Abstract
Introduction
PX-478 is a potent small-molecule inhibitor of HIF-1α. In preclinical studies, it had antitumor activity against various solid tumors in subcutaneous xenografts but had no measurable activity against a non-small cell lung cancer (NSCLC) xenograft. To determine the effectiveness of PX-478 against lung tumors, we investigated HIF-1α expression in several lung cancer cell lines, both in vitro and in vivo, and treated orthotopic mouse models of human lung cancer with PX-478.
Methods
Cells from two human lung adenocarcinoma cell models (PC14-PE6 and NCI-H441) or two human small cell lung cancer (SCLC) models (NCI-H187 and NCI-N417) were injected into the left lungs of nude mice and were randomized 16 to 18 days after injection with daily oral treatment with PX-478 or vehicle for 5 days.
Results
In the PC14-PE6 NSCLC model, treatment with 20 mg/kg PX-478 significantly reduced the median primary lung tumor volume by 87% (p = 0.005) compared with the vehicle-treated group. PX-478 treatment also markedly reduced mediastinal metastasis and prolonged survival. Similar results were obtained in a second NSCLC model. In SCLC models, PX-478 was even more effective. In the NCI-H187 model, the median primary lung tumor volume was reduced by 99% (p = 0.0001). The median survival duration was increased by 132%. In the NCI-N417 model, the median primary lung tumor volume was reduced by 97% (p = 0.008).
Conclusions
We demonstrated that the PX-478, HIF-1α inhibitor, had significant antitumor activity against two orthotopic models of lung adenocarcinomas and two models of SCLC. These results suggest the inclusion of lung cancer patients in phase I clinical trials of PX-478.
Keywords: Hypoxia, HIF-1α, PX-478, Orthotopic model, Lung cancer
The outcome of patients with lung cancer has not significantly changed for more than 2 decades; the disease causes more than 160,000 deaths annually in the United States alone.1 Metastatic and locoregional disease are both of concern: lung cancer commonly spreads within the lung and chest to the liver, bone, or brain and other metastatic sites.
Several targeted agents have been tested, but many of these have not fulfilled their early expectations. Until recently, combining targeted agents with chemotherapy in chemonaive patients have not resulted in a survival advantage. However, both gefitinib and erlotinib have resulted in dramatic responses and survival benefits in patients with clinical characteristics that lead to increased epidermal growth factor receptor mutations or copy numbers.2– 4 Unfortunately, no agent has demonstrated activity against small cell lung cancer (SCLC). However, the recent success of the antiangiogenic antibody, bevacizumab, in combination with chemotherapy in non-small cell lung cancer (NSCLC) provides proof-of-concept and illustrates the need for further research in this area.5
Hypoxia is a common feature in solid tumors, and cellular adaptation to hypoxia is critical to tumor progression and survival. The key regulator of the cellular response to hypoxia is the transcription factor hypoxiainducible factor-1 (HIF-1), which is composed of an oxygen-regulated HIF-1α subunit and a constitutively expressed HIF-1β subunit.6 Under normoxic conditions, the α subunit is rapidly hydroxylated at two proline residues and targeted for proteosomal degradation after ubiquitination by the von Hippel-Lindau factor. In addition, an asparagine residue in the HIF-1α transactivation domain is hydroxylated, inhibiting the binding of the cofactor p300/CBP.7 However, Under hypoxic conditions, oxygen-dependent hydroxylases are inhibited and the HIF-1α subunit is stabilized and then it interacts with the HIF-1β subunit and other cofactors and induces the transcription of many genes involved in metabolic adaptation, apoptosis resistance, angiogenesis, and tumor invasion and metastasis.8 Besides hypoxia, several cytokines, growth factors, oncogenes, and gene mutations can increase the expression and stability of HIF-1α, mostly through activation of the phosphoinositide 3-kinase/Akt, Ras/Raf/mitogen-activated protein kinase, and Smad pathways.9
HIF-1 is overexpressed in 30 to 62% of NSCLC and 43 to 46% of SCLC cases, and its overexpression has been associated with poorer overall survival.10 –15 This was confirmed recently, when HIF-1α mRNA expression was identified as one of three prognostic factors for overall survival in early-stage NSCLC.16
PX-478, a potent small-molecule inhibitor of HIF-1α, inhibits the subcutaneous growth of a wide variety of tumors, including SCLC; however, it was ineffective against A549 lung adenocarcinoma xenografts that showed only low expression of HIF-1α in vitro and in vivo.17 The drug is currently being evaluated in a phase I clinical trial, but the optimal treatment modalities in lung cancer and the biologic mechanism of resistance to radiation therapy and chemotherapy are not understood. Therefore, in this study, we investigated HIF-1α expression in a panel of human lung cancer cell lines in vitro and determined effectiveness of PX-478 against human HIF-1α-expressing SCLC and NSCLC tumors in vivo in orthotopic mouse models. We also determined the effectiveness of PX-478 alone and in combination with paclitaxel.
MATERIALS AND METHODS
Cell Culture
NCI-H187, NCI-N417, and NCI-H441 cells were purchased from American Type Culture Collection (Manassas, VA) and molecularly characterized by the University of Texas MD Anderson Cancer Center Cell Line Characterization Shared Resource. The NCI-H187 and NCI-N417 cells used for orthotopic injections were isolated from a mouse lung that had been orthotopically injected with parental NCI-H187 or parental NCI-N417 human SCLCs to improve tumor uptake. NCI-H187, NCIN417, and NCI-H441 cells were cultured in RPMI 1640 with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, l-glutamine, 2-fold vitamin solution, and penicillin-streptomycin (Invitrogen, Grand Island, NY). PC14-PE6 lung adenocarcinoma cells,18 a variant of PC14 human lung adenocarcinoma cells, were provided by Nagahiro Saijo (National Cancer Research Institute, Tokyo, Japan) and maintained in modified Eagle medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, l-glutamine, 2-fold vitamin solution, and penicillin-streptomycin.
Western Blotting
For Western blot analysis, cells were grown under standard conditions and incubated with or without 150 µM CoCl2 for 16 to 18 hours to mimic hypoxia.19 In experiments in which the HIF-1α antagonist PX-478 was used in vitro, cell were treated with 5 µM or 25 µM PX-478 with or without concurrent 150 µM CoCl2 for 16 hours.
Cells were homogenized in whole-cell extraction buffer (20 mM Tris-HCl, pH = 7.4; 150 mM NaCl; 2 mM ethylenediaminetetraacetic acid; 1 mM dithiothreitol; 1% [wt/vol] Triton X-100; and complete protease inhibitor cocktail, Roche, Nutley, NJ). After being centrifuged at 14,000g, the supernatant was collected, and the protein concentration was quantified (protein assay kit, Bio-Rad, Hercules, CA). Fifty micrograms of protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a Trans-Blot nitrocellulose membrane (Bio-Rad). After being blocked with 5% nonfat milk in washing buffer (10 mM Tris–HCl, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, and 0.1% [wt/vol] Tween 20, pH 8.0), the membranes were incubated with 1 µg/ml mouse antihuman HIF-1α antibody (BD Transduction Labs, San Jose, CA) and then goat antimouse IgG antibody coupled with horseradish peroxidase (1:3000, BD Pharmingen); they were visualized using an ECL Western blotting detection kit (Amersham Biosciences, Piscataway, NJ).
Orthotopic Lung Tumor Model
Human lung cancer cells were orthotopically injected into the left lungs of mice, as described previously,20 with slight modifications. In brief, 6- to-8-week-old male athymic nude mice (Taconic, Hudson, NY) were anesthetized using sodium pentobarbital (50 mg/kg body weight) and placed in the right lateral decubitus position. A 5-mm incision of the skin was made to visualize the left lung. Logarithmically growing NCI-H187, NCI-N417, NCIH441, or PC14-PE6 single-cell suspensions (1 × 106 cells with >90% viability and 50 µg of growth factor-reduced Matrigel [BD Biosciences] in 50 µL of Hanks balanced salt solution) were injected into the left lateral lungs of the mice. The incisions were closed with surgical clips. The mice were turned to the left lateral decubitus position and observed for 45 to 60 minutes until they had fully recovered. The mice were housed and maintained under specific pathogen-free conditions in accordance with the guidelines of the American Association for Laboratory Animal Care. All experiments met the current regulations and standards of the U.S. Department of Agriculture, the U.S. Department of Health and Human Services, the National Institutes of Health, and The University of Texas MD Anderson Cancer Center.
PX-478 (ProlX, Tucson, AZ) was dissolved on ice in vehicle containing saline. Paclitaxel (Taxol; Bristol-Myers Squibb, New York, NY) and cyclophosphamide (Cytoxan; Bristol-Myers Squibb) were dissolved in saline. For the first experiment, 18 days after the implantation of PC14PE6 tumor cells or 17 days after the implantation of NCI-H187 cells, mice (7–13 per group) were randomized and treated with 10 or 20 mg/kg oral PX-478 daily for 5 days; or 150 mg/kg body weight cyclophosphamide intraperitoneally, 3 days a week (Monday, Wednesday, and Friday) for 1 week; or vehicle. A second group (10 mice per group) was treated with 20 mg/kg PX-478 daily for 5 days or vehicle to determine survival. All mice except those in the survival experiment were killed and autopsied when the vehicle-treated animals became moribund; Then, we determined the primary left-lung tumor weight, tumor volume (dlong × dshort2/2), incidence, and volume of pleural effusion, and presence of metastasis. The mice in the survival experiment were closely monitored. Mice were killed when they became moribund, and the day of death was recorded. The experiment was terminated after 140 days.
In a similar but separate study, mice implanted with PC14PE6 or NCI-H187 lung tumors were randomly assigned to treatment (9 –12 per group) with 20 mg/kg oral PX-478 daily for 5 days, with or without 200 µg/mouse intraperitoneal paclitaxel once a week; 20 mg/kg PX-478 3 days a week (Monday, Wednesday, and Friday) until the end of the experiment, with or without paclitaxel weekly; 20 mg/kg PX-478 every third day until the end of the experiment, with or without paclitaxel weekly; 200 µg of paclitaxel weekly alone; or vehicle.
In studies using the NCI-H441, NSCLC cell line, and the NCI-N417, SCLC cell line, 16 days after tumor implantation, mice were randomly treated with 20 mg/kg oral PX-478 daily for 5 days or vehicle. All mice were killed when the vehicle-treated mice became moribund on day 62 (NCI-H441) or 43 (NCI-N417) after tumor implantation.
For the time course experiment to determine the kinetics of HIF-1α inhibition by PX-478, mice were orthotopically injected with PC14-PE6 cells and treated once with 20 mg/kg PX-478 on day 20 after tumor implantation. Four mice each were killed at 0, 2, 4, 6, 8, and 16 hours after treatment, and tumor tissues were collected for paraffin embedding.
Histologic and Immunohistochemical Analysis
Tumor tissue specimens were fixed with 10% formalin and embedded in paraffin or directly frozen in OCT compound (Miles, Elkhart, IN) and sectioned and stained with hematoxylin and eosin. Immunostaining for CD31 was performed on frozen tissues as described previously.21
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling was performed using a commercial apoptosis detection kit (Promega) with modifications.21 Cleaved caspase-3 staining was performed as described previously21 using a rabbit anticleaved caspase-3 antibody (#9661; Cell Signaling Technology, Danvers, MA).
For HIF-1α immunostaining, sections of tumor tissue were baked and dewaxed automatically and then stained using a Bond-maX autostainer and Homo-Mouse polyhorseradish peroxidase detection system (Leica BioSystems, Norwell, MA) combined with a monoclonal mouse antihuman HIF-1α antibody (clone 54; BD Transduction Laboratories). Slides were lightly counterstained with the onboard hematoxylin to visualize nuclei and then scanned with an Ariol SL-50 automated microscope; nuclear staining was quantified using a computerized image analysis system (Applied Imaging, San Jose, CA).
Statistical Analysis
Data were analyzed using GraphPad Prism5 software (GraphPad Software, Inc., La Jolla, CA). The Mann-Whitney U and log-rank (Mantel-Cox) tests were used when appropriate. The median survival duration was estimated using a Kaplan-Meier analysis. The values of p = 0.05 were considered statistically significant.
RESULTS
HIF-1α Expression in Human Lung Cancer Cells and Inhibition of HIF-1α Expression by PX-478 In Vitro
To determine the role of HIF-1α and its antagonism by PX-478 in lung cancer, we evaluated HIF-1α expression levels in a panel of NSCLC and SCLC cell lines in vitro under normoxic and hypoxia-mimicking conditions (CoCl2 treatment) by Western blot analysis, using an antibody specific to HIF-1α. HIF-1α expression levels varied between cell lines and were independent of cell histologic type, with the adenocarcinoma cell line PC14PE6 and the SCLC cell line NCI-H187 showing high expression of HIF-1α under hypoxia-mimicking conditions (Figure 1A). Next, we incubated the lung cancer cell lines, PC14PE6 and NCI-H187, with the HIF-1α antagonist PX-478 under normoxic or hypoxia-mimicking conditions and measured HIF-1α levels by Western blot analysis. As shown in Figure 1B, PX-478 inhibited hypoxia-induced HIF-1α expression protein in vitro at a dose of 25 µM in NCI-H187 cells and reduced HIF-1α protein expression in PC14PE6 cells by more than 50%. To confirm that HIF-1α expression was also present in lung cancer tissue, we performed an immunohistochemical analysis to determine HIF-1α expression in human lung cancer tumor tissue grown orthotopically in the lungs of nude mice. All tissues were obtained from tumors in a late stage of tumor progression. We observed HIF-1α expression in the tumor tissue of two SCLC cell lines, NCI-H187, and NCI-N417, and in tissue of the adenocarcinoma cell lines PC14PE6 and NCI-H441 (Figure 1C).
FIGURE 1.
HIF-1α is expressed in lung cancer cells. A, Western blot analysis of human NCLC (A427, A549, H226, H322, H358, H441, H460, H1299, and PC14PE6) and small cell lung cancer (SCLC; H187 and N417) cell lines. Cells were grown in media, with or without 150 µM CoCl2, for 18 hours. Total cell lysates (50 µg) were evaluated by Western blot analysis for HIF-1α protein expression. B, NCI-H187 and PC14PE6 cells were treated with PX-478, with or without 150 µM CoCl2, for 16 hours. Total cell lysates (50 µg) were evaluated by Western blot analysis for HIF-1α protein expression. C, Immunohistochemical analyses of representative sections obtained from different primary lung tumors grown orthotopically in mice stained with an antibody directed against human HIF-1α.
Together, these data demonstrate that HIF-1α is expressed at various levels in lung cancer cells in vitro under hypoxic conditions and in vivo in tumor tissues obtained from SCLC and NSCLC xenografts.
PX-478 Treatment Inhibits Growth and Metastasis of Orthotopic Human Lung Adenocarcinoma
On the basis of the observed expression of HIF-1α in lung cancer cells, both in vitro and in vivo, we orthotopically implanted PC14PE6 adenocarcinoma cells, which express high levels of HIF-1α, into the left lungs of mice. Mice were treated on day 18 with either 10 mg/kg or 20 mg/kg oral PX-478 for 5 days, or cyclophosphamide 3 days a week for 1 week, or vehicle. Mice were killed on day 29 after tumor implantation, when the mice in the vehicle control group became moribund. As shown in Table 1, treatment with PX-478 for 5 days led to a dose-dependent decrease in tumor growth and progression. In the 20 mg/kg PX-478 group, the median primary lung tumor volume was reduced by 87% and the median left lung weight was reduced by 80% (Table 1 and Figure 2A) compared with those in the vehicle group; these results were statistically significant (p = 0.005 and 0.006, respectively). Treatment with PX-478 also markedly reduced the occurrence of mediastinal lymph node metastasis and the production of pleural effusion.
TABLE 1.
PX-478 Treatment of PC14-PE6 and NCI-H441 Human Adenocaricomas Growing in the Lungs of Nude Mice
| Treatment | Body Weight, g | Tumor Incidence (n/Total) |
Left Lung Weight, mg |
Tumor Volume, mm3 |
Pleural Effusion, µl |
Medistinal Lymph Node Metastasis |
|---|---|---|---|---|---|---|
| PC14-PE6 | ||||||
| Vehicle | 24.6 (19.9–27.8) | 9/10 | 610 (252–1170) | 613 (120–921) | 150 (0–450) | 8/10 |
| PX-478 (10 mg/kg) | 23.8 (18.6–28.5) | 9/9 | 416 (53–935) | 338 (5–746) | 60 (0–400) | 3/9 |
| PX-478 (20 mg/kg) | 22.0 (20.1–23.2) | 12/12 | 124 (50–640) | 79 (98–6570) | 30 (0–450) | 3/12 |
| Cyclophosphamide | 25.2 (20.7–28.7) | 7/7 | 98 (72–368) | 46 (3–300) | 0 (0–180) | 2/7 |
| Vehicle | 22.3 (16.6–29.6) | 12/12 | 852 (64–1510) | 773 (22–1261) | 100 (0–250) | 8/12 |
| Paclitaxel | 24.6 (21.1–29.9) | 9/9 | 510 (85–1164) | 342 (19–447) | 120 (0–300) | 4/9 |
| PX-478 (QDx5) | 23.2 (16.1–26.0) | 11/11 | 290 (56–588) | 194 (15–447) | 30 (0–450) | 4/11 |
| PX-478 (QDx5) + paclitaxel | 23.2 (20.8–26.3) | 8/8 | 382 (65–758) | 170 (8–601) | 0 (0–570) | 2/8 |
| PX-478 (MWF) | 20.1 (15.7–23.6) | 10/10 | 222 (104–591) | 122 (47–488) | 0 (0–150) | 4/10 |
| PX-478 (MWF) + paclitaxel | 21.7 (16.1–25.0) | 9/9 | 199 (91–423) | 101 (6–354) | 0 (0–450) | 2/9 |
| PX-478 (Q3D) | 21.8 (19.6–24.9) | 9/10 | 290 (62–862) | 183 (0–666) | 0 (0–400) | 2/10 |
| PX-478 (Q3D) + paclitaxel | 19.8 (18.5–23.7) | 8/8 | 354 (65–631) | 180 (26–370) | 0 (0–170) | 4/8 |
| NCI-H441 | ||||||
| Vehicle | 32.6 (26.9–36.3) | 10/10 | 530 (150–740) | 591 (104–950) | 85 (0–500) | 9/10 |
| PX-478 (20 mg/kg) | 30.3 (26.5–34.5) | 10/10 | 110 (70–190) | 62 (22–146) | 0 (0–20) | 5/10 |
All the values are given as median (range).
FIGURE 2.
PX-478 treatment inhibits orthotopic growth (A, B) and increases survival (C) of human adenocarcinoma PC14PE6 and human small cell lung cancer (SCLC) NCI-H187 (D, E, F). A, PC14PE6 cells (1 × 106) were injected into the left lungs of nude mice; treatment with vehicle, PX-478 (10 or 20 mg/kg daily for 5 days), or cyclophosphamide (150 mg/kg 3 days a week for 1 week) was initiated 18 days after cell injection. Mice were killed when significant morbidity was observed, and the left lungs were weighed. B, A second experiment to evaluate different schedules of PX-478 and combination chemotherapy on tumor growth (left lung weight). Treatment was initiated 15 days after tumors had been injected with vehicle, paclitaxel (200 µg/mouse, weekly), or PX-478 (20 mg/kg, either daily for 5 days [QD × 5], 3 days a week [Monday, Wednesday, and Friday], or every third day [Q3D]), with or without paclitaxel, for the duration of the experiment. C, PC14PE6 (1 × 106) were injected into the left lungs of nude mice. Treatment with 20 mg/kg PX-478 or vehicle (daily for 5 days) was started 18 days after injection. Mice were monitored daily and killed when they became moribund. D, H187 cells (1 × 106) were injected into the left lungs of nude mice, and treatment with vehicle, PX-478 (10 or 20 mg/kg daily for 5 days), or cyclophosphamide (150 mg/kg 3 days a week for 1 week) was initiated 17 days after cell injection. Mice were killed when significant morbidity was observed, and the left lungs were weighed. E, A second experiment to evaluate different schedules of PX-478 and combination chemotherapy on tumor growth (left lung weight). Treatment was initiated 15 days after tumor injection with vehicle, paclitaxel (200 µg/mouse, weekly), or 20 mg/kg PX-478 daily for 5 days (QD × 5), 3 days a week (Monday, Wednesday, and Friday), or every third day (Q3D), with or without paclitaxel, for the duration of the experiment. F, H187 cells (1 × 106) were injected into the left lungs of nude mice. Treatment with 20 mg/kg PX-478 or vehicle (daily for 5 days) was started 17 days after injection. Mice were monitored daily and killed when they became moribund (Pac. = paclitaxel).
To confirm that the effect of PX-478 treatment on tumor growth inhibition in NSCLC was not cell-line specific, we used a second adenocarcinoma cell line, NCI-H441, in our orthotopic lung cancer model. In contrast to fast-growing PC14PE6 tumors, NCI-H441 tumors grew slowly; therefore, mice were killed on day 62 after tumor implantation, when mice in the vehicle group had become moribund. As shown in Table 1, treatment with 20 mg/kg PX-478 for 5 days decreased tumor volume by 90% (p = 0.0001) and left lung weight by 79% (p = 0.0004) in this tumor model. Furthermore, we observed a statistically significant reduction in pleural effusion (p = 0.0055).
Together, our results showed, for the first time, that PX-478 is effective against NSCLC in in vivo models of human adenocarcinoma.
PX-478 Treatment Inhibits Growth and Metastasis of Orthotopic Human Small Cell Lung Cancer
To determine the effectiveness of PX-478 in an orthotopic SCLC model, we injected NCI-H187 into the left lungs of mice. Mice were treated with 10 mg/kg or 20 mg/kg PX-478 daily for 5 days, or cyclophosphamide 3 days a week for 1 week, or vehicle control. PX-478 treatment led to a dose-dependent decrease in tumor growth and progression (Table 2). The high dose of PX-478 (20 mg/kg) led to near complete suppression of tumor growth, with a median tumor volume reduction of 99% (p = 0.0001) and a median left lung weight reduction of 91% (p = 0.0003, Table 2 and Figure 2D) compared with the vehicle control group. Moreover, in three of the 13 mice treated with 20 mg/kg PX-478, no lung tumors could be found on histologic analysis. Mediastinal lymph node metastasis was found in eight mice (73%) in the vehicle-treated group, whereas only one mouse (8%) had mediastinal lymph node metastasis in the group treated with 20 mg/kg PX-478, indicating a profound reduction in metastasis. To confirm that the effects of PX-478 on tumor growth inhibition were not cell-line specific, we used a second SCLC cell line, NCI-N417, in our orthotopic lung cancer model. Treatment with the higher dose of PX-478 (20 mg/kg) daily for 5 days decreased the median tumor volume by 97% (p = 0.008) and left lung weight by 87% (p = 0.002), indicating that PX-478 is highly active against SCLC (Table 2).
TABLE 2.
PX-478 Treatment of NCI-H187 and NCI-N417 Human SCLC Growing in the Lungs of Nude Mice
| Treatment | Body Weight, g | Tumor Incidence (n/Total) |
Left Lung Weight, mg |
Tumor Volume, mm3 |
Mediastinal Lymph Node Metastasis |
|---|---|---|---|---|---|
| NCI-H187 | |||||
| Vehicle | 25.9 (21.9–29.0) | 11/11 | 657 (55–1261) | 671 (9–1182) | 8/11 |
| PX-478 (10 mg/kg) | 28.3 (25.9–34.3) | 10/10 | 159 (57–300) | 105 (4–289) | 2/10 |
| PX-478 (20 mg/kg) | 27.0 (24.0–31.6) | 10/13 | 57 (50–158) | 2 (0–160) | 1/13 |
| Cyclophosphamide | 27.9 (25.4–31.8) | 7/7 | 70 (50–133) | 7 (1–93) | 1/7 |
| Vehicle | 27.0 (18.6–30.2) | 12/12 | 585 (77–1182) | 362 (4–951) | 6/12 |
| Paclitaxel | 27.1 (24.5–32.3) | 7/7 | 535 (164–1030) | 334 (56–601) | 5/7 |
| PX-478 (QDx5) | 26.4 (23.9–29.6) | 3/11 | 65 (45–77) | 0 (0–1) | 1/11 |
| PX-478 (QDx5) + paclitaxel | 26.5 (21.2–29.1) | 0/7 | 57 (42–66) | 0 (0–0) | 0/7 |
| PX-478 (MWF) | 20.4 (17.1–22.0) | 3/9 | 49 (40–65) | 0 (0–9) | 0/9 |
| PX-478 (MWF) + paclitaxel | 20.7 (17.2–23.4) | 8/10 | 55 (45–67) | 1 (0–23) | 1/10 |
| PX-478 (Q3D) | 22.1 (17.5–26.1) | 1/10 | 50 (43–59) | 0 (0–8) | 1/10 |
| PX-478 (Q3D) + paclitaxel | 22.9 (18.2–26.8) | 1/8 | 55 (51–74) | 0 (0–3) | 0/8 |
| NCI-N417 | |||||
| Vehicle | 29.9 (24.4–32.7) | 10/10 | 520 (70–940) | 564 (8–1059) | 8/10 |
| PX-478 (20 mg/kg) | 29.5 (23.7–34.6) | 12/12 | 70 (50–440) | 19 (4–462) | 5/12 |
All the values are given as median (range).
MWF, Monday, Wednesday, and Friday.
PX-478 Treatment Prolongs Survival in Mice with Orthotopic Human Adenocarcinoma or SCLC
To determine the effects of PX-478 on the survival duration of mice with human lung cancer, we treated mice implanted with PC14PE6 or NCI-H187 cells with PX-478 (20 mg/kg) orally for 5 days or vehicle as a control. PX-478 treatment significantly (p = 0.01) increased the survival duration of mice with human adenocarcinoma (PC14PE6) to a median of 41 days compared with 30.5 days in the vehicle-treated group (Figure 2C). The effects of PX-478 on the survival duration of mice with SCLC (H187) were even more pronounced: the median survival duration increased by 132% from a median of 45.5 days in the vehicle-treated group to 105.5 days in the PX-478-treated group (Figure 2F). Interestingly, two of 10 mice in the PX-478-treated group were healthy at the end of the experiment (day 140 after injection); three others had no primary lung tumors on the day they were killed. All seven mice that were killed before the end of the experiment had metastatic disease, some in multiple organs, including the lymph nodes (four mice), bone (3), brain (2), and liver (1). One mouse died of unknown reasons, with no visible tumor or metastases. In contrast, all nine mice killed in the vehicle-treated group had large lung tumors; one mouse in the vehicle-treated group was healthy at the end of the experiment, probably because of unsuccessful tumor uptake.
Different Treatment Schedules of PX-478 and Combination Chemotherapy
To determine the optimal schedule and chemotherapy combinations for more sustained tumor growth suppression, especially in the PC14PE6 model, we evaluated several different schedules, with or without paclitaxel. As shown in Table 1 and Figure 2B, treatment with 20 mg/kg oral PX-478 daily for 5 days, 3 days a week for 1 week, or every third day for 1 week led to a marked reduction in tumor growth in the PC14PE6 model. However, we observed no statistically significant differences between the different schedules. Combination treatment with paclitaxel did not further improve tumor growth reduction in this model.
In the NCI-H187 SCLC model, we observed nearly complete tumor growth reduction or cure (Table 2 and Figure 2E), regardless of schedule. Combination therapy with paclitaxel did not further improve the outcome, probably because of the high response rate obtained with PX-478 alone.
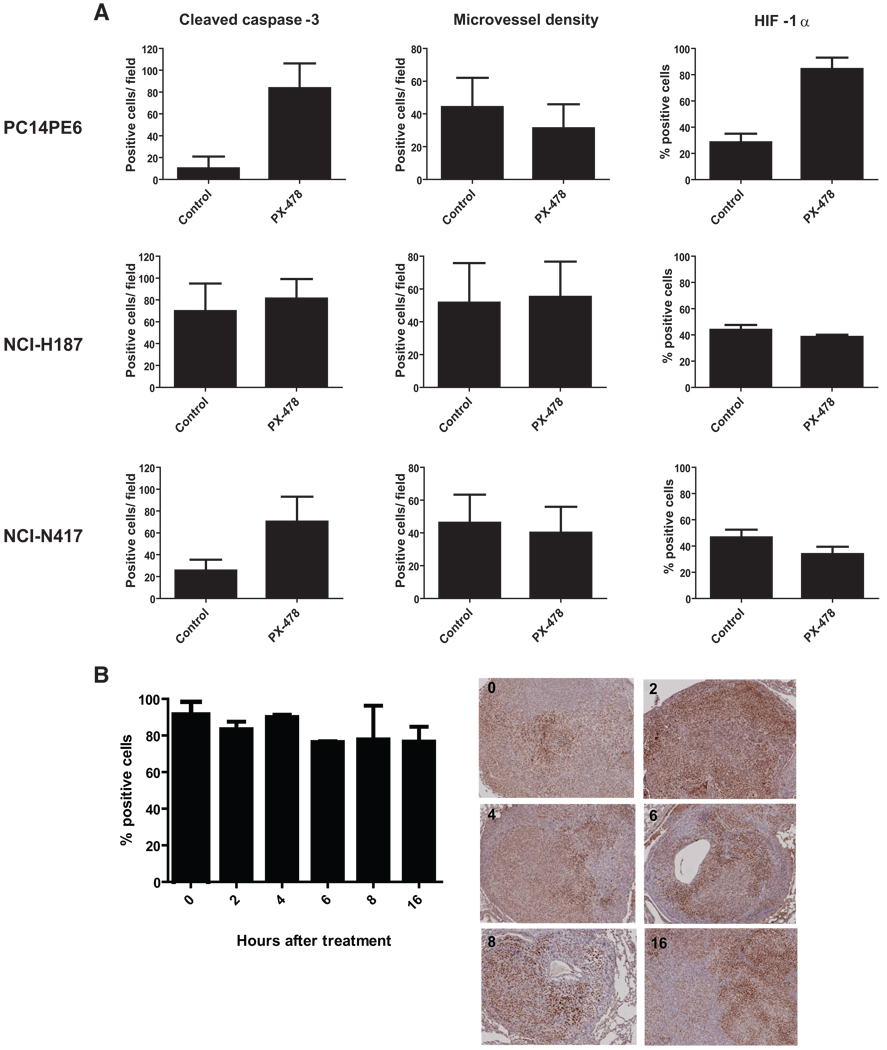
Effects of PX-478 on HIF-1α Expression, Cell Apoptosis, and Microvessel Density in Orthotopic Lung Tumor Tissue
Lung tumor tissues from mice with PC14PE6, NCI-H187, NCI-N417, and NCI-H441 tumors were obtained 4 hours after the fifth and final treatment with 20 mg/kg PX-478 (daily schedule) or vehicle and subjected to immunohistochemical analyses to determine apoptosis, microvessel density, and HIF-1α expression. As shown in Figure 3A, treatment with PX-478 for 5 days induced profound apoptosis in tumor cells, as determined by cleaved caspase-3 staining and the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay (data not shown); this was statistically significant in PC14PE6 and NCI-N417 tumors. In NCI-H187 tumors, a high background of apoptotic cells was visible in vehicle-treated cells; this was accompanied by necrotic areas. Microvessel density, as determined by CD31 staining, did not significantly differ between PX-478-treated tumors harvested after 5 days and vehicle-treated tumors. Tumors from the slow-growing NCI-H441 cell line were too small after 5 days of treatment (day 20 after tumor implantation) to allow an objective evaluation of apoptosis, microvessel density, or HIF-1α expression.
FIGURE 3.
Treatment with PX-478 induces apoptosis but does not alter microvessel density or HIF-1α staining. A, Immunohistologic quantification of apoptosis, microvessel density, or HIF-1α staining in tissue derived from PC14PE6, NCI-N417, and NCI-H187 tumors after the last treatment with PX-478 (day 5 of treatment). Tissue sections were stained with an anticleaved caspase-3 antibody, anti-CD31, or anti-HIF-1α antibody. For apoptosis and microvessel density, the number of positive cells was determined by counting 12–16 high-power fields at 200× magnification. For HIF-1α, the mean percentages of positive tumor cells from three or four tumors are shown. B, Time-course experiment of HIF-1α expression. Mice were killed 0, 2, 4, 6, 8, or 16 hours after a single dose of 20 mg/kg PX-478 at day 21 after injection of 1 × 106 PC14PE6 cells into the left lung. The mean percentages of HIF-1α-positive tumor cells from three or four tumors and representative stained tissue sections are shown.
Next, we compared HIF-1α expression in PX-478-treated tumors with that in vehicle-treated tumors. Surprisingly, we found no statistically significant reduction in HIF-1α expression after 5 days of treatment with the inhibitor in the 3 analyzed models (Figure 3A). We performed a time-course experiment to determine the kinetics of HIF-1α inhibition by PX-478. Mice were orthotopically injected with PC14PE6 cells and treated once with 20 mg/kg PX-478 on day 20 after tumor implantation; they were then killed at intervals. As shown in Figure 3B, we observed no significant reduction in HIF-1α expression.
DISCUSSION
PX-478 is a small-molecule inhibitor of HIF-1α that has antitumor activity against various human cancer cell lines in subcutaneous xenograft models.17 However, no scientific studies have demonstrated the activity of the compound in an orthotopic tissue environment. Furthermore, the drug was not effective against the NSCLC cell line A-549 in the panel used by Welsh et al.,17 indicating that NSCLC might be resistant to HIF-1α inhibition. Therefore, in our study, we determined the effectiveness of the HIF-1α inhibitor PX-478 in orthotopic models of human NSCLC and SCLC in mice. We found that PX-478 was effective in our models.
Welsh et al.17 suggested that the effectiveness of PX-478 is positively correlated with HIF-1α expression in tumors. Therefore, we used the adenocarcinoma cell line PC14PE6 for our NSCLC model because this cell line has high HIF-1α expression, both in vitro and in tumor tissue grown orthotopically in the lungs of mice. A 5-day course of PX-478 had marked antitumor activity in a dose-dependent manner and resulted in a longer median survival duration. Different schedules and the addition of paclitaxel did not lead to further tumor growth inhibition, indicating that paclitaxel targets the same cell population in the tumor. Because PX-478 treatment is effective in both this cell line and the NCI-H441 cell line, the nonresponsiveness of A-549 tumors to PX-478 is not specific to the NSCLC histologic type and may be due to yet unknown genetic alterations in this cell line.
PX-478 was particularly effective in our SCLC models. In the model using NCI-H187 cells, a 5-day course of PX-478 was curative in some mice, and in other mice, it led to dramatic tumor regression. Different schedules were equally effective. In the second SCLC model, based on NCI-N417 cells, we again observed a marked reduction in tumor growth; however, we observed no curative effects in the mice.
An important result that confirmed the benefits of PX-478 in SCLC treatment is the marked increase in the median survival duration after a 5-day course of PX-478 in the NCI-H187 model. Most mice in the treatment group had to be killed because of metastatic disease rather than impairment by the primary tumor. SCLC is known for spreading early to distant organs, and we frequently observe distant metastasis in our NCI-H187 model, but most mice have to be killed because of primary lung tumor burden, possibly before they develop visible metastases. However, in the PX-478-treated group, primary tumor growth was controlled, and the mice developed metastases later. Because we treated only mice with PX-478 for 5 days, early established micrometastases may be resistant to HIF-1α inhibition because of the lack of hypoxia and thus HIF-1α expression in these cells. To test this hypothesis, we are now planning to repeat this survival experiment and include a second 5-day treatment cycle 3 weeks after the first to target metastases in this model.
PX-478 had a more pronounced effect on tumor growth in the NCI-H187 model than in the PC14PE6 model, although IC50 values for PX-478 obtained in MTT assays conducted under normoxic and hypoxic (1% O2) conditions were similar in all four cell lines used in this study (data not shown), suggesting that sensitivity to the drug differs in vitro and in vivo. The differences in tumor growth may be because of different growth patterns observed in tumors based on these cell lines. We killed several mice before the start of treatment and 5 days later and found extended areas of necrosis and hypoxia in NCI-N417 and NCI-H187 tumors, whereas PC14PE6 tumors had only small necrotic and hypoxic areas (as measured by pimonidazole binding, data not shown). Furthermore, NCI-H187 tumors had a high background of apoptotic cells around the necrotic areas, even in the vehicle-treated group. In contrast, PC14PE6 vehicle-treated tumors had only a few apoptotic cells in a more scattered pattern. This observation and the profound effect of PX-478 on tumor growth suggest that NCI-H187 tumors are more sensitive, at least during an earlier stage of tumor growth, to apoptosis than the adenocarcinoma cell line PC14PE6, even in untreated tumors. We are planning to use an antiangiogenic drug in combination with PX-478 in the treatment of PC14PE6 tumors to inhibit vascularization and induce hypoxia, which may increase tumor growth inhibition.
An unexpected observation was that treatment with PX-478 did not significantly reduce HIF-1α expression or microvessel density in tumor tissues obtained from orthotopic models, although it inhibited expression in these cell lines in vitro. Interestingly, in NCI-H187 and NCI-N417 cells, HIF-1α expression levels were highest in hypoxic and necrotic areas. In PC14-PE6 tumors, HIF-1α levels were higher and random, and the tumors were not necrotic and less hypoxic. It is tempting to speculate that at least in PC14PE6 cells, HIF-1α expression is maintained by a hypoxia-independent mechanism in vivo that is not inhibited by PX-478. It is also likely that the microenvironment of the lung or different pharmacokinetics influences the effectiveness of PX-478 on HIF-1α inhibition; original studies were performed in subcutaneous models and the drug was given in higher doses intraperitoneally. It is possible that oral administration of PX-478 might lead to conversion of the drug into metabolites in the GI tract, which have cytotoxic effects or off-target effects, which may have contributed to tumor inhibition in our model. This cytotoxic or off-target effect would also explain why apoptosis seems to be the main mechanism of the drug in vivo under the condition tested in our experiments, and HIF-1α inhibition and reduction in microvessel density—through inhibition of HIF-1-dependent vascular endothelial growth factor expression—were not significant. In summary, off-target effects, which has also been described recently22 for this drug, may have contributed to tumor inhibition in our model. Further studies are necessary to determine the exact mechanism of tumor inhibition.
In summary, we showed that PX-478 is highly active against orthotopic models of human lung cancer. PX-478 treatment decreased tumor growth in a dose-dependent manner in the lung adenocarcinoma model and slightly but significantly improved survival when given as monotherapy for just 5 days. It also markedly reduced the rate of mediastinal lymph node metastasis. PX-478 was particularly effective against two orthotopic models of SCLC. In the NCI-H187 model, it had a curative effect in some mice and radically inhibited tumor growth in others. PX-478 treatment also dramatically increased the survival duration in this model. PX-478 was highly effective in the other SCLC model (NCI-N417), although curative effects were not observed. Our preclinical data indicate that PX-478 is active against HIF-1α-expressing human NSCLC and SCLC tumors and warrant further clinical investigation of the drug in lung cancer patients.
ACKNOWLEDGMENTS
We thank Ann Sutton for carefully editing the manuscript.
Supported by Lung Cancer Research Foundation grants (to J.J., R.H.), NIH grants R01-CA098920 and P01-CA017094 (to G.P.).
Footnotes
Disclosure: G. Powis named on patent of PX-478, holds stock in Oncothyreon and was a consultant. L. Kirckpatrick holds stocks in Oncothyreon and was a former employee. R. Herbst conducts the clinical trial of PX-478.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 4.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33:526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 9.Chan DA, Krieg AJ, Turcotte S, et al. HIF gene expression in cancer therapy. Methods Enzymol. 2007;435:323–345. doi: 10.1016/S0076-6879(07)35016-7. [DOI] [PubMed] [Google Scholar]
- 10.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. DEC1 (STRA13) protein expression relates to hypoxia- inducible factor 1-alpha and carbonic anhydrase-9 overexpression in non-small cell lung cancer. J Pathol. 2003;200:222–228. doi: 10.1002/path.1330. [DOI] [PubMed] [Google Scholar]
- 12.Enatsu S, Iwasaki A, Shirakusa T, et al. Expression of hypoxia-inducible factor-1 alpha and its prognostic significance in small-sized adenocarcinomas of the lung. Eur J Cardiothorac Surg. 2006;29:891–895. doi: 10.1016/j.ejcts.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Hung JJ, Yang MH, Hsu HS, et al. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax. 2009;64:1082–1089. doi: 10.1136/thx.2009.115691. [DOI] [PubMed] [Google Scholar]
- 14.Ioannou M, Papamichali R, Kouvaras E, et al. Hypoxia inducible factor-1 alpha and vascular endothelial growth factor in biopsies of small cell lung carcinoma. Lung. 2009;187:321–329. doi: 10.1007/s00408-009-9169-z. [DOI] [PubMed] [Google Scholar]
- 15.Karetsi E, Kerenidi T, Ioannou M, et al. Clinical significance of hypoxia inducible factor-1 alpha and VEGF expression in lung cancer. European Respiratory Society (ERS) Annual Meeting; Vienna. 2009. Abstract 283. [Google Scholar]
- 16.Lau SK, Boutros PC, Pintilie M, et al. Three-gene prognostic classifier for early-stage non small-cell lung cancer. J Clin Oncol. 2007;25:5562–5569. doi: 10.1200/JCO.2007.12.0352. [DOI] [PubMed] [Google Scholar]
- 17.Welsh S, Williams R, Kirkpatrick L, et al. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2004;3:233–244. [PubMed] [Google Scholar]
- 18.Yano S, Nokihara H, Hanibuchi M, et al. Model of malignant pleural effusion of human lung adenocarcinoma in SCID mice. Oncol Res. 1997;9:573–579. [PubMed] [Google Scholar]
- 19.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci US A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onn A, Isobe T, Itasaka S, et al. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin Cancer Res. 2003;9:5532–5539. [PubMed] [Google Scholar]
- 21.Wu W, Onn A, Isobe T, et al. Targeted therapy of orthotopic human lung cancer by combined vascular endothelial growth factor and epidermal growth factor receptor signaling blockade. Mol Cancer Ther. 2007;6:471–483. doi: 10.1158/1535-7163.MCT-06-0416. [DOI] [PubMed] [Google Scholar]
- 22.Palayoor ST, Mitchell JB, Cerna D, et al. PX-478, an inhibitor of hypoxia-inducible factor-1alpha, enhances radiosensitivity of prostate carcinoma cells. Int J Cancer. 2008;123:2430–2437. doi: 10.1002/ijc.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]