Abstract
Leucine is unique among the amino acids in its ability to promote protein synthesis by activating translation initiation via the mammalian target of rapamycin (mTOR) pathway. Previously, we showed that leucine infusion acutely stimulates protein synthesis in fast-twitch glycolytic muscle of neonatal pigs but this response cannot be maintained unless the leucine-induced fall in amino acids is prevented. To determine whether leucine can stimulate protein synthesis in muscles of different fiber types and in visceral tissues of the neonate in the long-term if baseline amino acid concentrations are maintained, overnight fasted neonatal pigs were infused for 24 h with saline, leucine (400 μmol kg−1 h−1), or leucine with replacement amino acids to prevent the leucine-induced hypoaminoacidemia. Changes in the fractional rate of protein synthesis and activation of mTOR, as determined by eukaryotic initiation factor 4E binding protein (4E-BP1) and S6 kinase 1 (S6K1) phosphorylation, in the gastrocnemius and masseter muscles, heart, liver, jejunum, kidney, and pancreas were measured. Leucine increased mTOR activation in the gastrocnemius and masseter muscles, liver, and pancreas, in both the absence and presence of amino acid replacement. However, protein synthesis in these tissues was increased only when amino acids were infused to maintain baseline levels. There were no changes in mTOR signaling or protein synthesis in the other tissues we examined. Thus, long-term infusion of leucine stimulates mTOR signaling in skeletal muscle and some visceral tissues but the leucine-induced stimulation of protein synthesis in these tissues requires sustained amino acid availability.
Keywords: Growth, Muscle, Translation initiation, Mammalian target of rapamycin, Eukaryotic initiation factor 4E binding protein, Ribosome protein S6 kinase
Introduction
Dietary amino acids are used efficiently for growth during the neonatal period because protein synthesis in neonatal muscle is profoundly sensitive to the stimulatory effect of feeding (Davis et al. 1993, 1996). Nonetheless, about 10% of babies born in developed countries are of low birth weight (LBW) and many show growth failure by discharge and remain small into adulthood (Ehrenkranz 2007; Saigal et al. 2001). To develop new strategies for the nutritional management of LBW infants, we have used the neonatal pig as a model of the human neonate so that the intracellular mechanism by which feeding regulates infant growth can be identified. Our studies have shown that the feeding-induced stimulation of protein synthesis in skeletal muscle of the neonate is independently mediated by the post-prandial rise in amino acids and insulin (Davis et al. 2002; Escobar et al. 2005; O’Connor et al. 2003a, b; Suryawan et al. 2009).
Previously, our laboratory showed that a short-term (1 h) parenteral infusion of leucine designed to raise blood leucine concentrations to within the post-prandial range, increased protein synthesis in the skeletal muscle and heart of neonatal piglets (Escobar et al. 2006), similar to other studies in adult rats where a gavage feed of leucine alone acutely increases rates of muscle protein synthesis (Anthony et al. 2000, 2002). Conversely, the removal of leucine from a complete meal prevents the stimulation of protein synthesis by feeding (Stipanuk 2007). This anabolic effect appears to be unique for leucine because when circulating levels of the other branched-chain amino acids, isoleucine and valine, are increased to fed concentrations by infusion, muscle protein synthesis in the neonatal pig is not increased (Escobar et al. 2006). However, this effect of leucine on neonatal muscle protein synthesis cannot be sustained for 2 h (Escobar et al. 2005) likely due to the leucine-induced reduction in circulating amino acids levels as they are utilized for protein synthesis. When the leucine-induced reduction in circulating amino acids is prevented by infusion of an amino acid mixture designed to maintain circulating levels of other amino acids at fasting baseline concentrations (Escobar et al. 2007), the leucine-induced stimulation of muscle protein synthesis is maintained for 2 h. Recently, we showed that a long-term infusion of leucine continues to stimulate protein synthesis in skeletal muscle containing fast-twitch glycolytic fibers if amino acids are provided at replacement concentrations (Wilson et al. 2010). However, the effects of long-term leucine infusion on protein synthesis in muscles of different fiber types and in peripheral tissues were not examined.
Leucine increases rates of protein synthesis by enhancing translation initiation, through activation of the mammalian target of rapamycin complex 1 (mTORC1) pathway (Kimball and Jefferson 2004). While the insulin signaling pathway that regulates mTORC1 has been well studied, amino acid induction of mTORC1 remains elusive. Leucine-induced mTORC1 activation phosphorylates the negative regulator of translation initiation, eukaryotic initiation factor 4E binding protein 1 (4E-BP1), allowing formation of the eukaryotic initiation factor (eIF) 4F complex that is required to mediate the binding of the mRNA to the ribosome (for a more detailed review see Davis and Fiorotto 2009). Additionally, mTORC1 phosphorylates ribosomal protein S6 kinase (S6K1), an activator of S6 (Gingras et al. 2001), a protein associated with enhanced protein synthesis. Previously, we showed that leucine stimulates this pathway in skeletal muscles containing primarily fast-twitch glycolytic fibers, irrespective of whether circulating amino acids are maintained at fasting concentrations for 1–2 h (Escobar et al. 2005, 2006, 2007; Suryawan et al. 2008) and up to 24 h (Wilson et al. 2010). The ability of leucine to stimulate mTORC1 activity, but not protein synthesis, when circulating levels of the other amino acids are allowed to fall below fasting concentrations is probably due to limited substrate availability for the synthesis of new proteins (Escobar et al. 2005, 2006, 2007).
To assess the potential value of leucine supplementation in the nutritional management of LBW infants, it is critical that the effectiveness of long-term (24 h) leucine supplementation on protein synthesis in tissues throughout the body be examined. We also wished to identify the intracellular mechanism that modulates the response to long-term leucine supplementation.
Materials and methods
Animals and design
Multiparous crossbred (Yorkshire × Landrace × Hampshire × Duroc) pregnant sows (Agricultural Headquarters of the Texas Department of Criminal Justice, Huntsville, TX) were housed in lactation crates in individually, environmentally controlled rooms prior to farrowing. After farrowing piglets remained with the sow and were not given supplemental creep feed. At least 3 days prior to infusion, piglets were anesthetized and catheters were surgically inserted into the jugular vein and carotid artery (Davis et al. 1996). Piglets were then studied at 5 days of age (2.6 ± 0.1 kg) following the protocol previously described (Wilson et al. 2010) and approved by The Animal Care and Use Committee of Baylor College of Medicine. This study was conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Treatments and infusions
The infusion procedure was described previously (Wilson et al. 2010). Briefly, overnight fasted 5-day-old piglets were randomly assigned to one of three treatment groups: saline, leucine, or leucine + amino acids (n = 6/treatment group) and infused for a period of 24 h. Animals assigned to the saline group were infused with sterile saline at 10 ml h−1 throughout the infusion period. Piglets assigned to the leucine group were infused with leucine at 400 μmol kg−1 h−1, while piglets in the leucine + amino acid group were also infused with a balanced amino acid mixture (Davis et al. 2002), prepared devoid of leucine, to maintain circulating amino acid concentrations at baseline fasting levels. The infusion rate of the amino acid mixture (devoid of leucine) was gradually increased at 10 min intervals from 0 to 0.4, 0.6, 0.85, 1.5, 1.85, 2.25, 2.7 and 2.85 ml h−1 kg−1, until the infusion rate of 2.85 ml h−1 kg−1 was achieved, and maintained constant throughout as previously calculated in our laboratory (Escobar et al. 2007).
Tissue protein synthesis in vivo
The fractional rate of protein synthesis was measured with a flooding dose of L-[4-3H] phenylalanine (1.5 mmol kg body wt−1, 0.5 mCi kg body wt−1, Amersham Bioscience, Piscataway, NJ) injected 30 min prior to the end of the infusion (Garlick et al. 1980). Piglets were killed at 24 h and samples were obtained from the gastrocnemius and masseter muscles, right and left chambers of the heart, liver, kidney, pancreas, and jejunum and immediately frozen in liquid nitrogen and stored at −70°C until analyzed as previously described (Davis et al. 1989). Protein content was measured by the Lowry et al. (1951) assay and total RNA by the method of Munro and Fleck (1969) for the determination of both protein synthetic capacity and efficiency.
Protein immunoblot analysis
Proteins from individual tissue homogenates were separated on polyacrylamide gels (PAGE). For each assay, all samples were run at the same time on triple-wide gels (C.B.S Scientific C., Del Mar, CA) to reduce inter-assay variation. Proteins were electrophoretically transferred to polyvinylidene difluoride transfer membranes (Pall Corporation, Pensacola, FL), which were incubated with appropriate primary antibodies, washed, and exposed to an appropriate secondary antibody as previously described (Davis et al. 2000).
For normalization, immunoblots performed with anti-phospho-specific antibodies were stripped in stripping buffer (Pierce Biotechnology, Rockford, IL) and reprobed with corresponding nonphospho-specific antibodies. Blots were developed using an enhanced chemiluminescence kit (GE Health Sciences, Buckinghamshire, UK), visualized, and analyzed using a ChemiDoc-It Imaging System (UVP, Upland, CA). Primary antibodies that were used in the immunoblotting were 4E-BP1 (total, Bethyl Laboratories, Montgomery, TX, and Thr70, Cell Signaling) and S6K1 (total and Thr398, Cell Signaling, Boston, MA).
Calculations and statistics
The fractional rate of protein synthesis (Ks, percentage of protein mass synthesized in a day) was calculated as Ks (%/day) = [(Eb/Ea) × (1,440/t)] × 100, where Eb (in dpm nmol−1) is the specific radioactivity of the protein-bound phenylalanine, Ea (in dpm nmol−1) is the specific radioactivity of the tissue-free phenylalanine at the time of tissue collection, corrected by the linear regression of the blood-specific radioactivity of the animal against time, t is the time of labeling in minutes, and 1,440 is the minutes-to-day conversion. Previous studies have demonstrated that, after a flooding dose of 3H-phenylalanine is administered, the specific radioactivity of tissue-free phenylalanine is in equilibrium with the aminoacyl-tRNA-specific radioactivity, and therefore the tissue-free phenylalanine is a valid measure of the tissue precursor pool-specific radioactivity (Davis et al. 1999). The majority of RNA in tissues is ribosomal, hence the RNA-to-protein ratio (mg RNA g protein−1) was used as an estimate of protein synthetic capacity (Cs). Protein synthetic efficiency (KRNA) was estimated as the total protein synthesized in a day per total RNA (g protein day−1 g RNA−1).
ANOVA analysis was carried out using Minitab using a general linear model to determine main statistical differences. Between group analysis was determined by TUKEY. Probability values of <0.05 were considered significant for all comparisons and data are presented as mean ± SE.
Results
Hormones and substrates
Circulating hormone and substrate concentrations during a 24 h infusion of leucine, with and without amino acid replacement, were reported previously (Wilson et al. 2010) and are presented here for reference. Circulating concentrations of glucose or insulin were not altered in response to a parenteral infusion of leucine during the 24 h period (Table 1). Circulating concentrations of leucine in the two leucine infused groups were increased about fourfold above saline-treated animals by the end of the 24 h infusion period (P < 0.05); these values were within the physiological range observed previously for formula fed newborn piglets (Burrin et al. 1995). An infusion of leucine alone reduced circulating levels of most essential amino acids compared to baseline values and those in the saline infused animals (P < 0.05). Circulating levels of the nonessential amino acids decreased in the saline infused animals compared to baseline (P < 0.05) and did not differ between leucine and saline infused animals. Concurrent infusion of leucine with an amino acid mixture (devoid of leucine) maintained circulating essential and nonessential amino acids at fasting baseline levels.
Table 1.
Circulating glucose, insulin, leucine, essential amino acid, and nonessential amino acid concentrations at baseline and after 24 h of infusion with saline or leucine, with and without replacement amino acids
| Treatment
|
||||
|---|---|---|---|---|
| Baseline | Saline | Leucine | Leu + AA | |
| Glucose (mg dl−1) | 87.0 ± 2.2 | 85.7 ± 3.2 | 82.7 ± 3.4 | 85.4 ± 2.4 |
| Insulin (μU ml−1) | 1.4 ± 0.4 | 0.7 ± 0.2 | 0.6 ± 0.2 | 1.8 ± 0.3 |
| Leucine (nmol ml−1) | 174.5 ± 6.7 | 214.3 ± 17.3 | 843.4 ± 47.8a,b | 859.1 ± 49.0a,b |
| EAA (nmol ml−1) | 1,059 ± 35.5 | 1,119 ± 80.9 | 502 ± 38.6a,b | 1,167 ± 71.5 |
| NEAA (nmol ml−1) | 2,281 ± 128 | 1,622 ± 154a | 1,342 ± 99.2a | 1,986 ± 81.5 |
Previously published data from Wilson et al. 2010
Values are mean ± SEM; n = 18 for baseline and 6 for treatment group. ANOVA indicated an effect of treatment for the leucine, EAA, and NEAA groups (P < 0.05)
Leu + AA leucine plus amino acid replacement, EAA essential amino acids, NEAA nonessential amino acids
Response differed from baseline
Response differed from saline-treated group at 24 h
Protein synthesis
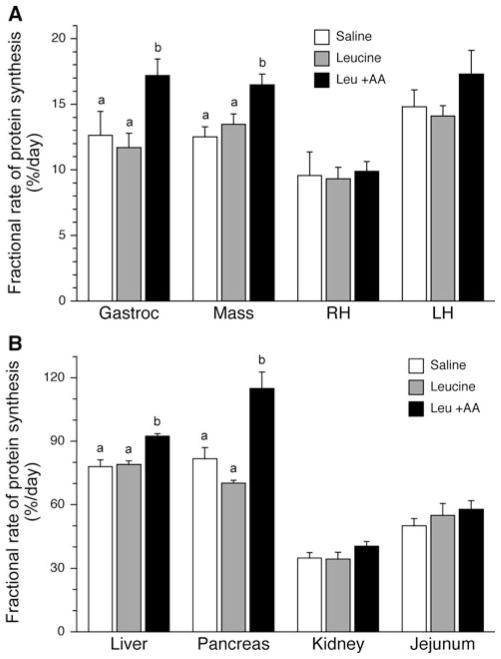
The fractional rate of protein synthesis in the gastrocnemius muscle showed a tendency towards an effect of the 24 h of treatment (P = 0.057; Fig. 1a), with pigs treated with leucine plus replacement amino acids having a higher rate of protein synthesis that those treated with saline (P = 0.083) or leucine alone (P = 0.011). Treatment with leucine plus replacement amino acids increased protein synthesis in the masseter muscle above that in pigs treated with saline (P = 0.007) or leucine alone (P = 0.024). Protein synthesis rates in the right heart and left heart, however, were not altered by treatment.
Fig. 1.
Fractional rates of muscle protein synthesis (Ks) in a muscle and b visceral tissues after a 24 h infusion of saline or leucine with and without replacement amino acids. Gastroc gastrocnemius muscle, Mass masseter muscle, RH right chamber of heart, and LH left chamber of heart. ANOVA indicates an effect of treatment in the gastrocnemius (P = 0.057), masseter (P = 0.009), liver (P < 0.001) and pancreas (P = 0.003). Results of TUKEY post hoc testing at P < 0.05 denoted by different letters. Values are mean ± SE; n = 6 per treatment
Fractional rates of protein synthesis in the liver were increased by infusion of leucine when amino acids were maintained at fasting concentrations (P < 0.001; Fig. 1b). Rates of protein synthesis in the pancreas were also increased in pigs treated with leucine plus replacement amino acids compared to those receiving either saline (P = 0.024) or leucine alone (P = 0.005) for 24 h. There was no effect of treatment in the kidney or the jejunum.
Protein synthetic capacity and efficiency
Measurement of protein synthetic capacity (Cs) showed no effect of treatment in any of the tissues examined (data not shown). However, infusion of leucine over a 24-h period, while maintaining amino acids at fasting concentrations, increased the efficiency with which the ribosomes synthesized protein in the gastrocnemius muscle by 26% (P = 0.014) compared to pigs treated with leucine alone, although there was no statistically significant difference from pigs infused with saline (Table 2). When the circulating amino acid concentrations were maintained at fasting levels, leucine increased translational efficiency in the masseter muscle by 26% (P = 0.033) and in liver by 19% (P = 0.004) compared to saline-treated animals. There was no statistically significant effect of treatment with leucine alone compared with pigs receiving saline on translational efficiency in any tissue. Leucine, with and without replacement amino acids, had no effect on translational efficiency in the right or left heart, jejunum, kidney, or pancreas.
Table 2.
Rates of translational efficiency (KRNA) in individual tissues of neonatal pigs after 24 h of infusion with saline or leucine, with and without replacement amino acids
| Treatment
|
|||
|---|---|---|---|
| Saline | Leucine | Leu + AA | |
| Gastrocnemius | 6.2 ± 1.0 | 5.5 ± 0.6 | 8.7 ± 0.8† |
| Masseter | 5.6 ± 0.4 | 6.5 ± 0.5 | 7.4 ± 0.6* |
| Right heart | 7.1 ± 0.5 | 7.5 ± 0.4 | 8.8 ± 0.5 |
| Left heart | 6.9 ± 0.6 | 7.0 ± 0.6 | 7.6 ± 0.9 |
| Liver | 11.1 ± 0.4 | 11.0 ± 0.2 | 13.5 ± 0.5*† |
| Pancreas | 10.5 ± 0.5 | 8.7 ± 0.6 | 11.7 ± 1.2 |
| Kidney | 7.4 ± 0.4 | 7.5 ± 0.5 | 8.4 ± 0.4 |
| Jejunum | 9.8 ± 0.7 | 10.4 ± 1.1 | 10.9 ± 1.1 |
Values are mean KRNA (g protein day−1 g RNA−1) ± SEM; n = 6/treatment group
P < 0.05 compared to saline group
P < 0.05 compared to leucine group
Translation initiation
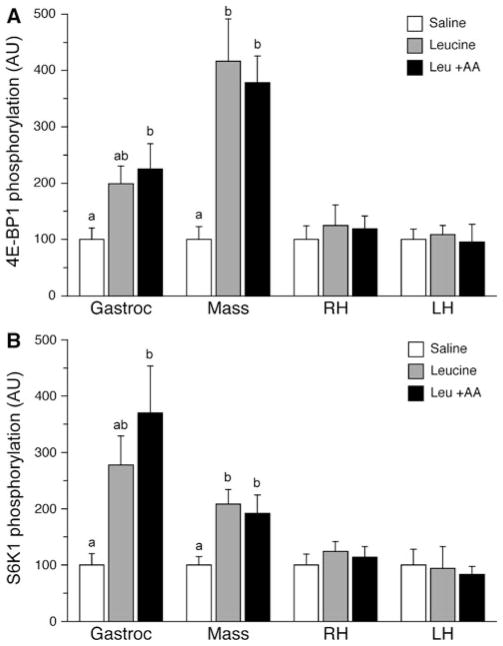
As a measure of the activation of mTORC1 and translation initiation, the phosphorylation of downstream targets of mTORC1 was assessed. The phosphorylation of both 4E-BP1 (Fig. 2a) and S6K1 (Fig. 2b) in the gastrocnemius muscle was altered by treatment (P = 0.045 and 0.025, respectively). Analysis of between group differences revealed that the phosphorylation of both 4E-BP1 and S6K1 in gastrocnemius muscle increased in pigs infused with leucine plus replacement amino acids compared with those infused with saline (P = 0.048 and 0.024, respectively). Phosphorylation of 4E-BP1 and S6K1 in the leucine only group did not differ from either the saline or the leucine plus replacement amino acid group. In the masseter muscle, treatment also altered the phosphorylation of 4E-BP1 (P = 0.001) and S6K1 (P = 0.022). Phosphorylation of 4E-BP1 in the masseter muscle was increased in pigs infused with leucine, both in the absence and presence of amino acid replacement (P < 0.01), with no differences detected between the two leucine groups. S6K1 phosphorylation in the masseter muscle was also higher in the leucine only (P = 0.030) and in the leucine plus amino acid replacement (P = 0.054) groups compared to saline. There was no effect of treatment on 4E-BP1 or S6K1 phosphorylation in the right heart or left heart.
Fig. 2.
Phosphorylation of 4E-BP1 (a) and S6K1 (b) in the gastrocnemius, masseter, right and left ventricles after a 24 h infusion of saline or leucine with and without replacement amino acids. ANOVA indicates an effect of treatment on 4E-BP1 for gastrocnemius (P = 0.045) and masseter (P = 0.001), and for S6K1 in the gastrocnemius (P = 0.025) and masseter (P = 0.022). Results of TUKEY post hoc testing at P < 0.05 denoted by different letters. Values are mean ± SE; n = 6 per treatment for all tissues
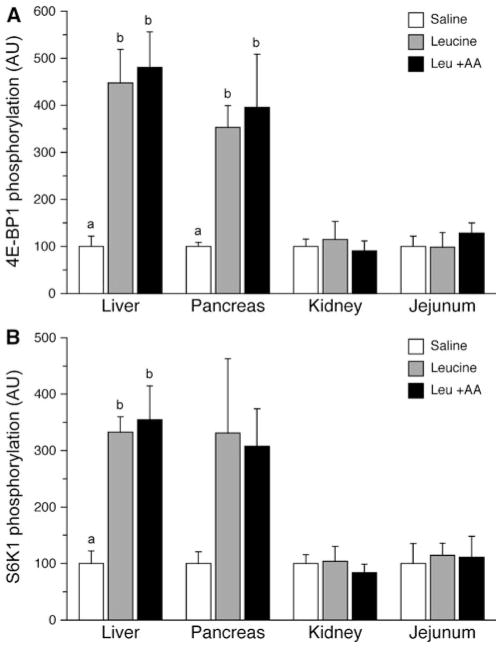
Phosphorylation of 4E-BP1 (Fig. 3a) and S6K1 (Fig. 3b) was altered in the liver in response to treatment (P = 0.002 and 0.003, respectively). Between group analysis revealed that the phosphorylation of both 4E-BP1 and S6K1 in liver was increased with leucine alone (P = 0.012 and 0.023, respectively) and leucine plus replacement amino acids (P = 0.002 and 0.003, respectively) compared to saline alone. No statistically significant differences were detected between the two leucine infusion groups for either target. For the pancreas, there tended to be an effect of treatment on the phosphorylation of 4E-BP1 (P = 0.052); however, no statistically significant effect of treatment was measured for S6K1 (P = 0.191). Between group analysis of 4E-BP1 showed that there tended to be an increase in the phosphorylation of this factor in the pancreas with both leucine alone (P = 0.099) and leucine in the presence of replacement amino acids (P = 0.058). No effect of treatment was found for either 4E-BP1 or S6K1 in the kidney (P = 0.814 and 0.758, respectively) or the jejunum (P = 0.658 and 0.943, respectively).
Fig. 3.
Phosphorylation of 4E-BP1 (a) and S6K1 (b) in the liver, pancreas, kidney and jejunum after a 24 h infusion of saline or leucine with and without replacement amino acids. ANOVA indicates an effect of treatment on 4E-BP1 for liver (P = 0.002) and pancreas (P = 0.052), and for S6K1 in the liver (P = 0.003). Results of TUKEY post hoc testing at P < 0.05 denoted by different letters. Values are mean ± SE; n = 6 per treatment for all tissues except pancreas n = 3 per treatment
Discussion
While considerable work has been conducted to investigate the role of leucine in the regulation of protein synthesis in skeletal muscle (Anthony et al. 2002; Crozier et al. 2005; Escobar et al. 2007; Koopman et al. 2008; Norton et al. 2009; Suryawan et al. 2008; Wilson et al. 2010), not much information is available on the effect of leucine on rates of protein synthesis and activation of translation initiation in other tissues in vivo, especially during long-term leucine treatment. Long-term administration of leucine may be useful in the nutritional management of LBW infants as a means of promoting protein synthesis, and hence growth. However, in order to assess the potential benefits of leucine supplementation on neonatal growth, it is essential to assess the effects of leucine on protein synthesis not only in skeletal muscle, but also in other tissues of the neonate as well. The results of the current study showed that a continuous infusion of leucine for 24 h increased the rates of protein synthesis in peripheral and visceral tissues in neonatal pigs, a response that appeared mediated by a corresponding increase in the rate of translation initiation, as measured by the phosphorylation of the downstream targets of mTORC1, i.e., 4E-BP1 and S6K1. However, the leucine-induced decline in the circulating concentrations of other amino acids had to be prevented to allow adequate substrate availability for the leucine-induced stimulation of protein synthesis. The effect of leucine was tissue specific, with leucine stimulating protein synthesis in muscles of different fiber types, liver, and pancreas but not in the right or left heart, kidney, or jejunum.
Most studies that have examined the effects of leucine have done so acutely, 30–60 min after leucine administration, and have not reported changes in circulating amino acid levels (Anthony et al. 2000, 2002; Crozier et al. 2005). Hypoaminoacidemia was observed in the current study in response to a 24 h infusion of leucine alone, as was previously reported by our laboratory following a 2 h infusion of leucine (Escobar et al. 2005) but not a 1 h leucine infusion (Escobar et al. 2005, 2006). The reduction in circulating essential amino acid concentrations with leucine infusion in the current study is consistent with studies in healthy humans subjects in which leucine levels were increased four- to sixfold for 2.5–7 h, resulting in a reduction in plasma concentrations of essential amino acids from 35 to 70% (Hagenfeldt et al. 1980; Nair et al. 1992). Results from the current and previous studies (Escobar et al. 2007; Wilson et al. 2010) suggest that the leucine-induced reduction in plasma essential amino acids is due to the increase in protein synthesis that occurs earlier during the infusion period, although a leucine-induced decrease in proteolysis and/or increase in amino acid oxidation may also occur.
Previously, we showed that leucine stimulates protein synthesis by enhancing translation initiation for up to 24 h in the longisimus dorsi muscle (Wilson et al. 2010), a skeletal muscle containing predominantly fast-twitch glycolytic fibers. In the current study, infusion of leucine for 24 h increased muscle protein synthesis in both the gastrocnemius, a muscle of mixed fiber type, and the masseter, which is mainly comprised of slow-twitch oxidative fibers. The increase in protein synthesis in both muscles was mediated by an increase in translation initiation, as indicated by an increased activation of the translation initiation factors, 4E-BP1 and S6K1, but the rise in protein synthesis was dependent upon circulating levels of other amino acids. These results suggest that leucine is capable of stimulating the synthesis of protein in all types of skeletal muscle irrespective of their composition. These results differ from that of Lynch et al. (2002a), where supplementation of the drinking water with leucine for 12 days to double circulating leucine concentrations increased protein synthesis in the gastrocnemius muscle in mature rats but had no effect on the signaling factors involved in the mTOR signaling pathway. Differences between the two studies may be due to duration of exposure to leucine. While acute increases in protein synthesis may be a consequence of enhanced efficiency of translation of mRNA via alterations in peptide chain initiation, more prolonged changes in protein synthesis are commonly accompanied by changes in ribosome abundance (Morgan and Beinlich 1997). Indeed, in the current study, treatment with leucine increased translational efficiency but not translation capacity, a parameter not measured in the study by Lynch et al. (2002a). Differences in developmental stage are unlikely to account for the alterations in the response since leucine has been shown to promote acute increases in protein synthesis by stimulating the mTOR signaling pathway in the gastrocnemius muscle of adult rats (Anthony et al. 2000; Crozier et al. 2005).
In contrast to the stimulatory effects of leucine in skeletal muscle, cardiac muscle was unresponsive to treatment with leucine in the current study. Both a stimulation (Vary 2009) and no change (Lynch et al. 2002b) in protein synthesis in the heart have been reported in response to acute supraphysiological doses of leucine in mature rats. Chronic supplementation of leucine in addition to the drinking water also had no effect on protein synthesis in the heart (Lynch et al. 2002a). Although we previously found no effect of parenteral infusion of physiological levels of an acute amino acid mixture on protein synthesis in the heart of neonatal pigs (Davis et al. 2002; Suryawan et al. 2009), more detailed study showed that while acute leucine infusion did not stimulate protein synthesis in the right ventricular wall, acute leucine infusion significantly increased protein synthesis in the left ventricular wall in this model (Escobar et al. 2006). This response was mediated by an increase in mTORC1 activation and hence, translation initiation, and was attributed to the greater hypertrophic growth of the left ventricular wall that occurs shortly after birth (Escobar et al. 2006). Since protein synthesis was also measured in the left ventricular wall in the current study, this suggests that while leucine can stimulate protein synthesis in the left heart of the neonate in the short-term via an enhancement of translation initiation, this effect is not prolonged.
The liver, like the skeletal muscle, showed a significant increase in the fractional rate of protein synthesis when leucine was infused for 24 h in the presence of replacement amino acids. The response appeared mediated by enhanced activation of the mTOR signaling pathway, as indicated by enhanced phosphorylation of 4E-BP1 and S6K1, resulting in increased efficiency of mRNA translation. However, there was no corresponding increase in liver protein synthesis when leucine was infused in the presence of hypoaminoacedemia, likely due to reduced substrate availability, even though mTOR signaling was activated. Although infusion of a physiological dose of a complete amino acid mixture acutely increased protein synthesis in the liver of neonatal pigs by increasing translation initiation (O’Connor et al. 2004; Suryawan et al. 2009), acute infusion of leucine in neonatal piglets (Escobar et al. 2005) and oral administration in mature rats (Anthony et al. 2001; Lynch et al. 2002b; Reiter et al. 2004) in the fasting state failed to demonstrate a stimulatory effect on protein synthesis in the liver, although 4E-BP1 and S6K1 were activated in some studies. By contrast, supplementing the drinking water of rats for a period of 12 days did lead to an increase in the rate of hepatic protein synthesis measured in the postprandial state (Lynch et al. 2002a). Therefore, the available data suggest that while leucine is unable to stimulate liver protein synthesis acutely, continued exposure does lead to an increase in liver protein synthesis when other amino acids are not limiting.
The pancreas also showed an increase in mTORC1 activity in response to a 24 h infusion of leucine, leading to an increase in the rate of protein synthesis, but only when hypoaminoacidemia was prevented. This supports a previous study in mature rats gavage fed leucine at a dose equivalent to a day’s intake and rates of protein synthesis in the pancreatic acini cells were measured up to 90 min thereafter (Sans et al. 2006). In that study, leucine stimulated protein synthesis and mTORC1 activity (Sans et al. 2006). Studies in pancreatic β cells have shown that leucine, but not valine nor isoleucine, increased 4E-BP1 and S6K1 phosphorylation (Xu et al. 1998, 2001). Taken together, this suggests that at least in the short-term and immediate long-term, leucine is capable of stimulating pancreatic protein synthesis.
Leucine did not alter the rate of protein synthesis in the kidney or the jejunum. In the case of the kidney, this supports previous data from the chronic leucine supplementation study of Lynch et al. (2002a), where no effect of treatment was observed although acute effects of leucine in the kidney have been reported (Lynch et al. 2002b). For the jejunum, not much information is available regarding the effect of leucine on protein synthesis. However, in intestinal epithelial cells in vitro, leucine has been reported to up-regulate 4E-BP1 and S6K1 activation (Ban et al. 2004; Rhodes et al. 2008). Although differences in the protein content of the diet have been shown to influence the rate of protein synthesis in both the kidney and small intestine (Frank et al. 2006), it is not possible to ascertain whether the increase in protein synthesis is due to leucine alone. As the small intestine is the first tissue to encounter a nutritional intervention if given enterally, determining the effects of a diet supplemented with leucine on translation and protein synthesis in this tissue is essential for future understanding of the role that leucine may play in neonatal growth.
Thus, the results of the current study showed that leucine infusion alone increased the activation of 4E-BP1 and S6K1 in skeletal muscles, liver, and pancreas but this was not sufficient to increase protein synthesis unless the leucine-induced hypoaminoacidemia was prevented by concurrent infusion of other amino acids to maintain their baseline levels. We postulate that the apparent discrepancy between the activation of translation initiation and protein synthesis with prolonged leucine infusion alone is a consequence of a reduction in the availability of amino acids. Thus, the rise in leucine levels in the blood increased the activation of mTOR effectors, 4E-BP1 and S6K1, in these tissues. However, this was not sufficient to stimulate protein synthesis unless the substrates for protein synthesis, amino acids, were sufficiently maintained. Moreover, leucine increased the activation of 4E-BP1 and S6K1 during hypoaminoacidemia, and there was no additional effect induced by euamionoacidemia in most tissues. This suggests that the leucine-induced activation of the mTOR signaling pathway was independent of circulating levels of other amino acids. In addition, the stimulation of protein synthesis was unlikely to be due to the infusion of the amino acid mixture (devoid of leucine) alone as the rate of infusion of the amino acid mixture (devoid of leucine) was about one-fifth of the infusion rate of the complete amino acid mixture previously shown to stimulate protein synthesis in the neonate (Davis et al. 2002; O’Connor et al. 2003a, 2004; Suryawan et al. 2009).
In summary, the results of the current and previous studies suggest that long-term stimulation of protein synthesis by leucine is tissue specific. The skeletal muscle shows a sustained increase in the rate of protein synthesis, by enhancing translational efficiency through an increase in the activation of translation initiation by mTORC1. This effect on protein synthesis can be maintained for 24 h if hypoaminoacidemia is prevented. Both liver and pancreas also respond to long-term infusion of leucine by activating mTOR signaling, and in the presence of euaminoacidemia, stimulating protein synthesis. The effects of leucine on mTORC1 activation in these tissues do not appear to be influenced by the circulating levels of other amino acids. Other tissues, such as the cardiac muscle, small intestine and kidney, are not responsive to long-term leucine administration. Importantly, the results suggest that, although supplemental leucine could lead to an increase in weight gain and muscle mass in the initial days of treatment, more prolonged leucine supplementation might also lead to asymmetric growth. Additional long-term studies are warranted to examine the effects of leucine supplementation for the neonate.
Acknowledgments
We thank Marta Fiorotto for helpful discussions, Jerome Stubblefield for care of animals, E. O’Brian Smith for statistical assistance, Adam Gillum for graphics and Linda Weiser for secretarial assistance. Funding for this research was received from the Ajinomoto Amino Acid Research Program, NIH R01 AR-44474, NIH K08 AR051563, and USDA/ARS Cooperative Agreement no. 58-6250-6-001. This work is a publication of the United States Department of Agriculture, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX.
References
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 2001;131:856S–860S. doi: 10.1093/jn/131.3.856S. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, Jefferson LS. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002;51:928–936. doi: 10.2337/diabetes.51.4.928. [DOI] [PubMed] [Google Scholar]
- Ban H, Shigemitsu K, Yamastsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomota Y. Arginine and leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537–543. [PubMed] [Google Scholar]
- Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res. 1995;37:593–599. doi: 10.1203/00006450-199505000-00006. [DOI] [PubMed] [Google Scholar]
- Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto MF. Regulation of muscle growth in neonates. Curr Opin Clin Nutr. 2009;12:78–85. doi: 10.1097/MCO.0b013e32831cef9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of sucking rats. Am J Physiol. 1989;257:R1141–R1146. doi: 10.1152/ajpregu.1989.257.5.R1141. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol. 1993;265:R334–R340. doi: 10.1152/ajpregu.1993.265.2.R334. [DOI] [PubMed] [Google Scholar]
- Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab. 1996;33:E802–E809. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol Endocrinol Metab. 1999;277:E103–E109. doi: 10.1152/ajpendo.1999.277.1.E103. [DOI] [PubMed] [Google Scholar]
- Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2000;279:E1226–E1234. doi: 10.1152/ajpendo.2000.279.6.E1226. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O’Connor PMJ. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab. 2002;282:E880–E890. doi: 10.1152/ajpendo.00517.2001. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol. 2007;31:48–55. doi: 10.1053/j.semperi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab. 2005;288:E914–E921. doi: 10.1152/ajpendo.00510.2004. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–E621. doi: 10.1152/ajpendo.00402.2005. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab. 2007;293:E1615–E1621. doi: 10.1152/ajpendo.00302.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E225–E233. doi: 10.1152/ajpendo.00351.2005. [DOI] [PubMed] [Google Scholar]
- Garlick PJ, Mcnurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980;192:719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L, Eriksson S, Wahren J. Influence of leucine on arterial concentrations and regional exchange of amino acids in healthy subjects. Clin Sci (Lond) 1980;59:173–181. doi: 10.1042/cs0590173. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Regulation of global and specific mRNA translation by oral administration of branched-chain amino acids. Biochem Biophys Res Commun. 2004;313:423–427. doi: 10.1016/j.bbrc.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJM, Kuipers H, van Loon LJC. Co-ingestion of leucine with protein does not further augment postexercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–580. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab. 2002a;283:E824–E835. doi: 10.1152/ajpendo.00085.2002. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Patson BJ, Anthony JC, Vaval A, Jefferson LS, Vary TC. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab. 2002b;283:E503–E513. doi: 10.1152/ajpendo.00084.2002. [DOI] [PubMed] [Google Scholar]
- Morgan HE, Beinlich CJ. Contributions of increased efficiency and capacity of protein synthesis to rapid cardiac growth. Mol Cell Biochem. 1997;176:145–151. [PubMed] [Google Scholar]
- Munro HN, Fleck A. Analysis of tissues and body fluids for nitrogenous constituents. In: Munro HN, editor. Mammalian protein metabolism. 3. Academic Press; New York: 1969. pp. 465–483. [Google Scholar]
- Nair KS, Matthews DE, Welle SL, Braiman T. Effect of leucine on amino acid and glucose metabolism in humans. Metabolism. 1992;41:643–648. doi: 10.1016/0026-0495(92)90057-h. [DOI] [PubMed] [Google Scholar]
- Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV, Garlick PJ. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J Nutr. 2009;139:1103–1109. doi: 10.3945/jn.108.103853. [DOI] [PubMed] [Google Scholar]
- O’Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003a;284:E110–E119. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- O’Connor PMJ, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003b;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of neonatal liver protein synthesis by insulin and amino acids in pigs. Am J Physiol Endocrinol Metab. 2004;286:E994–E1003. doi: 10.1152/ajpendo.00391.2003. [DOI] [PubMed] [Google Scholar]
- Reiter AK, Anthony TG, Anthony JC, Jefferson LS, Kimball SR. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int J Biochem Cell Biol. 2004;36:2169–2179. doi: 10.1016/j.biocel.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Rhodes JM, Liu Y, Niu X, Surendran S, Wu G. Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. J Nutr. 2008;138:1652–1657. doi: 10.1093/jn/138.9.1652. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf BL, Streiner DL, Burrows E. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics. 2001;108:407–415. doi: 10.1542/peds.108.2.407. [DOI] [PubMed] [Google Scholar]
- Sans MD, Tashiro M, Vogel NL, Kimball SR, D’alecy LG, Williams JA. Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. J Nutr. 2006;136:1792–1799. doi: 10.1093/jn/136.7.1792. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH. Leucine and protein synthesis: mTOR and beyond. Nutr Rev. 2007;65:122–129. doi: 10.1111/j.1753-4887.2007.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:868–875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, O’Connor PMJ, Bush JA, Nguyen HV, Davis TA. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. 2009;37:97–104. doi: 10.1007/s00726-008-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary TC. Oral leucine enhances myocardial protein synthesis in rats acutely administered ethanol. J Nutr. 2009;139:1439–1444. doi: 10.3945/jn.108.098707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr. 2010;140:264–270. doi: 10.3945/jn.109.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Kwon G, Marshall CA, Lin T, Lawrence JC, McDaniel MJ. Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic B-cells. J Biol Chem. 1998;273:28178–28184. doi: 10.1074/jbc.273.43.28178. [DOI] [PubMed] [Google Scholar]
- Xu G, Kwon G, Cruz WS, Marshall CA, McDaniel MJ. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic B-cells. Diabetes. 2001;50:353–360. doi: 10.2337/diabetes.50.2.353. [DOI] [PubMed] [Google Scholar]