Abstract
Purpose
Apolipoprotein E (APOE) has been studied for its potential role in osteoporosis risk. It is hypothesized that genetic variation at APOE locus, known as E2, E3, and E4, may modulate bone mineral density (BMD) through its effects on lipoproteins and vitamin K transport. The purpose of this study was to determine the association of the APOE-E4 gene polymorphism with bone-related phenotypes.
Methods
We conducted a meta-analysis that combined newly-analyzed individual data from two community-based cohorts, the Framingham Offspring Study (N=1,495) and the Vitamin K Clinical Trial (N=377), with fifteen other eligible published reports. Bone phenotypes included BMD measurements of the hip (total hip and trochanteric and femoral neck sites) and lumbar spine (from the L2 to L4 vertebrae) and prevalence or incidence of vertebral, hip and other fractures.
Results
In sex-pooled analyses, APOE4 carriers had a 0.018 g/cm2 lower weighted mean trochanteric BMD than non carriers (p=0.0002) with no evidence for between-study heterogeneity. A significant association was also detected with lumbar spine BMD (p=0.006); however, inter-study heterogeneity was observed. Associations with lumbar spine and trochanteric BMD were observed predominantly in women and became less significant in meta-regression (p=0.055 and 0.01, respectively). There were no consistent associations of APOE4 genotype with BMD at other skeletal sites or with fracture risk.
Conclusions
Based on these findings, there is insufficient evidence to support a strong and consistent association of the APOE genotype with BMD and fracture incidence.
Keywords: Apolipoprotein E, BMD, Fracture, meta-analysis, polymorphism
Introduction
Apolipoprotein E (APOE) is a ligand for the low density lipoprotein (LDL) receptor, as well as other triglyceride-rich lipoprotein receptors, and is therefore largely responsible for the cellular uptake of triglyceride rich lipoproteins [1]. In mice lacking APOE, there is increased bone formation which has been attributed to a decreased uptake of triglyceride-rich lipoproteins by the osteoblasts [2]. In humans, three common alleles, made up of amino acid substitutions at positions 112 and 158, are found in the population and are known as E2, E3, and E4 [1].
Given that bone mineral density (BMD) is a major determinant of bone strength acting under substantial genetic regulation (heritability estimates ranging from 0.5 to 0.9), variation in the APOE gene has been studied for its potential role in osteoporosis risk; however results have been inconsistent. Some association studies have found a lower BMD or increased risk of fracture in carriers of the E4 allele compared to those without the E4 allele[3–6], whereas others have not found this association [7–11]. Large-scale evidence is still lacking to clarify the role of APOE in bone metabolism.
In the current study, we tested the hypothesis that variability in BMD is associated with specific genotypes in APOE. We examined cross-sectional associations in two independent cohorts: a community-based sample of middle-aged men and women (the Framingham Offspring Study) [12] and a sample of healthy older men and women prior to randomization in a vitamin K supplementation clinical trial [13]. Since a considerable number of studies have already accumulated in the field, we combined the findings from these two studies with previously reported results in a meta-analysis to examine the associations of APOE4 genotype with both fracture and BMD of the hip and spine.
Material and Methods
Study Cohorts
Framingham Offspring Study
The Framingham Osteoporosis Study (FOS) is an ancillary study of the population-based Framingham Heart Study (FHS). The FHS is a community-based, prospective observational cohort of unrelated individuals of European descent described in detail elsewhere [12]. Between 1996 and 2001, eligible participants underwent routine medical history, physical examination, and BMD measures, as well as consented for genetic analysis and provided a blood sample. Unrelated participants in the current study were not selected on the basis of any trait, but are a subset from the Framingham Offspring cohort who provided blood samples for DNA and who had bone phenotypes. The detailed design of the Framingham Osteoporosis Study have been described previously [14].
Subjects were excluded if using osteoporosis medications (N=48) or the anticoagulant warfarin (N=30). Forty-seven subjects with the APOE 2/4 genotype were also excluded. The final sample consisted of 1,495 individuals.
Vitamin K clinical trial (VitK)
Free-living men and postmenopausal women, 60–80 years of age, were enrolled in a three-year, double-blind, randomized, placebo-controlled trial to assess the impact of vitamin K supplementation on age-related bone loss, as described elsewhere [13]. Exclusion criteria included a usual dietary calcium intake >1500 mg/d; a usual dietary vitamin D intake >1500 IU/d; women< 5 years postmenopausal; femoral neck bone density at screening >1.8 standard deviations above or below an age-and sex-matched reference mean; a 24-hour urine calcium to creatinine ratio >300mg/g for women or 350 mg/g for men; a terminal illness; renal or liver disease; a kidney stone in the past 5 years; current hyperparathyroidism; current warfarin use, and current treatment with an osteoporosis treatment medication or estrogen replacement. Of the 452 participants enrolled in this study, 36 subjects were excluded due to inability to obtain DNA and/or written consent for genotyping. Ten subjects with the APOE 2/4 genotype were also excluded. Given that BMD has been shown to differ by race and ethnicity groups [15], analyses were limited to Caucasians (93% of the subjects). The final sample consisted of 377 individuals. Data were collected at the baseline visit between 2000 and 2002, before randomization.
The Boston University, Hebrew Senior Life and Tufts Medical Center Institutional Review Boards reviewed the protocol for the Framingham Offspring Study and the Tufts Medical Center Institutional Review Board reviewed the protocol for the Vitamin K Clinical Trial, also registered with ClinicalTrial.gov (#NCT00183001).
Determination of Bone Mineral Density
In both studies, BMD was measured using dual-energy X-ray absorptiometry (model DPX-L, Lunar Radiation Corp). The BMD measures were taken at the hip (femoral neck and trochanter) and spine (L2-L4). Although total body BMD was measured in the Framingham Offspring Study, this anatomical site was not included in these analyses to be consistent in anatomical sites with the Vitamin K Clinical Trial. No data on fractures were available for these two studies.
Biochemical measurements
Plasma lipid profiles included enzymatic measurement of triglyceride concentrations [16] using a COBAS Mira (Roche Instruments). Plasma25-hydroxyvitamin D (25(OH)D) was measured by RIA (DiaSorin, Stillwater, MN). Samples from both studies were processed at the Jean Mayer United States Department of Agriculture Human Nutrition Research Center at Tufts University. Coefficients of variation ranged between 8.5% and 16.0%.
Additional participant characteristics
For both studies, information regarding current medication use, medical history and smoking status was collected. Height and weight were measured while the subjects stood. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. Current smokers were defined as subjects who reported smoking cigarettes on a regular basis during the previous year. Leisure, household, and occupational activities were estimated with use of the Physical Activity Scale for the Elderly (PASE) questionnaire [17].
Genotyping
Three common APOE alleles, made up of amino acid substitutions at positions 112 and 158, are found in the population and are known as E2, E3, and E4 [1]. In the Framingham Offspring Study, DNA was extracted from 5–10 mL whole blood with the method described by Miller et al.[18]. APOE genotypes were determined as described by Hixson and Vernier [19]. In VitK, genomic DNA was isolated from blood and purified for PCR analysis using the QIAamp DNA Mini Kit (Qiagen Inc., Chatsworth, CA). Genotyping was carried out using the ABI prism 7900 using single nucleotide polymorphism (SNP) discrimination assays designed by Applied Biosystems (ABI) (Applied Biosystems, Foster City, CA). Accuracy of genotype was tested by introducing 5% dummy duplicates and blank controls. The concordance rate for the duplicates was 100%.
Statistical Analysis
Association analysis of individual studies
Statistical analyses for both studies focused on the association between APOE genotype and BMD of the hip and spine. Individuals were defined E4 carriers if they carried at least one APOE4 allele corresponding to the 3/4 and 4/4 genotypes and non-carriers if they carried E2 or E3 alleles and their genotypes were 2/2, 2/3, or 3/3. Due to opposing effects of the E2 and E4 alleles reported for several traits, individuals with the 2/4 genotype were not included in the analyses.
The genotype frequencies were evaluated for Hardy-Weinberg Equilibrium (HWE) using the χ2 test. Multivariable regression analysis was used to evaluate the association between hip and spine BMD and APOE genotype, while adjusting for age, BMI, physical activity, triglycerides, serum 25(OH)D, and statin use. In the analysis of the Framingham Offspring cohort, additional adjustments were made for menopausal status and estrogen replacement therapy in women. All analyses of individual studies were conducted for men and women separately due to the known gender differences in BMD using SPSS for Windows (v.14). Tests with p-value <0.05 were considered statistically significant.
Meta-analysis
We searched PubMed for publications on the association of APOE genotypes with BMD or fractures using combinations of terms such as apolipoprotein E, APOE, bone mineral density, osteoporosis, dual X-ray absorptiometry, and fractures (last search October 16, 2009). The full search strategy is provided in Supplemental material. We also screened abstracts from the annual American Society for Bone and Mineral Research meetings (2007–2009) using the same terms. Eligible studies had a sample size of at least 10 people, 18 years of age or older, and were published as full articles in English-language journals. Eligible were BMD measurements of the lumbar spine (LS), femoral neck (FN), greater trochanter (or intertrochanter region), total hip, or total body with DXA. Fractures were identified either by questionnaire, medical records, or radiographic documentation. Longitudinal studies also had data available on incident fractures that had occurred during the follow-up period. Family-based studies were excluded.
For each eligible paper, we extracted bibliographic information, demographics (age, sex, ethnicity), study design (prospective or retrospective cohort, case-control), details of BMD measurements, definition of fractures (site; whether they were incident or prevalent and how they were diagnosed) and numerical data necessary for meta-analysis. Data extraction was performed by a single investigator (M.Y.) in electronic tables, and was further checked by two independent investigators.
For BMD outcomes, we calculated mean differences with standard errors, and for fractures risk, odds ratios with 95% confidence intervals between E4 allele carriers and non-carriers. We performed sex-pooled and sex-stratified meta-analyses for each outcome separately assuming a dominant model for the E4 allele (E4 carriers versus non-carriers) using the DerSimonian and Laird random effects model[20]. We tested for between-study heterogeneity with Cochran’s Q (considered significant when P<0.05) and quantified its extent with I2 [21]. I2 is expressed as the proportion of between-study variability that is attributable to heterogeneity rather than chance, with values larger than 50% indicative of high heterogeneity. Because of the way that primary studies reported the data, unadjusted effect sizes were synthesized.
As BMD measurements obtained from the same individuals at different sites are correlated, we performed multivariate (joint) meta-analysis of LS, FN, total hip, total body and trochanteric BMD (the phenotypes with sufficient number of studies) assuming that the correlation coefficients between the four phenotypes are the same as those that we calculated from the primary data in the FOS and VitK studies. The joint meta-analysis was fitted in a meta-regression framework, using a random effects model estimated with restricted maximum likelihood. The multivariate approach also allows phenotypes with more studies to inform the summary effects of phenotypes with fewer studies (borrowing strength). Such analyses were not possible for fracture data.
Finally, in sensitivity analyses we excluded the first-published [22], most extreme, or HWE-deviating studies [23], as well as studies conducted in different ethnic or racial groups, to assess the robustness of our results. We also repeated analyses by excluding the two studies that were analyzed in the current work. Meta-analyses were performed in Meta-analyst (Tufts EPC, Boston, MA) [24]and Stata SE 11 (Stata Corp, College Station, TX) using the user-written programs metan and mvmeta. Unless otherwise stated, all p-values are two-tailed and uncorrected for multiple comparisons. Nominal significance was set at 0.05.
Results
Association analysis of individual studies
The genotype frequencies conformed to HWE in both cohorts (p>0.05). Participant characteristics of the 809 women and 686 men from FOS and 222 women and 155 men from VitK stratified by APOE genotype are presented in Table 1. The overall mean age was 60 ± 9 years and 68 ± 5.5 years, respectively. Carriers of the E4 allele were more likely to be treated with statins (except the men from VitK). Also, male carriers of the E4 allele from FOS had higher total cholesterol levels.
Table 1.
Participant characteristics
| Framingham Offspring Study |
APOE genotype
|
|||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| E2/2, 2/3 or 3/3 | E3/4 or 4/4 | p | E2/2, 2/3 or 3/3 | E3/4 or 4/4 | p | |
| N=549 | N=137 | N=650 | N=159 | |||
| Age, years | 61±0.4 | 62±0.7 | 0.81 | 60±0.4 | 59±0.7 | 0.41 |
| BMI, kg/m2 | 29±0.2 | 28±0.3 | 0.43 | 28±0.2 | 28±0.4 | 0.43 |
| PASE | 156±7 | 161±8 | 0.53 | 140±3 | 127±5 | 0.04 |
| Total cholesterol, mg/dL | 191±1.5 | 195±3.2 | 0.23 | 207±1.5 | 210±2.9 | 0.43 |
| Triglycerides, mg/dL | 141±4.7 | 153±10 | 0.25 | 132±3.2 | 141±6.4 | 0.19 |
| Current smoker, % | 10 | 9 | 0.84 | 13 | 13 | 0.95 |
| Statin use, % | 20 | 27 | <0.01 | 11 | 20 | <0.01 |
|
| ||||||
| Vitamin K Clinical Trial | N=128 | N=27 | N=170 | N=52 | ||
|
| ||||||
| Age, years | 69±0.5 | 69±1.0 | 0.52 | 68±0.4 | 66±0.7 | 0.01 |
| BMI, kg/m2 | 28±0.4 | 28±0.8 | 0.68 | 28±0.4 | 27±0.6 | 0.15 |
| PASE | 133±5 | 124±12 | 0.42 | 121±4 | 127±8 | 0.42 |
| Total cholesterol, mg/dL | 188±3.0 | 185±5.3 | 0.65 | 214±3.0 | 217±4.4 | 0.63 |
| Triglycerides, mg/dL | 122±6.7 | 113±13.4 | 0.81 | 114±5.3 | 109±6.5 | 0.66 |
| Current smoker, % | 5 | 13 | 0.55 | 6 | 0 | 0.18 |
| Statin use, % | 25 | 22 | 0.48 | 23 | 40 | 0.02 |
Values are presented as Mean ± SEM unless otherwise noted; PASE – Physical Activity Scale for the Elderly. Continuous traits were compared using the t-test; smoking status and statin use were compared using the χ2 test.
In the FOS, after adjustment for covariates, women carriers of the APOE4 allele had a significantly lower lumbar spine BMD relative to those without the APOE4 allele; however this association was not found for BMD measures of the hip. APOE genotype was not a significant predictor of BMD in men in this cohort (Figure 1). No significant association between the APOE genotype and BMD was detected in the VitK sample (Figure 1).
Figure 1. Association of APOE4 genotypes with BMD (APOE E2/E2, E2/E3, and E3/E3 versus APOE E3/E4 or E4/E4 carrier status) in sex-stratified and pooled analyses.
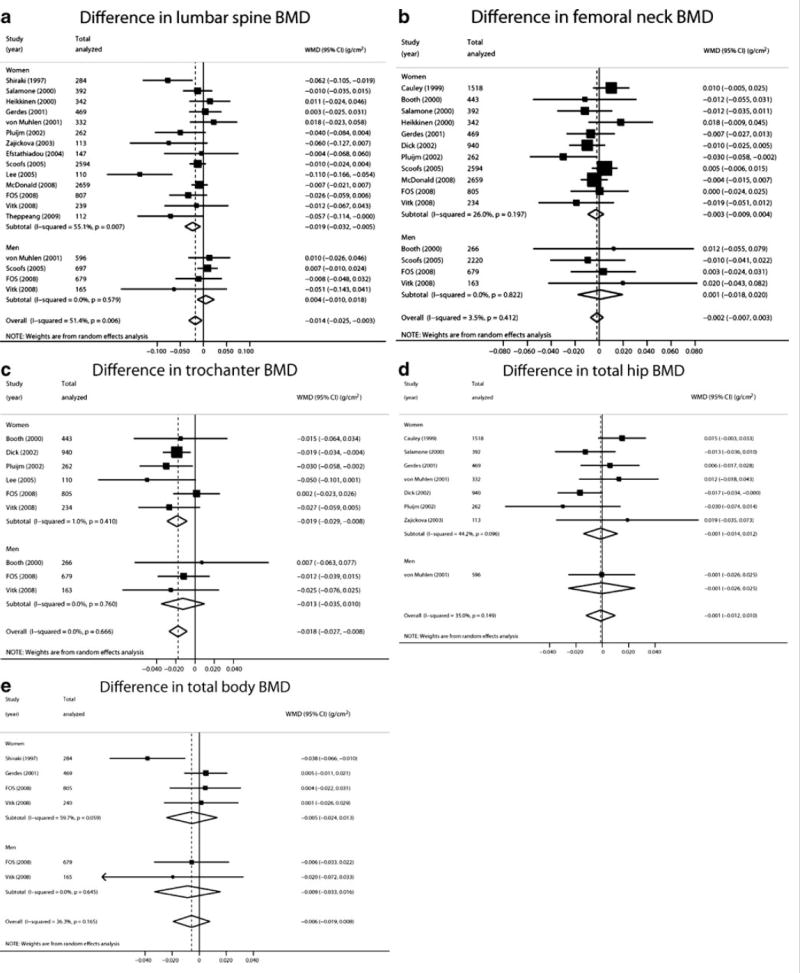
A. Lumbar spine; B. Femoral neck; C. Trochanter; D. Total hip, and E. Total body BMD.
Meta-Analysis
Forty-three studies were retrieved and reviewed in full text. Twenty-six were excluded because they did not have primary data (n=11), did not report outcomes of interest (n=11), or were published in languages other than English (n=4) (Supplemental Figure S1). We finally included seventeen studies (counting the two described in this manuscript) that provided data on 22 sex strata. The studies were performed in populations of European descent, with the exception of two studies conducted in Korean [25]and Japanese [4]women and a small study that also included women of African American descent and provided race-adjusted analyses[26]. Most studies excluded APOE 2/4 genotype carriers from the analyses. Table 2 summarizes the characteristics of these studies.
Table 2.
Characteristics of studies included in the meta-analysis
| Study, Year (reference) | Country | Design | Number, Population | Female (%); Mean age (y) | Phenotypes
|
BMD measurements | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LS | FN | Troch | T-Hip | T-Body | Fracta | ||||||
| VitK 2008 (13) | US | Clinical trial | 405, Free living men and postmenopausal women | 59; 69 | √ | √ | √ | – | √ | – | Lunar |
| Heikkinen 2000 (51) | Finland | Clinical trial | 464, Early postmenopausal women | 100; 53 | √ | √ | – | – | – | – | Lunar |
| Booth 2000 (52) | US | Cohort | 888, General | 62; 75 | – | √ | √ | – | – | – | Lunar |
| Cauley 1999 (53) | US | Cohort | 1750, Postmenopausal women | 100; 71 | – | √ | – | √ | – | √ | Hologic |
| Dick 2002 (54) | Australia | Cohort | 1332, Postmenopausal women | 100; 75 | – | √ | √ | √ | – | √ | Lunar, Hologic |
| Efstathiadou 2004 (10) | Greece | Cohort | 147, Peri-and postmenopausal | 100; 54 | √ | – | – | – | – | √ | Norland or Lunar |
| FOS 2009 (this study) | US | Cohort | 1495, General | 54; 62 | √ | √ | √ | – | √ | – | Lunar |
| Gerdes 2001 (55) | Denmark | Cohort | 479, Perimenopausal women | 100; 50 | √ | √ | – | √ | √ | – | Hologic |
| MacDonald 2008 (6) | UK | Cohort | 2721, Early postmenopausal women | 100; (49–54)b | √ | √ | – | – | – | – | Norland |
| Pluijm 2002 (7) | Netherlands | Cohort | 1406, Elderly men and women | 51; 76 | √ | √ | √ | √ | – | √ | Hologic |
| Salamone 2000 (56) | US | Cohort | 392, General | 100; 47 | √ | √ | – | √ | – | – | Hologic |
| Schoofs 2004 (9) | Netherlands | Cohort | 5857, Elderly men and women | 56; 70 | √ | √ | – | – | – | √ | Lunar |
| Von Muhlen 2001 (8) | US | Cohort | 928, Postmenopausal women not using estrogen | 36; 74 | √ | – | – | √ | – | √ | Hologic |
| Theppeang 2008 (26) | US | Cross-sectional | 112, General | 100; 60 | √ | – | – | – | – | – | Hologic |
| Lee 2005 (25) | Korea | Cross-sectional | 110, Postmenopausal women with rheumatoid arthritis | 100; (range 45, 82) | √ | – | √ | – | – | – | Lunar |
| Shiraki 1997 (4) | Japan | Cross-sectional | 284, Postmenopausal women | 100; 64 | √ | – | – | – | √ | – | Lunar |
| Zajickova 2003 (57) | Czech Republic | Cross-sectional | 113, Postmenopausal women | 100; 63 | √ | – | – | √ | – | – | Hologic |
| Bagger 2007 (30) | Denmark | Cross-sectional | 1176, Postmenopausal women | 100; 69 | – | – | – | – | – | √ | Hologic |
All fractures, vertebral fractures or hip fractures,
Visit 1-Visit 2. FN, femoral neck; LS, lumbar spine; Troch, trochanteric BMD; T-Hip, Total hip; T-Body, Total body BMD; VitK, Vitamin K Clinical Trial; FOS, Framingham Offspring Study.
BMD phenotypes
Among 18 sex-specific strata (14 reports in women and 4 reports in men totaling 8,862 and 2,137 individuals, respectively), BMD at the lumbar spine from the L2 to L4 vertebrae was on average 0.014 g/cm2 lower in carriers of APOE4 genotype compared to non-carriers (95%CI −0.025 to −0.003; p=0.006) (Figure 1a, Supplemental Table S1). This association was observed predominantly among women (0.019 g/cm2 difference, nominal p=0.006). Significant inter-study heterogeneity was observed in the sex-pooled and women-only analyses (I2=51% and 55%, respectively; p<0.01, Supplemental Table S1). FN BMD (15 sex-specific strata; 10,658 women, 3,328 men) was on average 0.002 g/cm2 lower among APOE4 carriers (p=0.47). These results were similar when women and men or different ethnicities were considered separately (Figure 1b, Supplemental Table S1). Trochanteric BMD was available for 9 strata (2,794 women and 1,108 men). The weighted mean difference between carriers and non-carriers was 0.018 g/cm2 (lower in carriers, 95%CI −0.027 to −0.008; p=0.0002), with no evidence for between-study heterogeneity (Figure 1c, Supplemental Table S1). As with LS BMD, this association was significant among women but not men (nominal p=0.0004 vs. p=0.27, Supplemental Table S1). Neither hip nor total body BMD were associated with APOE4 carrier status (Figure 1d and e), albeit based on fewer studies.
Results of meta-regression were very similar to those of the single-trait meta-analyses for four endpoints (total body BMD was not included because we did not have information on its correlation with other BMD phenotypes). Interestingly, the p-value for the association between LS BMD and APOE4 carrier status became non-significant (p=0.055)and the corresponding p-value for the trochanteric BMD became less extreme (p=0.011; Supplemental Table S2).
Fractures
In the sex-pooled analysis, no association was found between the incidence or prevalence of any fractures, hip or vertebral, and APOE4 carrier status (Figure 2). In men, APOE4 carriers showed a lower risk of vertebral fractures (OR=0.58; 95% CI 0.35–0.96) compared to non-carriers; this result was based on two studies (N=2,067).
Figure 2. Association of APOE4 genotypes with fractures (APOE E2/E2, E2/E3, and E3/E3 versus APOE E3/E4 or E4/E4 carrier status) in sex-stratified and pooled analyses.
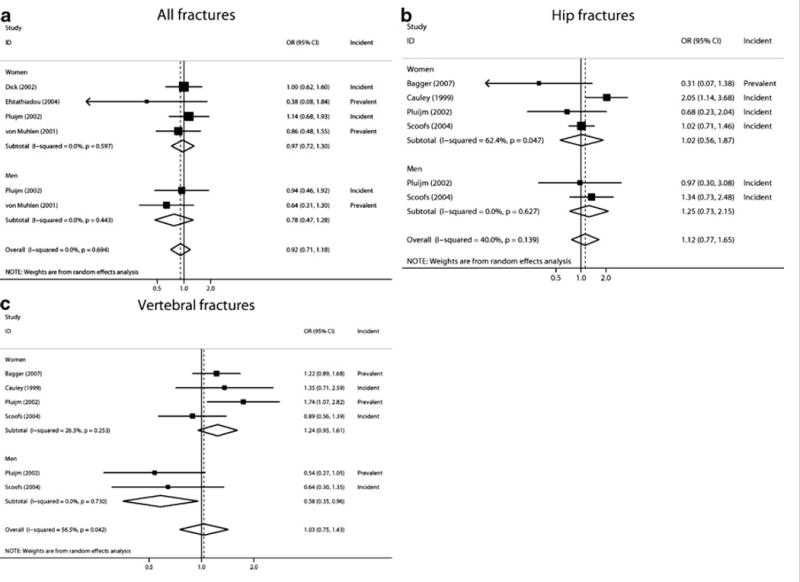
A. All fractures; B. Hip fractures, and C. Vertebral fractures.
Sensitivity analyses
The aforementioned results were similar in the sensitivity analyses. When we excluded the study showing the most extreme association [25](N=110), the LS BMD weighted mean difference between genotypes was attenuated (−0.009 g/cm2 [95% CI −0.018 to 0.000; p=0.04] from −0.014 g/cm2) and the initially observed high between-study heterogeneity became smaller (p for heterogeneity=0.13, I2=29%). This association and between-study heterogeneity disappeared when only studies performed in Caucasian populations were included (N=10,493; p=0.10). Apart from that, results of most sensitivity analyses were not remarkable (Supplemental Tables S1 and S3).
Discussion
This study addresses the association between the APOE4 gene polymorphism and osteoporosis-related phenotypes, a topic that has spurred controversy in the literature. Using meta-analysis that combined individual level data from two community-based cohorts, the Framingham Offspring Study and the Vitamin K Clinical Trial, and fifteen additional published reports, we found no consistent association of APOE4 genotype with BMD at most skeletal sites or with fracture risk. Although we observed associations of APOE4 with trochanteric and lumbar spine BMD, these results were not robust in sensitivity analyses.
Biological plausibility for the association between APOE and bone outcomes has been based on several lines of research. The expression of the APOE gene has been observed in osteoblasts [27]. The mouse model lacking the APOE gene (APOE−/−) has been shown to display a high bone mass phenotype caused by an increased bone formation rate [2], which can be significantly reduced through bone resorption in the presence of high-fat diet[28]. Moreover, in human studies, the APOE E4 allele, known for modulating effects on serum lipid profiles, has been found to be associated with high levels of total cholesterol, low-density lipoprotein (LDL) and ApoB and, as a result, with the development of atherosclerosis [29]. It has been suggested that obstructive vascular disease caused by atherosclerotic burden may hamper blood supply to the skeletal sites resulting in bone loss and fractures [30]. Also, vascular calcification has been shown to be associated with lower BMD, and greater bone turnover has been associated with increased risks for cardiovascular events[31]. Further, elevated levels of LDL in APOE4 allele carriers have been shown to accumulate oxidized lipids in subendothelial space of bone vasculature that can, in turn, lead to inhibition of osteoblast differentiation [32, 33]. Finally, it is thought that APOE mediates vitamin K transport, which is essential for carboxylation of glutamic acid residues of osteocalcin, an important bone protein[34, 35].
Our results are consistent with recent meta-analyses of genome-wide studies that did not identify genome-wide significant associations (i.e., associations with p-values <10−7) near the APOE gene for BMD and osteoporotic fractures[36, 37]. However, here we provide a detailed presentation of the specific association and examine phenotypes, such as trochanteric, total hip and total body BMD, that were not analyzed in the genome-wide meta-analyses. Interestingly, one of these additional phenotypes, trochanteric BMD, was associated with APOE4 carrier status with a low p-value (p=2*10−4). A nominal association was also identified for LS BMD (p=0.01), but not for FN or total hip BMD. This discrepancy in the results is puzzling. Only few studies investigating the association with APOE genotype have reportedly assessed trochanteric BMD, especially in men. Therefore, even through the sample size generated by the present meta-analysis was thousands of individuals (n=3,904 in sex-pooled analysis and n=1,110 in men), we cannot rule out a spurious association, or an association driven by selective reporting, a phenomenon originally described in randomized trials[38]. Further, in meta-regression that accounts for the correlations among the different BMD phenotypes, the significance of the aforementioned associations diminished. While we are cautious to report this association solely based on nominal statistical significance that may not pass the correction for multiple comparisons, it is interesting to note that the associations that we observed seemed to be specific to BMD of skeletal sites composed primarily of trabecular bone and not those composed of cortical bone. Recent evidence suggests that genetic factors contributing to BMD are site-specific and can be unique to different bone compartments[39, 40]. Moreover, it has also been shown that in women a period of accelerated trabecular bone loss begins at the time of menopause and plateaus 5–10 years later[41]. In men, however, a greater magnitude of trabecular bone loss compared to cortical bone loss occurs after the age of 70 years[42]. We can speculate that gender-specific differences in the patterns of trabecular bone loss with age can potentially mask genetic associations in cross-sectional studies and help explain inconsistencies in the results between men and women. Also, ethnical differences may play a role as the only two studies that individually reported significant associations with BMD were performed in Asian populations.
Whereas we observed a protective effect of APOE4 on the risk of vertebral fractures in men based on two studies only, we were unable to detect consistent associations between APOE4 genotype and incidence or prevalence of bone fractures at other anatomical sites. It may be that an APOE4 effect on fracture risk is observed only in the presence of suboptimal nutritional status. That is, it has been suggested that renal failure patients undergoing hemodialysis have an increased risk of fracture if they carry the APOE4 allele due to their tendency to have lower circulating concentrations of vitamin K[43]. Moreover, given the established association between the APOE4 allele and Alzheimer’s disease, cardiovascular disease and stroke[44–46], it is plausible that the projected higher risk of fractures in carriers is a consequence of the presence of other underlying health conditions rather that the direct effect of APOE4 on the bone.
This study’s limitations include the fact that, like all meta-analyses, the present study is susceptible to reporting biases. When publication bias and outcome reporting bias operate, negative (statistically non-significant) associations are not published at all or are not reported in sufficient detail for inclusion in analyses. This results in an over-representation of statistically significant findings in the meta-analyses, and potentially spurious results. In our case, most analyses were statistically non-significant, and only trochanteric (and marginally LS) BMD were nominally significant. It is impossible to deduce whether reporting biases are responsible for the observed association of trochanteric BMD with APOE4 status or whether the association is genuine. Further, we could not perform meta-analyses adjusted for a range of effect modifiers or confounders, such as physical activity, age, diet, hormonal status in women (menopausal status, hormone replacement therapy), because these require individual level data. Although we adjusted for statin use, we were unable to include information on the dose or duration of use. Some studies have implicated statins as protective for hip fracture[47]. However, the oral route of these agents is unlikely to produce meaningful peripheral concentrations in the skeleton as their action requires contact with bone cells, where as statins passing through the liver reach the skeleton. Moreover, APOE plasma concentrations may be responsible for some of the effects. Whereas the APOE4 allele is associated with lower plasma concentrations of APOE compared to APOE3 and APOE2 alleles, its affinity for the LDL receptor is higher compared to either APOE3 or APOE2. While not well understood, these are two opposing effects which may cancel any effect due to the allele [48]. However, blood APOE levels are not usually measured in population studies so we cannot address this potential confounding effect. Nevertheless, in previous meta-analyses of numerous genetic determinants of BMD and fractures based on individual patient data, various adjustments did not affect the results in any appreciable way[49, 50].
In summary, we find suggestive evidence for associations between LS and trochanteric BMD and APOE4 carrier status. However these results were either based on a subset of the identified studies or are sensitive to single influential studies, and do not attain the stringent levels of statistical significance that are typically required for hypothesis forming research. The results of our study do not support the hypothesis that the APOE4 allele exerts direct effects on BMD or fracture risk. Therefore it is unlikely that carriers of the APOE4 allele are at greater risk for bone loss or require more aggressive therapies to prevent osteoporosis compared to those who are not carriers of the allele.
Supplementary Material
Acknowledgments
Supported by the U.S. Department of Agriculture, Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707, National Institutes of Health (AG14759, HL69272, HL54776 and T32-DK62032), National Institute of Arthritis Musculoskeletal and Skin Diseases and the National Institute on Aging (R01 AR/AG 41398), the Framingham Heart Study of the NIH-NHLBI (Contract No. N01-HC-25195) and the Japanese Ministry of Education, Culture, Sports and Technology (MY). Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Footnotes
The original publication is available at springerlink.com: http://www.springerlink.com/content/j8h25263132lv26r/fulltext.pdf
Conflict of Interest
All authors have no conflict of interest. They have full control of all primary data and agree to allow the journal to review their data if requested.
References
- 1.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–95. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 2.Schilling AF, Schinke T, Munch C, Gebauer M, Niemeier A, Priemel M, Streichert T, Rueger JM, Amling M. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res. 2005;20:274–82. doi: 10.1359/JBMR.041101. [DOI] [PubMed] [Google Scholar]
- 3.Long JR, Liu PY, Liu YJ, Lu Y, Shen H, Zhao LJ, Xiong DH, Deng HW. APOE haplotypes influence bone mineral density in Caucasian males but not females. Calcif Tissue Int. 2004;75:299–304. doi: 10.1007/s00223-004-0034-z. [DOI] [PubMed] [Google Scholar]
- 4.Shiraki M, Shiraki Y, Aoki C, Hosoi T, Inoue S, Kaneki M, Ouchi Y. Association of bone mineral density with apolipoprotein E phenotype. J Bone Miner Res. 1997;12:1438–45. doi: 10.1359/jbmr.1997.12.9.1438. [DOI] [PubMed] [Google Scholar]
- 5.Johnston JM, Cauley JA, Ganguli M. APOE 4 and hip fracture risk in a community-based study of older adults. J Am Geriatr Soc. 1999;47:1342–5. doi: 10.1111/j.1532-5415.1999.tb07436.x. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald HM, McGuigan FE, Lanham-New SA, Fraser WD, Ralston SH, Reid DM. Vitamin K1 intake is associated with higher bone mineral density and reduced bone resorption in early postmenopausal Scottish women: no evidence of gene-nutrient interaction with apolipoprotein E polymorphisms. Am J Clin Nutr. 2008;87:1513–20. doi: 10.1093/ajcn/87.5.1513. [DOI] [PubMed] [Google Scholar]
- 7.Pluijm SM, Dik MG, Jonker C, Deeg DJ, van Kamp GJ, Lips P. Effects of gender and age on the association of apolipoprotein E epsilon4 with bone mineral density, bone turnover and the risk of fractures in older people. Osteoporos Int. 2002;13:701–9. doi: 10.1007/s001980200096. [DOI] [PubMed] [Google Scholar]
- 8.von Muhlen DG, Barrett-Connor E, Schneider DL, Morin PA, Parry P. Osteoporosis and apolipoprotein E genotype in older adults: the Rancho Bernardo study. Osteoporos Int. 2001;12:332–5. doi: 10.1007/s001980170124. [DOI] [PubMed] [Google Scholar]
- 9.Schoofs MW, van der Klift M, Hofman A, van Duijn CM, Stricker BH, Pols HA, Uitterlinden AG. ApoE gene polymorphisms, BMD, and fracture risk in elderly men and women: the Rotterdam study. J Bone Miner Res. 2004;19:1490–6. doi: 10.1359/JBMR.040605. [DOI] [PubMed] [Google Scholar]
- 10.Efstathiadou Z, Koukoulis G, Stakias N, Challa A, Tsatsoulis A. Apolipoprotein E polymorphism is not associated with spinal bone mineral density in peri-and postmenopausal Greek women. Maturitas. 2004;48:259–64. doi: 10.1016/j.maturitas.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Stulc T, Ceska R, Horinek A, Stepan J. Bone mineral density in patients with apolipoprotein E type 2/2 and 4/4 genotype. Physiol Res. 2000;49:435–9. [PubMed] [Google Scholar]
- 12.Cupples LA, D’Agostino RB, Anderson K, Kannel WB. Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med. 1988;7:205–22. doi: 10.1002/sim.4780070122. [DOI] [PubMed] [Google Scholar]
- 13.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93:1217–23. doi: 10.1210/jc.2007-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 15.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18:943–53. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 16.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 17.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–40. [PubMed] [Google Scholar]
- 18.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–8. [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–9. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 23.Trikalinos TA, Salanti G, Khoury MJ, Ioannidis JP. Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am J Epidemiol. 2006;163:300–9. doi: 10.1093/aje/kwj046. [DOI] [PubMed] [Google Scholar]
- 24.Wallace B, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Research Methodol. 2009 doi: 10.1186/1471-2288-9-80. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SI, Lee SY, Yoo WH. Association of apolipoprotein E polymorphism with bone mineral density in postmenopausal women with rheumatoid arthritis. Rheumatology (Oxford) 2005;44:1067–8. doi: 10.1093/rheumatology/keh675. [DOI] [PubMed] [Google Scholar]
- 26.Theppeang K, Glass TA, Bandeen-Roche K, Todd AC, Rohde CA, Links JM, Schwartz BS. Associations of bone mineral density and lead levels in blood, tibia, and patella in urban-dwelling women. Environ Health Perspect. 2008;116:784–90. doi: 10.1289/ehp.10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachner D, Schroder D, Betat N, Ahrens M, Gross G. Apolipoprotein E (ApoE), a Bmp-2 (bone morphogenetic protein) upregulated gene in mesenchymal progenitors (C3H10T1/2), is highly expressed in murine embryonic development. Biofactors. 1999;9:11–7. doi: 10.1002/biof.5520090103. [DOI] [PubMed] [Google Scholar]
- 28.Hirasawa H, Tanaka S, Sakai A, Tsutsui M, Shimokawa H, Miyata H, Moriwaki S, Niida S, Ito M, Nakamura T. ApoE gene deficiency enhances the reduction of bone formation induced by a high-fat diet through the stimulation of p53-mediated apoptosis in osteoblastic cells. J Bone Miner Res. 2007;22:1020–30. doi: 10.1359/jbmr.070330. [DOI] [PubMed] [Google Scholar]
- 29.Wilson PW, Schaefer EJ, Larson MG, Ordovas JM. Apolipoprotein E alleles and risk of coronary disease. A meta-analysis. Arterioscler Thromb Vasc Biol. 1996;16:1250–5. doi: 10.1161/01.atv.16.10.1250. [DOI] [PubMed] [Google Scholar]
- 30.Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tanko LB. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007;18:505–12. doi: 10.1007/s00198-006-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szulc P, Samelson EJ, Kiel DP, Delmas PD. Increased Bone Resorption is Associated with Increased Risk of Cardiovascular Events in Men - the MINOS Study. J Bone Miner Res. 2009 doi: 10.1359/JBMR.090531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol. 2000;20:2346–8. doi: 10.1161/01.atv.20.11.2346. [DOI] [PubMed] [Google Scholar]
- 33.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 34.Kohlmeier M, Salomon A, Saupe J, Shearer MJ. Transport of vitamin K to bone in humans. J Nutr. 1996;126:1192S–6S. doi: 10.1093/jn/126.suppl_4.1192S. [DOI] [PubMed] [Google Scholar]
- 35.Knapen MH, Hamulyak K, Vermeer C. The effect of vitamin K supplementation on circulating osteocalcin (bone Gla protein) and urinary calcium excretion. Ann Intern Med. 1989;111:1001–5. doi: 10.7326/0003-4819-111-12-1001. [DOI] [PubMed] [Google Scholar]
- 36.Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41:1199–206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Zillikens MC, Wilson SG, Mullin BH, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra BA, Pols HA, Sigurdsson G, Thorsteinsdottir U, Soranzo N, Williams FM, Zhou Y, Ralston SH, Thorleifsson G, van Duijn CM, Kiel DP, Stefansson K, Uitterlinden AG, Ioannidis JP, Spector TD. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151:528–37. doi: 10.7326/0003-4819-151-8-200910200-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291:2457–65. doi: 10.1001/jama.291.20.2457. [DOI] [PubMed] [Google Scholar]
- 39.Yerges LM, Klei L, Cauley JA, Roeder K, Kammerer CM, Ensrud KE, Nestlerode CS, Lewis C, Lang TF, Barrett-Connor E, Moffett SP, Hoffman AR, Ferrell RE, Orwoll ES, Zmuda JM. Candidate Gene Analysis of Femoral Neck Trabecular and Cortical Volumetric Bone Mineral Density in Older Men. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yerges LM, Klei L, Cauley JA, Roeder K, Kammerer CM, Moffett SP, Ensrud KE, Nestlerode CS, Marshall LM, Hoffman AR, Lewis C, Lang TF, Barrett-Connor E, Ferrell RE, Orwoll ES, Zmuda JM. A High-Density Association Study of 383 Candidate Genes for Volumetric Bone Density at the Femoral Neck and Lumbar Spine among Older Men. J Bone Miner Res. 2009 doi: 10.1359/JBMR.090524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eastell . Pathogenesis of postmenopausal osteoporosis. In: Favus, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism American. Society for Bone and Mineral Research; Washington DC: 2003. pp. 314–316. [Google Scholar]
- 42.Rochira V, Balestrieri A, Madeo B, Zirilli L, Granata AR, Carani C. Osteoporosis and male age-related hypogonadism: role of sex steroids on bone (patho)physiology. Eur J Endocrinol. 2006;154:175–85. doi: 10.1530/eje.1.02088. [DOI] [PubMed] [Google Scholar]
- 43.Kohlmeier M, Saupe J, Schaefer K, Asmus G. Bone fracture history and prospective bone fracture risk of hemodialysis patients are related to apolipoprotein E genotype. Calcif Tissue Int. 1998;62:278–81. doi: 10.1007/s002239900430. [DOI] [PubMed] [Google Scholar]
- 44.Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer’s disease connection. FASEB J. 1996;10:1485–94. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 45.Contois JH, Anamani DE, Tsongalis GJ. The underlying molecular mechanism of apolipoprotein E polymorphism: relationships to lipid disorders, cardiovascular disease, and Alzheimer’s disease. Clin Lab Med. 1996;16:105–23. [PubMed] [Google Scholar]
- 46.Bersano A, Ballabio E, Bresolin N, Candelise L. Genetic polymorphisms for the study of multifactorial stroke. Hum Mutat. 2008;29:776–95. doi: 10.1002/humu.20666. [DOI] [PubMed] [Google Scholar]
- 47.Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Ensrud KE, van der Klift M, Pols HA. Use of statins and fracture: results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med. 2004;164:146–52. doi: 10.1001/archinte.164.2.146. [DOI] [PubMed] [Google Scholar]
- 48.Greenow K, Pearce NJ, Ramji DP. The key role of apolipoprotein E in atherosclerosis. J Mol Med. 2005;83:329–42. doi: 10.1007/s00109-004-0631-3. [DOI] [PubMed] [Google Scholar]
- 49.Langdahl BL, Uitterlinden AG, Ralston SH, Trikalinos TA, Balcells S, Brandi ML, Scollen S, Lips P, Lorenc R, Obermayer-Pietsch B, Reid DM, Armas JB, Arp PP, Bassiti A, Bustamante M, Husted LB, Carey AH, Perez Cano R, Dobnig H, Dunning AM, Fahrleitner-Pammer A, Falchetti A, Karczmarewicz E, Kruk M, van Leeuwen JP, Masi L, van Meurs JB, Mangion J, McGuigan FE, Mellibovsky L, Mosekilde L, Nogues X, Pols HA, Reeve J, Renner W, Rivadeneira F, van Schoor NM, Ioannidis JP. Large-scale analysis of association between polymorphisms in the transforming growth factor beta 1 gene (TGFB1) and osteoporosis: the GENOMOS study. Bone. 2008;42:969–81. doi: 10.1016/j.bone.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 50.van Meurs JB, Trikalinos TA, Ralston SH, Balcells S, Brandi ML, Brixen K, Kiel DP, Langdahl BL, Lips P, Ljunggren O, Lorenc R, Obermayer-Pietsch B, Ohlsson C, Pettersson U, Reid DM, Rousseau F, Scollen S, Van Hul W, Agueda L, Akesson K, Benevolenskaya LI, Ferrari SL, Hallmans G, Hofman A, Husted LB, Kruk M, Kaptoge S, Karasik D, Karlsson MK, Lorentzon M, Masi L, McGuigan FE, Mellstrom D, Mosekilde L, Nogues X, Pols HA, Reeve J, Renner W, Rivadeneira F, van Schoor NM, Weber K, Ioannidis JP, Uitterlinden AG. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:1277–90. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heikkinen AM, Kroger H, Niskanen L, Komulainen MH, Ryynanen M, Parviainen MT, Tuppurainen MT, Honkanen R, Saarikoski S. Does apolipoprotein E genotype relate to BMD and bone markers in postmenopausal women? Maturitas. 2000;34:33–41. doi: 10.1016/s0378-5122(99)00084-5. [DOI] [PubMed] [Google Scholar]
- 52.Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, Wilson PW, Ordovas J, Schaefer EJ, Dawson-Hughes B, Kiel DP. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr. 2000;71:1201–8. doi: 10.1093/ajcn/71.5.1201. [DOI] [PubMed] [Google Scholar]
- 53.Cauley JA, Zmuda JM, Yaffe K, Kuller LH, Ferrell RE, Wisniewski SR, Cummings SR. Apolipoprotein E polymorphism: A new genetic marker of hip fracture risk--The Study of Osteoporotic Fractures. J Bone Miner Res. 1999;14:1175–81. doi: 10.1359/jbmr.1999.14.7.1175. [DOI] [PubMed] [Google Scholar]
- 54.Dick IM, Devine A, Marangou A, Dhaliwal SS, Laws S, Martins RN, Prince RL. Apolipoprotein E4 is associated with reduced calcaneal quantitative ultrasound measurements and bone mineral density in elderly women. Bone. 2002;31:497–502. doi: 10.1016/s8756-3282(02)00851-7. [DOI] [PubMed] [Google Scholar]
- 55.Gerdes LU, Vestergaard P, Hermann AP, Mosekilde L. Regional and hormone-dependent effects of apolipoprotein E genotype on changes in bone mineral in perimenopausal women. J Bone Miner Res. 2001;16:1906–16. doi: 10.1359/jbmr.2001.16.10.1906. [DOI] [PubMed] [Google Scholar]
- 56.Salamone LM, Cauley JA, Zmuda J, Pasagian-Macaulay A, Epstein RS, Ferrell RE, Black DM, Kuller LH. Apolipoprotein E gene polymorphism and bone loss: estrogen status modifies the influence of apolipoprotein E on bone loss. J Bone Miner Res. 2000;15:308–14. doi: 10.1359/jbmr.2000.15.2.308. [DOI] [PubMed] [Google Scholar]
- 57.Zajickova K, Zofkova I, Hill M, Horinek A, Novakova A. Apolipoprotein E 4 allele is associated with low bone density in postmenopausal women. J Endocrinol Invest. 2003;26:312–5. doi: 10.1007/BF03345178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


