Abstract
Introduction
Innate immune responses likely contribute to synovial inflammation in rheumatoid arthritis (RA). Of the innate receptors implicated in RA, TLR3 activates several signaling cascades, including -interferon regulatory factors 3 and 7 (IRF), resulting in production of viral-stress IFN-inducible genes. The present study was designed to investigate the contributions of IRF3 and IRF7 to the type I IFN response as well as the expression of other cytokines, chemokines, and degradative enzymes in synoviocytes.
Methods
Fibroblast-like synoviocytes (FLS) were stimulated with poly (I-C) after transfection with IRF3 or IRF7 siRNA to knockdown transcription factor expression. Western blots, luciferase assay after transfection with reporter constructs, Q-PCR, and AP-1 DNA binding ELISA was performed to evaluate the role of IRF3 and IRF7 in poly (I-C)-induced signaling and synoviocyte gene expression.
Results
IRF3 and IRF7 knockdown showed that IRF3 regulates IFN-stimulated response element (ISRE) promoter activity as well as IFNβ, IRF5, IRF7, RANTES, IP-10, MCP-1, and MIP1α gene expression in response to poly (I-C). IRF7 knockdown modestly decreased a subset of genes and ISRE activity, although the results were not significant. Surprisingly, IRF3 knockdown almost completely blocked expression of additional genes in which the ISRE is not traditionally considered a dominant promoter site in FLS, including MMP3, MMP9, IL-6 and IL-8. We then investigated a possible role for IRF3 in c-Jun activation and AP-1 binding because its promoter site is present in all four of the non-IFN regulated genes. IRF3 deficiency significantly decreased AP-1 binding of activated c-Jun compared with control.
Conclusions
In contrast to immune cells, IRF3 rather than IRF7 regulates TLR3-mediated type I IFN responses in human synoviocytes. IRF3 activates IFN-response gene expression by increasing ISRE promoter activity. In addition, IRF3 regulates other cytokines, chemokines, and MMPs through a novel mechanism that involves c-Jun and the AP-1 promoter site. Because the signaling pathway modulated by IRF3 plays a crucial role in synoviocytes, targeting IRF3 represents a potential approach to suppress diverse mediators while limiting suppression of IRF7-mediated immune responses.
Keywords: Rheumatoid arthritis, signal transduction, transcription factors, interferon
Introduction
Innate immune responses play a critical role in cell activation and recruitment into the rheumatoid joint [1]. Toll-like receptor (TLR) recognition of viral and bacterial products can potentially contribute to this sequence of events. In fact, the gene expression profile in rheumatoid arthritis (RA) synovium reflects exposure to TLR ligands and displays characteristic features of the type I interferon (IFN) signature [2–5]. While the proximal mechanisms involved are not known, the IKK-related kinase IKKε appears to participate as a component of the key signaling pathway that transduces TLR3 activation [6]. However, IKKε can phosphorylate numerous substrates, and the downstream transcription factors that control expression of type I IFN-regulated genes in RA have not been defined. One possibility is that the interferon regulatory factor (IRF) family, especially IRF3 and IRF7, is responsible for the IFN signature in RA.
Activation of the TLR-IRF-type I IFN system could contribute to the pathogenesis of many autoimmune diseases, including SLE. The function and relative hierarchy of kinases and transcription factors that integrate innate responses after an extracellular stimulus is cell lineage dependent and varies with the type of stimulus. The synthetic TLR3 ligand poly (I-C) and viral infection activate the IKK-related kinases, resulting in phosphorylation, nuclear translocation, and dose dependent promoter binding of IRF3 and IRF7, NF-kB, and ATF2/cJun [7, 8]. Formation of a transcriptional complex, or enhanceosome, results in activation of pro-inflammatory and IFN-regulated gene expression. In murine embryonic fibroblasts and plasmacytoid dendritic cells, IRF7 is the master regulator of type I IFN immune responses [9]. Constitutive IRF3 mainly controls IFNβ expression while IRF7 is required not only for IFNα expression, but also production of the full IFN signature. Key cell-lineage specific responses to poly (I-C) stimulation of synoviocytes, macrophages, dendritic cells, and endothelial cells have been recently described and are more complex than noted in the murine cell systems [10].
Most data on TLR-dependent and independent signaling pathways are derived from murine knockout cells and immortalized cell lines, and does not necessarily reflect responses in primary human cells. We have begun dissecting the TLR3 signaling pathway in human fibroblast-like synoviocytes (FLS); initial studies showed that the IKKrelated kinase IKKε regulates IRF3 phosphorylation in cultured RA FLS [6]. While IRF3 is expressed in rheumatoid synovial tissue, the relative contribution of IRF3 and IRF7 to synovial gene expression is not known. The present study was designed to determine whether IRF3 or IRF7 is the primary regulator of type I IFN responses in FLS. In contrast to many other cell types, IRF3 is the dominant transcription factor in primary synoviocytes while the contribution of IRF7 is relatively modest. In addition, IRF3 regulates other cytokines, chemokines, and MMPs through a novel mechanism that involves c-Jun and the AP-1 promoter site.
Materials and Methods
Preparation of human FLS
Synovial tissue and FLS were obtained from patients after informed consent was obtained with osteoarthritis (OA) and RA at the time of total joint replacement or synovectomy as previously described [11]. Our ethics committee approved this protocol (071819) and all human research was performed in compliance with the Helsinki Declaration. The diagnosis of RA conformed to ACR 1987 revised criteria [12]. Synovium was minced and incubated with 1 mg/ml collagenase type VIII (Sigma Chemicals, St. Louis, MO) in serum-free RPMI 1640 (Gibco BRL, Grand Island, NY) for 1h at 37°C, filtered, extensively washed, and cultured in DMEM (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) (Gemini Bio Products, Calabasas, CA), penicillin, streptomycin, gentamicin, and glutamine in a humidified 5% CO2 atmosphere. Cells were allowed to adhere overnight and FLS were split at 1:3 when 70–80% confluent. FLS were used from passage 3 through 9 during which time they are a homogeneous population of cells (<1% CD11b positive, <1% phagocytic, and <1% FcγRII and FcγRIII receptor positive). FLS were cultured and used at 80% confluence. Cells were synchronized in 0.1% FBS for 24h before the addition of cytokines or TLR ligands.
Reagents
Monoclonal anti-IRF7 (sc-74472) and anti-actin Ab (sc-1616) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Dr. Michael David (UCSD La Jolla, CA) generously provided the IFNβ and polyclonal rabbit anti-IRF3 serum. IL-1 was purchased from R&D Systems (Minneapolis, MN). Poly I-C, peptidoglycan (PGN), and lipopolysaccharide (LPS) were obtained from Sigma-Aldrich (St. Louis, MO).
siRNA transfection
5×105 cells FLS (passages 4 to 6) were transfected with 5μg of IRF3, IRF7, or scramble (sc) control Smartpool small interfering RNA (siRNA; Dharmacon, Lafayette, CO, USA), using NHDF Nucleofector Kit according to the manufacturer’s instruction (Amaxa, Gaithersburg, MD, USA). Approximately 75–95% decrease in protein expression is achieved using this method. Transfected FLS were allowed to recover overnight, synchronized 24h, and then stimulated overnight with poly (I-C) prior to lysis on day 5 post-transfection.
Western blot analysis
Western blot was performed as described previously [13]. FLS were incubated with 0.1% FBS medium, TNFα (100 ng/ml), IL-1 (1 ng/ml), LPS (1 μg/ml), PGN (50 μg/ml), poly I-C (20 μg/ml), or IFNβ (1000 U/ml) for various time points up to 24h. Cells were washed with cold PBS, and protein was extracted using kinase lysis buffer (50 mM HEPES, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM MgCl2, 1.5 mM EDTA (pH 8.0), 20 mM β-glycerophosphate, 50 mM NaF, 1 mM Na3VO4, 10 μg/ml aprotinin, 1 μM pepstatin A, and 1 mM PMSF). The protein concentrations of tissue and FLS were determined using the Micro BCA protein assay kit (Thermo Scientific, Rockford, IL). Samples containing 50 μg of protein from cultured FLS or 75 μg of protein from synovial tissue were resolved via 15% SDS PAGE and transferred to a PVDF membrane. The membranes were blocked, incubated with primary Ab at 4°C overnight, followed by HRP conjugated secondary Ab for 1h. Proteins were visualized with chemiluminescence using Kodak X-AR film (Eastman Kodak, Rochester, NY).
Reporter gene assays
After siRNA transfection, FLS were incubated for 5 days and subsequently 5×105 cells were transfected with 1 μg of reporter plasmid DNA, ISRE-luc, which has five repeats of the ISRE sequence from ISG15 promoter and 0.1 μg of Renilla reniformis luciferase construct as internal control for transfection efficiency (kind gift of Dr. Michael David, UCSD, La Jolla, CA). After overnight incubation, transfected cells were stimulated with 20 μg/ml of poly (I-C) for 24h. Luciferase activity was measured using a dual luciferase assay kit (Promega, Madison, WI).
Quantitative real time PCR
After siRNA transfection, FLS were cultured in DMEM with 10% FBS at 37°C for 5 days. The cells were incubated in fresh media for 48h and subsequently serum starved (0.1% FBS/DMEM) for 48h. FLS were then treated with either medium or poly (I-C) (20 μg/ml). RNA isolation and RT-PCR were performed as previously described using TaqMan PCR analysis and the GeneAmp 7300 Sequence Detection System [14]. Forward and reverse primers as well as fluorogenic TaqMan FAM/TAMRA-labeled hybridization probes were used (Assays on Demand, Applied Biosystems). To control for sample cellularity, human GAPDH forward and reverse primers and labeled probe were included in separate PCR reactions. The threshold cycle C(t) was determined for each sample using GeneAmp software. Standard curves are generated by linear regression using log (C(t)) versus log (cell number). The cell equivalent (CE) number for samples was calculated using the standard curve. Data are expressed as the ratio between gene of interest CE and GAPDH CE, yielding the Relative Expression (RE).
Quantitative phospho-c-Jun binding AP-1 promoter site
After transfection with 5 μg of scramble control or IRF3 siRNA (Dharmacon) using the NHDF Nucleofector Kit (Amaxa), FLS were cultured for 2 days. On day 3, cells were synchronized in 0.1% FBS for 48h. On day 5, FLS were treated with 20μg/mL of poly (I-C) or 100ng IL-1 for 1h. After treatment, cells were harvested using the Nuclear Extraction Kit (Chemicon International) according to the protocol. Nuclear extracts were analyzed using the TransAM transcription factor ELISA (Active Motif) according to manufacturer’s instruction. This method can be used for quantitative analysis of transcription factor activation rather than phospho-c-Jun Western blot, EMSA, or reporter gene assay. Nuclear extract is added to an AP-1 oligonucleotide coated plate followed by phospho-c-Jun and secondary antibody incubation. The results are quantified by spectrophotometry and the ELISA can detect <1.25μg of nuclear extract/well.
Statistical Analysis
Statistics were generally performed using the paired Student’s t test. A comparison was considered significant if p<0.05.
Results
Activation of IRF3 and IRF7 in human FLS
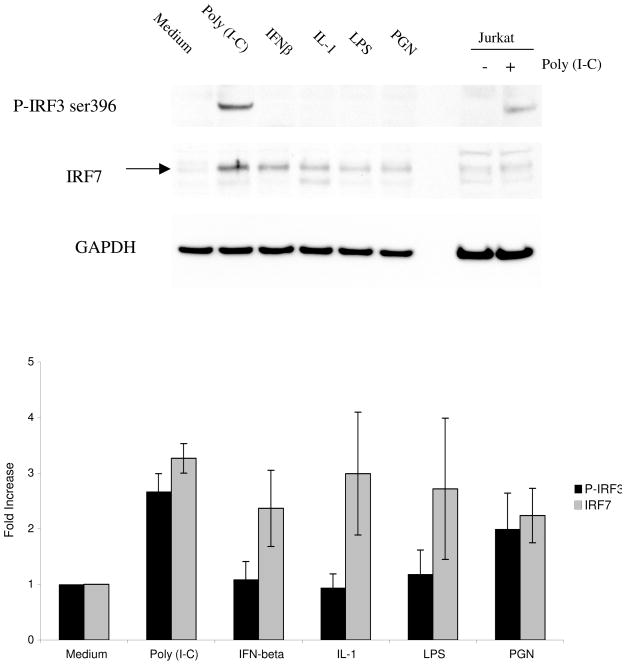
We previously showed that IKKε and IRF3 are constitutively expressed by cultured FLS and that IKKε can regulate IRF3 phosphorylation [6]. To characterize the relative functional hierarchy of IRF3 and IRF7 in synoviocyte innate immune responses, FLS were stimulated with cytokines or TLR ligands followed by Western blot analysis to detect IRF3 phosphorylation (Figure 1, top panel). Because IRF7 is inducible rather than constitutively expressed, we also measured total IRF7 protein expression. Quantification of P-IRF3 and IRF7 expression in FLS stimulated with each ligand is also shown in Figure 1 (bottom panel). Poly (I-C), IFNβ, IL-1, LPS, and PGN induced IRF7 expression in FLS, and poly (I-C) was the most potent. Based on these results, this synthetic TLR3 ligand poly (I-C) was used for subsequent studies of the type I IFN response.
Figure 1.
Western blot analysis of IRF3 phosphorylation and IRF7 induction. FLS were stimulated for 18h with poly (I-C), IFNβ, IL-1, LPS, or PGN. Lysates were then analyzed by Western blot using anti-P-IRF3 ser 396, anti-IRF7, and anti-GAPDH antibodies. Jurkat cell lysate was included as a positive control. Stimulation with poly (I-C) resulted in a significant increase in phosphorylation of IRF3 (2.67 ± 0.32, n=4). Induction of IRF7 was also increased by poly (I-C) stimulation (3.27 ± 0.27, n=4) and this TLR3 ligand was used for stimulation of the type I IFN response by cultured FLS. Top panel shows a representative Western blot and the bottom panel shows combined results for 3 separate FLS lines.
Kinetics of IRF activation in cultured human FLS. To determine the time course of IRF3 and IRF7 activation, we stimulated human FLS with poly (I-C) for up to 24h (Figure 2). Phosphorylation of IRF3 was detected within 2h of poly (I-C) exposure and persisted for 24h. IRF7 induction began within 4h and continued to increase during the 24h culture period. As anticipated, total IRF3 protein did not change significantly during the experiment.
Figure 2.
Time course of IRF activation in poly (I-C)-stimulated FLS. Cells were incubated with poly (I-C) for up to 24h and analyzed by Western blot analysis. Poly (I-C) increased phosphorylated IRF3 levels within 2–4h, which persists for at least 24h. IRF7 induction was detected between 4–6h and increased for at least 24h time. Total IRF3 and GAPDH levels were constant. Figure is representative of two independent experiments.
Targeted knockdown of IRF3 and IRF7
The relative contribution of IRF3 and IRF7 to the type I IFN response was evaluated by transfecting FLS with IRF3, IRF7, or IRF3+IRF7 (IRF3/7) siRNA or control siRNA (sc) followed by poly (I-C) stimulation. Western blot analysis confirmed effective knockdown of IRF3 and IRF7 protein expression. IRF3 siRNA decreased IRF3 without decreasing IRF7 protein levels below basal levels. Of interest, knockdown of IRF3, IRF7, or a combination of IRF3 and IRF7 prevented IRF7 induction. Decreased phosphorylation of IRF3 was observed in cultures treated with combined IRF3/7 siRNA or IRF3 siRNA alone, but not with IRF7 or control siRNA.
Role of IRF3 and IRF7 in ISRE promoter activity
The upstream binding of IRF3 and IRF7 to the ISRE in the promoter of the classic IFN-response gene ISG15 was evaluated using an ISRE luciferase reporter construct. ISRE promoter activity in poly (I-C) stimulated FLS was significantly lower in IRF3 and IRF3/7 deficient FLS (p<0.03, Figure 4, n=3 separate experiments). IRF7 siRNA modestly decreased ISRE activity, but the effect of IRF3 and IRF7 siRNA together was the same as IRF3 siRNA alone. These data demonstrate a primary role for IRF3 in ISRE transcriptional activity in human FLS stimulated with poly (I-C).
Figure 4.
Effect of IRF3 and IRF7 deficiency on ISRE promoter activity. IRF3, IRF7, IRF3+IRF7 (IRF3/7) or scrambled (sc) siRNA-treated FLS were co-transfected with an ISRE luciferase reporter construct. Synoviocytes were stimulated with poly (I-C) (20μg/ml) overnight and cell lysates were assayed for luciferase activity normalized to R. reniformis luciferase. FLS transfected with sc siRNA were used as control. ISRE promoter activity was decreased to baseline by IRF3 and IRF3/7 siRNA (p<0.03, n=3), but no additional effect was detected when comparing IRF3 and IRF3/IRF7 double knockdown FLS. A trend towards modest suppressive effect of IRF7 knockdown was observed but this was not statistically significant. These data confirm the key role of IRF3, with no additional decrease in promoter activity by IRF7 knockdown. The values are the mean ± SEM from 3 independent experiments.
Decreased IFN-regulated gene expression in IRF3 deficient synoviocytes
We then studied whether the differences in IRF3 and IRF7 transcriptional activity were reflected in TLR3- induced gene expression. IRF3, IRF7, and combined IRF3 + IRF7 siRNA knockdown was performed and cells were stimulated with poly (I-C). Q-PCR was used to measure IFNregulated genes IFNβ, IRF5, IRF7, RANTES, IP-10, MCP-1 and MIP1α mRNA levels. These genes are considered IFN-regulated because they are primarily controlled by the upstream ISRE although other promoter sites are present. Figure 5A shows that IRF3 deficiency profoundly decreased IFNβ, IRF5, IRF7, RANTES, IP-10, MCP-1 and MIP1α gene expression (90–100% inhibition; p<0.01). IRF7 deficiency decreased IRF7 mRNA levels as expected, but did not result in significant inhibition of other IFN-stimulated genes. Of interest, RANTES and IRF5 gene expression was not decreased by IRF7 knockdown. The combination of IRF3 + IRF7 knockdown was similar to IRF3 siRNA alone.
Figure 5.

Figure 5A. Inhibition of IFN-response gene expression by IRF3 knockdown. Q-PCR was performed to determine relative expression of IFN-regulated genes (ISRE is the dominant promoter element) after IRF3, IRF7, or IRF3+IRF7 (IRF3/7) siRNA knockdown. After transfection, cells were stimulated for 18h with poly (I-C) followed by quantitative analysis of IFNβ, IRF5, IRF7, RANTES, IP-10, MCP-1 and MIP1α mRNA. Percent inhibition for IRF siRNA compared with scrambled siRNA was calculated (see Material and Methods). IRF3 deficiency markedly decreased IFNβ, IRF5, IRF7, RANTES, IP-10, MCP-1 and MIP1α gene expression (p<0.04 for each gene, n=3 separate FLS lines). IRF7 inhibition decreased IRF7 expression levels but did not significantly decrease other IFN-regulated genes compared with IRF3.
Figure 5B. Inhibition of pro-inflammatory and MMP gene expression by IRF3 knockdown. Q-PCR was performed to determine relative expression of cytokine and MMP genes after IRF3, IRF7, or IRF3+IRF7 (IRF3/7) siRNA knockdown. Surprisingly, IRF3 inhibition decreased gene expression of poly (I-C)-induced MMP3 and MMP9, as well as IL-6 and IL-8 (p<0.03 for each gene, n=3 separate FLS lines), while IRF7 siRNA had minimal effect.
Decreased pro-inflammatory and MMP gene expression in IRF3 deficient synoviocytes
We then evaluated the contribution of IRF3 and IRF7 to expression of MMP3 and MMP9, and the cytokines IL-6 and IL-8, because of their role in inflammation and joint destruction in RA (Figure 6). The upstream regulatory regions of these genes contain multiple promoter sites. The dominant promoter element involved in MMP gene expression is AP-1 in most cell types including human FLS. IL-6 and IL-8 gene expression is more directly under the control of NF-κB. Interestingly, IRF3 inhibition decreased poly (I-C) induced expression of MMPs, IL-6, and IL-8 gene expression while the effect of IRF7 knockdown was not significant. Because we have previously demonstrated that IKKε, directly upstream of IRF3, can regulate production of MMPs in synoviocytes [15], we hypothesized that IRF3 contributed to MMP production through modulation of c-Jun binding AP-1.
Figure 6.
IRF3 inhibition decreases activated c-Jun binding to AP-1. We investigated a role for IRF3 in AP-1 activation because this promoter is present in all four of the genes (IL-6, IL-8, MMP3, MMP9) inhibited by IRF3 knockdown. IRF3 deficiency decreased AP-1 binding by 52% compared with control (n=3, p <0.02). The figure shows the mean ± SEM from 3 independent experiments.
IRF3 knockdown decreases activated phosphorylated c-Jun binding to AP-1
Because MMP3 and MMP9 were inhibited by IRF3 knockdown, we investigated a role for IRF3 in AP-1 promoter binding. AP-1 is the dominant promoter element involved in regulation of MMP gene expression. Transcription factor ELISA (TransAM) using an AP-1 oligonucleotide is a quantitative and sensitive method for measuring phosphorylated c-Jun binding to the AP-1 promoter site. This approach can be used for quantitative analysis of transcription factor activation rather than EMSA or reporter gene assay. IRF3 deficiency decreased poly (I-C)- induced AP-1 binding by 52% compared with control (n=3, p <0.02).
Discussion
TLR ligands can potentially activate viral and stress-inducible gene expression of chemokines and cytokines that promote inflammation, cell recruitment, and joint destruction in RA. These signaling pathways have been implicated in inflammatory arthritis, and the interferon signature induced by this pathway has been observed in diverse autoimmune diseases. In addition to the synovium, an interferon profile has been reported in peripheral blood cells of a subset of RA patients [16]. The relevance of this observation to disease activity and progression in RA is unknown, as the IFN pathway can be either detrimental or beneficial depending on the relative balance of IFNβ, IL-1RA, and pro-inflammatory chemokines [17]. To determine the contribution of this signaling cascade in RA, we previously examined how the IKK-related kinase IKKε controls distal transcription factors like IRF3 and c-Jun in human synoviocytes [6]. In the present study, we extended these observations by dissecting how two key IRFs, namely IRF3 and IRF7, contribute to the synoviocyte type I IFN response. These experiments identified IRF3, rather than IRF7, as a pivotal transcription factor in RA synoviocytes.
The specific TLR ligands or cytokines that activate the type I IFN response in RA synovium or peripheral blood cells have not been identified. While virus exposure or infection could participate, it is also possible that endogenous TLR ligands, necrotic debris, and cytokines known to be present in the rheumatoid joint contribute to synovial inflammation [18–20]. In other cell types such as murine embryonic fibroblasts (MEF) and transformed cell lines, poly (I-C) stimulation or viral infection results in IKK-related kinase IKKε or TBK1 activation of IRF3 phosphorylation, dimerization, nuclear localization, and DNA binding to the ISRE to produce IFNβ [21, 22]. Assembly of a transcription factor complex in the enhancer region, including c-Jun/ATF2, NF-kB, and IRFs, amplifies and expands IFN-stimulated gene expression [7, 8]. This initial response is followed by activation of IFNα/β receptor signaling, inducing IRF7 transcription [23]. This powerful amplification loop can proceed through the IKK-related kinases, especially IKKε [24]. For in vitro synoviocyte studies that could mimic the in vivo environment, we focused on the TLR3 ligand poly (I-C) because it was the most potent activator of IRF3 and IRF7 in cultured FLS. Previous synoviocyte studies indicate that IKKε regulates c-Jun activation of MMP expression and IRF3 induced transcription of IFNβ and RANTES [6, 15]. The sequence of events in RA synoviocytes is similar to other cell types because targeted inhibition of IRF3, IRF7, as well as IRF3 plus IRF7 blocked IRF7 induction. Thus, IRF7 induction requires IRF3 in primary human synoviocytes. No synergy is apparent when comparing IRF3 with the combination IRF3 and IRF7 siRNA.
IRFs bind to the ISRE and regulate transcription of IFN-stimulated genes that are expressed in rheumatoid joints, including IFNβ, RANTES, and IP-10, [2–4]. Many of these proteins contribute to cell recruitment in RA due to their chemotactic activity. IFNβ, however, might play a more complex role and could potentially suppress inflammation. Some investigators have suggested that IFNβ itself could be used as a therapeutic agent in RA because it decreases MMP, IL-1, and TNF production by synoviocytes [25]. Mice with collagen-induced arthritis (CIA) injected with fibroblasts expressing IFNβ have less severe disease and decreased bone and cartilage destruction [26]. However, a clinical trial using IFNβ in patients with RA showed minimal efficacy despite decreased synovial IL-1, IL-6, and MMP1 [27]. An alternative approach in a murine model combined IKKε deficiency with low dose “replacement” IFNβ, which amplified the anti-inflammatory effects of this pathway [17]. Thus, careful dissection of the TLR signaling pathways that regulate the type I IFN response and identification of the key regulatory IFN-response genes could shed light on news ways to enhance anti-inflammatory effects without markedly suppressing host defense.
We also evaluated the hierarchy of IRF3 and IRF7 in synoviocyte gene expression. In contrast to bone marrow derived cells and murine embryonic fibroblasts where IRF7 is paramount, IRF3 is the master regulator of the type I IFN response in human synoviocytes. One caveat is that IRF3 could potentially act by blocking IRF7 induction. However, this is unlikely because selective IRF7 knockdown had only a modest effect on expression of IFNstimulated genes, whereas IRF3 deficiency profoundly blocked all of these same genes. Of interest, IRF3 also controlled IRF5 expression. This particular IRF is interesting because polymorphisms have been associated with SLE [28]. However, the genetic contribution of IRF5 variants to RA is controversial [29, 30].
IRF3 deficiency also suppressed expression of some genes implicated in RA that are predominantly regulated by AP-1 (MMPs) or NF-κB (IL-8, IL-6) promoter elements rather than an ISRE site. As with the classical IFN response genes, IRF7 was less effective than IRF3 in the regulation of these cytokine and MMP genes. Although all three sites are present in the positive regulatory domains of IFN-stimulated genes and interact to amplify responses via formation of a transcription factor enhanceosome, we hypothesized that predominantly AP-1 regulated genes such as MMPs might be directly induced by IRF3 via activation of c-Jun. The quantitative binding of phosphorylated c-Jun to the AP-1 site was inhibited by IRF3 knockdown. The mechanism is uncertain, but could be due to the ability of type I IFN to activate IKKε. Our previous studies show that IKKε activates AP-1 by phosphorylating c-Jun, thereby initiating MMP transcription. The effect of IRF3 knockdown on IL-6 and IL-8 gene expression might also be a result of decreased AP-1 activation in the promoter elements of these genes.
Because IRF3 is constitutively expressed and involved in immediate antiviral responses, inhibition might impair early innate immunity. IRF7 is expressed in a more limited fashion and is induced in most cells after IFNβ production is initiated. IRF7 can also serve as a critical checkpoint in adaptive immune responses and antigen presentation. Thus, targeting the TLR signaling pathways and IFN signature can be complex and requires a detailed understanding of each component. Considering the potential pathogenic role of IFN in autoimmune disease and the delicate balance between anti- and pro-inflammatory effects, dissecting the IFN response could have important therapeutic and safety implications. The overall effect of blocking IRF3 might depend on cell lineage and microenvironment specific responses. Our data suggest that targeting IRF3 in synoviocytes could decrease synovial inflammation while sparing the critical functions of IRF7 in immune cells.
Conclusions
IRF3 regulates poly (I-C)-induced type I IFN responses in human synoviocytes by binding to the ISRE promoter site to increase IFN-regulated gene expression. IRF3 regulates other cytokines, chemokines, and MMPs through a novel mechanism that involves c-Jun and the AP-1 promoter site. Targeting synoviocyte IRF3 could suppress diverse mediators while limiting suppression of IRF7-mediated immune responses.
Figure 3.
Effect of siRNA knockdown on IRF3 and IRF7 protein expression. Cultured FLS were transfected with Smartpool control siRNA (sc), IRF3, IRF7, or IRF3 + IRF7 (IRF3/7). Three days later, cells were stimulated with poly (I-C) for 18 h. Western blot analysis shows knockdown of IRF3 and P-IRF3 and decreased IRF7 accumulation by IRF3 siRNA. IRF7 siRNA only decreased IRF7, with no effect on IRF3. Figure is representative of three independent experiments.
Acknowledgments
The work was supported by NIH grant K08 AR052800 (SES, TBK; manuscript preparation) and R01 AI067752 (GSF, TBK).
List of abbreviations
- AP-1
Activator protein 1
- CE
Cell Equivalent
- EMSA
Electrophoretic mobility shift assay
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- FBS
Fetal bovine serum
- FLS
Fibroblast-like synoviocyte
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenasen
- IKK
I kappa B kinase
- ISRE
IFN-stimulated response element
- IL
Interleukin
- IL-1RA
IL-1 receptor antagonist
- IFN
Interferon
- IRF
Interferon regulatory factors
- IP-10; CXCL-10
Interferon gamma induced protein 10
- LPS
Lipopolysaccharide
- MMP
Matrix metalloproteinase
- MCP-1
Monocyte chemotactic protein-1
- MEF
Murine embryonic fibroblast
- NF-κB
Nuclear factor-kappa-B
- PGN
Peptidoglycan
- poly I-C
Polyinosinic and polycytidylic acid
- Q-PCR
Quantitative polymerase chain reaction
- RE
Relative Expression
- RA
Rheumatoid arthritis
- RANTES
Regulated upon Activation, Normal T-cell Expressed, and Secreted
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SLE
Systemic lupus erythematosus
- TBK1
TANK binding kinase 1
- TNF
Tumor necrosis factor
- TLR
Toll-like receptor
Footnotes
Competing interests
The author(s) declare that they have no competing interests
Authors’ contributions
SES conceived the study, designed and coordinated the experiments, analyzed and interpreted the data, drafted and revised the manuscript. TBK acquired and analyzed data and contributed figures and methods to the manuscript. GSF participated in the design and coordination of the study and helped revise the manuscript. All authors have approved the final version of the manuscript to be published.
References
- 1.Brentano F, Kyburz D, Schorr O, Gay R, Gay S. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol. 2005;233(2):90–96. doi: 10.1016/j.cellimm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 2.van Holten J, Smeets TJ, Blankert P, Tak PP. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Ann Rheum Dis. 2005;64(12):1780–1782. doi: 10.1136/ard.2005.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haringman JJ, Smeets TJ, Reinders-Blankert P, Tak PP. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65(3):294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Vicuna R, Gomez-Gaviro MV, Dominguez-Luis MJ, Pec MK, Gonzalez-Alvaro I, Alvaro-Gracia JM, Diaz-Gonzalez F. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblastlike synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004;50(12):3866–3877. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- 5.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, Burdick MD, Pope RM, Strieter RM. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweeney S, Mo L, Firestein G. Antiviral gene expression in rheumatoid arthritis: role of IKK epsilon and interferon regulatory factor 3. Arthritis Rheum. 2007;56(3):743–752. doi: 10.1002/art.22421. [DOI] [PubMed] [Google Scholar]
- 7.Kim TK, Kim TH, Maniatis T. Efficient recruitment of TFIIB and CBP-RNA polymerase II holoenzyme by an interferon- enhanceosome in vitro. Proc Natl Acad Sci U S A. 1998;95(21):12191–12196. doi: 10.1073/pnas.95.21.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439–454. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, et al. IRF-7 is the master regulator of type-I interferondependent immune responses. Nature. 2005;434(7034):772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg A, Drexler S, Monaco C, Williams L, Sacre S, Feldmann M, Foxwell B. Key differences in TLR3/poly I:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood. 2007;110(9):3245–3252. doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]
- 11.Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990;86(6):1790–1798. doi: 10.1172/JCI114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.Hammaker DR, Boyle DL, Chabaud-Riou M, Firestein GS. Regulation of JNK1 by MEKK2 and MAP kinase kinase kinases in rheumatoid arthritis. J Immunol. 2004;172(3):1612–1618. doi: 10.4049/jimmunol.172.3.1612. [DOI] [PubMed] [Google Scholar]
- 14.Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5(6):R352–360. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweeney SE, Hammaker D, Boyle DL, Firestein GS. Regulation of c-Jun phosphorylation by the I kappa B kinase-epsilon complex in fibroblast-like synoviocytes. J Immunol. 2005;174(10):6424–6430. doi: 10.4049/jimmunol.174.10.6424. [DOI] [PubMed] [Google Scholar]
- 16.Olsen N, Sokka T, Seehorn C, Kraft B, Maas K, Moore J, Aune T. A gene expression signature for recent onset rheumatoid arthritis in peripheral blood mononuclear cells. Ann Rheum Dis. 2004;63(11):1387–1392. doi: 10.1136/ard.2003.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corr M, Boyle D, Ronacher L, Flores N, Firestein G. Synergistic benefit in inflammatory arthritis by targeting I kappaB kinase epsilon and interferon beta. Ann Rheum Dis. 2009;68(2):257–263. doi: 10.1136/ard.2008.095356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zare F, Bokarewa M, Nenonen N, Bergstrom T, Alexopoulou L, Flavell RA, Tarkowski A. Arthritogenic properties of double-stranded (viral) RNA. J Immunol. 2004;172(9):5656–5663. doi: 10.4049/jimmunol.172.9.5656. [DOI] [PubMed] [Google Scholar]
- 19.Roelofs MF, Joosten L, Abdollahi-Roodsaz S, van Lieshout AW, Sprong T, van den Hoogen FH, van den Berg WB, Radstake TR. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of tolllike receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52(8):2313–2322. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 20.Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52(9):2656–2665. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKK epsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300(5622):1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 23.Borden E, Sen G, Uze G, Silverman R, Ransohoff R, Foster G, Stark G. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6(12):975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenoever B, Ng S, Chua M, McWhirter S, García-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315(5816):1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- 25.Smeets TJ, Dayer JM, Kraan MC, Versendaal J, Chicheportiche R, Breedveld FC, Tak PP. The effects of interferon-beta treatment of synovial inflammation and expression of metalloproteinases in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43(2):270–274. doi: 10.1002/1529-0131(200002)43:2<270::AID-ANR5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.van Holten J, Reedquist K, Sattonet-Roche P, Smeets TJ, Plater-Zyberk C, Vervoordeldonk MJ, Tak PP. Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2004;6(3):R239–249. doi: 10.1186/ar1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Holten J, Pavelka K, Vencovsky J, Stahl H, Rozman B, Genovese M, Kivitz A, Alvaro J, Nuki G, Furst D, et al. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64(1):64–69. doi: 10.1136/ard.2003.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham R, Kozyrev S, Baechler E, Reddy M, Plenge R, Bauer J, Ortmann W, Koeuth T, González Escribano M, et al. Argentine and Spanish Collaborative Groups . A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38(5):550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- 29.Dieguez-Gonzalez R, Calaza M, Perez-Pampin E, de la Serna A, Fernandez- Gutierrez B, Castañeda S, Largo R, Joven B, Narvaez J, Navarro F, et al. Association of interferon regulatory factor 5 haplotypes, similar to that found in systemic lupus erythematosus, in a large subgroup of patients with rheumatoid arthritis. Arthritis Rheum. 2008;58(5):1264–1274. doi: 10.1002/art.23426. [DOI] [PubMed] [Google Scholar]
- 30.Sigurdsson S, Padyukov L, Kurreeman F, Liljedahl U, Wiman A, Alfredsson L, Toes R, Rönnelid J, Klareskog L, Huizinga T, et al. Association of a haplotype in the promoter region of the interferon regulatory factor 5 gene with rheumatoid arthritis. Arthritis Rheum. 2007;56(7):2202–2210. doi: 10.1002/art.22704. [DOI] [PubMed] [Google Scholar]