Abstract
Background:
Individuals with spinal cord injury (SCI) develop premature cardiovascular disease. Regular exercise reduces the incidence and symptoms of cardiovascular disease in able-bodied individuals; these salutary effects of exercise have not been documented in persons with SCI.
Objective:
To evaluate the effects of functional electrical stimulation leg cycle ergometry (FES-LCE) exercise training on platelet aggregation and blood coagulation in persons with SCI.
Participants:
Subjects (n = 14) with stable chronic (>1 year) paraplegia (T1–T10) or tetraplegia (C4–C8).
Methods:
Blood samples were collected before and after the first and eighth sessions (2 sessions per week for 4 weeks) of FES exercise.
Results:
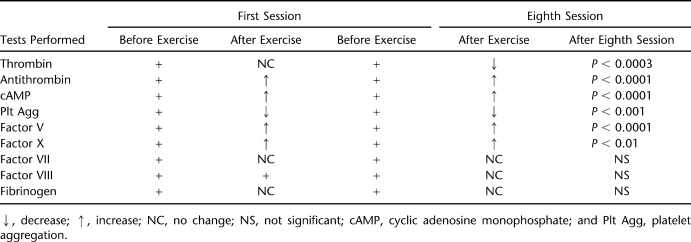
Platelet aggregation was inhibited by 20% after the first session and by 40% (P < 0.001) after the eighth session. Thrombin activity was unchanged after the first session (10.7 ± 0.85 s to 10.43 ± 0.56 s) and decreased after the eighth session (12.5 ± 1.98 s to 11.1 ± 1.7 s; P < 0.0003). Antithrombin III activity increased after the first (103.8% ± 8.9% to 110% ± 6.9%; P < 0.0008) and eighth sessions (107.8% ± 12.1% to 120.4% ± 13.1%; P < 0.0001). Cyclic adenosine monophosphate increased after the first (9.9% ± 2.5% to 15.8% ± 3%; P < 0.001) and eighth sessions (17.8% ± 4.2% to 36.5% ± 7.6%; P < 0.0001). After the eighth session, factors V and X increased significantly (88% ± 27% to 103% ± 23%, P < 0.0001; 100% ± 40% to 105% ± 7%, P < 0.01, respectively); factors VII and VIII and fibrinogen did not change significantly. A significant reduction in platelet activation/aggregation was demonstrated in response to FES-LCE. The decrease in thrombin level was caused by the simultaneous increase in antithrombin activity.
Conclusion:
These findings provide new insight into the potential protective effects of FES-LCE against the risk of cardiovascular disease.
Keywords: Spinal cord injuries, Functional electrical stimulation, Leg cycle ergometry, Cardiovascular disease, Tetraplegia, Paraplegia, Paralysis, Exercise regimen, Hypercoagulability, Blood coagulation, Thrombin, Antithrombin
INTRODUCTION
In the United States, 13.6% (31.3 million) of the total noninstitutionalized population report some limitation in physical activity as a result of chronic health conditions; 3.8% (8.8 million) are unable to perform any major activity, ∼6% (13.6 million) have restricted major activity, and 4.4% (10.1 million) have some limitation of activity (1). Immobilization or prolonged inactivity as a result of any condition, including spinal cord injury (SCI), is associated with hyperinsulinemia and impaired glucose tolerance; both factors are associated with increased cardiovascular risk (2,3).
In cross-sectional studies, persons with SCI have been placed at the lowest end of the fitness range. Persons with paraplegia have been shown to be poorly conditioned and are only marginally more fit than those with tetraplegia. Nearly 25% of otherwise healthy young subjects with paraplegia fail to achieve a fitness level (peak oxygen consumption after arm ergometry) that is sufficient for independent living (4–7). It is highly unlikely that this level of fitness can improve without some form of exercise regimen (8). Of the 179,000 persons with SCI in the United States, 40% are at least 45 years old and 1 in 4 has lived 20 or more years after injury. SCI is no longer a static medical condition but a medical condition associated with constantly changing needs, abilities, and limitations (9–12).
A clustering of risk factors associated with cardiovascular disease (CVD) has been demonstrated in individuals with SCI, including dyslipidemia (low levels of high-density lipoprotein and high triglyceride levels), hypertension, diabetes mellitus, and hyperinsulinemia. Additional risk factors include a high prevalence of cigarette smoking and physical inactivity and the fact that the majority of the people with SCI are men. However, these risk factors account for only half of the observed risk for CVD. The emerging role of acute thrombosis in the etiology of CVD suggests additional novel risk factors that could mediate risk associated with SCI (3). The deleterious effects of SCI-induced paralysis might be reduced by the introduction of well-designed exercise programs at an appropriate interval after injury.
Able-bodied individuals exercise for many reasons. Exercise is performed to increase muscle mass, strength, and endurance to recondition and reduce the risk of CVD by decreasing blood coagulation factors (13), lowering body fat, reversing insulin resistance, and reducing physical stress. In the short term, exercise has been shown to reduce blood coagulation and platelet aggregation (14–16). Persons with SCI have limited options for exercise that may be attributed not only to external factors but also lack of desire. Therefore, it is imperative that the selected exercise activities effectively reduce the risks of physical dysfunction without increasing the risks of physical injury (whether muscular or skeletal).
Although many forms of exercise have been shown to be beneficial in persons with SCI, for those unable to perform voluntary motor movement exercise, functional electrical stimulation (FES) exercise induced by electrically stimulated contraction has been used to train individual body segments and/or invoke cycling movements, with or without arm movement (17,18). Electrical stimulation of local muscle sites increases muscle mass and circulation and favorably alters muscle fiber composition (19). In tetraplegia, electrically stimulated cycling improves fitness and lower-extremity circulation, associated with reversal of cardiac muscle atrophy (20,21). Arm and wheelchair ergometry have been shown to increase arm endurance, decrease CVD risks associated with hyperlipidemia, improve body composition with increased lean mass and decreased fat mass, and improve whole-body uptake of insulin-stimulated glucose transport in the quadriceps muscles (22,23). In addition, there are reports of increased expression of GLUT 4, a muscle/adipose tissue–specific glucose transport protein, and decreased platelet aggregation (5,14,15,23).
Individuals with SCI have been reported to have premature and accelerated CVD (2,24). An epidemiologic study showed that CVD was the leading cause of death more than 30 years after injury (46% of all deaths) and among those above 60 years of age (35% of all deaths) (3,25–27). Currently, the knowledge is very limited regarding the effect of exercise on hemostatic factors and fibrinolysis in subjects with chronic SCI.
Biochemical markers for hypercoagulability are associated with increased risk for CVD among able-bodied populations (28). Hypercoagulability may result from elevated levels of fibrinogen, Factor VII, or von Willebrand factor or a decreased fibrinolytic system, including elevated levels of plasminogen activator inhibitor-1 antigen and decreased tissue plasminogen activator (15,29). Hypercoagulability may partially explain the increased risk of CVD in people with SCI.
As in able-bodied individuals, regular physical exercise of moderate intensity decreases platelet aggregability by increasing levels of tissue plasminogen activator and decreasing plasminogen activator inhibitor-1 activity (15,29). In addition, the exercise-associated improvement in the lipid profile and the reduction in fat mass may improve glucose utilization, increase sensitivity of platelets, which decreases platelet aggregability and blood coagulation, and increase fibrinolysis (13–15). Thus, it can be hypothesized that physical exercise has a beneficial impact on blood coagulation and fibrinolysis.
Because prolonged inactivity following SCI has been shown to be associated with an increased prevalence of cardiovascular risk factors (3), the purpose of the present study was to determine the effects of functional electrical stimulation leg cycle ergometry (FES-LCE) on blood coagulation factors in persons with SCI (1,24).
MATERIALS AND METHODS
Subject Selection
Subjects (n = 14) with stable chronic (>1 year) paraplegia (T1–T10) or tetraplegia (C4–C8) enrolled in an FES-LCE training program (electrically induced lower-extremity ergometer manufactured by Therapeutic Alliance Inc, New York, NY) were recruited for study participation. Two subjects were terminated from the study; one subject fractured her femur, and another developed a pressure ulcer (neither event was related to the FES-LCE program).
The subjects were asked to abstain from aspirin and other medications known to affect platelet aggregation for 2 weeks prior to blood donation. None of the subjects were prescribed oral anticoagulants.
FES-LCE was performed for 8 sessions. Before and after exercise (sessions 1 and 8), blood samples were collected in sodium citrate (13 mM final concentration). The study was approved by the Institutional Review Boards for Clinical Research, Mount Sinai School of Medicine, and the James J. Peters Veterans Affairs Medical Center, Bronx, New York.
Exercise Prescription
Computerized FES allowed active LCE exercise of limbs paralyzed by upper motor neuron lesions. Previously untrained subjects with SCI who required FES for leg movement were recruited for this study. Individuals with SCI participated in an FES-LCE exercise program for 2 sessions per week for 4 weeks. The lower-extremity ergometer used in the study employs 6 channels of electrical stimulation delivered to 12 carbon surface electrodes. The stimulation was applied to the quadriceps, hamstrings, and gluteal muscle groups bilaterally with a constant current of 0 to 132 mA at a sequential frequency of 30 Hz and a pulse width of 350 microseconds, resulting in stationary bicycling.
The initial workload was zero and resistance was increased in increments up to 0.0125 kilopond based on the subject's tolerance. Each exercise session consisted of multiple exercise bouts of discontinuous training, terminating in a total exercise time of up to 30 minutes, with a 5-minute rest period between bouts. Each exercise bout was preceded by a 2-minute warm-up and followed by a 2-minute cool-down. During the warm-up and cool-down, the legs were passively pedaled while receiving low-level electrical stimulation. Fatigue was defined as the point at which the subjects could no longer maintain a minimum pedaling speed of 35 rpm.
Blood samples were obtained at baseline (first session) and at the completion of training (eighth session), before and after each training session. Future studies will address the duration of the effects of FES-LCE exercise after termination of exercise training.
Collection of Blood
Blood (20 mL) was drawn from each subject and anticoagulated by mixing 9 volumes of blood with 1 volume of 0.13 M sodium citrate. Platelet-rich plasma (PRP) was prepared by centrifuging blood at 200g for 15 minutes at 23°C. Platelet-poor plasma was prepared by centrifuging PRP at 3,000g for 15 minutes at 23°C (30).
Platelet Aggregation
Platelet aggregation was determined by placing 0.5 mL PRP in a silicon-coated cylindric curette containing a Teflon-coated magnetic stirring bar. Aggregation of platelets was initiated by adding adenosine diphosphate (2 to 4 µM) as an aggregating agent in an aggregometer (Chronolog, Broomall, PA) and stirring the PRP at 37°C at 1,200 rpm (31,32).
Determination of Coagulation Factors, Thrombin, Antithrombin III, and Fibrinogen Activity in Plasma
Hemostatic factor analysis was performed in blood anticoagulated with sodium citrate (13 mM final concentration) and kept on ice until centrifugation and separation of plasma at 3,000g for 20 minutes at 4°C. Plasma was separated from the red blood cells and aliquots were frozen and stored at −70°C for subsequent analysis (32).
Screens for factors V, VII, VII, X, thrombin, antithrombin III (AT III), and fibrinogen were run on an automated IL ACL Advance #2 instrument. Factors (V, VII, VII, X) in the coagulation cascade (intrinsic and extrinsic pathway) were tested. Prothrombin time (PT) measures the extrinsic pathway and was tested by adding tissue factor (TF; here, thromboplastin was used) and Ca2+, whereas the intrinsic pathway (or activated partial thromboplastin time (APTT)) was initiated with a substance that mimics platelet factor (microsilica glass beads and cephalin) and Ca2+ was added. This test activates Factor VII, which starts the common pathway; the instrument detects the start of fibrin formation. When all reagents were assembled in the test tube, the time to form a clot was recorded in seconds.
The percent activity of a coagulation factor in the experimental plasma was compared with normal plasma. This was performed by running a single factor assay. A calibration curve was created with each run, using the Calibration Plasma tool (Instrument Laboratory Company, Lexington, MA).
To assay thrombin and fibrinogen, the reagents were reconstituted with the recommended water and placed into 0.5-mL sample cups. The specimens were placed in the sample tray and analysis was started.
Assay of Cyclic Adenosine Monophosphate
Washed platelets (2 × 109) in Tyrode's buffer (pH 7.5) containing 5.0 mM magnesium chloride (MgCl2) were incubated with 10 mM theophylline for 2 minutes, in a total volume of 200 µL, at 23°C. Prostaglandin E1 (1.0 µM) then was added to the cell suspension. After incubation for an additional 1 minute, ice-cold trichloroacetic acid (5%) and 1.0 N hydrochloric acid were added to the mixture. The concentration of cyclic adenosine monophosphate (cAMP) in the extract was determined (30).
Statistical Analysis
Results are reported as means ± SDs. The statistical analysis for normally distributed data was performed with paired Student t test with Bonferroni correction for multiple comparisons to test differences between the groups. Differences were considered significant at P < 0.0125.
RESULTS
Effect of FES-LCE Exercise on Thrombin and Antithrombin III
Thrombin
After the first session of FES-LCE exercise, plasma thrombin level decreased marginally from baseline value of 10.7 ± 0.85 s to 10.43 s ± 0.56 s (not significant). The basal level of thrombin was within the normal range. Pre-exercise plasma thrombin activity increased by 20% from session 1 to session 8 (Figure 1). After the eighth session, thrombin activity decreased significantly, from 12.5 ± 1.98 s to 11.1 ± 1.7 s (P < 0.001) (Table 1, Figure 1).
Figure 1.
Effect of FES exercise training on thrombin. Blood samples for thrombin levels were collected before and after the first and eighth FES exercise sessions. Results are expressed as means ± SDs.
Table 1.
The Effect of FES-LCE Exercise on the Various Thrombogenic Factors and Platelet Function in Subjects Trained Twice a Week for 30 Minutes for 4 Weeks (8 Sessions)
Antithrombin III
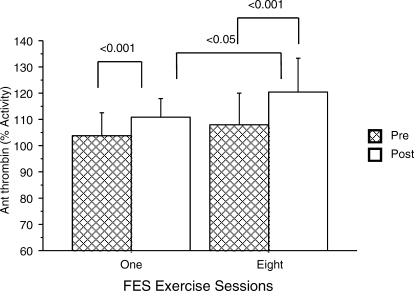
The basal level of AT was within the normal range (80%–130%). Following FES-LCE exercise, in parallel with the aforementioned decrease in thrombin activity, there was a concomitant increase in AT. After the first session of FES-LCE exercise training, AT activity increased from 103.8% ± 8.9% to 110% ± 6.9% (P < 0.001); AT activity increased after the eighth session, from 107.8% ± 12.1% to 120.4% ± 13.1% (P < 0.001) (Table 1, Figure 2). There was no significant change in the pre-exercise values. AT activity was significantly increased after the eighth session compared with after the first session.
Figure 2.
Effect of FES exercise on AT activity. The percent activity of AT III was determined before and after the first and eighth FES exercise sessions for comparison. AT activity post–FES exercise was compared after the first and eighth sessions. Results are expressed as means ± SDs.
Effect of FES-LCE Exercise on Fibrinogen
Fibrinogen was assayed because it has been identified as an independent cardiovascular risk factor. Fibrinogen levels did not increase significantly after FES-LCE exercise training. After the first session of FES-LCE training, the fibrinogen level increased slightly from the basal level of 410 ± 78 mg/dL to 425 ± 97 mg/dL (change not significant). Normal levels of fibrinogen vary from 150 to 400 mg/dL. After the eighth session of FES-LCE exercise, the fibrinogen level had decreased slightly from 439 ± 104 mg/dL to 427 ± 65 mg/dL (change not significant) (Tables 1 and 2).
Table 2.
Effect of FES-LCE Exercise Training on Blood Coagulation Factors VII, VIII, and Fibrinogen
Effect of FES-LCE Exercise Training on Blood Coagulation Factors
Before the initiation of FES-LCE exercise training, basal plasma levels of factors V, VII, VIII, and X were determined to be 96% ± 19%, 109% ± 23%, 140% ± 24%, and 97% ± 5%, respectively (Table 2, Figures 3 and 4). (The normal plasma levels of factors V, VII, VIII, and X range from 60% to 150%).
Figure 3 and 4.
Determination of activity of coagulation factors V and X. Results are expressed as means ± SDs.
After the first session of FES-LCE exercise, plasma levels of factors, V, VII, VIII, and X were determined to be 99% ± 18%, 109% ± 27%, 149% ± 25%, and 98% ± 6%, respectively (changes not significant) (Table 2, Figures 3 and 4). After the eighth FES-LCE exercise session, factor V and factor X increased significantly from 88% ± 27% to 103% ± 23% (P < 0.0001) and 100% ± 40% to 105% ± 7% (P < 0.01), respectively (Figures 3 and 4). The activity of plasma factors VII and VIII did not change significantly after the eighth session of FES-LCE exercise and were determined to vary from 112% ± 24% to 113% ± 27% and 136% ± 7% to 137% ± 10%, respectively (Table 2).
Effect of FES-LCE Exercise Training on cAMP
After the first session of FES exercise, the cAMP level in platelets increased, from 9.9% ± 2.5% to 15.8% ± 3% (P < 0.001). Basal cAMP levels vary from 2 to 4 picomol/108 platelets. After the eighth session of FES-LCE exercise, the cAMP levels in platelets were significantly increased, from 17.8% ± 4.2% to 36.5% ± 7.6% (P < 0.001) (Figure 5). Pre-exercise and postexercise levels of platelet cAMP were significantly higher at the eighth session compared with the first session (Figure 5).
Figure 5.
Effect of FES exercise on cAMP. The formation of cAMP was determined by adding 100 nM PGI2 to platelet suspensions. All comparisons (eg, before vs after exercise, after the first and eighth sessions, before exercise at each time point, and after exercise at each time point) were significant. Results are expressed as means ± SDs.
Effect of FES-LCE Exercise Training on Platelet Aggregation
Platelet aggregation after the first session of FES-LCE exercise was not significantly inhibited. However, adenosine diphosphate–induced aggregation of platelets was significantly inhibited by 40% (P < 0.001) after the eighth session of FES-LCE exercise.
DISCUSSION
The results presented herein demonstrate that subjects with SCI had a significant reduction in platelet activation after their first session of FES-LCE exercise. Platelet hyperactivity, among other factors, stimulates atherosclerosis and may lead to CVD in persons with SCI (30). Previously, it had been demonstrated that the platelets from subjects with SCI developed resistance to the inhibitory effect of prostaglandin I2 (PGI2) in controlling platelet-stimulated thrombin generation, thereby increasing excess thrombin production and the release of platelet-derived growth factor (PDGF, a cytokine) (33–37). Thrombin and PDGF are atherogenic mitogens and should be controlled, because if they are not inhibited by the normal counterregulatory mechanism(s), atherosclerosis would be expected to be accelerated (35).
Thrombin is the most potent agonist of platelet aggregation; it promotes platelet activation, via activation of the dual protease-activated receptors (PAR) PAR 1 and PAR 4, on the platelet, proceeding through the actions of Gi-proteins that are inhibitory to cAMP (38,39). Thrombin has an essential role in blood coagulation by activating factor XI, factor V, and factor VIII; positive feedback of the end products of this pathway accelerates the production of thrombin. Additionally, thrombin converts fibrinogen to fibrin and plays a critical role in the development of arterial thrombosis. However, there are no therapeutic strategies that effectively target PAR 1 and PAR 4 receptors (39). Thrombin is also a potent mitogenic agent for arterial smooth muscle cells, inducing the proliferation of human mesangial cells that encodes the mRNA synthesis of PDGF, and it has been implicated in atherosclerotic plaque formation (33,34). The effects of thrombin are mediated through thrombin's interaction with platelets and inhibited by PGI2 binding to specific receptors on the platelet surface, which activates membrane adenylate cyclase and increases cAMP, leading to the inhibition of platelet function (30,39). Intraplatelet cAMP was shown to be significantly increased after the first and eighth FES-LCE exercise sessions. These values were also higher pre- and postexercise at the eighth session compared to those at the first session.
Basal levels of PDGF in SCI plasma have previously been shown to be 3 times higher than normal levels; release of platelet-stimulated thrombin and PDGF from SCI platelets was not inhibited by the PGI2-stimulated increase of cAMP formation, suggesting that in nonexercising individuals with SCI, the potential for thrombin generation was significant and not directly counterbalanced by normal regulatory mechanisms (36). Physical training has been shown to reverse endothelium dysfunction by releasing arachidonic acid metabolites, especially PGI2, from contracting muscles; it also produces increased levels of the vasodilators PGE2 and PGI2 while reducing the vasoconstrictor thromboxane A2 in the interstitial fluid of the muscles in non-SCI individuals (37,38,40,41). The increase in platelets cAMP (pre-exercise vs postexercise training) after the first and eighth sessions of FES-LCE exercise training in subjects with SCI was presumably caused by increases in PGI2 production, which would inhibit thrombin generation. Exercise training has been reported to improve glucose utilization and enhance insulin action in individuals with SCI (29), and insulin has been shown to increase the level of platelet PGI2 and inhibit thrombin generation (35,36).
Furthermore, FES-LCE exercise induced a significant decrease in thrombin activity with a concomitant increase in AT activity. AT III inhibits thrombin and other enzymes involved in the clotting cascade. AT remarkably reduces infarction volume in mice (41). The physiologic target proteases of AT are those of the intrinsic coagulation system, namely the activated forms of factor X (Xa), factor IX (IXa), factor VII (VIIa), factor XI (XIa), factor XII (XIIa), and factor II (thrombin) (IIa). AT III has a potent inhibitory effect on the proinflammatory and procoagulant processes (42–44). After FES-LCE exercise, the increase in AT generation diminished the potential for activation of blood coagulation factors VII, VIII, X, and thrombin, as well as reducing fibrin formation that would enhance blood clot dissolution, thereby further diminishing the outcomes of thrombosis.
AT exerts anti-inflammatory activity by promoting endothelial release of PGI2 (45), a potent vasodilator and inhibitor of platelet aggregation (31,45,46). In individuals with SCI, 8 sessions of FES-LCE exercise training inhibited platelet aggregation by 40% through an increase in platelet cAMP activity, without a concomitant activation of blood coagulation. FES-LCE exercise, therefore, may be beneficial in controlling an enhancement in platelet activation that may lead to a thrombotic episode.
Although after the eighth session, and before the bout of exercise, the thrombin level was increased by about 20% over the baseline value; this was expected because exercise training is associated with modest increases in thrombin levels (14–16). However, it should be appreciated that despite the modest increase, the thrombin levels remained within the normal range. Although it may not be intuitive, especially if one is focused on the absolute values for thrombin, that coagulation had been favorably modified after FES exercise training; this is strongly suggested from the highly favorable response of platelet aggregation, which was significantly reduced at the eighth session before exercise, regardless of previous AT III levels.
Factor V has a dual function in procoagulation and anticoagulation pathways (47). In individuals with SCI, after the eighth session of FES-LCE exercise training, Factor V activity increased significantly, acting as a cofactor to reduce Factor VIII activity and thrombin generation. In addition, a significant increase in Factor V and Factor X after FES-LCE exercise may suggest that, on the platelet surface, a Factor Va-Xa complex was formed and resulted in increased prostaglandin (PGI2) synthesis, inhibiting platelet aggregation. As previously shown, a 2% increase in Factor X inhibits thromboxane A2 synthesis in platelets, and this inhibition requires Factor V (47).
Factor VII (tissue factor) has been associated with coronary heart disease and plays a vital role in the initial activation of the coagulation cascade (extrinsic pathway) when it complexes with TF from a leaking or ruptured atherosclerotic plaque. The present evidence suggests that the extrinsic pathway is critical to the initiation of fibrin formation (40,42). In one report, in persons with SCI, the plasma procoagulant activities of Factor VII and fibrinogen concentrations were significantly increased, demonstrating the increased activity of the extrinsic and common pathways at baseline (48). These data suggest that SCI may, via as-yet unknown mechanisms, lead to the alteration of the extrinsic and common coagulation pathways and several factors of the hemostatic and fibrinolytic systems (16,49–51). The effects of physical exercise in 56 patients after myocardial infarction before and after 4 weeks of training showed that the levels of Factor VII before treadmill test had decreased significantly (by 10.6%) (50). However, Factor VII levels were unchanged and remained normal after 8 sessions of FES-LCE exercise training. Although Factor VII levels were not significantly reduced by FES-LCE exercise training, perhaps its activation may have been diminished by elevated levels of AT.
Increased plasma levels of fibrinogen are associated with a risk of various CVDs that is roughly double that of normal (51). Fibrinogen is the final substrate of the coagulation system, is converted to fibrin through thrombin generation, and promotes platelet aggregation, thrombosis, and early atherogenesis (49,51). Also, fibrinogen and Factor VII appear to be as effective as total cholesterol in predicting future risk of coronary heart disease (40). In individuals with SCI after FES-LCE exercise training, fibrinogen activity remained unchanged. Because Factor VII and fibrinogen are associated with CVD, and because these factors did not increase, this indicates that the common blood coagulation pathway was not activated.
Compared with able-bodied individuals, SCI subjects with injuries above the level of T1 have been shown to have lower circulating catecholamine levels at rest, and levels may increase only slightly with exercise. Catecholamines are known to affect platelet function and blood coagulation. In the present article, there was no difference in the inhibition of platelet aggregation or of coagulation factors after FES exercise.
CONCLUSION
In conclusion, 8 sessions of FES-LCE exercise training induced favorable hemostatic changes in individuals with SCI. Nine parameters were analyzed (thrombin, AT, cAMP, platelet aggregation, fibrinogen, and coagulation factors V, VII, VIII, and X). There were no significant changes in 3 parameters (fibrinogen, factors VII and VIII); however, favorable changes were observed for the 6 other determinations. Our findings for thrombin, AT III, fibrinogen, and FVII are consistent with previous observations in sedentary men evaluated for changes in blood coagulation before and after exercise on a treadmill.
This is the first study to demonstrate that FES-LCE exercise training may suppress platelet aggregation. It appeared that this effect was caused by greater activity in the platelets' intracellular cAMP. Cyclic AMP production is known to be stimulated by PGI2 and to cause the inhibition of platelet aggregation in individuals with SCI. In individuals with SCI, FES-LCE exercise training did not activate the 2 separate blood coagulation pathways: the intrinsic pathway and the extrinsic pathway, both of which contain Factor VII, Factor VIII, thrombin, and fibrinogen. Factor X, Factor V, and AT were activated after FES-LCE exercise. These results indicate an additional possible protective effect against cardiovascular disease of FES-LCE exercise training, independent of the known favorable changes in lipoprotein concentrations associated with exercise. FES-LCE exercise training, compared to alternative treatments, such as surgery or pharmacotherapy, is not only cost effective but also may have a beneficial impact on the quality-adjusted life expectancy of persons with SCI. Thus, FES-LCE exercise training should be considered as an essential component of the medical management of persons with SCI to improve their hemostatic profile and reduce the risk of CVD.
Acknowledgments
The authors are indebted to Dr Kristjan Ragnarsson, Professor and Chairman of the Department of Rehabilitation Medicine of Mount Sinai, School of Medicine for allowing us to conduct the study in the Department of Rehabilitation.
REFERENCES
- Laplante MP. Data on Disability from the National Interview Survey, 1983–85. Washington, DC: US Dept. of Education, National Institute on Disability and Rehabilitation Research; 1988. [Google Scholar]
- Bauman WA, Adkins RH, Spungen AM, et al. Is immobilization associated with an abnormal lipoprotein profile? Observations from a diverse cohort. Spinal Cord. 1999;37(7):485–493. doi: 10.1038/sj.sc.3100862. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Kahn NN, Grimm DR, Spungen AM. Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord. 1999;37(9):601–616. doi: 10.1038/sj.sc.3100911. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and cause of deaths in persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80(11):1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Dearwater SR, Laporte RE, Robertson RJ, Brenes G, Adams LL, Becker D. Activity in the spinal cord injury patients: an epidemiological analysis of metabolic parameters. Med Sci Sports Exerc. 1986;18(5):541–544. [PubMed] [Google Scholar]
- Bostom AG, Toner MM, McArdle WD, Montelione T, Brown CD, Stein RA. Lipid and lipoprotein profiles relate to peak aerobic power in spinal cord injured men. Med Sci Sports Exerc. 1991;23(4):409–414. [PubMed] [Google Scholar]
- Noreau L, Shephard RJ, Simard C, Pare G, Pomerleau P. Relationship of impairment and functional ability to habitual activity and fitness following spinal cord injury. Int J Rehabil Res. 1993;16(4):265–275. doi: 10.1097/00004356-199312000-00002. [DOI] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Gerhart KA, Bergstom E, Charlifue SW, Menter RR, Whiteneck GG. Long-term spinal cord injury: functional changes over time. Arch Phys Med Rehabil. 1993;74(10):1030–1034. doi: 10.1016/0003-9993(93)90057-h. [DOI] [PubMed] [Google Scholar]
- Phillips WT, Kiratli BJ, Sarkarati M, et al. Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol. 1998;23(11):641–716. doi: 10.1016/s0146-2806(98)80003-0. [DOI] [PubMed] [Google Scholar]
- Menter RR. Aging and spinal cord injury: implication for existing model systems and future federal, state and local healthcare policy. In: Apple DF, Hudson LM, editors. Spinal Cord Injury: The Model. Proceedings of the National Consensus Conference on Catastrophic Illness and Injury. The Spinal Cord Injury Model: Lessons Learned and New Applications. Atlanta, GA: Georgia Regional Spinal Cord Injury Care System, Shepherd Center for Treatment of Spinal Injuries; 1990. [Google Scholar]
- Walters RL, Sie IH, Adkins RH. The musculoskeletal system. In: Whiteneck GG, editor. Aging With Spinal Cord Injury. New York, NY: Demos Publications; 1993. pp. 53–57. [Google Scholar]
- Sloan RP, Shapiro PA, Demeersman RE, et al. Aerobic exercise attenuates inducible TNF production in humans. J Appl Physiol. 2007;103(3):1007–1011. doi: 10.1152/japplphysiol.00147.2007. Epub 2007 Jul 12. [DOI] [PubMed] [Google Scholar]
- El-Sayed MS, Younesian A, Rahman K, Ismail FM, Aliz El-Sayed. The effect of and training on platelet aggregation in male spinal cord individuals. Thromb Res. 2004;113(2):129–136. doi: 10.1016/j.thromres.2004.02.014. [DOI] [PubMed] [Google Scholar]
- El-Sayed MS, Sale C, Jones PG, Chester M. Blood hemostasis in exercise and training. Med Sci Sports Exerc. 2000;32(5):918–925. doi: 10.1097/00005768-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yamauchi K, Yamada C, et al. Blood coagulation and fibrinolytic activity before and after physical training during the recovery phase of acute myocardial infarction. Clin Cardiol. 1992;15(5):358–364. doi: 10.1002/clc.4960150510. [DOI] [PubMed] [Google Scholar]
- Figoni SF, Glaser RM, Rodgers MM, et al. Acute hemodynamic responses of spinal cord injured individuals to functional neuromuscular stimulation induced knee extension exercise. J Rehabil Res Dev. 1991;28(4):9–18. doi: 10.1682/jrrd.1991.10.0009. [DOI] [PubMed] [Google Scholar]
- Scott TR, Peckham PH, Keith MW. Upper extremity neuroprostheses using functional electrical stimulation. Baillieres Clin Neurol. 1995;4(1):57–75. [PubMed] [Google Scholar]
- Scremin AM, Kurta L, Gentili A, et al. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil. 1999;80(12):1531–1536. doi: 10.1016/s0003-9993(99)90326-x. [DOI] [PubMed] [Google Scholar]
- Nash MS, Bilsker S, Marcillo AE, et al. Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia. 1991;29(9):590–599. doi: 10.1038/sc.1991.87. [DOI] [PubMed] [Google Scholar]
- Nash MS, Montalvo BM, Applegate B. Lower extremity blood flow and responses to occlusion ischemia differ in exercise-trained and sedentary tetraplegic persons. Arch Phys Med Rehabil. 1996;77(12):1260–1265. doi: 10.1016/s0003-9993(96)90190-2. [DOI] [PubMed] [Google Scholar]
- Hjeltnes N, Aksnes AK, Birkeland KI, et al. Improved body composition after 8 wk of electrically stimulated cycling in tetraplegic patients. Am J Physiol. 1997;273(3 Pt 2):R1072–R1079. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- Hjeltnes N, Galuska D, Bjornholm M, et al. Exercise-induced over-expression of key regulatory proteins involved in glucose uptake and metabolism in tetraplegic persons: molecular mechanisms for improved glucose homestasis. FASEB J. 1998;12(15):1701–1712. doi: 10.1096/fasebj.12.15.1701. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Figoni SF. High density lipoprotein cholesterol in individuals with spinal cord injury: the potential role of physical activity. Spinal Cord. 1999;37(10):685–695. doi: 10.1038/sj.sc.3100917. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Raza M, Spungen AM, Machac J. Upper ergometry cardiac stress testing with thallium201 imaging in paraplegia. Arch Phys Med Rehabil. 1994;75(9):946–950. [PubMed] [Google Scholar]
- Budoff MJ, Lane KL, Bakhsheshi H, et al. Rates of progression of coronary calcium by electron beam tomography. Am J Cardiol. 2000;86(1):8–11. doi: 10.1016/s0002-9149(00)00820-1. [DOI] [PubMed] [Google Scholar]
- Whiteneck GG, Charlifue SW, Frankel HL. Mortality, morbidity and psychosocial outcomes of person with spinal cord injury more than 20 years ago. Paraplegia. 1992;30(9):617–630. doi: 10.1038/sc.1992.124. [DOI] [PubMed] [Google Scholar]
- Koenig W. Haemostatic risk factors for cardiovascular disease. Eur Heart J. 1998;19(suppl C):C39–C43. [PubMed] [Google Scholar]
- Jeon JY, Weiss CB, Steadward RD, et al. Improved glucose tolerance and insulin sensitivity after electrical stimulation-assisted cycling in people with spinal cord injury. Spinal Cord. 2002;40(3):110–117. doi: 10.1038/sj.sc.3101260. [DOI] [PubMed] [Google Scholar]
- Kahn NN, Bauman WA, Sinha AK. Demonstration of a novel circulating anti-prostacyclin receptor antibody. Proc Natl Acad Sci USA. 1997;94(16):8779–8782. doi: 10.1073/pnas.94.16.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn NN, Sinha AK. Inhibition of prostaglandin E1 induced activation of adenylate cyclase in human blood platelet membrane. Biochim Biophys Acta. 1988;972(1):45–53. doi: 10.1016/0167-4889(88)90101-2. [DOI] [PubMed] [Google Scholar]
- Kahn NN, Sinha AK, Spungen AM, Bauman WA. The effects of an anabolic steroid, oxandrolone, on hemostasis. Am J Hematol. 2006;81(2):95–100. doi: 10.1002/ajh.20532. [DOI] [PubMed] [Google Scholar]
- Cimminiello CG, Arpaia M, Aloisio M, et al. Platelet-derived growth factor (PDGF) in patients with different degrees of chronic arterial obstructive disease. Angiology. 1994;45(4):289–293. doi: 10.1177/000331979404500405. [DOI] [PubMed] [Google Scholar]
- DiCorleto PE. Cellular mechanism of atherogenesis. Am J Hypertens. 1993;6(11 Pt 2):314S–318S. [PubMed] [Google Scholar]
- Kahn NN. Platelet-stimulated thrombin generation and PDGF are normalized by insulin and Ca2+ channel blockers. Am J Physiol. 1999;276(5 Pt 1):E856–E862. doi: 10.1152/ajpendo.1999.276.5.E856. [DOI] [PubMed] [Google Scholar]
- Kahn NN, Bauman WA, Sinha AK. Loss of high-affinity prostacyclin receptors in platelets and the lack of prostaglandin-induced inhibition of platelet-stimulated thrombin generation in subjects with spinal cord injury. Proc Natl Acad Sci. 1996;93(1):245–249. doi: 10.1073/pnas.93.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn NN, Sinha AK, Bauman WA. Impaired platelet prostacyclin receptor activity: a monozygotic twin study discordant for spinal cord injury. Clin Physiol. 2001;21(1):60–66. doi: 10.1046/j.1365-2281.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- Kahn NN. Insulin-induced expression of prostacyclin receptors on platelets is mediated through ADP-ribosylation of Giμ protein. Life Sci. 1998;63(22):2031–2038. doi: 10.1016/s0024-3205(98)00481-0. [DOI] [PubMed] [Google Scholar]
- Leger AJ, Jacques SL, Badar J, et al. Blocking the protease-activated receptor 1–4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113(9):1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. Epub 2006 Feb 27. [DOI] [PubMed] [Google Scholar]
- Meade TW, Ruddock V, Stirling Y, Chakrabarti R, Miller GJ. Fibrinolytic activity, clotting factors and long term incidence of ischemic heart disease in the Northwick Park Heart Study. Lancet. 1993;342(8879):1076–1079. doi: 10.1016/0140-6736(93)92062-x. [DOI] [PubMed] [Google Scholar]
- Cuomo O, Pignataro G, Gala R, et al. Antithrombin reduces ischemic volume, ameliorates neurologic deficits, and prolongs animal survival in both transient and permanent focal ischemia. Stroke. 2007;38(12):3272–3279. doi: 10.1161/STROKEAHA.107.488486. [DOI] [PubMed] [Google Scholar]
- Heinrich J, Balleisen L, Schulte H, Assmann G, van de Loo J. Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb. 1994;14(1):54–59. doi: 10.1161/01.atv.14.1.54. [erratum 1994 Aug;14(8):1392]. [DOI] [PubMed] [Google Scholar]
- Olson ST, Bjork I. Regulation of thrombin activity by antithrombin and heparin. Sem Thromb Hemost. 1994;20(4):373–409. doi: 10.1055/s-2007-1001928. [DOI] [PubMed] [Google Scholar]
- Karamouzis M, Karamouzis I, Vamvakoudis E, et al. The response of muscle interstitial prostaglandin E(2)(PGE(2)), prostacyclin (2)(PGI2) and thromboxane A(2) (TXA(2)) levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandin Leukot Essent Fatty Acids. 2001;64(4–5):259–263. doi: 10.1054/plef.2001.0269. [DOI] [PubMed] [Google Scholar]
- Dahlback B, Villoutreix B. Regulation of blood coagulation by the protein c anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol. 2005;25(7):1311–1320. doi: 10.1161/01.ATV.0000168421.13467.82. [DOI] [PubMed] [Google Scholar]
- Siegl AM, Smith JB, Silver MJ, Nicolau KC, Ahern D. Selective binding sites for 3H-prostacyclin on platelets. J Clin Invest. 1979;63(2):215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha AK, Rao AK, Willis J, Colman RW. Inhibition of thromboxane A2 synthesis in human platelets by coagulation Factor Xa. Proc Natl Acad Sci USA. 1977;80(19):6086–6090. doi: 10.1073/pnas.80.19.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Winer RL, Khani S, et al. Extrinsic and common coagulation pathways in end-stage renal disease associated with spinal cord injury. Paraplegia. 1986;24(3):154–158. doi: 10.1038/sc.1986.20. [DOI] [PubMed] [Google Scholar]
- Scrutton MC, Ross-Murphy SB, Bennett GM, Stirling Y, Meade TW. Changes in clot deformability—a possible explanation for the epidemiological association between plasma fibrinogen concentration and myocardial infarction. Blood Coagul Fibrinol. 1994;5(5):719–723. doi: 10.1097/00001721-199410000-00007. [DOI] [PubMed] [Google Scholar]
- Katz RT, Green D, Sullivan T, Yarkony G. Functional electric stimulation to enhance systemic fibrinolytic activity in spinal cord injury patients. Arch Phys Med Rehabil. 1987;68(7):423–426. [PubMed] [Google Scholar]
- Lee MH, Vosburgh E, Anderson K, McDonagh J. Deficiency of plasma plasminogen activator inhibitor 1 results in hyperfibrinolytic bleeding. Blood. 1993;81(9):2357–2362. [PubMed] [Google Scholar]