Abstract
Only little is known about how cells coordinately behave to establish functional tissue structure and restore microarchitecture during regeneration. Research in this field is hampered by a lack of techniques that allow quantification of tissue architecture and its development. To bridge this gap, we have established a procedure based on confocal laser scans, image processing, and three-dimensional tissue reconstruction, as well as quantitative mathematical modeling. As a proof of principle, we reconstructed and modeled liver regeneration in mice after damage by CCl4, a prototypical inducer of pericentral liver damage. We have chosen the regenerating liver as an example because of the tight link between liver architecture and function: the complex microarchitecture formed by hepatocytes and microvessels, i.e. sinusoids, ensures optimal exchange of metabolites between blood and hepatocytes. Our model captures all hepatocytes and sinusoids of a liver lobule during a 16 days regeneration process. The model unambiguously predicted a so-far unrecognized mechanism as essential for liver regeneration, whereby daughter hepatocytes align along the orientation of the closest sinusoid, a process which we named “hepatocyte-sinusoid alignment” (HSA). The simulated tissue architecture was only in agreement with the experimentally obtained data when HSA was included into the model and, moreover, no other likely mechanism could replace it. In order to experimentally validate the model of prediction of HSA, we analyzed the three-dimensional orientation of daughter hepatocytes in relation to the sinusoids. The results of this analysis clearly confirmed the model prediction. We believe our procedure is widely applicable in the systems biology of tissues.
Keywords: agent based model, image processing and analysis, mathematical tissue modeling, systems biology, morphogenesis
The liver is the main metabolic organ which removes drugs and toxins from the blood. One of the outstanding features of the liver is its capacity to regenerate hepatocyte loss of up to 70% of its mass within a relatively short period of time (1). Hepatic parenchyma is organized in repetitive functional units called liver lobules, which besides its main constituents, hepatocytes, consists of sinusoidal endothelial cells, Kupffer, stellate, and bile duct cells. Branches of the hepatic artery and portal vein guide blood to the periportal regions of the lobules (Fig. 1A). From there, it flows through microvessels, the sinusoids, along hepatocyte columns that are lined with endothelial cells (generally known as sinusoidal cells), and drains into the central vein. This complex lobule architecture ensures a maximal exchange area between blood and hepatocytes in healthy liver. In liver disease, such as hepatocellular cancer, the contact surface between hepatocytes and sinusoidal cells decreases and contributes to compromised liver function (Fig. 1F). Recent research on liver regeneration has focused on molecular pathways and the mechanisms involved (2). Little is known about how cells coordinately behave to restore the complex functional lobule architecture. What are the fundamental mechanisms underlying this complex regeneration process? In the present study, we analyzed liver regeneration in mice after intoxication with CCl4. CCl4 causes hepatocytes close to the central vein to die (Fig. 2B) since only these cells express CYP2E1, which metabolically activates CCl4 to the toxic entity (3). This pattern of toxicity is similar to that caused in humans by an overdose of acetaminophen. Nevertheless, after only about 10 d this central necrotic lesion is closed and the lobule architecture is completely restored (Fig. 2D). To shed light on the underlying processes, we established a three-step procedure based on confocal laser scans visualizing hepatocytes and sinusoidal cells (Fig. 1B), image processing and 3D tissue reconstruction (Fig. 1 C–E), and quantitative mathematical modeling (Fig. 3 and Movies S1, S2, S3, S4, S5, and S6). Our procedure uses three parameter types: (lobule) architectural parameters to quantify the static liver lobule, (regeneration) process parameters to quantify the regeneration process, and (mathematical) modeling parameters to characterize the mathematical simulation model. We combined architectural and process parameters to set up a detailed mathematical computer model of liver lobule regeneration after toxic damage. For determination of the process parameters, we complemented conventional techniques, such as BrdU incorporation, with techniques of processing and analyzing experimentally obtained 3D confocal images. This enabled us to extract quantitative information that would otherwise be inaccessible, such as the 3D spatial-temporal proliferation pattern of hepatocytes and the contact area between hepatocytes and sinusoidal cells during the regeneration process (Fig. 3F). We identified possible mechanisms underlying the observed regeneration process by analyzing a wide range of mathematical model variants within plausible physiological model parameter ranges, followed by a quantitative comparison of the simulation results with the experimental observations, using the same process parameters for both experiments and simulations. Finally, we used the mathematical model to guide further experiments by predicting the most informative experiments to select the most appropriate mechanisms underlying regeneration. Using this strategy, we identified a mechanism, “hepatocyte-sinusoid alignment” (HSA), whereby daughter cells from hepatocyte division align along the closest sinusoid. Model simulations and experiments demonstrated that HSA is a key mechanism necessary for the regeneration of the functional architecture of the liver lobule that cannot be replaced by alternative mechanisms. Quantitative spatio-temporal mathematical modeling of tissue organization represents a generic way to merge information from different sources to synergistically obtain quantitative and qualitative insights into tissue organization processes.
Fig. 1.
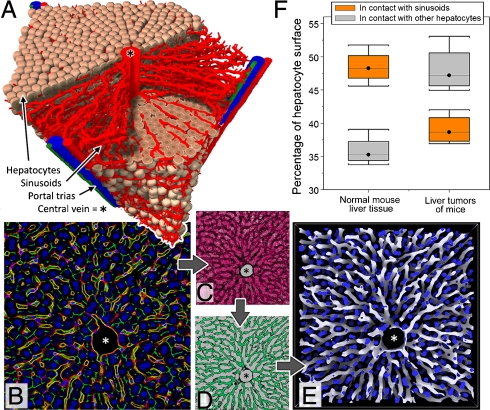
(A) Concrete liver lobule inferred from experimental data by the image processing chain shown in (B)–(E) and successive image analysis. Reconstructed lobules served as an initial state for the mathematical model. (B) A typical image obtained by confocal microscopy after adaptive histogram equalization filtering. Blue: DAPI (hepatocyte nuclei); yellow: ICAM + DPPIV (sinusoids); red: ICAM; green: DPPIV. (C) Effect of generalized erosion filtering (all red pixels will be removed). (D) Effect of generalized dilatation filtering (all green pixels are added). (E) Result of image processing chain in three dimensions. Blue: Hepatocyte nuclei; white: sinusoids. Note the complex architecture that links the periportal zone with the central vein in the middle of the lobule. (F) Fraction of the surface area of hepatocytes in contact with sinusoids (orange) and other hepatocytes (gray) in normal liver tissue and liver carcinomas. Details in SI Appendix.
Fig. 2.
Representative examples of mouse liver lobules visualized under light microscopy. (A) Control, (B) 2 d, (C) 4 d, and (D) 8 d after administration of CCl4, illustrating the emergence and regeneration of the central dead cell area. The examples shown in (A)–(D), employing a suboptimal peroxidase block, allowed an automated differentiation between the paler central dead cell area (B2, green striped area) and the darker surviving hepatocytes, while, at larger magnification, BrdU-positive and BrdU-negative cells could still be clearly distinguished. Hand-drawn lines in (A)–(D), (A2) and (B2) show the approximate extension of the liver lobules. (A2) Central veins were identified by immunostaining for GS. The process parameters for quantification of the regeneration process were (E) distribution of BrdU-positive cells in a liver lobule. Three mice were used per time point, namely, 0, 1, 2, 3, 4, 8, and 16 d after administration of CCl4. At least two liver lobules were analyzed per mouse: (F) average hepatocyte density, (G) area of central necrosis, and (H) hepatocyte-sinusoid contact area over time.
Fig. 3.
Regeneration in the simulation model, starting with a representative liver lobule. (A)–(C) partly show cross sections (compared to Fig. 1A) of model simulations: (A) simulation result from model 1 after 10 d, (B) simulation result from model 2 after 10 d, and (C) illustration of the regeneration process (after t = 0, 1, 2, 4, and 10 d) using model 3 (Movies S1–S3). (D)–(F) A quantitative comparison of experimental data with each model: (D) average hepatocyte density, (E) area of central necrosis, and (F) hepatocyte-sinusoid contact area.
Results
Three-Dimensional Reconstruction of the Liver Lobule.
In order to establish a quantitative spatial-temporal model of the regenerating liver lobule, we first reconstructed and quantitatively described its architecture. For this purpose, we used confocal laser scans of approximately 150 µm thick liver tissue slices. Sinusoidal cells appear red after staining with ICAM (intercellular adhesion molecule) antibodies; whereas the apical side of hepatocytes appears green after staining with DPPIV (Dipeptidylpeptidase IV) antibodies (Fig. 1B). Based on the confocal laser scans (SI Appendix), the full 3D structure of the sinusoidal network was reconstructed using filtering, segmentation, and morphological restoration steps (Fig. 1 C–E and SI Appendix). The same confocal laser scans have been used to determine the position of hepatocyte nuclei after staining with DAPI (Fig. 1E). From the image processing and analysis steps, we obtained statistical distributions for the architectural parameters. These were the average vessel diameter and density (including branching lengths that quantify the sinusoidal network and the position), volume, density, and shape of the hepatocytes (SI Appendix). In order to capture variations among different liver lobules, we studied 26 lobules from different mice. Additionally, the results were validated using transgenic mice in which differentiation between hepatocytes and sinusoidal cells was based on EGFP expression under the control of an albumin/alpha-fetoprotein (Alfp)-promoter, and sinusoidal cells were visualized by CD31 immunostaining (SI Appendix). From the obtained architectural parameter distributions, we generated a representative liver lobule as an initial configuration for the mathematical model (Fig. 3C).
Quantification of the Destruction and Regeneration Process.
Next, we defined process parameters to quantify the regeneration process. These were (i) the spatial BrdU incorporation pattern within the liver lobule as a measure for cell proliferation, (ii) the number of cells in a lobule section as a measure of the lobule mass, (iii) the area of the central necrosis, and (iv) the hepatocyte-sinusoid contact area, defined as the percentage of the hepatocyte surface that is in contact with an adjacent sinusoid as a measure of lobule architecture. These process parameters were determined from light microscopy images and confocal laser scans 0, 1, 2, 3, 4, 8, and 16 d after intoxication with CCl4 (1.6 g/kg body weight). The BrdU incorporation pattern was used as an input parameter to determine the proliferation pattern in our mathematical model. The central necrotic area was clearly distinguished from the surviving hepatocytes and was maximal 1–2 d after CCl4 administration (Fig. 2G), at which time the average hepatocyte density within the lobule was minimal (Fig. 2F). BrdU incorporation into hepatocytes peaked 2–3 d after CCl4 administration (Fig. 2E). The distribution of proliferating hepatocytes over the lobule was not homogeneous and preferentially occurred in the hepatocyte layers next to the dead cell area (Fig. 2E). The order of cell layers was determined and visualized in Fig. 2B2 (for details, see SI Appendix). The pericentral hepatocyte marker, glutamine synthetase (GS), transiently decreased; an effect detected by mRNA expression, immunostaining and enzyme activity analysis (Figs. S1–S3). This demonstrates that the dynamics of destruction and regeneration of the pericentral hepatocytes is in agreement with previously reported observations (1, 4). The number of macrophages increased in the necrotic zone and was concomitant with an increase in CD68 mRNA (Fig. S4). This may explain the rapid disappearance of the dead cell mass. A number of observations were in agreement with a destruction and regeneration process. These included a transient decrease of ATP content (Fig. S5), as well as albumin, CYP3A11, and BSEP (ATP binding cassette, subfamily B, member 11) mRNA expression (factors responsible for differentiated liver functions); a transient increase of AFP (alphafetoprotein) and ubiquitin mRNA expression (Fig. S1); and also the macroscopic appearance of the analyzed livers (Fig. S6). Within 8 d, the central necrotic area was closed (Fig. 2 A–D and G) and the average liver lobule hepatocyte density restored (Fig. 2F). As soon as the central dead cell areas were regenerated it became difficult to histologically identify the central veins, which were visible by GS immunostaining in neighboring slices (Fig. 2A2). It is important to note that the structure of the sinusoidal network remained essentially unaffected by CCl4 (Fig. 4F). In order to quantify the liver lobule microarchitecture, we measured the hepatocyte-sinusoid and the hepatocyte-hepatocyte contact areas. This measurement was validated by comparing healthy liver and liver carcinoma tissues (Fig. 1F). In normal liver, 35.8 ± 2.3% (mean ± standard deviation) of the hepatocyte surface was in contact with other hepatocytes and 48.5 ± 2.5% with sinusoids. In liver carcinomas, the contact areas were 48.1 ± 3.6% (with hepatocytes) and 39.1 ± 2.3% (with sinusoids), illustrating the significant (unpaired t-test: p = 0.0015) decrease in hepatocyte-sinusoid contact and a corresponding increase of hepatocyte-hepatocyte-contact in liver tumors. Since both measures are complementary, we considered only the hepatocyte-sinusoid contact area at which the exchange of metabolites between blood and hepatocytes occurred. Also, during the regeneration process after CCl4 damage, the hepatocyte-sinusoid contact transiently decreased with a minimum at day 2 and subsequent recovery up to day 16 (Fig. 2H).
Fig. 4.
Sinusoidal cells survive in the central dead cell area of the liver lobule after CCl4 poisoning and activate the tie-2 promoter. (A) Constructs of the triple transgenic tie-2-reporter mice. (B) Liver tissue of an untreated tie-2-reporter mouse. Green fluorescence (EGFP) indicates positive tie-2 promoter activity in the endothelial cells of a vein (white arrow in upper right image). Sinusoidal cells are visualized by CD31 immunostaining (light red in the lower left image) and nuclei by DAPI (blue in the lower right image). The merged picture (upper left image) demonstrates that endothelial cells of the vein, but not the sinusoidal cells, express EGFP (yellow). (C) and (D) Two days after CCl4 administration, some of the sinusoidal cells start to express EGFP. (C) EGFP green fluorescence and (D) green, red, and blue merged fluorescence. The central dead cell area is characterized by loss of nuclei and increased red background fluorescence. A substantial fraction of the sinusoidal cells within the central dead hepatocyte area survives and starts to express EGFP as a reporter of tie-2-promoter activity. 3D reconstructed lobule (E) before and (F) after CCl4 administration. As hepatocytes (blue) die, the sinusoids (gray) remain largely intact.
Establishment of the Spatial-Temporal Simulation Model.
Having measured 3D changes in liver structure during and after liver damage, we established a mathematical model of a single liver lobule simulating the 16 d process after treatment with CCl4. The basic units of the model are the single hepatocytes and the sinusoids. We modeled each hepatocyte as an individual homogeneous, isotropic elastic and adhesive object capable of migration, growth, division, and death. The interactions among hepatocytes, between hepatocytes and sinusoids, and hepatocytes and extra-cellular matrix were modeled using a previously validated force model (5). It includes central forces between cells resulting from adhesion by cell surface receptors and repulsion from cell compression and deformation. The hepatocyte movement was modeled by a stochastic equation of motion for the position of each hepatocyte. The equation included all forces exerted on that hepatocyte at each point in time as well as a random term mimicking the hepatocyte micromotility. Each sinusoid of the sinusoidal network was modeled as a chain of linked spheres characterized by its extensibility. The ends of the sinusoidal network were fixed at the central vein and the periportal field. Its movement was modeled similarly as for hepatocytes but with no micromotility. Our mathematical model was parameterized by measurable biophysical and cell-biological parameters (for details, see SI Appendix). Moreover, we considered a possible influence of morphogenes transported either via blood into the liver lobules or secreted by the necrotic cells close to the central vein. We iteratively developed our final model which was driven by direct comparisons with the process parameters in Fig. 3 D–F. A detailed description of the spatial-temporal simulation model, the used variants, and the model parameters can be found in SI Appendix. We considered two starting configurations: (i) a representative liver lobule generated by averaging the architectural parameters of 26 liver lobules, and (ii) a concrete liver lobule reconstructed from a specific confocal dataset to avoid possible artifacts that may arise from averaging. For the representative initial configuration shown in Fig. 3C, the hepatocyte-sinusoid contact area was 51 ± 1.2% of the hepatocyte surface which was close to the experimental value of 48.5 ± 2.5%. Note that these parameters cannot be directly tuned as they are fully determined by the architectural parameters.
Initial analyses started with a model variant (model 1) that was successfully used in previous studies to quantitatively mimic the growth dynamics of growing cell populations in vitro (6). In model 1, we assumed random orientation of cell division, an absence of morphogen influencing the direction of cell movement, and an unspecific homogeneous isotropic adhesion of hepatocytes to other hepatocytes (Fig. 3A and Movie S1). Despite the fact that this model was in agreement with the experimental findings regarding the average hepatocyte density (Fig. 3D), it did not explain the experimentally observed dynamics of the regeneration process, since closure of the central necrotic lesion was too slow (Fig. 3E). Furthermore, the hepatocyte-sinusoid contact area did not agree with the experimental data (Fig. 3F). We verified this finding by running simulations over a wide range of physiological parameters modifying cellular micromotility, hepatocyte-hepatocyte and hepatocyte-sinusoid adhesion, hepatocyte-hepatocyte and hepatocyte-matrix friction, and changed the biophysical properties of hepatocytes and sinusoids. For example, strongly increased micromotility resulted in detachment of hepatocytes migrating individually into the necrotic lesion (SI Appendix). However, a detachment of single cells from the regeneration front was not observed in our experiments. Therefore, the micromotility must not exceed a threshold value beyond which hepatocytes detach and migrate individually into the lesion. We concluded that the lesion cannot be closed at the necessary speed without leading to this detachment of single hepatocytes, in the absence of a mechanism that directs hepatocyte migration into the necrotic zone. We tested the ability of morphogen and mechanical force gradients to direct migration of the hepatocytes towards the necrotic area.
In addition, we introduced anisotropic cell-cell adhesion effects since hepatocytes are polar, with an apical or bile canalicular side oriented towards neighboring hepatocytes and a basolateral blood side oriented towards sinusoids. We modified cell-cell adhesion in the model such that adjacent polar hepatocytes only formed adhesive bonds at their apical sides (SI Appendix). The best data fit was obtained with a model (model 2) that integrated polar hepatocytes with a micromotility that was biased in the direction of the necrotic area and thereby directed cell migration. Model 2 (Fig. 3B and Movie S2) was in agreement with the experimental observations regarding hepatocyte density (Fig. 3D) and successfully mimicked the regeneration dynamics (Fig. 3E). A bias in the micromotility may be caused by a local mechanical or morphogen gradient, as long as both affect a layer thickness of only 2–5 cells at the edge of the necrotic lesion. We found that if all hepatocytes in a lobule were affected, the lobule architecture would be distorted. We modeled the influence of gradients caused by cytokine secretion by dead or dying hepatocytes from the central necrotic region, or cytokine transport via the blood. However, none of those model variations was able to successfully restore the lobule microarchitecture (Fig. 3F). After 16 d, the representative model 2 showed a hepatocyte-sinusoid contact area of only 37.1 ± 1.1% which was significantly lower than the experimental situation (48.5 ± 2.5%).
Hepatocyte Alignment Along Sinusoidal Cells Is a Key Mechanism to Restore Liver Microarchitecture.
Because neither model 1 nor model 2 were able to fully explain the experimentally observed data, we incorporated a further mechanism into our model, namely, HSA. As already mentioned, in the process of HSA, daughter hepatocytes from cell division align themselves along the closest sinusoid, such that the line connecting the centers of the two daughter cells is parallel to the local orientation of the closest sinusoid. The first experimental evidence that sinusoids may serve as an aid to orientation of regenerating hepatocytes came from our tie-2-reporter mice. Sinusoidal cells survive after administration of CCl4, even in the central region of the lobule where almost all hepatocytes die. However, since sinusoidal cells are very thin they may easily be overlooked in the central dead cell mass when paraffin slices are prepared and stained by conventional techniques (Fig. S7). We first noticed their presence in the central dead cell mass using tie-2-reporter mice where EGFP is expressed under control of a tie-2 promoter (Fig. 4A and SI Appendix). A relatively high fraction of sinusoidal cells, especially in the dead cell area, expressed EGFP (Fig. 4 C and D and Fig. S8). This result shows that the sinusoidal cells in the dead hepatocyte area survive, but may be stressed and begin to show tie-2 promoter activity, which is known to be involved in vessel remodeling (7). In order to visualize the state of the sinusoidal network, we immunostained sinusoidal cells with ICAM antibodies and reconstructed the network in healthy livers 2 d after CCl4 administration. Although the sinusoidal network showed some degree of destruction, the basic network structure remained intact (Fig. 4 E and F). Analysis of the numbers of hepatocytes and sinusoidal cells showed that the majority of sinusoidal cells survived, even in the central area where almost all hepatocytes were destroyed by CCl4 (Fig. S9). This prompted us to study the hypothesis that sinusoidal cells may play a role during the regeneration process and to include the HSA mechanism into our model. As a result, we found model 3 (Fig. 3C and Movie S3) to be in excellent agreement with all experimental observations, including hepatocyte density (Fig. 3D) and regeneration dynamics (Fig. 3E). Furthermore, the lobule architecture was restored after 16 d and the hepatocyte-sinusoid contact area was 50.4%, corresponding to the experimental situation (48.5 ± 2.5%) (Fig. 3F). Fig. 3C illustrates a typical computer simulation with model 3. In summary, our model simulations strongly suggested that HSA may be the key mechanism in regeneration of the liver architecture. From a sensitivity analysis, our model predicts that the alignment of daughter cells along the closest sinusoid must occur within a maximum of 2 h after cell division.
Experimental Validation of Hepatocyte-Sinusoid Alignment.
To further enhance our model, we experimentally tested the prediction of the mathematical model by determining the degree of alignment of hepatocytes after cell division along sinusoids. For this purpose, we reconstructed the 3D sinusoidal network for resting hepatocytes (BrdU-negative) and hepatocytes after cell division (BrdU-positive) using confocal laser scans (Fig. 5D). BrdU-positive nuclei appeared as green fluorescence; whereas, cell borders appeared red due to phalloidine staining. We applied an experimental design whereby BrdU was injected 48 h after CCl4 administration, when hepatocyte proliferation was close to its maximum. Livers were prepared at time intervals between 8 h and 14 d after BrdU injection (for detailed design, see Fig. S10). Two-dimensional analysis of paraffin slices suggested that daughter cells were aligned in the direction of the sinusoid (Fig. 5 A–C). However, in 2D analyses, the result may be compromised by the choice of the cutting plane. Therefore, we reconstructed and analyzed the full 3D structure of the lobules and identified all pairs of BrdU-positive neighboring hepatocytes. For each of these pairs, we calculated the line connecting the cell centers and determined the angle between that line and the tangent to the adjacent sinusoid. The closer this angle is to zero, the better the alignment. Eight hours after BrdU injection (the earliest analyzed time period), the majority of daughter cells showed a good alignment with the neighboring sinusoid (Fig. 5E and Fig. S11). The simulation result with model 3 showed an excellent agreement with the experimentally observed angle distribution, while in models 1 and 2, the orientation angle was uniformly distributed in [0,π/2] (Fig. 5E).
Fig. 5.
Experimental validation of HSA. (A) Immunohistochemistry staining in light microscopy. BrdU-positive nuclei are dark brown. (B) Confocal microscopy image, green: BrdU-positive cells; blue: nonproliferating hepatocytes; red: lectin (cell boundaries; sinusoids). Note the pair of BrdU-positive cells indicated by the white arrow is oriented in parallel to the neighboring sinusoid indicated by a yellow arrow. (C) 3D reconstruction of two daughter cells that are oriented in the direction of the neighboring sinusoid. (D) 3D distribution of BrdU-positive cells and sinusoids. The inset shows the connecting line of daughter hepatocytes (red) and their orientation (angle α) with regard to the closest sinusoid (blue line). (E) Density-distribution for α in experiments and models 1–3.
Discussion
Development, architecture and function of tissues depend on interactions between cells that can vary in time and space (8). Such interactions occur primarily by direct contact or secretion of soluble factors. In particular, liver function and dysfunction depends on its microarchitecture. Blood flows through the sinusoids thereby coming into contact with hepatocytes before it flows out into the central vein (Fig. 1A). Quantitative analysis of the liver lobule microarchitecture suggests that, during evolution, an optimal sinusoidal architecture has formed to ensure an efficient exchange between blood and hepatocytes. Liver function is compromised if the hepatocyte-sinusoid contact area decreases. Obviously, the two most abundant cell types of the liver, hepatocytes and sinusoidal cells, are crucial for the maintenance of liver microarchitecture. However, analysis of hepatocyte-sinusoid interactions and their influence on liver microarchitecture is experimentally challenging. Conventional techniques are insufficient in describing 3D spatial-temporal processes; therefore, there were no techniques available to quantify liver microarchitecture. As an alternative, we established a process chain that utilized the synergies from combined experimental assays, image analysis, and direct spatial-temporal modeling. As a starting point for modeling, we reconstructed liver lobules from confocal laser scans such that the position of all individual hepatocytes and sinusoidal cells, as well as all further relevant information on lobule architecture, were correctly captured. We introduced architectural parameters to quantify lobule mass and structure. The architectural parameters served to define the initial state of our mathematical model. In order to quantify the regeneration process after CCl4 induced necrosis of hepatocytes close to the central vein, and to permit a quantitative comparison with the simulation results of our mathematical model, we introduced a number of process parameters. These parameters were experimentally determined in regenerating mouse liver, covering a period of 0.5–16 d after treatment with CCl4, and included a measure of (i) the spatial-temporal pattern of cell proliferation, (ii) the average liver lobule hepatocyte density, (iii) the area of the necrotic lesion, and (iv) the liver lobule microarchitecture, namely, the hepatocyte-sinusoid contact area reflecting liver function. Using model simulations, we have demonstrated that if any of these parameters had not been taken into account, we would have failed to correctly identify the key mechanisms involved in liver regeneration after CCl4 intoxication. The first parameter (i) served as an input parameter and—together with our abstract but still realistic description of a cell—ensured that the average lobule hepatocyte density was restored [parameter (ii)]. However, if cell migration was completely dictated by physical interaction forces, the cells would accumulate in the periportal zone and the lesion would not be closed (Movie S1). The lesion is only closed if hepatocytes actively migrate towards the necrotic zone [parameter (iii)]. Indeed, several lines of evidence suggested that dead or dying hepatocytes caused surviving hepatocytes to migrate in their direction: (I) Time-lapse videos of cultured mouse hepatocytes demonstrated that viable hepatocytes were attracted by dead hepatocytes (Movie S1 and S2), (II) filopodia extended several micrometers in front of the hepatocytes at the edge of the dead cell area (SI Appendix), and (III) some hepatocytes show stress fiber formation, as evidenced by phalloidine staining, similar to hepatocytes in vitro showing a high scattering activity (Fig. S12). We also included the known polarity of hepatocytes into the model by assuming polar hepatocyte-hepatocyte adhesion and considered no adhesion with sinusoids as adhesion was found to slow down regeneration. However, none of the mechanisms resulted in correct hepatocyte alignment. They formed local double-cell, instead of single-cell, columns by pushing apart adjacent sinusoids, thereby increasing the hepatocyte-hepatocyte contact area at the expense of the hepatocyte-sinusoid contact area.
The simulated tissue architecture was in agreement with the experimentally obtained data only when we introduced a unique mechanism i.e. the alignment of daughter hepatocytes in the direction of the closest sinusoid (HSA) a so-far unrecognized process. Importantly, by using a model parameter sensitivity analysis within all model variants, we could show that HSA could not be substituted by including any other likely mechanisms into the model. Therefore, the model unambiguously predicted that HSA must take place and that complete regeneration is not possible without HSA. In order to experimentally validate the model prediction of HSA, we reconstructed and analyzed the 3D orientation of daughter hepatocytes in relation to the sinusoids. The results of this analysis (Fig. 5E) confirmed the model prediction.
As previously already recognized, sinusoidal cells are central to triggering hepatocyte proliferation (9–12). An important mechanism of CCl4 toxicity is that it causes a greater than 5-fold increase of hepatocyte growth factor (HGF) in sinusoidal cells, leading to increased proliferation of hepatocytes (10). In addition to HGF, IL-6 and TNF-alpha are also secreted by sinusoidal cells and contribute to the proliferative stimulus (12). The same cells also secrete the mito-inhibitor, transforming growth factor-beta1, which, after a spectacular phase of hepatocyte proliferation, terminates liver regeneration (1). Because of the influence of sinusoidal cells on hepatocyte proliferation, we speculated whether cytokines secreted by sinusoidal cells might also explain HSA. Some explorative experiments did indeed support this hypothesis. When hepatocytes and sinusoidal cells were cocultured under the time-lapse microscope, hepatocytes were attracted by sinusoidal cells and tended to maximize the hepatocyte-sinusoidal contact area (Movies S3, S4, S5, and S6 and Fig. S13). This is plausible because HGF does not only induce proliferation but also serves as a chemoattractant for hepatocytes (1) and thus may provide a mechanism that contributes to the proposed HSA. In this case, the hepatocyte alignment along the sinusoid should not occur during but subsequent to cell division. In order to test this hypothesis, we performed pilot experiments to study the orientation of mitotic spindles by staining tubulin in 2D slices from the same liver samples as those analyzed for daughter cell orientation. In contrast to the above described BrdU-positive daughter cells, the mitotic spindles were not systematically aligned in the direction of the sinusoids (Fig. S14). This data suggests that although the orientation of the mitotic spindles of hepatocytes may be random, the daughter cells still realign themselves in the direction of the closest sinusoid within a short period of time. An analysis of mitotic spindle 3D orientation was challenging and is currently under investigation. However, we were able to mimic the mechanism in computer simulations by replacing the alignment of dividing cells along the closest sinusoid in model 3 with the two following submechanisms: (1) cell division in a random direction, corresponding to a random orientation of the mitotic spindle, and (2) attraction of hepatocyte cells by a short-range morphogen secreted by the sinusoids. We found that morphogen-induced attraction of hepatocytes by sinusoids could explain HSA if it is additionally assumed that the reestablishment of hepatocyte polarity occurs after cell division. Without reestablishment of polarity, hepatocytes formed columns with at least two cell layers between the sinusoids, induced by local energy minima.
In conclusion, we have shown that HSA represents a so-far unrecognized mechanism which is essential for the restoration of liver microarchitecture. It will be interesting to investigate the role of HSA in liver diseases such as cirrhosis and hepatocellular carcinoma where microarchitecture is also compromised.
Materials and Methods
Experiments.
A detailed description of the applied experimental techniques is given in the SI Appendix. A standard protocol with CCl4 was applied to induce liver damage in male C57BL/6N mice. Tie-1 and Alfp-Cre transgenic reporter mice were used to visualize sinusoidal cells and hepatocytes, respectively. Immunostaining and confocal laser scanning microscopy were performed according to published techniques (SI Appendix).
Image Processing and Analysis.
In order to reconstruct and analyze the sinusoidal network from confocal images a complex image processing chain was applied. We used adaptive histogram equalization (AHE), morphological operators and a medial axis transform-like process to geometrically represent the sinusoidal network as an undirected graph in 3D that allowed us to investigate properties of the sinusoidal network. A similar image processing chain that included AHE, median filtering, and cell shape reconstruction based on Voronoi space decomposition was shown to lay a solid foundation to investigate hepatocyte properties. For details see SI Appendix.
Mathematical Modeling.
Our model included hepatocytes, sinusoids, the central vein, and the periportal triads, but does not take into account size and morphology variations between individual hepatocytes, nonparenchymal cell types, such as stellate, Kupffer, and bile duct cells. Cell division is separated into G1, S, and G2-phases, during which a cell grows and doubles its volume, and mitosis where the cell deforms at a constant volume until two daughter cells have emerged. For the diffusion, secretion, and dissociation of the morphogenes, a reaction-diffusion equation was used. For details see SI Appendix.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. G. Schütz and Professor U. Deutsch for providing the Alfp-mice and tie-2 driver mice, respectively. This study was supported by the BMBF (Federal Ministry of Education and Research) network HepatoSys (projects 0313081A and 0313081F), and the (European Union) projects CancerSys (HEALTH-F4-2008-223188) and Passport (No: 223894). We dedicate this work to our co-author Alexander Bauer, who passed away shortly before his PhD-defense, to bear in remembrance his valuable contribution.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0909374107/-/DCSupplemental/.
References
- 1.Michalopoulos GK, DeFrances M. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Juskeviciute E, Vadigepalli R, Hoek JB. Temporal and functional profile of the transcriptional regulatory network in the early regenerative response to partial hepatectomy in the rat. BMC Genomics. 2008;9:527–541. doi: 10.1186/1471-2164-9-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gómez MID, et al. Liver nuclear and microsomal cyp2e1-mediated metabolism of xenobiotics in rats chronically drinking an alcohol-containing liquid diet. Toxicol Ind Health. 2006;22:367–374. doi: 10.1177/0748233706070982. [DOI] [PubMed] [Google Scholar]
- 4.Hoehme S, et al. Mathematical modelling of liver regeneration after intoxication with ccl4. Chem Biol Interact. 2007;168:74–93. doi: 10.1016/j.cbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Chu YS, et al. Johnson-Kendall-Roberts theory applied to living cells. Phys Rev Lett. 2005;94:028102. doi: 10.1103/PhysRevLett.94.028102. [DOI] [PubMed] [Google Scholar]
- 6.Drasdo D, et al. On the role of physics in the growth and pattern formation of cellular systems: What can we learn from individual-cell-based models? J Stat Phys. 2007;128:287–345. [Google Scholar]
- 7.Saharinen P, et al. Angiopoietins assemble distinct tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 8.Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci USA. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeCouter J, et al. Angiogenesis-independent endothelial protection of liver: Role of vegfr-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 10.Maher JJ. Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91:2244–2252. doi: 10.1172/JCI116451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ping C, et al. Hepatic sinusoidal endothelial cells promote hepatocyte proliferation early after partial hepatectomy in rats. Arch Med Res. 2006;37:576–583. doi: 10.1016/j.arcmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425–431. doi: 10.1016/s1084952102001301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.