Abstract
Understanding the relationship between external load and bone morphology is critical for understanding adaptations to load in extant animals and inferring behavior in extinct forms. Yet the relationship bony anatomy and load is poorly understood, with empirical studies often producing conflicting results. It is widely assumed in many ecological and paleontological studies that bone size and strength reflects the forces experience by the bone in-vivo. This study examines that assumption by providing preliminary data on gait mechanics in a hypermuscular myostatin-deficient mouse model with highly mineralized and hypertrophied long bones. A small sample of hypermuscular and wild-type mice were videorecorded while walking freely across a force platform. Temporal gait parameters, peak vertical and transverse (mediolateral) ground reaction forces, vertical impulse, and loading rates were measured. The only gait parameters that differed between the two groups were the speeds at which the animals traveled and the transverse forces on the hind limb. The myostatin-deficient mice move relatively slowly and experienced the same magnitude of vertical forces on all limbs and transverse forces on the forelimb as the wild-type mice, though the myostatin-deficient mice did experience lower mediolateral forces on their hindlimbs compared to the wild-type mice. These preliminary results call into question the hypothesis that skeletal hypertrophy observed in hypermuscular mice is a result of larger ground reaction forces experienced by the animals’ limbs during locomotion and calls for further analysis and a cautious approach to inferences about locomotor behavior derived from bony morphology in extant and fossil species.
Keywords: myostatin, locomotor biomechanics, skeletal morphology, mouse model
Introduction
Understanding the relationship between the mechanical properties of animal bones and the forces generated during locomotion has long been a goal of morphologists, particularly those interested in understanding the selective pressures on bony anatomy in extant and extinct animals. Although there is extensive debate about the factors that influence bone anabolism (see Martin and Burr, ‘82; Bertram and Swartz, ‘91; Rubin et al. ‘94, 2001; Turner et al., ‘95; Jacobs et al., ‘98; Robling et al. 2000, 2002; Turner, 2002; Turner and Beamer, 2002; Lieberman et al., 2003; Zumwalt, 2006; Main and Biewener, 2007), it is clear that ground reaction forces generated during locomotion influence the loading environment for any long bone. External ground reaction forces are not a direct surrogate for the strain experienced by bone tissue, but the magnitude and orientation of these forces must play a significant role in determining the strain and bending moments experienced by the bone. The actual strain experienced by the bone tissue is influenced by complex interactions between bone shape, bone mechanical properties, limb position, and ground reaction force magnitude and orientation (see Biewener, ‘89, ‘91).
Despite this complexity, many studies of fossil animals rely on an assumption that bone shape reflects in-vivo loading environment generated by many factors including ground reaction forces generated during locomotion, which are in turn assumed to reflect locomotor activity (see Thomason, ′85; Trinkaus et al., ′91, ‘94, ′99; Heinrich et al., ‘93; Casinos, ′96; Skerry, 2000; Churchill and Schmitt, 2003; Ruff, 2005 for a number of examples). This assumption explicitly underlies many paleontological reconstructions both in anthropology and broader areas of vertebrate paleontology. For example, Casinos (′96) specifically states that the shape of the long bones in Megatherium reflect stresses induces by loads associated with bipedalism. Similarly, Heinrich et al. (′93) use cross-sectional properties to infer the onset of bipedalism in Dryosaurus by assuming a relationship between forces and bone shape. Trinkhaus and colleagues (′91:272) assert that the shape of the Neanderthal femur reflects mediolateral bending stress whereas the shape of the femur of early modern humans reflect the loads “generated during a normal human striding gait.” In a 1995 book dealing with fossil reconstruction, Weishampel (′95:38) argues explicitly that cross-sectional properties provide information on in-vivo mechanical loadings. In the same volume, Thomason (′95:260) discusses the complexities of the issue but still concludes that bone structure encodes information useful in “…reconstructing patterns of force that probably acted on fossil bones in-vivo.”
As we discuss below there are good theoretical and empirical reasons to critically consider the assumption that bone responds to “changes in mechanical strain in a precise and systematic manner.” (Heinrich et al. ′93:179). Therefore, investigating the validity of the presumed correlation between bony morphology and peak ground reaction forces is critical to evaluating the strength of such inferences about the relationship between bone mechanical properties and load as well as reconstructions of locomotion in fossil animals.
It is difficult to collect the kind of data that is necessary to illuminate these complicated issues. Ideally, in order to infer locomotor behavior from long bone morphology, one should collect data on the local strain environment of the bone tissue, bone mechanical properties, limb movements, and ground reaction forces. Numerous studies of large and medium-sized animals such as horses, dogs, primates, and squirrels have provided important insights into the relationship between bone strength and loading (Biewener, ‘82, ‘83a, ‘83b; ‘89, ‘91; Budsberg et al. ‘87; Merkens et al. ‘93a, ‘93b; Riggs et al. ‘93; Demes et al. ‘98, 2001; Blob and Biewener, 2001). But until recently, it was exceedingly difficult to explore strain-based variation in load response in small animal models, despite the ready availability of rodent models of bone growth (Hamrick et al., 2000, 2002, 2003, 2007; Judex, et al. 2002, 2004a, 2004b; Amblard et al. 2003; Hamrick, 2003; Kelly et al. 2006; Carlson and Judex, 2007; Plochoki et al. 2008). A number of recent studies have explored gait patterns in mouse strains and mouse models of disease (Hampton et al. 2004; Herbin et al., 2004; 2006, 2007; Lepicard et al., 2005). The recent development of a small, durable, sensitive force plate (Fig. 1) and inexpensive high-speed cameras now allows collection of accurate ground reaction force values for small rodents (Zumwalt et al. 2006). The availability of a hypermuscular mouse model allowed this study to explore the loading environment of the rodent limb in order to examine the relationship between muscle mass, bone mineralization, and ground reaction forces. The study presented here represents a preliminary dataset utilizing this unique mouse model.
Figure 1.

A) Schematic diagram of the set-up for the mouse ground reaction force data collection. The runway covers but does not touch the force platform (black). Steps only register on the force platform when mouse paws touch the strip of wood covering the center of the plate (*). (B) Image of a mouse making a single forelimb contact with the wood strip (*).
The hypermuscular mice used in this study are deficient in myostatin, a growth factor that is a negative regulator of skeletal muscle growth (McPherron et al., ‘97). Because they lack myostatin, these animals have twice as much muscle in their limbs than do wild-type mice, and it has also been found that myostatin deficiency increases bone rigidity and biomineralization throughout the skeleton, including the limbs (Fig. 2), spine, and jaw (Hamrick et al., 2000, 2002, 2007; Hamrick, 2003; Ravosa et al., 2007). It has been proposed that the increased size and rigidity of the limb bones in these mice is a result of the mechanical influences of their large muscles (Hamrick et al., 2000; Hamrick et al., 2002), but the mechanism by which this relationship may occur has never been explored. It is presently unclear whether the large limb bones of myostatin-deficient mice develop as a response to large external loads experienced during locomotion, or to the action of the large muscles directly on the bones. It is also possible that the skeletal hypertrophy may be the result of a non-mechanical mechanism, such as the expression of osteogenic factors from the muscles or direct effects of myostatin deficiency on the differentiation or activity of osteoprogenitor cells.
Figure 2.

(a) Faxitron radiographs of the humerus in wild type (top row) and myostatin-deficient mice (bottom row) showing the expanded deltoid crest (asterisk) and transverse dimension of the diaphysis (arrows) in the mice lacking myostatin. (b) Radiographs of the femur in normal (top row) and myostatin-deficient mice (bottom row) showing the expanded third trochanter (asterisk) and transverse dimension of the diaphysis (arrows) in mice lacking myostatin.
In addition to the increased muscularity and overall bone rigidity, the diameters of some long bones in these mice are relatively expanded in the transverse plane. In the humerus, this mediolateral expansion is associated with increased periosteal circumference of the diaphysis (Figure 2a) (Hamrick et al., 2002). In the femur, Hamrick and his colleagues noted increased mediolateral diameter and reduced anteroposterior diameter of the diaphysis (Figure 2b) (Hamrick et al., 2003).
If a functional morphologist or paleontologist were to examine the bones of these mice, they might conclude that this animal experienced high peak ground reaction forces with a specific emphasis on vertical and transverse loads. Such a conclusion would lead to the development of postural and behavioral scenarios that would match the assumption of high ground reaction forces. This kind of thinking especially regarding mediolateral bending is evident in studies of fossil mammals (Casinos, ′96) and early humans (Trinkhaus et al. ′99), just as two examples. These reconstructions are ultimately based on the poorly tested assumption that limb biomechanical properties and shape reflect the external loading environment of the limb during locomotion. The mouse model presented here allows us to begin to test this assumption by examining the in vivo loading patterns of an animal that demonstrates clear skeletal hypertrophy.
Background
Vertical ground reaction forces reflect in large part an animal’s body mass and running speed. Thus, researchers interested in understanding the mechanical principles and the underlying selective pressures that influence limb design have examined the relationship between bone mechanical properties, posture, and body size (see Alexander et al., ‘79; Biewener, ‘82, ‘83a, ‘83b; ‘89, ‘91; Bertram and Biewener, ‘88, ‘90; Bertram and Swartz, ‘91; Heinrich et al., ‘93; Christiansen, 2002). The fundamental finding of these studies is that bone cross-sectional properties (a measure of strength expressed as an area) generally scale isometrically or moderately positively with body mass (expressed as a volume). However, bone cross-section size does not increase proportionally (i.e. with enough positive allometry) with increases in mass, so larger animals have relatively weaker long bones than do smaller ones. Large animals compensate for this phenomenon by using standing and locomotor postures that reduce the bending moments on their limbs incurred by ground reaction forces (Biewener, ‘83a, ‘83b, ‘89, ‘91). As a result, many researchers interested in inferring load in bones and joints in extant animals or in the reconstruction of posture and locomotor patterns in extinct animals (see Heinrich et al., ‘93; Trinkaus et al., ‘94, ‘99; Skerry, 2000; Churchill and Schmitt, 2003; Ruff, 2005) argue that increases in cortical area, bone cross-sectional area and bone mineral content reflect increased magnitudes of peak1 loading that occurs during locomotion (see Bertram and Swartz, ‘91; Pearson and Lieberman, 2004; Ruff, 2005 for reviews of this complex issue).
However, detailed experimental studies have suggested that this relationship is not straightforward in all cases, and is often quite complex (see Rubin and Lanyon, ‘85; Demes et al., ‘98, 2001; McLeod et al., ‘98; Lieberman et al., 2003; Lieberman et al., 2004; Main and Biewener, 2007). Although it must be the case that forces generated by accelerating body and limb mass influence bone strain and bone mechanical properties, there remains no clear consensus concerning which aspect (peak magnitude, impulse, frequency, rate) of these ground reaction forces plays the most critical role, or the mechanism(s) by which this occurs (see Martin and Burr, ‘82; Bertram and Swartz, ‘91; Jacobs et al., ‘98; Robling et al., 2000, 2002a, 2002b; Skerry, 2000; Robling and Turner, 2002; Rubin et al., 2001; Lieberman et al., 2003, 2004; Zumwalt et al., 2006; Judex et al. 2007).
Although peak vertical and fore-aft forces2 have figured prominently in previous analyses of limb design (e.g. Biewener, ‘83a, ‘83b), the transverse (mediolateral) component of the ground reaction force remains poorly studied primarily because this component is highly variable and relatively small, often less than 10% of an animal’s body weight (Budsberg et al. ‘87; Ishida et al. ‘90; Merkens et al. ‘93a, ‘93b; Riggs et al., ‘93). However, although these forces are relatively low magnitude during average walking speeds chosen by the animal under relaxed conditions, they can still have a significant role in determining diaphyseal morphology. Studies of some non-mammalian bipeds such as penguins (Griffin and Kram, 2000), primate quadrupeds (Schmitt, 2003; Carlson et al. 2005) and quadrupeds with a sprawling limb posture, such as alligators (Blob and Biewener, 2001; Willey et al., 2004) have examined the transverse components of ground reaction forces with interesting results. For example, studies of primate quadrupeds have also suggested that transverse forces play an important part in maintaining stability during locomotion (Kimura et al., ‘79; Schmitt, ‘95, 2003; Demes et al ‘98, 2001, 2007; Carlson et al. 2005) and may play an important role in determining limb morphology (Schmitt, ‘95, 2004; Demes et al. ‘98, 2001, 2007; Carlson et al., 2005). It is also likely that the limb is subjected to significant transverse forces during turning (Carlson et al. 2005, Demes et al.2007). Several studies have documented the kinematics of turning in horses, primates, dogs, and mice (Walter, 2003; Carlson et al. 2005; Davies and Merritt, 2004) and some have argued that the anatomy of the lower limbs is influenced by transverse loads during turning (Carlson et al. 2005; Carlson and Judex, 2007).
There are few studies of the vertical forces applied by limbs of rodents, and no data on the transverse forces. Clarke and colleagues (Clarke and Still, ‘99, 2001; Clarke et al., 2001; Rochester and Clarke, ‘94) have collected peak vertical ground reaction force data on rats and mice, and Lee et al (2002) collected strain data from a single strain gauge on the mouse ulna. Experimental data on the loading environment of rodent limbs has recently been expanded to more complex questions such as the influence of turning on the rodent skeleton (Carlson and Judex, 2007; Walter, 2003), highlighting the importance of collecting transverse forces.
The study will document the vertical and transverse forces experienced by the limb in mice. We will compare these values in wild-type and myostatin-deficient mice, whose limbs have extra-large muscles and highly mineralized and hypertrophied limb bones in order to offer a preliminary test of the hypothesis that limbs of the myostatin-deficient mice are stronger because they experience higher peak vertical and transverse ground reaction forces, higher vertical loading rates, and greater vertical impulses than wild-type mice.
This project does not assume that increased muscularity increases forces. That is they hypothesis being explored in this study. There are a number of potential mechanisms by which the hypermuscular limbs might create higher vertical ground reaction forces during a normal stride. But before discussing this further, it is worth noting that we chose to test the hypothesis that there is a relationship between bone mechanical properties, muscle mass, and the external forces exerted during normal moderate-speed locomotion, rather than extreme forms of lomotion or broad activity patterns, for several reasons. First, this is the first step in a process and if this hypothesis is rejected we can explore additional areas including more extreme forms of locomotion. Secondly, McPherron and Lee (2002) have shown that myostatin knockouts do not differ from normal mice (relative to body weight) in metabolic rate, food consumption, or body temperature. Thus the myostatin-deficient mice do not appear to differ from wild-type mice in any significant physiological parameters and may differ only in muscle mass and bone mechanical properties. In addition, Hamrick (unpublished data) has shown, using an infrared in-cage monitoring system, that normal and myostatin-deficient mice do not differ significantly in activity level (p=.75). Third, as mentioned above, many of the arguments regarding the functional morphology of extinct animals rely on the assumption that bone mechanical properties reflect normal locomotor load levels.
In that light we recognize the fact that we are testing a specific hypothesis which is one of many possible ideas about what is reflected by different bone mechanical properties. Moreover, the mechanisms by which locomotor behavior might influence bone mechanical properties articulated below are based on mechanical models rather than specific data on limb forces in rodents.
As described above, ground reaction forces are affected by many factors, including the weight of the limbs, the force generated by the muscles during stance phase, and the posture adopted during locomotion. Since most of the muscle hypertrophy in these animals is localized to their limbs (Hamrick et al., 2002), The mechanism for increased load on heavier limbs is unclear and may have to do with higher muscle force generate by larger muscles during stance. It is also possible that the muscles themselves generate relatively high forces when firing to swing the limb forward, causing the limbs to strike the ground with higher initial loading rates and peaks of initial loads as the heavy limbs touch down and begin to bear weight. Additionally, in two animals with the same body mass, an animal with a relatively shorter stance phase for a given stride length (i.e., a shorter duty factor) experiences higher peak vertical forces during its stance phase (Schmitt, ‘99). It is possible that in the myostatin-deficient mice, swinging heavy limbs may increase the duration of their swing phases and concomitantly reduce stance phase, thereby leading to higher peak vertical forces.
The way in which differences in transverse (mediolateral) forces across strains of mice might be generated can also vary. It is possible that the hypertrophied muscles of these mice adduct the limb more forcefully than they do in wild-type mice and thus generate higher transverse ground reaction forces than those experienced by wild-type mice. It is also possible that increased muscle mass induces a sprawling gait that may require strong adduction forces and induce transverse forces that are at least somewhat higher than during upright posture (Blob and Biewener, 2001). Therefore, there are too many potential mechanisms by which large GRFs might be created by hypertrophied limbs (or vice versa) for this project to clarify the mechanism at work in this model. Instead, this study can use force data to test the commonly held assumption that hypertrophied limb bones indicate high locomotor GRFs.
Materials and Methods
This is a preliminary study involving a small number of wild-type and myostatin-deficient mice walking on a newly developed force platform designed to collect data on small animals. The myostatin-deficient mice used in this study were bred on a CD1 background at the Medical College of Georgia (Augusta, Georgia) and the CD1 wild-type mice were obtained from Charles River Laboratories (Wilmington, MA). The animals used in this study were four months old and were housed at Duke University. The methods for kinetic and kinematic data collection are modified from Schmitt (‘99) and Zumwalt et al.(2006). All data were collected at the Animal Locomotion Laboratory in the Department of Biological Anthropology and Anatomy at Duke University. All methods were consistent with the standards of the Institutional Animal Care and Use Committee of Duke University (IACUC protocol #A398-03-12).
The force platform is a small (15cm x 15cm), precise Hall-effects force platform capable of accurately recording small animals’ ground reaction forces in all three directions, as well as impulse and loading rate in the vertical direction (Advanced Mechanical Technology, Inc; Watertown, MA). This plate was modified for collection of single-limb contacts and was tested for accuracy with mice as described previously (Zumwalt et al. 2006) (Figure 1). The mice (n=4 myostatin-null; n=3 wild-type) were allowed to walk freely along a 60cm x 15cm wooden runway. The force platform was positioned below the runway and isolated from contact except for a thin (15cm x 1cm x 1cm) strip of wood positioned to isolate single-limb contacts as the animals walked along the runway (Figure 1). Force data were collected into a personal computer using Bioanalysis with NetForce software (Advanced Mechanical Technology, Inc; Watertown, MA) and filtered with a 25Hz low pass filter using Autosignal wave analysis software (Systat Software Inc, Point Richmond, CA).
Subjects were filmed in lateral view at 125Hz with a Redlake Motionscope digital camera (Redlake, Tucson, AZ). Digital video data were processed in Motus 2000 and the x-y coordinate position of a marker on the body was collected for every frame and instantaneous and average horizontal velocity was calculated. Only walking (no aerial phase) strides showing no significant (less than 25%) acceleration or deceleration were used in the analysis. In addition footfall contacts were noted for each stride. Contact times (time of contact of the relevant limb) and stride times (time from one touch-down to the subsequent touch-down for the same limb) were from these data. Duty factors (contact time / stride time) were then calculated from these data. A complete stride was on average 31 frames long.
Force data were filtered with a 25Hz low pass filter using Autosignal wave analysis software (Systat Software Inc, Point Richmond, CA). Data were then processed in Kaleidagraph (Synergy Software; Reading, PA) and peak vertical (Fig. 3 points B and C) and transverse (Fig. 4) ground reaction force, vertical force loading rate (Fig. 3 point A), and vertical force impulse (area under the force curve) (Fig. 3 point D) were measured from these force traces. Loading rate was calculated by taking the first derivative of the force trace curve, which is equivalent to finding the slope of the curve at every point in time. The beginning of this period was defined as the point of foot or hand contact. The end of this period was defined when the slope became equal to or less than zero (Fig. 3 point B). The peak loading rate (peak slope) within this initial loading phase of the limb contact was identified (Fig. 3, peak slope indicated by line A) and the average of all rates within 50% of this peak was calculated (modified from Gerlach et al., 2005). This value is an estimate of the average loading rate for the initial part of limb contact. The peak vertical force at the end of this initial loading period (Fig. 3, point B) and the peak vertical force for the entire limb contact period (Fig. 3, point C) were also recorded. Absolute peak forces are reported in Table 1. In addition, the vertical forces were standardized for body mass by dividing them by the animal’s weight in Newtons.
Figure 3.

Demonstration of the various parameters measured on the vertical ground reaction force curve in this study. Shown is a force trace of the vertical ground reaction force of a single forelimb contact of a walking mouse, measured in Newtons. Parameters measured are (A) loading rate: the average slope of the initial loading period as defined in the text, (B) the peak vertical force at the end of the initial loading period, (C) the peak vertical force for the entire contact time, and (D) impulse: the area under the curve (shaded gray).
Figure 4.
A representative mediolateral (ML) force (N) trace for the forelimb (FL) and hind limb (HL) from a single individual walking at 0.18 m/s.
Table 1.
Summary of the kinetic and kinematic data collected during this study. Means ± standard deviations are reported, followed by the range of observed data in parentheses. The reported significance results are from analyses of covariance, which controlled for the confounding effect of speed (α=0.05; n.s. = not significant). N = newtons, BW = body weight (N), s = seconds
| Mstn−/− | Wild Type | Signif at α=0.05 | |
|---|---|---|---|
| Individuals | 4 | 3 | |
| Body weight (N) | 0.48 ± 0.03 (0.45 – 0.53) |
0.46 ± 0.06 (0.39 – 0.49) |
n.s. |
| Speed (m/s) | 0.24 ± 0.06 (0.16 - 0.38) |
0.33 ± 0.06 (0.18 - 0.66) |
p<0.003 |
| Forelimb | |||
| Number of Steps | 22 | 24 | |
| Duty Factor (contact time [s] / stride time [s]) |
0.63 ± 0.05 (0.51 – 0.74) |
0.61 ± 0.07 (0.47 – 0.74) |
n.s. |
| Loading Rate (milliseconds) | 16.6 ± 6.3 (6.1 – 30.6) |
16.2 ± 5.5 (7.2 – 30.4) |
n.s. |
| Peak vertical force / BW at end of initial load (N) |
0.46 ± 0.12 (0.2 – 0.71) |
0.48 ± 0.14 (0.23 – 0.72) |
n.s. |
| Peak vertical force | 0.30 ± 0.05 (0.20 – 0.40) |
0.27 ± 0.05 (0.18 – 0.42) |
n.s. |
| Peak vertical force (N) / BW | 0.60 ± 0.1 (0.42 – 0.77) |
0.64 ± 0.08 (0.47 – 0.82) |
n.s. |
| Impulse (area under curve) | 0.07 ± 0.02 (0.05 – 0.14) |
0.06 ± 0.02 (0.04 – 0.09) |
n.s. |
| Peak transverse force (N) |
0.11 ± 0.03 (0.07 – 0.17) |
0.10 ± 0.04 (0.05 – 0.2) |
n.s. |
| Peak transverse force (N) / BW | 0.22± 0.04 (0.14 – 0.35) |
0.22± 0.055 (0.1 – 0.43) |
n.s. |
| Hind Limb | |||
| Number of Steps | 17 | 14 | |
| Duty Factor (contact time [s] / stride time [s]) |
0.67 ± 0.07 (0.51 – 0.84) |
0.64 ± 0.1 (0.47 – 0.87) |
n.s. |
| Loading Rate (milliseconds) | 17.9 ± 7.8 (4.8 – 31.7) |
19.1 ± 6.3 (11.7 – 31.2) |
n.s. |
| Peak vertical force / BW at end of initial load (N) |
0.51 ± 0.14 (0.23 – 0.86) |
0.5 ± 0.13 (0.35 – 0.74) |
n.s. |
| Peak vertical force (N) | 0.28 ± 0.06 (0.20 – 0.44) |
0.24 ± 0.06 (0.17 – 0.34) |
n.s. |
| Peak vertical force / BW (N) | 0.56 ± 0.12 (0.39 – 0.86) |
0.55 ± 0.11 (0.36 – 0.74) |
n.s. |
| Impulse (area under curve) | 0.06 ± 0.02 (0.04 – 0.11) |
0.08 ± 0.09 (0.02 – 0.39) |
n.s. |
| Peak transverse force (N) | 0.07 ± 0.02 (0.05 – 0.13) |
0.11 ± 0.04 (0.06 – 0.19) |
p=0.007 |
| Peak transverse force (N) / BW | 0.14± 0.05 (0.1 – 0.27) |
0.23± 0.05 0.13 – 0.41 |
P=0.007 |
All statistics were performed using the statistical package SPSS (SPSS Inc, Chicago, IL). All force data were divided by the animal’s body mass to standardize for differences in body size. Student’s t-tests were used to compare the group means for body mass. Analyses of covariance (ANCOVAs) were used to control for the influence of the potentially confounding factor of speed when comparing the group means of peak transverse forces experienced by the animals’ limbs.
Results
There was no significant difference in body weights across the two groups (Table 1). The myostatin-deficient mice walked more slowly than the wild-type mice (p=0.008) (Table 1; Figs. 6 - 7). Despite the difference in speed, the two groups had similar duty factors (Table 1).
Figure 6.

Bivariate plot of peak vertical ground reaction force (divided by body weight) on the forelimb versus velocity (m/s) for the wild-type mice (dark gray triangles) and mysotatin-deficient knock-out mice (light gray circles).
Figure 7.
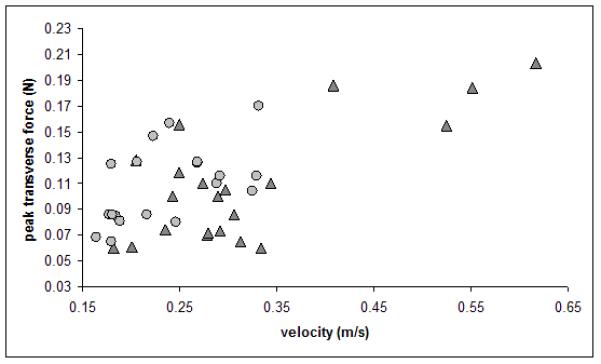
Bivariate plot of peak transverse ground reaction force (in Newtons) on the forelimb versus velocity (m/s) for the wild-type mice (dark gray triangles) and mysotatin-deficient knock-out mice (light gray circles).
There was no difference in absolute or body-weight adjusted peak vertical forces between the groups of mice (Table 1). Once speed and body weight were accounted for, the two groups of mice produce statistically similar peak vertical ground reaction forces in both forelimbs and hind limbs (Table 1, Figs. 5 and 6) at a given speed during normal symmetrical walking chosen by the animal Nor were there any statistical differences for loading rate, vertical force at end of initial load, vertical impulse (Table 1).
Figure 5.

Comparisons of some of the locomotor parameters for the wild-type (WT) and myostatin-knockout (KO) mice. The mean and one standard deviation for the walking speeds and peak vertical forces for forelimbs and hind limbs of the two groups of mice are compared. Significance values are indicated in the upper left corner. The designation “n.s.” indicates that the values are not significantly different from each other. Note that the myostatin-deficient (KO) mice walk significantly more slowly but the peak vertical forces are not significantly different from that of the wild-type (WT) mice.
Peak transverse forces on the forelimb were the same across the two groups of mice (Table 1, Figure 7). This was not the case for the hind limb, where the transverse forces for the wild-type mice were significantly higher than those of the myostatin-deficient mice (p=0.007) (Table 1).
Discussion
The goal of this study was to collect preliminary data on the footfall patterns and ground reaction forces in wild-type and hypermusclar mice and to use these data to test the hypothesis that there is a relationship between external loading patterns and skeletal morphology. The walking velocity, footfall pattern, and duty factors found in the mice in this study are similar to those reported for other mice using symmetrical gaits (Herbin et al., 2004; 2006, 2007; Lepicard et al. 2005) and for most other animals (Hildebrand, ‘67, ‘76; Cartmill et al. 2002). Thus, although this is a small sample, it appears to reasonably represent the gait patterns of small rodents.
One of the significant difference between the wild-type and myostatin deficient mice documented in this study was the speed at which they walked (Table 1; Figs. 5 - 7). The myostatin-deficient mice in this study walked on average more slowly than their wild-type counterparts. In most animals, duty factor correlates strongly with walking velocity showing higher duty factors at lower walking speeds eventually shifting below 0.5 as they animals break into a run (Hildebrand, ‘67, ‘76; Biewener, ‘83b; Demes et al. ‘94; Cartmill et al. 2002). However, despite a significantly lower walking velocity, there was no significant difference in duty factor across groups. This suggests that the hyper-muscular mice may actually have relatively shorter contact times at a given speed compared to the wild-type mice. This could be a result of restricted joint yield due to increased limb muscularity, but this cannot be verified without a detailed kinematic study.
The kinetic data reported here are also consistent with those for most other mammals. The peak vertical forces, which were consistent with those previously reported for mice and rats (Clarke et al., 2001; Clarke and Still, ‘99; Clarke and Still, 2001; Rochester and Clarke, ‘94) were roughly 60% of body weight with slightly higher values on the forelimb compared to the hind limb, as is the case in most nonprimate mammals (Kimura et al. ‘79; Demes et al. ‘94; Schmitt and Lemelin, 2002). The transverse forces were between 5% and 20% of body weight, as is the case with most other mammals recorded to date (Budsberg et al. ‘87; Ishida et al. ‘90; Merkens et al. ‘93a, ‘93b; Riggs et al., ‘93; Schmitt, ‘95, 2003; Demes et al., 2007; Carlson et al. 2005).
The mechanical properties of an animal’s skeleton is often theorized to be strongly influenced by the external loading environment experienced by the animal’s limbs during locomotion. Thus we predicted that the myostatin-deficicient mice that have have increased rigidity in their long bones would also experience higher external loads. But in this study, after accounting for velocity, there were no significant differences in loading of the limbs between the two groups. The myostatin-deficient mice do not appear to experience higher external loads on their limbs than do their wild-type counterparts. The limbs of the two groups were not loaded at different rates, nor did they experience higher vertical ground reaction forces at the end of initial loading periods (Table 1). Additionally, the peak forces (Table 1, Fig. 5 and 6) and vertical impulses (Table 1) experienced by the limbs of both groups were similar. The finding that peak vertical forces are the same in both groups is consistent with an earlier finding using a less sensitive force platform (Zumwalt et al. 2005). In addition the hypermusclar mice did not generate higher peak transverse ground reaction forces than the limbs of the wild-type mice did (Table 1, Fig. 7). In fact, the peak transverse forces in the hind limbs were actually lower in the knockout mice than in the normal mice (Table 1). Of course future studies with a larger sample and a wider range of gaits at higher speeds is needed to fully explore these interesting results.
At a given speed myostatin-deficient and wild-type mice experience the same magnitude vertical forces (Table 1, Fig. 5 and 6). However, because they adopt relatively slow speeds, the myostatin-deficient mice are able to habitually subject their limbs to lower peak vertical and transverse forces than they would experience were they to walk at the speeds wild-type mice use.
These results suggest that the increased bone formation in the limbs of these mice (Hamrick et al. 2007) is not the product of excessively large ground reaction forces created during normal locomotion. There are other aspects of gait-related loading patterns that may influence the bone and its response to load that were not explored in detail here. For instance, bones are subjected to bending strains during gait because joints are characteristically maintained in somewhat bent postures (Biewener, ‘83a, ‘83b, ‘89, ‘91). It is possible that the myostatin-null mice are adopting a more flexed-limb posture, and therefore creating greater moments around their joints and greater bending strains across their bones. Preliminary examinations of their shoulder and hip heights during gait indicate that these animals do not adopt more flexed joint positions during standing or locomotion (D. Schmitt, unpublished). However, it is impossible to precisely identify joint centers with the video data we have collected. A more thorough investigation, possibly using cineradiography, is needed in order to more accurately document the joint angles used by these animals during locomotion. Along the same lines, it is possible that the large agonist and antagonist muscles may stimulate increased bone robusticity by countering each other, without affecting the GRF (see Blob et al. 2008). Unfortunately, directly measuring the force created by muscles on bone is extremely challenging, especially in small animals.
One possible explanation for the expanded transverse anatomy in the limb bones of hypermuscular mice in the absence of high transverse ground reaction forces is that the presence and/or action of muscles and muscle tissue may have a direct, local influence on bone formation and modification. Much of the mediolateral expansion of the myostatin-deficient mouse humeri and femora exists at or near muscle attachment sites (Figure 2) (Hamrick et al. 2002, 2003). Although a recent study suggests that a direct relationship between muscle mass and attachment site size does not necessarily exist in quadrupeds (Zumwalt, 2006), it is possible that in the case of these hypermuscular mice, the bones hypertrophy simply to provide surface area for the attachment of the large and numerous muscle fibers (Hamrick et al. 2000).
It is also possible that other physiological mechanisms also play an important role in regulating bone apposition. However, McPherron and Lee (2002) have shown that myostatin knockouts do not differ from normal mice in metabolism, food consumption, or body temperature. In addition, Hamrick (unpublished data) has shown no difference between the two groups in in-cage activity level. An interesting alternative explanation is that the large muscles of the myostatin-deficient mice function as paracrine organs that secrete critical growth factors along the periosteal surface and stimulate bone growth (Zacks and Sheff, ‘82; Montgomery et al., 2005). Muscle tissue is also likely to serve as a local source of muscle satellite cells capable of undergoing skeletal differentiation (Asakura et al., 2001), and myostatin deficiency itself may have direct effects on osteoprogenitor cells that increases their osteogenic potential (Hamrick et al., 2007). Recent work indicates that circulating myostatin may have directs effects on bone formation. The myostatin-receptor is expressed in bone-marrow stromal (stem) cells, and bone marrow stromal cells from mice lacking myostatin show increased osteogenic differentiation and enhanced mechanosensitivity (Hamrick et al., 2007). Mice injected with a decoy myostatin receptor, which binds circulating myostatin so that it cannot reach its target cells, show increased bone mass (Bialek et al., 2008). Thus, factors such as myostatin that influence muscle mass may also directly effect bone formation as well, suggesting that the integrated growth and development of these two tissues is regulated by a common molecular signals. Clearly further studies are still needed to elucidate the primary mechanism through which the skeletal hypertrophy observed in these animals is generated.
There are also myriad other mechanical influences on bone remodeling. These include the magnitude, frequency, and rate of strains experienced locally at and within the bone tissue, and the influence of local factors such as bone curvature ( Lanyon et al., ‘82; Rubin and Lanyon, ‘85; Biewener and Bertram, ‘93; Mosley et al., ‘97; Judex and Zernicke, 2000; Fritton et al., 2000; Hsieh et al., 2001; Burr et al., 2002; Robling and Turner, 2002; Robling et al., 2002b; Warden and Turner, 2004).
It is also clear that muscle-derived stimuli are critical for the proper growth and development of limb bones, and we do not mean to suggest that this is not the case. For example, joints will not form in the absence of movement in utero, and mechanical loading stimulates chondrocyte proliferation in articular regions as well as in the growth plate. The key issue here is mechanism, specifically the role of peak locomotor forces in driving morphology as opposed to other stimuli, such as high frequency, low magnitude muscle-derived signals (see Rubin and Lanyon, ‘85; Fritton et al. 2000; Judex and Zernicke, 2000, Rubin et al. 2001; Judex et al. 2007 or changing intramedullary fluid pressure in bone emanating from muscle contraction working as a “pump” (Qin et al. 2003). Our data simply suggest that the unique skeletal phenotype of hypermuscular,myostatin-deficient mice is unlikely related to large forces encountered during locomotion, but rather reflects other aspects of the muscle-bone relationship.
The role that all of these factors (external loads, strain, bone-muscle interactions etc.) play in bone formation in these mice will only be answered with more detailed study using bone strain gauges and other techniques on these mice. This was beyond the scope of this study, which focused on ground reaction forces and gait because many functional morphologists use bone morphology to infer locomotor behavior, often with a focus on peak ground reaction forces experienced during locomotion (e.g., Churchill and Schmitt, 2003; Heinrich et al., ‘93; Ruff, 2005; Trinkaus et al., ‘94; Trinkaus et al., ‘99). This study suggests that the assumptions used in such studies should be carefully considered when interpreting their results. The many alternative explanations for the skeletal morphology we observe in these mice reinforce the concept that the relationship between limb bone anatomy and in vivo loading is extremely complex. Although there is no doubt that external loads can influence skeletal morphology (Martin and Burr, ‘82; Frost, ‘87; Bertram and Swartz, ‘91; ; Turner et al., ‘95; Jacobs et al., ‘98; Robling et al., 2000, 2002b; Rubin et al., 2001; Turner, 2002; Turner and Beamer, 2002; Lieberman et al., 2003; Lieberman et al., 2004), this study reinforces the growing realization that this relationship is complex and difficult to predict.
The availability of novel animal models and improved and more accessible force plate technology has made it possible to investigate these questions in new ways. The preliminary data reported in this study call into question one commonly assumed mechanism for the moderation of bone morphology: a straightforward influence of locomotor ground reaction forces on skeletal morphology. The results of this study emphasize the need for further exploration of ground reaction forces in these unusual animals models as well as broader investigations into the myriad in vivo mechanisms regulating muscle-bone interactions.
Acknowledgements
These data were collected with the help of a number of talented undergraduate students. We also are grateful to Rick Blob, Damiano Marchi, Matthew Oneill, Chris Wall, and Megan Wilson for invaluable discussions and comments on the project and this manuscript.
Funding for this research was provided by a grant from the National Institutes of Health (AR 049717) to MWH and DOS.
Footnotes
The ground reaction forces (GRF) that occur during locomotion may be broken down into three directional components (vertical, fore-aft and mediolateral). The vertical component of the GRF is the largest component applied by the limb (Winter, 1990; Biewener, 2003).
We did not collect fore-aft forces for this study because the filtering methods used with this platform (Zumwalt et al. 2006) do not allow confidence in the output of braking and propulsive forces.
Literature Cited
- Alexander RM, Jayes AS, Maloiy GMO, Wathuta EM. Allometry of limb bones of mammals from shrews (Sorex) to elephant (Loxodonta) J Zool Lond. 1979;189:305–314. [Google Scholar]
- Amblard D, Lafange-Proust MH, Laib A, Thomas T, Ruegesegger P, Alexander C, Vito L. Tail suspension induces bone loss in skeletally mature mice in the C57 BL/6J strain but not in the C3H/HeJ strain. J Bone Mineral Res. 2003;18:561–569. doi: 10.1359/jbmr.2003.18.3.561. [DOI] [PubMed] [Google Scholar]
- Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- Bertram JEA, Biewener AA. Bone curvature: sacrificing strength for load predictability? J Theor Bio. 1988;131:75–92. doi: 10.1016/s0022-5193(88)80122-x. [DOI] [PubMed] [Google Scholar]
- Bertram JEA, Biewener AA. Differential scaling of the long bones in the terrestrial carnivora and other mammals. J Morph. 1990;204:157–169. doi: 10.1002/jmor.1052040205. [DOI] [PubMed] [Google Scholar]
- Bertram JEA, Swartz SM. The ‘Law of Bone Transformation’: A case of crying Wolff? Biologicial Reviews. 1991;66:245–273. doi: 10.1111/j.1469-185x.1991.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Scaling body support in mammals: Limb posture and muscle mechanics. Science. 1989;245:45–8. doi: 10.1126/science.2740914. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Bone strength in small mammals and bipedal birds: do safety factors change with body size? J Exp Biol. 1982;98:289–301. doi: 10.1242/jeb.98.1.289. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Allometry of quadrupedal locomotion: The scaling of duty factor, bone curvature and limb orientation to body size. J Exp Biol. 1983a;105:147–171. doi: 10.1242/jeb.105.1.147. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Locomotory stresses in the limb bones of two small mammals: The Ground Squirrel and Chipmunk. J Exp Biol. 1983b;103:131–154. doi: 10.1242/jeb.103.1.131. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Musculoskeletal design in relation to body size. J Biomech. 1991;24(Suppl. 1):19–29. doi: 10.1016/0021-9290(91)90374-v. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Bertram JEA. Skeletal strain patterns in relation to exercise training during growth. J Exp Biol. 1993;185:51–69. doi: 10.1242/jeb.185.1.51. [DOI] [PubMed] [Google Scholar]
- Bialek P, Parkington J, Warner L, St. Andre M, Jian L, Gavin D, Wallace C, Zhang J, Yan G, Root A, Seeherman H, Yaworsky P. Mice Treated with a Myostatin/GDF-8 Decoy Receptor, ActRIIB-Fc, Exhibit a Tremendous Increase in Bone Mass. Bone. In press.
- Blob RW, Biewener AA. Mechanics of limb bone loading during terrestrial locomotion in the green iguana (Iguana iguana) and American alligator (Alligator mississippiensis) J Exp Biol. 2001;204:1099–1122. doi: 10.1242/jeb.204.6.1099. [DOI] [PubMed] [Google Scholar]
- Budsberg SC, Verstraete MC, Soutas-Little RW. Force plate analysis of the walking gait in healthy dogs. Am J Vet Res. 1987;48:915–918. [PubMed] [Google Scholar]
- Butcher MT, Blob RW. Mechanics of limb bone loading during terrestrial locomotion in river cooter turtles (Pseudemys concinna) J Exp Biol. 2008;211:1187–1202. doi: 10.1242/jeb.012989. [DOI] [PubMed] [Google Scholar]
- Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781–786. doi: 10.1016/s8756-3282(02)00707-x. [DOI] [PubMed] [Google Scholar]
- Cartmill C, Lemelin P, Schmitt D. Support polygons and symmetrical gaits in mammals. Zool J Linn Soc. 2002;136:401–420. [Google Scholar]
- Carlson KJ, Demes B, Franz TM. Mediolateral forces associated with quadrupedal gaits of lemurids. J. Zool., Lond. 2005;266:261–273. [Google Scholar]
- Carlson KJ, Judex S. Increased non-linear locomotion alters diaphyseal bone shape. J Exp Biol. 2007;210:3117–25. doi: 10.1242/jeb.006544. [DOI] [PubMed] [Google Scholar]
- Casinos A. Bipedalism and quadrupedalism in Megatheria: an attempt at biomechanical reconstruction. Lethaia. 1996;29:87–96. [Google Scholar]
- Christiansen P. Mass Allometry of the Appendicular Skeleton in Terrestrial Mammals. Journal of Morphology. 2002;25:195–209. doi: 10.1002/jmor.1083. [DOI] [PubMed] [Google Scholar]
- Churchill SE, Schmitt D. Biomechanics in paleoanthropology: engineering and experimental approaches to the investigation of behavioral evolution in the genus Homo. In: Harcourt C, Crompton R, editors. New Perspectives in Primate Evolution and Behavior. Linnaean Society; London: 2003. pp. 59–90. [Google Scholar]
- Clarke KA, Smart L, Still J. Ground reaction force and spatiotemporal measurements of the gait of the mouse. Behavior Research Methods, Instruments, & Computers. 2001;33:422–426. doi: 10.3758/bf03195396. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Still J. Gait analysis in the mouse. Physiol. and Behavior. 1999;66:723–729. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Still J. Development and consistency of gait in the mouse. Physiol. and Behavior. 2001;73:159–164. doi: 10.1016/s0031-9384(01)00444-9. [DOI] [PubMed] [Google Scholar]
- Davies HMS, Merritt JS. Surface strains around the midshaft of the third metacarpal bone during turning. Equine Vet. J. 2004;36:689–692. doi: 10.2746/0425164044848109. [DOI] [PubMed] [Google Scholar]
- Demes B, Qin Y, Stern JT, Larson SG, Rubin CT. Patterns of strain in the macaque tibia during functional activity. Am J Phys Anthropol. 2001;116:257–265. doi: 10.1002/ajpa.1122. [DOI] [PubMed] [Google Scholar]
- Demes B, Stern JT, Hausman MR, Larson SG, McLeod KJ, Rubin CT. Patterns of strain in the macaque ulna during functional activity. Am J Phys Anthropol. 1998;106:87–100. doi: 10.1002/(SICI)1096-8644(199805)106:1<87::AID-AJPA6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Fritton S, McLeod K, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33:317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- Frost H. The mechanostat: A proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone and Mineral. 1987;2:73–85. [PubMed] [Google Scholar]
- Griffin TM, Kram R. Penguin waddling is not wasteful. Nature. 2000;408:929. doi: 10.1038/35050167. [DOI] [PubMed] [Google Scholar]
- Gerlach KE, White SC, Burton HW, Dorn JM, Leddy JJ, Horvath PJ. Kinetic changes with fatigue and relationship to injury in female runners. Med Sci Sports Exerc. 2005;37:657–63. doi: 10.1249/01.mss.0000158994.29358.71. [DOI] [PubMed] [Google Scholar]
- Hampton TG, Stasko MR, Kale A, Amende I, Costa AC. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiol Behav. 2004;82:381–389. doi: 10.1016/j.physbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, McPherron AC, Lovejoy CO, Hudson J. Femoral morphology and cross-sectional geometry of adult myostatin-deficient mice. Bone. 2000;27:343–349. doi: 10.1016/s8756-3282(00)00339-2. [DOI] [PubMed] [Google Scholar]
- Hamrick MW. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Record. 2003;272A:388–391. doi: 10.1002/ar.a.10044. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, McPherron AC, Lovejoy CO. Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcified Tissue International. 2002;71:63–68. doi: 10.1007/s00223-001-1109-8. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Byron CD. Bone architecture and disc degeneration in the lumbar spine of mice lacking GDF-8 (myostatin) J Orthop Research. 2003;21:1025–1032. doi: 10.1016/S0736-0266(03)00105-0. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, Kang B, Isales CM, Fulzele S, Wenger KH. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;23:23. doi: 10.1016/j.bone.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich RE, Ruff CB, Weishampel DB. Femoral ontogeny and locomotor biomechanics of Dryosaurus lettowvorbecki (Dinosauria, Iguanodontia) Zool J Linn Soc. 1993;108:179–196. [Google Scholar]
- Herbin M, Gasc JP, Renous S. Symmetrical and asymmetrical gaits in the mouse: patterns to increase velocity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;11:895–906. doi: 10.1007/s00359-004-0545-0. [DOI] [PubMed] [Google Scholar]
- Herbin M, Gasc JP, Renous S. How does a mouse increase its velocity? A model for investigation in the control of locomotion. Comptes Rendus Palevol. 2006;5:531–540. [Google Scholar]
- Herbin M, Hackert R, Gasc JP, Renous S. Gait parameters of treadmill versus overground locomotion in mouse. Behav Brain Res. 2007;181:173–9. doi: 10.1016/j.bbr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hildebrand M. Symmetrical gaits of primates. Am. J. Phys. Anthropol. 1967;26:119–130. [Google Scholar]
- Hildebrand M. Analysis of tetrapod gaits: general considerations and symmetrical gaits. In: Herman RM, Grillner S, Stein PSG, Stuart DC, editors. Neural control of locomotion. Plenum Press; New York: 1976. pp. 203–236. [Google Scholar]
- Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo: The strain threshold for osteogenic response varies with location. J Bone and Mineral Res. 2001;16:2291–2297. doi: 10.1359/jbmr.2001.16.12.2291. [DOI] [PubMed] [Google Scholar]
- Ishida H, Jouffroy F, Nakano Y. Comparative dynamiocs of pronograde and upsidedown quadrupedalism in the slow loris (Nycticebus coucang) In: Jouffroy F, Stack M, Niemitz C, editors. Gravity, Posture, and Locomotion in Primates. Sendicesimo; Florence: 1990. pp. 209–220. [Google Scholar]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–76. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judex S, Zernicke R. High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. J Appl Physiol. 2000;88:2183–2191. doi: 10.1152/jappl.2000.88.6.2183. [DOI] [PubMed] [Google Scholar]
- Judex S, Donahue LR, Rubin CT. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16:1280–1282. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- Judex S, Garman R, Squire M, Donahue LR, Rubin CT. Genetically based influences on the site-specific regulation of trabecular and cortical bone morphology. J Bone Miner Res. 2004;19:600–6. doi: 10.1359/JBMR.040101. [DOI] [PubMed] [Google Scholar]
- Judex S, Garman R, Squire M, Busa B, Donahue LR, Rubin CT. Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res. 2004;19:607–13. doi: 10.1359/JBMR.040110. [DOI] [PubMed] [Google Scholar]
- Judex S, Lei X, Han D, Rubin CT. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Czech PP, Wight JT, Blank KM, Garland T. Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J Morph. 2006;267:360–374. doi: 10.1002/jmor.10407. [DOI] [PubMed] [Google Scholar]
- Kimura T, Okada M, Ishida H. Kinesiological characteristics of primate walking: its significance in human walking. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, behavior and morphology: dynamic interactions in primates. Gustav Fischer; New York: 1979. pp. 297–312. [Google Scholar]
- Lanyon LE, Goodship AE, Pye CJ, MacFie JH. Mechanically adaptive bone remodelling. J Biomech. 1982;15:141–154. doi: 10.1016/0021-9290(82)90246-9. [DOI] [PubMed] [Google Scholar]
- Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31:407–12. doi: 10.1016/s8756-3282(02)00842-6. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Venault P, Abourachid A, Pellé E, Chapouthier G, Gasc JP. Spatio-temporal analysis of locomotion in BALB/cByJ and C57BL/6J mice in different environmental conditions. Behav Brain Res. 2006;167:365–372. doi: 10.1016/j.bbr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Pearson OM, Polk JD, Demes B, Crompton AW. Optimization of bone growth and remodeling in response to loading in tapered mammalian limbs. J Exp Biol. 2003;206:3125–3138. doi: 10.1242/jeb.00514. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Polk JD, Demes B. Predicting long bone loading from cross-sectional geometry. Am J Phys Anthropol. 2004;123:156–71. doi: 10.1002/ajpa.10316. [DOI] [PubMed] [Google Scholar]
- Main RP, Biewener AA. Skeletal strain patterns and growth in the emu hindlimb during ontogeny. J Exp Biol. 2007;210:2676–90. doi: 10.1242/jeb.004580. [DOI] [PubMed] [Google Scholar]
- Martin RB, Burr DB. A hypothetical mechanism for the stimulation of osteonal remodelling by fatigue damage. J Biomech. 1982;15:137–9. doi: 10.1016/s0021-9290(82)80001-8. [DOI] [PubMed] [Google Scholar]
- McLeod K, Rubin CT, Otter M, Qin Y. Skeletal cell stresses and bone adaptation. Am J Med Sci. 1998;316:176–183. doi: 10.1097/00000441-199809000-00005. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Merkens HW, Schamhardt HC, vanOsch G, Hartman W. Ground reaction force patterns of Dutch Warmbloods at the canter. Am J Vet Res. 1993;54:670–674. [PubMed] [Google Scholar]
- Merkens HW, Schamhardt HC, vanOsch G, van den Bogert AJ. Ground reaction force patterns of Dutch warmblood horses at normal trot. Equine Vet. J. 1993;25:134–137. doi: 10.1111/j.2042-3306.1993.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Montgomery E, Pennington C, Isales C, Hamrick MW. Muscle-bone interactions in dystrophin-deficient and myostatin-deficient mice. Anat Record. 2005;286A:814–822. doi: 10.1002/ar.a.20224. [DOI] [PubMed] [Google Scholar]
- Mosley JR, March BM, Lynch J, Lanyon LE. Strain magnitude related changes in whole bone architechture in growing rats. Bone. 1997;20:191–198. doi: 10.1016/s8756-3282(96)00385-7. [DOI] [PubMed] [Google Scholar]
- Pearson OM, Lieberman DE. The aging of Wolff’s “Law”: Ontogeny and responses to mechanical loading in cortical bone. Yrbk Phys Anthropol. 2004;47:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- Plochocki JH, Rivera JP, Zhang C, Ebba SA. Bone modeling response to voluntary exercise in the hindlimb of mice. J Morph. 2008;269:313–318. doi: 10.1002/jmor.10587. [DOI] [PubMed] [Google Scholar]
- Qin Y-X, Kaplan T, Saldahna A, Rubin CT. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J. Biomech. 2003;36:1427–1437. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Ravosa MJ, Klopp EB, Pinchoff J, Stock SR, Hamrick MW. Plasticity of mandibular biomineralization in myostatin-deficient mice. J Morph. 2007;268:275–282. doi: 10.1002/jmor.10517. [DOI] [PubMed] [Google Scholar]
- Riggs CM, DeCamp CE, Soutas-Little RW, Braden TD, Richter MA. Effects of subject velocity on force plate-measured ground reaction forces in healthy greyhounds at the trot. Am J Vet Res. 1993;54:1523–1526. [PubMed] [Google Scholar]
- Robling AG, Burr DB, Turner AS. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Mineral Res. 2000;15:1596–1602. doi: 10.1359/jbmr.2000.15.8.1596. [DOI] [PubMed] [Google Scholar]
- Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greates if loading is separated into short bouts. J Bone Mineral Res. 2002a;17:1545–1554. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Med Sci Sports & Exercise. 2002b;34:196–202. doi: 10.1097/00005768-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Robling AG, Turner CH. Mechanotransduction in bone: genetic effects on mechanosensitivity in mice. Bone. 2002;31:562–9. doi: 10.1016/s8756-3282(02)00871-2. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Clarke KA. Gait analysis in the rat as a model for the study of peripheral vascular disease. Physiol and Behavior. 1994;55:723–6. doi: 10.1016/0031-9384(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Sommerfeldt DW, Judex S, Qin YX. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. DDT. 2001;6:848–858. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calc Tissue Internl. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- Ruff CB. Mechanical determinants of bone form: insights from skeletal remains. J Musculoskelet Neuronal Interact. 2005;5:202–12. [PubMed] [Google Scholar]
- Schmitt D. Ph.D. Dissertation. State University of New York at Stony Brook; Stony Brook: 1995. A kinematic and kinetic analysis of forelimb use during arboreal and terrestrial quadrupedalism in Old World monkeys. [Google Scholar]
- Schmitt D. Compliant walking in primates. J Zool. 1999;248:149–160. [Google Scholar]
- Schmitt D. The relationship between forelimb anatomy and mediolateral forces in primate quadrupeds: Implications for interpretation of locomotor behavior in extinct primates. J Hum Evol. 2004;44:49–60. doi: 10.1016/s0047-2484(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Schmitt D, Lemelin P. Origins of primate locomotion: gait mechanics of the woolly opossum. Am J Phys Anthropol. 2002;118:231–238. doi: 10.1002/ajpa.10048. [DOI] [PubMed] [Google Scholar]
- Skerry T. Biomechanical influences on skeletal growth and development. In: O’Higgins P, Cohn MJ, editors. Development, growth, and evolution. Implications for the study of the hominid skeleton. Academic; New York, NY: 2000. pp. 29–40. [Google Scholar]
- Thomason JJ. Estimation of locomotory forces and stresses in limb bones of recent and extinct equids. Paliobiology. 1985;1:209–220. [Google Scholar]
- Thomason JJ. To what extent can the mechanical environment of bone be inferred from its internal architecture? In: Thomason JJ, editor. Functional Morphology in Vertebrate Paleontology. Cambridge University Press; Cambridge: 1995. pp. 249–263. [Google Scholar]
- Trinkaus E, Churchill SE, Villemeur I, Riley KG, Heller JA, Ruff CB. Robusticity versus shape: The functional interpretation of neanderthal appendicular morphology. J. Anthropol. Soc. Nippon. 1991;99:257–278. [Google Scholar]
- Trinkaus E, Churchill SE, Ruff CB. Postcranial robusticity in Homo. II: Humeral bilateral asymmetry and bone plasticity. Am J Phys Anthropol. 1994;93:1–34. doi: 10.1002/ajpa.1330930102. [DOI] [PubMed] [Google Scholar]
- Trinkaus E, Stringer CB, Ruff CB, Hennessy RJ, Roberts MB, Parfitt SA. Diaphyseal cross-sectional geometry of the Boxgrove 1 Middle Pleistocene human tibia. J Hum Evol. 1999;37:1–25. doi: 10.1006/jhev.1999.0295. [DOI] [PubMed] [Google Scholar]
- Turner CH. Biomechanics of bone: Determinants of skeletal fragility and bone quality. Osteoporosos Int. 2002;13:97–104. doi: 10.1007/s001980200000. [DOI] [PubMed] [Google Scholar]
- Turner CH, Beamer WG. Is skeletal mechanotransduction under genetic control? J Musculoskelet Neuronal Interact. 2002;2:237–8. [PubMed] [Google Scholar]
- Turner CH, Owan I, Takano Y. Mechanotransduction in bone: role of strain rate. Am J Physiol. 1995;269:E438–E442. doi: 10.1152/ajpendo.1995.269.3.E438. [DOI] [PubMed] [Google Scholar]
- Vinyard CJ, Schmitt D. New technique for studying reaction forces during primate behaviors on vertical substrates. Am J Phys Anthropol. 2004;125:343–351. doi: 10.1002/ajpa.10395. [DOI] [PubMed] [Google Scholar]
- Walter RM. Kinematics of 90 degrees running turns in wild mice. J Exp Biol. 2003;206:1739–49. doi: 10.1242/jeb.00349. [DOI] [PubMed] [Google Scholar]
- Warden SJ, Turner CH. Mechanotransduction in cortical bone is most efficient at loading frequencies of 5-10Hz. Bone. 2004;34:261–270. doi: 10.1016/j.bone.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Weishampel DB. Fossils, function, and phylogeny. In: Thomason JJ, editor. Functional Morphology in Vertebrate Paleontology. Cambridge University Press; Cambridge: 1995. pp. 34–54. [Google Scholar]
- Willey JS, Biknevicius AR, Reilly SM, Earls KD. The tale of the tail: limb function and locomotor mechanics in Alligator mississippiensis. J Exp Biol. 2004;207:553–563. doi: 10.1242/jeb.00774. [DOI] [PubMed] [Google Scholar]
- Zacks SI, Sheff MF. Periosteal and metaplastic bone formation in mouse minced muscle regeneration. Lab Invest. 1982;46:405–12. [PubMed] [Google Scholar]
- Zumwalt AC. The effect of exercise on the morphology of muscle attachment sites. Journal of Experimental Biology. 2006;209:444–454. doi: 10.1242/jeb.02028. [DOI] [PubMed] [Google Scholar]
- Zumwalt AC, Schmitt D, McCormick J, Hamrick MW. Locomotor biomechanics and muscle-bone interactions in myostatin-deficient mice. FASEB J. 2005;19:199.4. [Google Scholar]
- Zumwalt AC, Hamrick MW, Schmitt D. Force plate for measuring the ground reaction forces in small animal locomotion. J Biomech. 2006;39:2877–81. doi: 10.1016/j.jbiomech.2005.10.006. [DOI] [PubMed] [Google Scholar]



