efficacy of these cells when used in adoptive cancer immunotherapy. In this study, we used both the human IL-2-independent NK cell line YT as well as freshly isolated human NK cells to investigate the effect of IL-2 on NK cell migration and MMP expression.
Materials and Methods
Cell culture
The human immature NK cell line YT, established from an Asian boy diagnosed with thymus lymphoma (15) was obtained from DSMZ (Braunschweig, Germany) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 50 units/mL penicillin and 50 µg/mL streptomycin (all obtained from GIBCO®, Paisley, UK). Cell culture procedures were performed under sterile conditions and the cells were propagated at 37°C with 5% CO2 in humidified air. Cell cultures were negatively screened for mycoplasma contamination using PCR technique and all medium filtered through 0.22 µm pore size membranes before use. Recombinant human IL-2 (Proleukin®, Novartis Vaccines & Diagnostics, Emeryville, CA, USA) was diluted in RPMI-1640 to obtain a stock solution of 100 000 Cetus Units (U)/mL.
Isolation of human NK cells
Human NK cells were separated from buffy coats obtained from three healthy donors either as described by Jonges et al. (16) (for semi-quantitative RT-PCR) and the purity always determined to be > 90% CD56+ and < 1% CD3+ cells, or by Ficoll/Hypaque centrifugation followed by an immunomagnetic purification using the MACS NK cell separation kit from Miltenyi Biotech (Bergisch Gladbach, Germany) (for real-time RT-PCR). A high purity of the eluted NK cells (> 99% CD56+ and < 0.1% CD3+ cells) was determined using fluorescence-activated cell sorting (FACS) using FITC-conjugated anti-CD3 and PE-conjugated anti-CD56 (both obtained from BD Biosciences San Diego, CA, USA)
IL-2 and IFN-γ ELISA
YT cells, 5×105 cells per mL in opti-MEM medium (2 mM L-glutamine and antibiotics) (GIBCO®, Paisley, UK) supplemented with 1, 10, 100 or 1000 U IL-2 were cultured for 24h. Control cells were cultured without added IL-2. Incubation in Phorbol-12-myristate 13-acetate (PMA) (Sigma, St. Louis, MO, USA) was used as positive control. Supernatants were removed and centrifuged for 45 minutes at 5000×G at 4°C and samples aliquoted and stored at −80°C until analysis. Levels of endogenously produced IL-2 were quantified using BD OptEIA Human IL-2 ELISA kit II (BD Biosciences, San Diego, CA, USA). Quantification of human IFN-γ present in supernatants was performed using BD OptEIA Human IFN-γ ELISA kit II (BD Biosciences, San Diego, CA, USA). The results are expressed as the mean ± SEM of at least three separate experiments.
Matrigel invasion assay
The invasive potential of IL-2 stimulated freshly isolated NK cells and YT cells was measured using the Matrigel invasion assay as previously described by us (17). A total of 5×105 cells re-suspended in 0.3 mL opti-MEM medium (including L-glutamine and antibiotics), with or without (w/wo) IL-2 (for YT cells) or with 10 or 100 U IL-2 (for freshly isolated NK cells), were loaded into each insert well and 1.5 mL of the same medium (w/wo IL-2) added to the lower chambers. For inhibition studies, 10 µM of the broad-spectrum MMP inhibitor GM6001 (Calbiochem, Nottingham, UK) was included in the assay. The DMSO-vehicle alone was used in control wells. After incubation at 37°C for 48h the number of transmigrated cells was quantified by fluorescent labelling using alamarBlue (Serotec, Oxford, UK). All determinations were performed in triplicate and data are expressed as number of invaded cells.
Semi-quantitative reverse transcriptase (RT)-PCR
Freshly isolated human NK cells, 2×106 cells per mL, were cultured in AIM-V medium (Life Technologies, Grand Island, NY, USA), supplemented with 10% human AB-serum, 2 mM L-glutamine, 50 µg/mL streptomycin and 50 U/mL penicillin for 2 days in the presence of IL-2 (10 or 1000 U, Strathmann Biotech GMBH, Hannover, Germany). RNA was extracted using TRIzol reagent (Life Technologies, NY, USA) according to the manufacture’s instructions. cDNA synthesis was performed using murine Moloney leukemia virus (M-MLV) reverse transcriptase (Promega, Madison, WI, USA). RNA was reverse transcribed using random hexamer or oligo (dT)16 primers. The PCR primers for the detection of MMP-2 (fwd 5’-GAGGACTACGACCGCGGACAA-3’and rev 5’-CCAAATGAACCGGTCCTTGA-3’) and MMP-9 (fwd 5’-TTGACAGCGACAAGTGG-3’, and rev 5’-CCTGATGTGGGTGAATACAA-3 ’ ) were used as described previously (12). Glyceraldehyde-3-phosphatedehydrogenase (GAPDH) (fwd 5’-TAGACGGGAAGCTCACTGGC-3’, and rev 5’-AGGTCCACCACCCTGTTGCT-3’) was used as internal control. PCR was performed for 25–30 cycles in a Perkin Elmer thermocycler 2400 (Perkin Elmer, Norwalk, CT, USA). PCR products were separated on 1% agarose gels and stained with ethidium bromide. The density of each band was measured using a scanning densimeter and relative pixel densities, corrected to the internal control, were used to evaluate the effect of IL-2.
Quantitative Real-time RT-PCR
YT cells, 2×106, were cultured for 30h in serum-free opti-MEM medium on plastic or on Matrigel coated plastic (Matrigel diluted 1:10 in sterile filtered RPMI-1640 medium) with or without 100 U IL-2. 1×106 YT cells were in addition mixed 3:1 with Matrigel and grown in Matrigel droplets for 30h w/wo 100 U IL-2. Freshly isolated NK cells were either prepared immediately or cultured with 10 or 100 U IL-2 for 2 and 24h. RNA was isolated using the RNeasy® Fibrous Tissue Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. For YT cell samples, RNA concentrations were determined using NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and 3 µg RNA from each sample synthesised to cDNA using Cloned AMV First-strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). cDNA quality was visually verified using 1% agarose gels. For human NK cell samples, RNA from 0.5×106 freshly isolated NK cells were synthesised to cDNA using the same kit as above. Real-time RT-PCR was performed as previously described by us using forward and a backward primers plus a specific [6-carboxy-fluorescein/6-carboxy-tetramethyl-rhodamine (FAM/TAMRA)] or [6-carboxy-fluorescein/2,5-di-tert-butylhydroquinone-1 (FAM/BHQ1)] double-labelled probe (all obtained from Isogen, Maarsen, The Netherlands) (17). MMP expression levels were normalized against GAPDH expression using a VIC-labelled GAPDH primer-probe combination (Applied Biosystems, Nieuwerkerk aan de Ijssel, The Netherlands) and compared to unstimulated control cells. Fold change values from at least three independent experiments were used to determine changes in MMP expression.
MMP-2 and -9 ELISA
YT cells cultured in complete medium were washed and re-suspended at a concentration of 4×105 cells per mL in opti-MEM medium (0.2% FBS, 2 mM L-glutamine and antibiotics) w/wo 100 U IL-2 and cultured for definite times. Culture supernatants were removed at different time points, particulates removed by centrifugation and samples aliquoted and stored at −80°C until analysis. MMP-2 and -9 ELISA kits (Calbiochem, La Jolla, CA, USA), were used according to the manufacturers’ protocol. The results are expressed as the mean ± SEM of at least three separate experiments.
Statistical analysis
Statistical significance was assessed by unpaired Student's t-test except for the real-time RT-PCR data concerning YT cells which were analysed using factorial ANOVA as well as Welch Modified Two-Sample t-test.
Results
YT cells
Effect of IL-2 stimulation on YT cell migration
Firstly, YT cell supernatants were analysed using IL-2 ELISA to rule out any significant endogenous production of IL-2. No production of IL-2 was found (< 1pg/mL/0.5×106 cells/24h). However, IL-2 was produced dose-dependently in response to PMA stimulation (data not shown). Production of IFN-γ was furthermore quantified to ensure an adequate response to IL-2 stimulation. The YT cells demonstrated a basal production of approximately 150 pg IFN-γ/mL/0.5×106 cells, and stimulation with IL-2 for 24h resulted in a dose-dependent increase (Figure 1).
Fig. 1.
IFN-γ produced in response to IL-2 stimulation. YT cells (5×105 cells/mL) were stimulated with 0, 1, 10, 100 and 1000 U IL-2 for 24h and levels of IFN-γ were measured in supernatants. Results are described as mean values of at least three separate experiments using IFN-γ ELISA kit (OptEIA, BD Biosciences). A dose-response relationship was shown with an increase in IFN-γ production with increasing levels of IL-2.
The Matrigel invasion assay was used to determine the influence of IL-2 on YT cells ability to invade and pass through a basement membrane equivalent (Matrigel). YT cells (5×105) were subjected to the assay in the presence of 100 U IL-2 and/or an MMP inhibitor (10 µM GM6001). Stimulation with IL-2 significantly increased the amount of invaded cells by 52% (P < 0.05) (Figure 2A). YT cell invasion was furthermore studied using different amounts of IL-2 but a dose-response relationship could not be found (data not shown). Furthermore, in presence of GM6001 migration was unsignificantly decreased by 21% when the YT cells were stimulated with 100 U IL-2 and only reduced by 8% without IL-2 present (data not shown). GM6001 was found to have no effect on either cell viability or proliferation by Trypan blue exclusion and alamarBlue (data not shown). In order to mimic the in vitro procedure for NK cell production for adoptive transfer, YT cells were pre-cultured for 48h with IL-2. The IL-2 pre-cultured YT cells migrated approximately 40% less than cells with no prior IL-2 stimulation (P < 0.05) (Figure 2B). Together, these results demonstrate that while YT cell migration through Matrigel increase with addition of IL-2, longer stimulation with IL-2 decrease their migration.
Fig. 2.
Interleukin 2 stimulation of YT cells migration across Matrigel membranes. YT cells (5×105) were placed in the top well of Matrigel Invasion chambers and subjected to invasion assay for 48h with either no IL-2 or 100 U IL-2. Results are expressed as the number of invaded cells and each bar represents mean values (± SEM) from at least three separate experiments. Statistical significance was assessed using Student’s t-test, P < 0.05 (*two-tailed; #one-tailed). A) IL-2 stimulation significantly increased the amount of invaded cells by 52% (P < 0.05). B) YT cells pre-cultured for 48h with 100 U IL-2 prior to migration through Matrigel inserts invaded to a lesser degree than YT cells not pre-cultured with IL-2.
Expression of MMPs in YT cells in response to IL-2 stimulation
Next, we investigated the impact of IL-2 stimulation on the expression of MMP-2, -9, -13, and the membrane type MT1-, MT3- and MT6-MMP. The finding of message for MT3-MMP and MT6-MMP (MMP-16 and -25) has not previously been reported for NK cells. We also wanted to evaluate any possible effects of culture environment, therefore, the mRNA expression was measured after 30h of culture on plastic, on Matrigel-coated plastic and enclosed in Matrigel droplets with or without 100 U IL-2. The effect of culture conditions and IL-2 was analysed using factorial ANOVA. Irrespective of the various culture conditions the MMP expression decreased when IL-2 was present in the culture medium. The expression of MMP-2, -9, -13, MT3- and MT6-MMP were all found to decrease significantly in response to IL-2 (Figure 3).
Fig. 3.
The effect of IL-2 treatment on the expression of MMP-2, -9, -13, MT1-, MT3- and MT6-MMP in YT cells. Results were normalized to GAPDH expression and compared to unstimulated control cells. Significance of differences was tested with Welch Modified Two-Sample t-test. Stimulation with IL-2 significantly down regulated the expression of MMP-2, -9, -13, MT3- and MT6-MMP.
Levels of MMP-9 in supernatants from IL-2 stimulated YT cells
Following the expression analysis, the effect of IL-2 on MMP-2 and -9 protein levels in YT cell supernatants were quantified using ELISA technique. Stimulation with IL-2 resulted in a significant increase in MMP-9 levels at 2h (P < 0.05), followed by a decrease at 4 and 10h (P < 0.05) (Figure 4A). By 24h, the level had returned back to baseline. The amount of MMP-9 produced at 24h by YT cells cultured with IL-2 was 50% lower than that produced by YT cells in culture without IL-2 (Figure 4B). Levels of MMP-2 were undetectable in all YT cultures (data not shown).
Fig. 4.
Production of MMP-9 in response to IL-2 stimulation. Each bar represents mean values (± SEM) from at least three independent experiments. Statistical significance was assessed by two-tailed Student's t-test (*P < 0.05). A) YT cells (4×105) were stimulated with 100 U IL-2 for 1, 2, 4, 10 and 24h. Stimulation with IL-2 resulted in an increase in levels of MMP-9 in supernatants with a peak at 2h (P < 0.05), followed by a decrease to 10h (P < 0.05), and returning back to baseline by 24h. B) Levels of MMP-9 in supernatant from YT cells cultured with or without 100 U IL-2 for 24h were also compared. Lower levels (50 %) of MMP-9 were found in supernatants from YT cells cultured for 24h with 100 U IL-2 compared to YT cells without IL-2.
Freshly isolated human NK cells
Effect of IL-2 stimulation on human NK cell migration
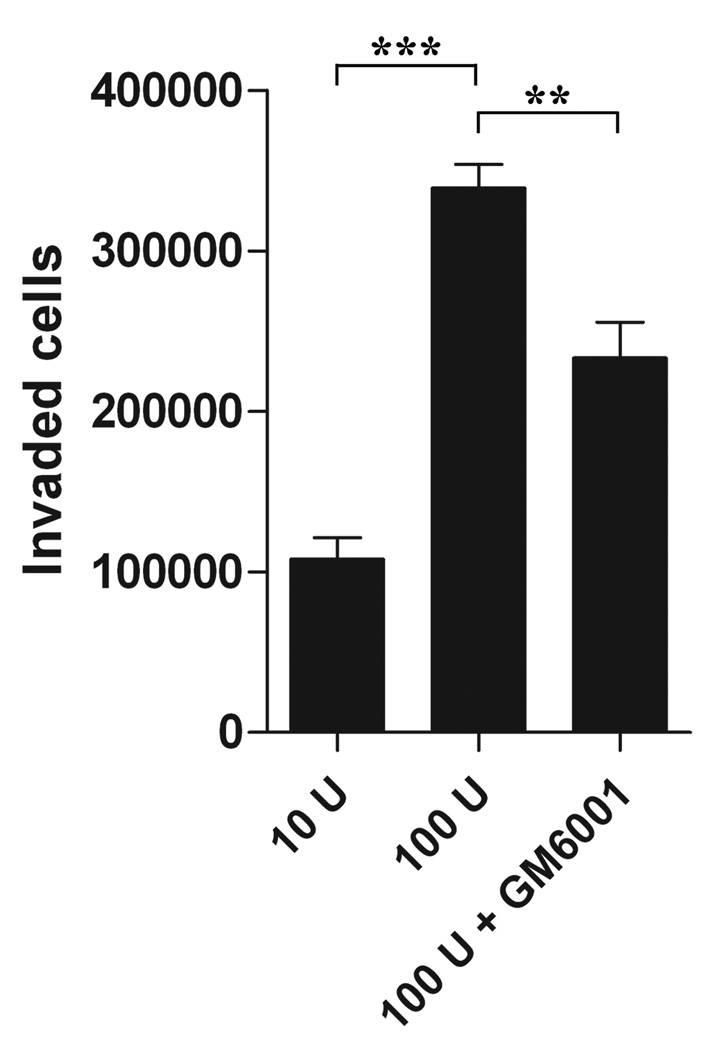
The effect of IL-2 on the migratory ability of freshly isolated human NK cells was analysed using Matrigel invasion assay. A concentration of 10 U IL-2 was added to control wells, this was necessary for the IL-2 dependent human NK cells to survive the incubation period. Addition of 100 U IL-2 significantly increased the number of invaded NK cells by 68% (P < 0.0001) (Figure 5). The use of the MMP inhibitor GM60001 was shown to decrease human NK cell migration by more than 30% (P < 0.01).
Fig. 5.
Interleukin 2 stimulation of human NK cell migration. Freshly isolated human NK cells (0.5×106) were placed in the top well of Matrigel Invasion chambers and subjected to invasion assay for 48h with either 10 U IL-2 (as a control) or 100 U IL-2 with and without 10 µM GM6001. Results are expressed as number of invaded cells (± SEM). Statistical significance was assessed using two-tailed Student’s t-test (**P < 0.01; ***P < 0.0001). A concentration of 100 U IL-2 significantly increased the amount of invaded cells by 68% (P < 0.0001). Addition of GM6001 inhibited the migratory ability with 31% (P < 0.01).
Expression of MMPs in human NK cells in response to IL-2 stimulation
Expression of MMP-2 and MMP-9 was respectively compared between freshly isolated and IL-2-cultured NK cells, using semi-quantitative RT-PCR. Experiments were performed in triplicates using NK cells isolated from healthy donors. While IL-2 stimulation had a down-regulatory effect on MMP-9 expression, it rather increased the expression of MMP-2. However, no significant effect could be demonstrated (data not shown). We thereafter used real-time RT-PCR to analyse the expression of MMP-2, -9, -13, and the membrane type MT1-, MT3- and MT6-MMP in freshly isolated as well as IL-2-cultured human NK cells. While expression of all analysed MMPs was detected, most at low levels, expression of MMP-2 could only be found in some samples (Figure 6A). Notably, stimulation with IL-2 was found to increase the expression of MT6-MMP significantly, both after 2 and 24h stimulation (Figure 6B).
Fig. 6.
MMP expression analysis. Expression of MMP-2, -9, -13, MT1-, MT3- and MT6-MMP was analysed in freshly isolated as well as IL-2 stimulated human NK cells. Results, normalized to GAPDH expression, and compared to unstimulated control cells are expressed as fold change values. Statistical significance was assessed using two-tailed Student’s t-test (*P < 0.05; **P < 0.01). A) MMP-9, -13, MT1-, MT3- and MT6-MMP were found to be expressed in low levels. B) Stimulation with IL-2 (100 U and 1000 U) for both 2 and 24h led to a significant increase in the expression of MT6-MMP.
Discussion
Quantitative measurements of the amounts of MMPs secreted by human NK cells are scarce and very little is known about how MMPs are regulated in NK cells (1). It has been shown that IL-2 stimulation leads to an increased accumulation of endogenous NK cells into tumour nodules (1). There are, however, no reports describing the direct effects of IL-2 on NK cell matrix degradation and related MMP expression. In this study we wanted to examine the effect of IL-2 stimulation on; i) NK cell migration through the basement membrane equivalent Matrigel, ii) the expression levels of several MMPs and, iii) the production of MMP-2 and MMP-9, known to have high activity on collagen IV in basement membranes. We used both freshly isolated human NK cells as well as the NK cell line YT. Given that the survival of the YT NK cell line is independent of exogenous IL-2, we regard them as an excellent model to study the effects of this important cytokine in cell migration, even though they have a low infiltrative capacity compared to other NK cell lines (17).
The YT cells were shown to lack measurable amounts of endogenous IL-2 production. However, they were indeed responsive to IL-2 stimulation as measured by IFN-γ production and an anticipated dose-dependent increase in IFN-γ production/release was found. We furthermore examined the influence of IFN-γ on YT cells production of MMP-9 using ELISA. However, no demonstrable effect was observed (data not shown), thus making it unlikely that the effects of IL-2 in YT cells are mediated by the increased IFN-γ.
The ability of YT cells to invade Matrigel was found to increase significantly in response to de novo stimulation with IL-2. A similar cytokine, IL-15, which is also of interest in the context of NK cell invasion into tumour tissue, was tested for its effect on YT cell invasion but no clearly enhanced invasion could be found using up to 100 ng/mL IL-15 (data not shown). The effect of IL-2 on NK cell migration was in addition analysed using freshly isolated human NK cells. Since IL-2 is ubiquitous for the survival of freshly isolated human NK cells, it was not possible to exclude IL-2 completely when testing these cells. We have previously observed signs of an IL-2-dependent migration in the Matrigel invasion assay using human NK cells (9) and IL-2 stimulation significantly increased the ability of human NK cells to migrate through Matrigel in this present study. The human NK cell line NK-92 that resembles freshly isolated human NK cells being IL-2-dependent in culture, respond similar to fresh NK cells and the YT cell line with an increased migration through Matrigel in response to 100 U IL-2 (data not shown). We could also demonstrate, as has been shown for NK cells of mouse and rat, that MMPs are involved in human NK cell migration which is evident by the significantly decreased migration by the broad-spectrum MMP inhibitor GM6001 (Figure 5). It is noteworthy that this MMP inhibition did not completely inhibit migration as is also the case for rodent NK cells inhibited by BB94 (5, 6). Therefore, the uPA/uPAR system, which is involved in rat NK cell migration (18), could also be important for human NK cell migration. It cannot, however, be excluded that the inhibitors used have only partial or no inhibitory activity against one or more of the multiple MMPs found and therefore cannot totally block invasion. Further, the possibility of protease-independent migration or other yet poorly understood mechanisms of locomotion could also be implicated (17, 19, 20).
A quantification of the YT cell lines MMP-9 production using ELISA showed a time-dependent increase within the first 2 to 4h, and also higher MMP-9 levels at 24h from unstimulated YT cells. We therefore suggest that IL-2 stimulation of YT cells is mostly effective short-term. Accordingly, YT cells pre-cultured without IL-2 migrated significantly better in the migration assay. We therefore speculate that an early stimulatory effect of IL-2 releases a burst of MMP-9, from a possible pre-storage location, while long-term IL-2 stimulation seemingly exhausts the YT cell MMP-9 production. Supporting this view, we found in the MMP expression analysis of the YT cell line that no early (up to 4h) changes in MMP mRNA expression was found while the expression of most MMP analysed decreased following a 30h IL-2 stimulation (Figure 3). Thus, in YT cells, a continuous IL-2 stimulation leads to a down-regulation of the MMPs needed for their migration which correlates with a decrease in Matrigel invasion.
Expression of all the MMPs investigated in this study was detected in freshly isolated human NK cells using real-time RT-PCR. Thus we confirm that freshly isolated human NK cells as well as the YT NK cell line express MMP-13, MT3-MMP and MT6-MMP, previously not described in human NK cells. The effect of IL-2 stimulation on human freshly isolated NK cell MMP expression was in addition analysed, and IL-2 stimulation was found to clearly increase expression levels of MT6-MMP but not of the other MMPs included in this study. Similarly, expression of MMP-2 and -9 was not found to be significantly affected by IL-2 when analysed using semi-quantitative RT-PCR. The observed increase in MT6-MMP expression in response to IL-2 makes it, for now, the most interesting MMP member and its specific importance for NK cell migration needs further investigation. An increase in proteolytically active membrane-bound MMPs on the NK cell surface may lead to enhanced degradation of extracellular matrix components at the cell invasive front and thus enhance locomotion in the extra vascular space.
In summary, we demonstrate that freshly isolated human NK cells have an MMP-dependent ability to invade Matrigel. This invasion was found to increase in response to IL-2. Stimulation with IL-2 furthermore increased the human NK cells expression of MT6-MMP. Using the IL-2-independent NK like cell line YT, a more complex role of IL-2 is suggested, where IL-2 stimulation shows an early increase in MMP-9 secretion and an enhanced Matrigel transmigration, but longer stimulation leads to down-regulation of several MMPs, and importantly, to a decrease in invasion capacity.
Acknowledgement
We wish to thank J.H. Lindeman at TNO (Applied Scientific Research, Leiden, The Netherlands) for help with the Real-time PCR analysis, H.M. Kim for technical assistance and Szilard Nemes at Epistat, University of Gothenburg, for statistical advice. We also want to thank Samuel Lundin at the Department of Medical Microbiology and Immunology at the University of Gothenburg, for performing the FACS separation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Swedish Cancer Foundation supported this study, grants 05 0582 and 06 0007, together with the King Gustav V Jubilee Clinic Cancer Research Foundation. PHB was supported in part by a grant from the US-NIH (PO1-CA101944-P1). There are no conflicts of interests to report.
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Albertsson PA, Basse PH, Hokland M, et al. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603–609. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Hagenaars M, Ensink NG, Basse PH, et al. The microscopic anatomy of experimental rat CC531 colon tumour metastases: consequences for immunotherapy? Clin Exp Metastasis. 2000;18:189–196. doi: 10.1023/a:1006774602360. [DOI] [PubMed] [Google Scholar]
- 3.Kuppen PJ, van der Eb MM, Jonges LE, et al. Tumor structure and extracellular matrix as a possible barrier for therapeutic approaches using immune cells or adenoviruses in colorectal cancer. Histochem Cell Biol. 2001;115:67–72. doi: 10.1007/s004180000224. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Hokland ME, Bryant JL, et al. Tumor-localization by adoptively transferred, interleukin-2-activated NK cells leads to destruction of well-established lung metastases. Int J Cancer. 2003;105:512–519. doi: 10.1002/ijc.11119. [DOI] [PubMed] [Google Scholar]
- 5.Kim MH, Kitson RP, Albertsson P, et al. Secreted and membrane-associated matrix metalloproteinases of IL-2-activated NK cells and their inhibitors. J Immunol. 2000;164:5883–5889. doi: 10.4049/jimmunol.164.11.5883. [DOI] [PubMed] [Google Scholar]
- 6.Kitson RP, Appasamy PM, Nannmark U, Albertsson P, Gabauer MK, Goldfarb RH. Matrix metalloproteinases produced by rat IL-2-activated NK cells. J Immunol. 1998;160:4248–4253. [PubMed] [Google Scholar]
- 7.Zeng L, An S, Goetzl EJ. Selective regulation of RNK-16 cell matrix metalloproteinases by the EP4 subtype of prostaglandin E2 receptor. Biochemistry. 1996;35:7159–7164. doi: 10.1021/bi960036x. [DOI] [PubMed] [Google Scholar]
- 8.Kim MH, Albertsson P, Xue Y, Kitson RP, Nannmark U, Goldfarb RH. Expression of matrix metalloproteinases and their inhibitors by rat NK cells: inhibition of their expression by genistein. In Vivo. 2000;14:557–564. [PubMed] [Google Scholar]
- 9.Albertsson P, Kim MH, Jonges LE, et al. Matrix metalloproteinases of human NK cells. In Vivo. 2000;14:269–276. [PubMed] [Google Scholar]
- 10.Goda S, Inoue H, Umehara H, et al. Matrix metalloproteinase-1 produced by human CXCL12-stimulated natural killer cells. Am J Pathol. 2006;169:445–458. doi: 10.2353/ajpath.2006.050676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida Y, Migita K, Izumi Y, et al. The role of IL-18 in the modulation of matrix metalloproteinases and migration of human natural killer (NK) cells. FEBS Lett. 2004;569:156–160. doi: 10.1016/j.febslet.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Chuang SS, Kim MH, Johnson LA, et al. 2B4 stimulation of YT cells induces natural killer cell cytolytic function and invasiveness. Immunology. 2000;100:378–383. doi: 10.1046/j.1365-2567.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnatty RN, Taub DD, Reeder SP, et al. Cytokine and chemokine regulation of proMMP- 9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol. 1997;158:2327–2333. [PubMed] [Google Scholar]
- 14.Maenpaa A, Jaaskelainen J, Carpen O, Patarroyo M, Timonen T. Expression of integrins and other adhesion molecules on NK cells; impact of IL-2 on short- and long-term cultures. Int J Cancer. 1993;53:850–855. doi: 10.1002/ijc.2910530524. [DOI] [PubMed] [Google Scholar]
- 15.Yodoi J, Teshigawara K, Nikaido T, et al. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells) J Immunol. 1985;134:1623–1630. [PubMed] [Google Scholar]
- 16.Jonges LE, Albertsson P, van Vlierberghe RL, et al. The phenotypic heterogeneity of human natural killer cells: presence of at least 48 different subsets in the peripheral blood. Scand J Immunol. 2001;53:103–110. doi: 10.1046/j.1365-3083.2001.00838.x. [DOI] [PubMed] [Google Scholar]
- 17.Edsparr K, Johansson BR, Goldfarb RH, et al. Human NK cell lines migrate differentially in vitro related to matrix interaction and MMP expression. Immunol Cell Biol. 2009 doi: 10.1038/icb.2009.35. [DOI] [PubMed] [Google Scholar]
- 18.al-Atrash G, Kitson RP, Xue Y, Goldfarb RH. Cooperation of urokinase plasminogen activator and matrix metalloproteinases in NK cell invasion. In Vivo. 2000;14:565–570. [PubMed] [Google Scholar]
- 19.Albertsson P, Basse PH, Edsparr K, et al. Differential locomotion of long- and short-term IL-2-activated murine natural killer cells in a model matrix environment. Scand J Immunol. 2007;66:402–409. doi: 10.1111/j.1365-3083.2007.01956.x. [DOI] [PubMed] [Google Scholar]
- 20.Friedl P, Wolf K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem Soc Symp. 2003:277–285. doi: 10.1042/bss0700277. [DOI] [PubMed] [Google Scholar]