Abstract
Cytochrome P450 3A4 (CYP 3A4) is the most abundant cytochrome P450 enzyme in human liver and metabolizes more than 60% of prescribed drugs in human body. Patients with liver conditions such as cirrhosis show increased secretion of cytokines (e.g, interleukin-6) and decreased capacity of oxidation of many drugs. In this study, we provided molecular evidence that cytokine secretion directly contributed to the decreased capacity of oxidative biotransformation in human liver. After human hepatocytes were treated with IL-6, the expression of CYP3A4 decreased at both mRNA and protein levels, so did the CYP3A4 enzymatic activity. Meanwhile, the repression of CYP3A4 by IL-6 occurred after the decrease of pregnane X receptor (PXR) in human hepatocytes. The PXR-overexpressed cells (transfected with human PXR) increased the CYP3A4 mRNA level, and the repression of CYP3A4 by IL-6 was greater in the PXR-overexpressed cells than in the control cells. Further, PXR-knockdown (transfected with siPXR construct) decreased the CYP3A4 mRNA level with less repression by IL-6 than in the control cells transfected with corresponding vector. Collectively, our study suggests that PXR is necessary for IL-6-mediated repression of the CYP3A4 expression in human hepatocytes.
Keywords: cytochrome P450 3A4 (CYP3A4), interleukin 6 (IL-6), pregnane X receptor (PXR), transcription regulator, transcription repression
1. Introduction
Liver is the major organ for the biotransformation of drugs, and expresses enzymes including phase I enzymes such as cytochrome P450 (CYP), phase II enzymes such as UDP – glucuronosyltransferase (UGT), and phase III enzymes such as various transporters. CYP450s are critical players in metabolizing xenobiotics including the vast majority of clinically used drugs, environmental procarcinogens and toxins ([Nelson et al., 1996] and [Moon et al., 2006]). Cytochrome P450 3A4 (CYP3A4) is the most important human CYP450 in the liver and small intestine and plays a major role in the biotransformation of many drugs. It has been suggested that CYP3A4 is responsible for the oxidative metabolism of more than 60% of all pharmaceuticals ([Guengerich et al., 1999] and [Cooper et al, 2008]). And its activity shows a wide inter-individual variability, which forms a basis for clinically significant drug interactions and toxicities ([Lehmann et al., 1998] and [Shou et al., 2008]). The existence of polymorphic CYP genes may contribute to the variation (Meyer et al., 1997). However, other factors such as age, diet, hormonal status, disease, and exposure to drugs or xenobiotics may also contribute to the wide variability of certain CYPs (e.g., CYP3A4)([Wilkinson, 1997] and [Lin and Lu, 2001]), since the low-frequency of polymorphisms was observed in these CYPs(Lamba et al., 2002). Certain pathological states, particularly those involving a host inflammatory response (e.g., bacterial and viral infection), have also been associated with lower drug metabolism in the body with decreased hepatic CYP content (Morgan, 1997; 2001), and may ultimately influence the therapeutic efficacy and toxicity of many drugs.
In an inflammatory response, cytokines (mainly tumor necrosis factor α or TNF-α, and Interleukin-6, or IL-6) are produced and released into the systemic circulation to initiate a so-called acute-phase response (APR) ([Baumann and Gauldie, 1994] and [Koj, 1996]). Hepatocytes are influenced by these inflammatory cytokines, which dramatically alter the synthesis of a number of plasma proteins known as acute-phase proteins (APPs) (Castell et al., 1990). IL-6 is recognized as the most important cytokine in the hepatic response during inflammation and the major regulator of hepatic APP synthesis ([Castell et al., 1989] and [Heinrich et al., 1990]).
Recent studies have demonstrated that transcriptional activation of CYP3A4 is mediated by nuclear receptor pregnane X receptor (PXR)([Kojima et al., 2007] and [Liu et al., 2008]). PXR regulates the expression of target genes by binding with its obligate partner RXR to form a heterodimer. The heterodimer is further bound to the PXR response elements in the promoters of target genes that are involved in metabolizing and transporting endogenous and exogenous molecules ([Goodwin et al., 2004] and [Kullak-Ublick et al., 2004]). Besides CYP3A4, other important target genes are multiple drug-resistant genes including multiple drug resistance 1 (MDR1) (Synold et al., 2001) and multidrug resistant protein 2 (MRP2)(Kast et al., 2002). Gu et al.(Gu et al., 2006) have demonstrated that lipopolysaccharide (LPS), proinflammatory cytokines (TNFα) induced the production of NF-κB. When NF-κB was translocated into the nucleus and combined with RXRα to interfere the formation of PXR-RXRα, it suppressed transcription. Therefore, the consumption of PXR partner, RXRα, by NF-κB during LPS- and TNFα-mediated inflammation leads to the decrease of CYP3A4. However, the role of PXR in the IL-6-mediated down regulation of CYP3A4 has not been characterized. In this study, we provide, for first time, the evidence to show the role of PXR in the IL-6-mediated down-regulation of CYP3A4 in human hepatocytes.
2. Materials and methods
2.1. Chemicals and supplies
IL-6 was purchased from R&D Systems (Minneapolis, MN, USA). Hank’s balanced salt solution, 5,6-Dichlororibosidylbenzimidazole(DRB), Williams’E medium were from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium, high-fidelity platinum Taq DNA polymerase, and insulin-transferrin-selenium G supplement were from Invitrogen (Carlsbad, CA, USA). Dual-luciferase reporter assay system was from Promega (Madison, WI, USA). Fetal bovine serum was from Hyclone Laboratories (Logan, UT, USA). The antibody against glyceradehyde-3-phosphate dehydrogenase (GAPDH) was from Abcam (Cambridge, UK). The goat anti-rabbit IgG conjugated with horseradish peroxidase was from Pierce Chemical (Pierce, Rockford, IL, USA). Nitrocellulose membrane was from Bio-Rad Laboratories (Hercules, CA, USA). All other reagents were from Fisher Scientific (Fair Lawn, NJ, USA).
2.2. Culture and treatment of human primary hepatocytes and cell line (HepG2 cells)
Human primary cultured hepatocytes in 6-well plates were obtained from the Liver Tissues Procurement and Distribution System (University of Minnesota, Minneapolis, MN or CellzDirect, Pittsboro, NC, USA). The ten hepatocyte donors were all non-smokers of four males (21–65 years old) and six females (35–72 years old) with seven white and three black. Upon the arrival of the hepatocytes, the culture media were replaced with Willians’E medium containing insulin-transferrin-selenium supplement and penicillin/streptomycin (Yang and Yan, 2007). After incubation at 37°C with 5% CO2 for 24h, the hepatocytes were treated with 10 ng/ml IL-6 for 24 h(for mRNA level) or 48h(for protein level and enzymatic assay) (Yang and Yan, 2007). Hepatoma (HepG2) cells were purchased from American Type Culture Collection (Mannassas, VA, USA), and maintained in the Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, penicillin/streptomycin, and 1 ×nonessential amino acids. HepG2 cells were seeded at the density of 2.5 ×105 cells/well (12-well plates) in a regular medium, and the treated cells were cultured in a 1% serum-reduced medium.
2.3. Quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated by using a RNA-Bee (Tel-Test Inc., Friendswood, TX, USA) according to the manufacturer’s instruction and checked by formaldehyde gel electrophoresis for quality control. The first-strand cDNA was synthesized using total RNA (1µg) at 25°C for 10 min, 42°C for 50 min, and 70°C for 10 min by using random primers and moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI,USA). The cDNAs were then diluted eight times and quantitative PCR was conducted with TaqMan Gene Expression Assay kits (Applied Biosystems, Foster City, CA, USA). The TaqMan assay identification numbers are: CYP3A4, Hs00604506_m1; PXR (NR1I2), Hs00243666_m1; GAPDH, 4352934E. A 20 µl PCR mix contained 10µl of universal PCR master mixture, 1 µl of gene-specific TaqMan assay mixture (probe), 6 µl of diluted cDNA as template and 3 µl of water. The PCR amplification and quantification were done in an Applied Biosystems 7900 real-time PCR system (Applied Biosystems, Foster City, CA, USA) with one cycle at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The signals from each target gene were normalized based on the signal from GAPDH.
2.4. Plasmid constructs and site-directed mutagenesis
Human PXR expression constructs (Wild type), CYP3A4-DP and CYP3A4P promoter reporters (−7836/−6093 to −362/+53, −362/+53) were prepared as previously described ([Song et al., 2005] and [Yang and Yan, 2007]). The siPXR construct was kindly provided by Dr. Kemper (Bhalla et al., 2004). The PXR natural variants were made by site-directed mutagenesis as described previously (Li et al., 2004). Complementary oligonucleotides were synthesized to introduce substitution. The primers were annealed to the human expression construct and subjected to 15 cycles of PCR. The resultant constructs were digested with DpnI to remove the non-mutated parental construct. The PCR-amplified mutant constructs were used to transform XL1-Blue bacteria. And the sequences of all PXR mutant constructs were verified by direct DNA sequencing.
2.5. Transient co-transfection experiment
HepG2 cells were plated in 48-well plates in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum at the density of 8×104 cells/well. Transfection was conducted by FUGENE HD (Roche Diagnostics, Indianapolis, IN, USA). Transfection mixtures contained 50 ng of PXR plasmid, 50 ng of a reporter plasmid, and 5 ng of Null-Renilla reniformis luciferase plasmid. HepG2 cells were transfected for 12 h and the medium was replaced with fresh medium supplemented with 1% fetal bovine serum with or without IL-6(10 ng/ml). After another 24 h of treatment, the cells were washed once with phosphate-buffer saline (PBS) and collected by scraping. The collected cells were subjected to two cycles of freeze/thaw. The reporter enzyme activities were assayed with Dual-Luciferase reporter assay system. This system used two substrates, the firefly luminescence and the Renilla luminescence to determine the activities of two luciferases sequentially. The firefly luciferase activity, which reflected the reporter activity, was evaluated by mixing an aliquot of lysates (10 µl) with Luciferase Assay Reagent II (Promega, Madison, WI). Then, the firefly luminescence was quenched and the Renilla luminescence was activated simultaneously by adding Stop & Glo reagent (Promega) to the sample tubes. The firefly luminescence signal intensity was normalized based on the intensity of Renilla luminescence signal.
2.6. The modulation of PXR expression by RNAi and PXR overexpression
To define the role of PXR in the IL-6-mediated down-regulation of CYP3A4, the expression of PXR was regulated by RNAi and overexpression. In the RNAi experiment, HepG2 cells were plated in 6-well plates at a density of 5×106 and transfected with the siPXR construct (800 ng/well) or the corresponding vector for 72 h with a change of fresh medium at 36 h. The same procedure was used for overexpression experiment except for the replacement siPXR construct with the human PXR construct or the corresponding vector for 48h. The transfected cells were treated with IL-6(10 ng/ml) or the same volume of PBS for 24 h, and the expressions of CYP3A4 and PXR were monitored by real-time PCR and regular PCR.
2.7. Microsomes isolation
HepG2 cells (108) were washed once with PBS and re-suspended in 1 ml ice-cold homogenization buffer (50mM Tris-HCl, 150mM KCl, 2mM EDTA, pH 7.4), then, homogenized on ice using a motor-driven homogenizer. The samples were sonicated on ice 6 times (15s each time) and then centrifuged at 10000 g for 20 min at 4°C. The supernatant (S9 fraction) was decanted into clean ultra-centrifuge tubes and centrifuged at 105,000 g for 70 min. After being washed for once, the microsomal pellets were re-suspended and homogenized in 100 µl of 250 mM sucrose. The microsomes of each sample were aliquoted and stored at −80°C until use.
2.8. Western analysis
Human hepatocytes and HepG2 cell lysates (8µg) or HepG2 cells microsomes (20µg) were resolved by 7.5% SDS- polyacrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. After nonspecific binding sites were blocked with 5% nonfat milk, the blots are incubated with an antibody against CYP3A4 (1:2500), PXR (1:2500) and GAPDH (1:4000). The preparation of the antibody against human PXR and CYP3A4 was described elsewhere ([Sachdeva et al., 2003] and [Lindley et al., 2002]). The primary antibodies were subsequently localized with goat anti-rabbit IgG conjugated with horseradish peroxidase. Horseradish peroxidase activity was detected with a chemiluminescent kit (Pierce, Rockford, IL, USA). The chemiluminescent signal was captured by KODAK Image Station 2000 (Estman Kodak, Rochester, NY, USA), and the relative intensities were quantified by KODAK Image Analysis software (Estman Kodak, Rochester, NY, USA).
2.9. Enzymatic assay
Primary hepatocytes and HepG2 cells were treated with IL-6 (10 ng/ml) or the same volume of PBS for 48 h, and the cell lysates were prepared in 100 mM potassium phosphate buffer. The activity of CYP3A4 was determined with a P450-Glo kit (CYP3A4) (Promega, Madison, WI, USA) (Yang and Yan, 2007) according to the manufacturer’s manual. Briefly, cell lysates (16.5 µg in 12.5 µl) were mixed with 12.5 µl of CYP3A4 substrate Luciferin-BE (4×). After a 10-min pre-incubation at 37°C, the NADP regeneration mixture (25 µl containing 400 mM KPO4) was added to initiate enzymatic reaction. The reaction lasted for 30 min at 37°C and was terminated by adding 50 µl of Luciferin Detection Reagent (Promega, Madison WI). After another 10 min of incubation at room temperature, the luminescent signal intensity was determined by EGΣG BERTHOLD Microplate Luminometer (PerkinElmer, Waltham, MA, USA). Several controls were performed including incubation without cells lysates or regeneration system.
2.10. Other analyses
Protein concentrations were determined with BCA assay (Pierce, Rockford, IL, USA) based on albumin standard (Yang and Yan, 2007). Data are presented as mean ± SD from at least three independent experiments. Statistical analysis was performed using SAS software version 9.1, (SAS Institute, Cary, NC. USA). The significant difference between treatments was claimed at P < 0.05 based on one-way analysis of variance followed Duncan’s multiple comparison tests.
3. Results
3.1. IL-6 reduces the CYP3A4 activity by decreasing the expression of CYP3A4 in primary cultured human hepatocytes
Primary cultured human hepatocytes from various donors differed in the basal levels of CYP3A4 mRNA (Fig.1A). The basal level of CYP3A4 mRNA was the highest in donor 2 and the lowest in donors 1 and 5. It was about six times higher in donor 2 than that in donor 1 and 5. However, the addition of IL-6 consistently reduced the CYP3A4 mRNA level in the hepatocytes from all donors, despite the variation in basal levels among the donors. IL-6 almost inhibited all the CYP3A4 mRNA transcription in donors 1,3,4,5 and reduced 64% of the CYP3A4 mRNA in donor 2 in comparison with the corresponding control hepatocytes (Fig. 1A).
Fig. 1. Repression effects of IL-6 on CYP3A4 expression and enzymatic activity in primary human hepatocytes and HepG2 cells.
A, effect of IL-6 treatment on the level of CYP3A4 mRNA in five donors’s primary hepatocytes. Human primary hepatocytes were treated with IL-6(10ng/ml) or the same volume of PBS for 24 h. Total RNA was isolated and subjected to the qRT-PCR analysis for the level of CYP3A4 mRNA probe as described under Materials and Methods. The qPCR Cts were 24 for CYP3A4 and 20 for GAPDH. B, effects of IL-6 treatment on the CYP3A4 enzymatic activity (top) and the CYP3A4 protein expression (bottom) in human primary hepatocytes. C, effects of IL-6 treatment on the CYP3A4 enzymatic activity (top) and the CYP3A4 protein expression (bottom) in HepG2 cell. Human hepatocytes or HepG2 cells were treated with IL-6(10ng/ml) or the same volume of PBS for 48 h, and cell lysates or microsomes were prepared and assayed for the activity of CYP3A4 as described in Materials and Methods. To determined the content of CYP3A4 protein, human hepatocytes lysates(8µg) or HepG2 cell microsomes(20µg) was subjected to Western analyses with an antibody against CYP3A4 or GAPDH. All the experiments were repeated at least three times, and the data were expressed as mean ± S.D. * p<0.05, a statistically significant decrease by IL-6 treatment.
To determine whether the decrease of CYP3A4 mRNA can be translated into the decrease in the oxidative activity, primary hepatocytes were treated with IL-6, and the cell lysates were analyzed for the CYP3A4 oxidative activity. As expected, the CYP3A4 oxidative activity significantly decreased in IL-6 treated cells (Fig. 1B), which agrees with the decrease of CYP3A4 mRNA. To determine whether the decreased CYP3A4 oxidative activity is due to the decrease in CYP3A4 protein, the same lysates were analyzed by Western blotting. The result showed that the level of the CYP3A4 protein also decreased markedly, and the percentage of the reduction of the CYP3A4 protein was much more than that of the decrease in its oxidative activity (Fig.1B). However, neither mRNA nor protein levels of glyceradehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene, changed in the same experiment. A similar trend was observed in HepG2 cells as in human hepatocytes(Fig.1B & 1C).
3.2. Transcription involvement in CYP3A4 repression by IL-6
To determine the cause of the decrease in the CYP3A4 mRNA level, a transcriptional inhibition assay was performed by using a RNA synthesis inhibitor, 5,6-dichlororibosidyl-benzi-midazole (DRB), to inhibit the RNA synthesis(Clement and Wilkinson, 2000). Both human hepatocytes and HepG2 cells were treated with IL-6 and DRB for 9 h separately and together. Quantitative RT-PCR results indicated that DRB abolished the IL-6-mediated transcriptional repression of CYP3A4 in both human hepatocytes (Fig.2A) and HepG2 cells (data not shown).
Fig. 2. Transcriptional involvement in suppression of CYP3A4 by IL-6.
A, effect of DRB on the suppression of CYP3A4 mRNA in primary human hepatocytes. Human hepatocytes were treated with 10ng/ml IL-6 for 9 h in the absence or presence of 5µM DRB. Total RNA was prepared and analyzed for the levels of CYP3A4 and GAPDH by qRT-PCR. The qPCR Cts were 24 for CYP3A4 and 20 for GAPDH. B, repression of CYP3A4-DP-Luc promoter reporter. HepG2 cells were transiently transfected by FuGENE HD with a mixture containing 50 ng of CYP3A4-DP-Luc, along with 5 ng of the Null-Renilla reniformis luciferase plasmid in the presence and absence of PXR. The transfected cells were treated with 10 ng/ml IL-6 or the same volume of PBS for 24 h. Luciferase activities were determined with a Dual-Luciferase reporter assay system, and the reporter activity was normalized based on the Null-Renilla reniformis luminescence signal. C, differential repression of CYP3A4-DP-Luc and CYP3A4-P-Luc reporters mediated by IL-6 in the presence of PXR. HepG2 cells were transfected and treated with various concentrations of IL-6(0–10ng/ml) for 24 h. The reporter activities were determined as described above. All the experiments were repeated at least three times, and the data were expressed as mean ± S.D. p<0.05, a statistically significant decrease by IL-6 treatment.
The abolition of IL-6-mediated suppression of CYP3A4 by a RNA synthesis inhibitor suggests that IL-6 decrease the expression of CYP3A4 through repressing its promoter. To test this hypothesis, co-transfection was performed using a CYP3A4 promoter reporter and an hPXR construct in HepG2 cells. Two CYP3A4 promoter reporters were used, CYP3A4-DP-luc containing both proximal and distal promoter regions that respond to many CYP3A4 inducers (Song et al., 2005) and CYP3A4-P-luc containing only the proximal promoter region. Hepatoma cells (HepG2) were transfected with CYP3A4-DP-luc or CYP3A4-P-luc and Renilla plasmid with or without hPXR, and then treated with 10 ng/ml of IL-6 or PBS. After 24 h incubation, the cells were lysed and used to determine luciferase activities. The cells treated with IL-6 significantly reduced the activity of CYP3A4-DP promoter reporter with or without hPXR (Fig. 2B). Furthermore, the inhibition of CYP3A4 promoter reporter by IL-6 in the cells transfected with hPXR (74.8%) was higher than that without hPXR (30.8%), and the basal activity of CYP3A4-DP-luc co-transfected with hPXR was much higher than that without hPXR (Fig. 2B). Additionally, although the basal activity of CYP3A4-DP-luc was much higher than that of CYP3A4-P-luc, IL-6 suppressed the expression of both CYP3A4-DP-luc and CYP3A4-P-luc in a dose-dependent manner (Fig. 2C). These data suggest that IL-6-mediated repression of CYP3A4 occur at the transcriptional level and PXR promote the IL-6-mediated down regulation of CYP3A4 in hepatocytes.
3.3. Requirement for PXR in the IL-6-mediated suppression of CYP3A4 expression
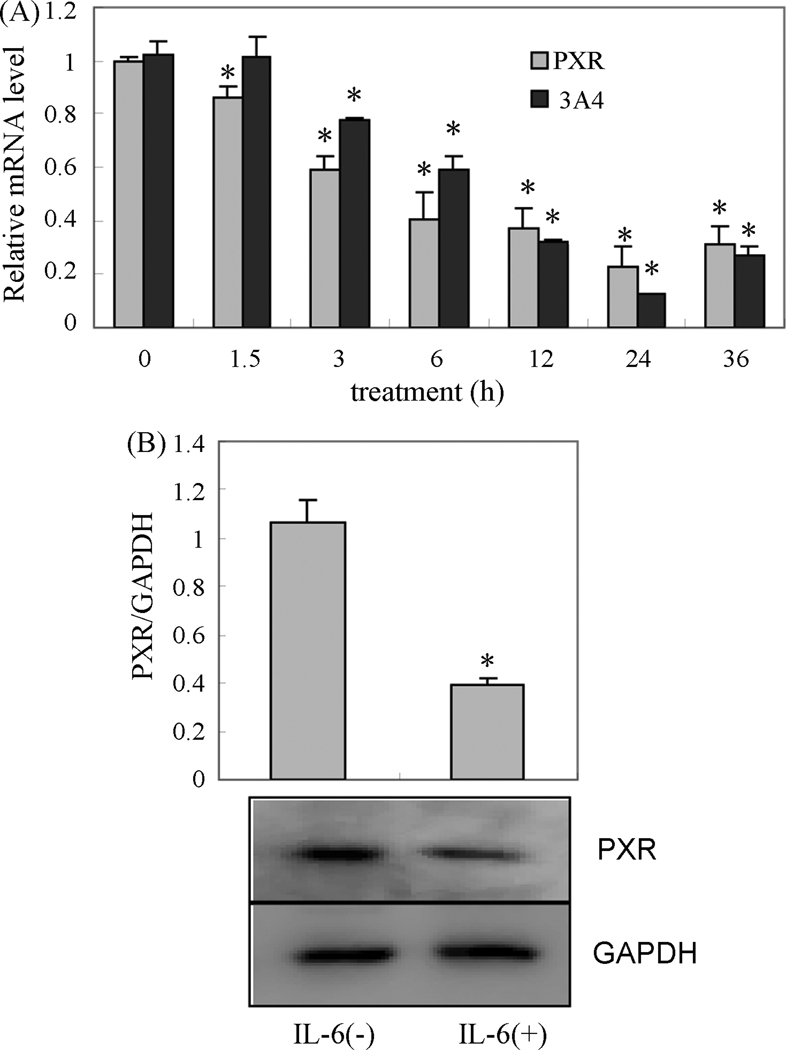
The inhibition of CYP3A4 promoter reporter by IL-6 was stronger in the presence of PXR than that in the absence of PXR, which suggests that PXR is involved in the IL-6-mediated repression of CYP3A4. This notion is supported by the observation that both the expression of PXR and CYP3A4 mediated by IL-6 simultaneously changed in primary cultured human hepatocytes. Human hepatocytes were treated with IL-6 (10ng/ml) or PBS for 1.5h, 3h, 6h, 12h, 24h and 36h, and then analyzed for the PXR and CYP3A4 mRNA content using qRT-PCR. The PXR protein of human hepatocytes was also analyzed with Western blotting after treatment with IL-6 (10ng/ml) for 24 h. A continuous decrease in PXR expression was observed after 1.5 h of treatment with IL-6, but the significant continuous decrease in CYP3A4 occurred after 3 h of treatment (Fig.3A), which indicated that the decrease in the CYP3A4 expression occurred after the decrease of PXR mediated by IL-6 in human hepatocytes (Fig.3A). The decrease in PXR protein was confirmed by Western blot (Fig.3B).
Fig. 3. Repression effect of IL-6 on PXR expression in primary human hepatocytes.
A, a time-course effect of IL-6 on the levels of PXR and CYP3A4 in primary human hepatocytes. Human hepatocytes were treated with IL-6(10ng/ml) or the same volume of PBS for 24 h. Total RNA was isolated and subjected to the qRT-PCR analysis for the levels of PXR and CYP3A4. The qPCR Cts were 25 for PXR, 24 for CYP3A4 and 20 for GAPDH. B, repression effect of IL-6 on the PXR protein level in primary hepatocytes. Human hepatocytes were treated with IL-6(10ng/ml) or the same volume of PBS for 48 h, and cell lysates(10µg) were prepared and analyzed by Western blotting. All the experiments were repeated at least three times, and the data were expressed as mean ± S.D. p<0.05, a statistically significant decrease by IL-6 treatment.
To determine the role of PXR in the down regulation of CYP3A4 in response to IL-6, we performed knockdown and overexpression experiments to selectively modulate the expression of PXR. In the knockdown experiment, HepG2 cells were transfected with siPXR construct for 72 h using corresponding vector as control. After being treateded with either IL-6 (10 ng/ml) or PBS for 24 h, the CYP3A4 and PXR levels in cells were analyzed using qRT-PCR and regular RT-PCR. The result indicated that PXR knockdown alone significantly decreased the CYP3A4 expression (Fig.4A right). And IL-6 significantly decreased CYP3A4 mRNA in the cells transfected with the vector, but, not in the cells transfected with the siPXR construct (Fig. 4A right). The PXR mRNA level in the cells transfected with siPXR was about 60% lower than that in the control cells transfected with the corresponding vector using qRT-PCR and regular RT-PCR (Fig. 4A, left and 4A, middle).
Fig. 4. Suppression of CYP3A4 as a function of PXR.
A, effect of SiPXR on the suppression of CYP3A4. HepG2 cells were transiently transfected with the siPXR construct or the corresponding vector for 72 h, and the transfected cells were treated with IL-6(10ng/ml) or the same volume of PBS for 24 h. Total RNA was prepared and analyzed for levels of CYP3A4, PXR and GAPDH by qRT-PCR and RT-PCR. B, effect of PXR overexpression on the repression of CYP3A4 mediated by IL-6. The same procedure as the above was used for overexpression experiment except for the replacement siPXR construct with the human PXR construct or the corresponding vector for 48h. The qPCR Cts were 27 for PXR, 32 for CYP3A4 and 16 for GAPDH. All the experiments were repeated at least three times, and the data were expressed as mean ± S.D. p<0.05, a statistically significant decrease by IL-6 treatment.
In the overexpression experiment, the construct encoding hPXR was used instead of the siPXR construct in the previous experiment. After being treated with IL-6 (10 ng/ml) or PBS for 24 h, the cells transfected with hPXR were analyzed for CYP3A4 using qRT-PCR. Contrary to the PXR knowndown experiment, the PXR overexpression significantly increased CPY3A4 and IL-6 markedly decreased the CYP3A4 expression both in the PXR overexpression and normal cells (Fig.4B). Moreover, the inhibition of CYP3A4 expression was higher in the cells transfected with hPXR (65.2%) than those transfected with vector alone (34.5%) (Fig. 4B). The results suggest that PXR is required for the IL-6-mediated repression of CYP3A4 and the PXR decrease is likely to lead to the CYP3A4 decrease in hepatocytes.
3.4. Inhibition of human PXR variants mediated by IL-6
To further investigate the variation mediated by IL-6 among human PXR variants, wild type human PXR and its natural variants of human PXR were tested for response to IL-6. As shown in Fig. 5, among all the PXR variants (including the wild type), higher inhibitions of CYP3A4-DP-luc by IL-6 were detected with hPXRR148Q (~80%), hPXRE18K, hPXRG36R (~79%) and wild type hPXR(~65%), and lower inhibitions were detected with hPXRW223A (~11%), hPXRW223A/Y225A (~23%) and hPXRY225A(~45%) although some hPXR variants (such as hPXRR98C, hPXRS208F, hPXRW223A) showed very low basal activities. It should be noted that PXR and its variants were expressed to a comparable extent (Fig. 5, bottom). These findings suggest that the variation in IL-6-mediated repression exists among human PXR variants.
Fig. 5. The repression of wild type and 15 natural human PXR variants mediated by IL-6.
HepG2 cells were transiently transfected with a mixture containing 50 ng of CYP3A4-DP-Luc, 50 ng wide type or nature human PXR variant, along with 5ng of the Null-Renilla reniformis luciferase plasmid. The transfected cells were treated with 10ng/ml IL-6 or the same volume of PBS for 24 h. Luciferase activities were determined as described in the above. To determine whether the expression is altered with certain variants, cell lysates(8µg) were analyzed by Western blotting(Anti-hPXR) for the expression level(bottom). Three independent experiments were performed, and the data were expressed as mean ± S.D.
4. Discussion
It has been reported that drug biotransformation was impaired in patients with liver conditions such as hepatitis and cirrhosis ([Shibuya et al., 2003] and [Qu et al., 2007]). In these conditions, as the production of various proinflammatory cytokines (e.g., IL-6) is markedly increased ([Eriksson et al., 2004] and [Zhang et al., 2002]), the metabolisms of many drugs are decreased ([Thom et al., 2007], [Aitken and Morgan, 2007] and [Jover et al., 2002]). To understand the relationship between the increased cytokines production and the decreased drugs biotransformation, we tested whether IL-6 suppresses the CYP3A4 expression, the most important CYP450 enzyme, and studied the possible mechanism. Here, we show that both human hepatocytes and hepatoma cells treated with IL-6 showed a significant decrease in the CYP3A4 expression. The decreased CYP3A4 expression occurred at both mRNA and protein levels (Fig.1A, 1B and 1C), and its function was also confirmed by enzymatic assay (Fig.1B and 1C). The data showed that the reduction at the CYP3A4 protein and mRNA levels were much higher than that in its oxidative activity (Fig.1A,1B and 1C). It is possible that human hepatocytes may compensate part of the function of CYP3A4 when CYP3A4 expression is decreased. The reduction at the CYP3A4 mRNA and protein levels might result from suppressing the expression of CYP3A4 by transcriptional repression and/or increasing the degradation of CYP3A4 mRNA. In this study, the decrease of CYP3A4 mediated by IL-6 was prevented by adding a RNA synthesis inhibitor (Fig. 2A), suggesting that the repression of CYP3A4 by IL-6 occurrs at the transcriptional level, which was further confirmed by CYP3A4 promoter experiments (Fig.2B and 2C). It is well-known that inflammation decreases the drug metabolism enzymes, but the mechanism(s) of such action is(are) still controversial. Several previous studies proposed the possible mechanisms as increasing the signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein kinases (MAPKs), or nuclear factor-κB (NF-κB), which can directly impair the action of nuclear receptors involved in the regulation of CYP3A4 (Robertson et al., 2008). For example, if the actions of PXR and constitutive androstane receptor (CAR), which are responsible for the induction of CYP3A4 by xenobiotics (Jover et al., 2002) are impaired, the expression of their target genes such as CYP3A4 will be affected. Gu et al (Gu et al., 2006) found that IL-6 increased the level of NF-κB that can form dimmers with retinoid X receptor-α (RXR-α). The formation of NF-κB RXR-α heterodimer consumes the RXR-α to disrupt the combination of PXR and RXR-α and therefore represses the CYP3A4 transcription. Another potential mechanism that may contribute to the CYP3A4 repression is the disruption of the balance between the two isoforms of the CAAT/enhancer binding protein-β (C/EBP-β)—liver activating protein (LAP) and liver inhibitory protein (LIP). The ratio between LAP and LIP in turn impacts the action of C/EBP-α required for the basal expression of CYP3A4 (Jover et al., 2002).
Pregnane X receptor (PXR) was abundantly expressed in liver and intestine, which are predominately responsible for the entry and metabolism of chemicals ([Kliewer et al., 1998] and [Lehmann et al., 1998]). Pregnane X receptor has been recognized as a key regulator that mediates the induction of many chemical elimination genes including CYP3A ([Kliewer et al., 1998] and [Kliewer and Willson, 2002]). However, how PXR as a regulator mediates the repression of the target genes remains unknown. Structurally, PXR belongs to a super-family of nuclear receptors ([Honkakoski et al., 2003] and [Jetten et al., 2001]), including hormone receptors (e.g., steroid receptors), vitamin receptors (e.g., vitamin D receptor), and the so-called orphan nuclear receptors (e.g., peroxisome proliferator-activated receptor). All the nuclear receptors consist of an N-terminal highly conserved DNA-binding domain, a hinge region of varying lengths, and a multifunctional C-terminal ligand-binding domain ([Greschik and Moras, 2003] and [Shiau et al., 2001]). The major portion of the ligand-binding domain has a helical structure, and the C-terminal helix (helix 12) is directly involved in switching from repressing to activating the status of a target gene. Binding to an agonist, PXR induces the conformational changes of this helix to form a platform that favors association with coactivators (e.g., steroid receptor coactivator-1, SRC-1)([Weiss and Ramos, 2004] and [Xu and Li, 2003]). Some studies have suggested that the down-regulation of CYP450 (CYP2B, CYP3A) by cytokines is due to the down-regulation of PXR and constitutive androstane receptor (CAR)(Beigneux et al., 2002), while other studies argue that the down-regulation of P450 during inflammation does not require the nuclear receptors PPARalpha and PXR based on the data from the knockout experiments of PPARalpha and PXR in mice (Richardson and Morgan, 2005). In the present study, we demonstrated that the PXR knockdown decreased the CYP3A4 expression, and IL-6 decreased CYP3A4 significantly in normal cells but not in the PXR knockdown cells (Fig.4A right). And opposite to the PXR knockdown experiment, the PXR overexpression increased the CYP3A4 expression and the repression of CYP3A4 expression by IL-6 in the PXR overexpression cells was much higher than that in the regular HepG2 cells (Fig.4B). Therefore, PXR is essential for the IL-6-mediated down regulation of the CYP3A4 expression in human hepatocytes. These results agree with the animal data coming from normal mice (Beigneux et al., 2002), but different from other data coming from PXR-null-mice (Richardson and Morgan, 2005). One of the reasons for the discrepancy between the results from this study and Richardson et al.’s may be that some substituted function occurred in the PXR-null mice(in vivo for long time) but did not in the PXR knockdown human hepatoma cells (in vitro for short time).
Among the wild type human PXR and 15 PXR variants containing substitution in DNA-binding domain (such as hPXRE18K, hPXRG36R) and ligand-binding domain (such as hPXRS208F, hPXRW223A), three of the variants that had substitution in the ligand-binding domain (hPXRW223A, hPXRY225A and hPXRW223A/Y225A) showed significant differences in response to IL-6 from the wild type of hPXR (Fig.5). The precise mechanism of three of the hPXR variants with low response to IL-6 remains to be determined. It is very interesting to note that the tryptophan-223, tyrosine-225 and the double sites in these hPXR variants were replaced by alanine. Conserved tryptophan and tyrosine residues locked across the dimmer interface and provided the first tryptophan-zipper (Trp-Zip) interaction as observed in a native protein, and such interaction is likely to eliminate the receptor’s recruitment of transcriptional factors including co-repressor (Noble et al., 2006). These findings suggest that hPXR variants have different responses to IL-6, which lead to individuals to metabolize the drugs in inflammation. These findings also support the notion that PXR is very critical in the IL-6 –mediated down regulation of CYP3A4 in human hepatocytes.
In summary, this study leads to several important conclusions. First, IL-6 can repress the CYP3A4 expression and its function, and the repression of CYP3A4 mediated by IL-6 is required for PXR in human hepatocytes. Therefore, decreased CYP3A4 is likely to reduce the metabolism of drugs during inflammation. Second, the repression of CYP3A4 by IL-6 is achieved through decreasing PXR expression in human hepatocytes, thus IL-6 is likely to decrease the expression of all PXR target genes (such as MDR1). It is possible to alter the functions of PXR target genes in inflammation to change the metabolism and transport of many drugs. Third, the repression by IL-6 varies with human natural PXR variants, and the repression is likely to exhibit individual variants. Pharmacologically, the critical role of PXR in IL-6-mediated repression suggests that this important cytokine causes a broad range of drug-drug interactions, an alteration in the therapeutic effect and the toxicity of many drugs in inflammation, and the differential action of natural PXR variants suggests that the magnitude of such interactions varies from person to person.
Acknowledgement
The authors would like to thank Drs Dolan of the University of Chicago and Kemper of the University of Illinois for providing plasmid constructs. This work is supported by Natural Science Foundation of China 30772616, Foundation of “Liu-Da-Ren-Cai”, Jiangsu Province (CHINA) and by National Institutes of Health grants (USA) F05AT003019, RO1ES07965, RO1GM61988.
Abbreviations
- IL-6
interleukin-6
- CYP450
cytochrome P450
- PXR
pregnane X receptor
- hPXR
human pregnane X receptor
- LPS
lipopolysaccharide
- TNF-α
tumor necrosis factor α
- DRB
5,6-dichlororibosidyl-benzi-midazole
- GAPDH
glyceradehyde-3-phosphate dehydrogenase
- PCR
polymerase chain reaction
- DMEM
Dulbecco’s modified Eagle’s medium
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–1693. doi: 10.1124/dmd.107.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR(pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun. 2002;293:145–149. doi: 10.1016/S0006-291X(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Bhalla S, Ozalp C, Fang S, Kemper JK. Ligand-activated pregnane X receporter interes with HNF-4 signaling by targeting a common coactivator PGc-1alpha. Functional implication in hepatic cholesterol and glucose metabolism. J Biol Chem. 2004;279:45139–45147. doi: 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- Castell JV, Gomez-Lechon M, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PP. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Castell JV, Gomez-Lechon MJ, David R, Fabra Trullenque R, Heinrich PC. Acute phase response of human hepatocytes: regulation of acute phase protein synthesis by interleukin 6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- Clement JQ, Wilkinson MF. Rapid induction of nuclear transcripts and inhibition of intron decay in response to the polymerase II inhibitor DRB. J Mol Biol. 2000;299:1179–1191. doi: 10.1006/jmbi.2000.3745. [DOI] [PubMed] [Google Scholar]
- Cooper BW, Cho TM, Tompson PM, Wallace AD. Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of PXR expression. Toxicol Sci. 2008;103:268–677. doi: 10.1093/toxsci/kfn047. [DOI] [PubMed] [Google Scholar]
- Eriksson AS, Gretzer C, Wallerstedt S. Elevation of cytokines in peritoneal fluid and blood in patients with liver cirrhosis. Hepatogastroenterology. 2004;51:505–509. [PubMed] [Google Scholar]
- Goodwin B, Moore JT. CAR: detailing new models. Trends Pharmacol Sci. 2004;25:437–441. doi: 10.1016/j.tips.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Greschik H, Moras D. Structure-activity relationship of nuclear receptor-ligand interactions. Curr Top Med Chem. 2003;3:1573–1599. doi: 10.2174/1568026033451736. [DOI] [PubMed] [Google Scholar]
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB. Role of NF-κB in Regulation of PXR-mediated Gene Expression. J Biol Chem. 2006;281:17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkakoski P, Sueyoshi T, Negishi M. Drug-activated nuclear receptors CAR and PXR. Ann Med. 2003;35:172–182. doi: 10.1080/07853890310008224. [DOI] [PubMed] [Google Scholar]
- Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- Jover R, Rort R, Gomez-Lechon MJ, Castell JV. Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: molecular mechanism and transcription factors involved. FASEB J. 2002;16:1799–1801. doi: 10.1096/fj.02-0195fje. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, Mckke DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Willson TM. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J Lipid Res. 2002;43:359–364. [PubMed] [Google Scholar]
- Koj A. Initiation of acute phase response and synthesis of cytokines. Biochim Biophys Acta. 1996;1317:84–94. doi: 10.1016/s0925-4439(96)00048-8. [DOI] [PubMed] [Google Scholar]
- Kojima K, Nagata K, Matsubara T, Yamazoe Y. Broad but distinct role of pregnane x receptor on the expression of individual cytochrome p450s in human hepatocytes. Drug Metab Pharmacokinet. 2007;22:276–286. doi: 10.2133/dmpk.22.276. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Lamba JK, Lin YS, Schuetz EG, Thummel RE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Song X, Ma Y, Liu J, Yang D, Yan B. DNA binding but not interaction with Bmal 1, is responsible for DEC1-mediated transcription regulation of the circadian gene mper1. Biochem J. 2004;382:895–904. doi: 10.1042/BJ20040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Lu AY. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu Rev Pharmacol Toxicol. 2001;41:535–567. doi: 10.1146/annurev.pharmtox.41.1.535. [DOI] [PubMed] [Google Scholar]
- Lindley C, Hamilton G, McCune JS, Faucette S, Shord SS, Hawke RL, Wang H, Gillert D, Jolley S, Yan B, Lelluyse EL. The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug Metab Dispos. 2002;30:814–822. doi: 10.1124/dmd.30.7.814. [DOI] [PubMed] [Google Scholar]
- Liu F, Song X, Yang D, Deng R, Yan B. The far and distal enhancers in the CYP3A4 gene co-ordinate the proximal promoter in responding similarly to the pregnane X receptor but differentially to hepatocyte nuclear factor-4alpha. Biochem J. 2008;409:243–250. doi: 10.1042/BJ20070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer UA, Zanger UM. Molecular mechanisms of genetic polymorphisms of drug metabolism. Annu Rev Pharmacol Toxicol. 1997;37:269–296. doi: 10.1146/annurev.pharmtox.37.1.269. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- Morgan ET. Regulation of cytochrome P450 by inflammatory mediators: why and how? Drug Metab Dispos. 2001;29:207–212. [PubMed] [Google Scholar]
- Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Noble SM, Carnahan VE, Moore LB, Luntz T, Wang H, Ittoop OR, Stimmel JB, Davis-Searles PR, Watkins RE, Wisely GB, LeCluyse E, Tripathy A, McDonnell DP, Redinbo MR. Human PXR forms a tryptophan zipper-mediated homodimer. Biochemistry. 2006;45:8579–8589. doi: 10.1021/bi0602821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu ZQ, Li XD, Liu HL, He P, Zhang X, Wu M. Impaired clearance of phenacetin in hepatic cirrhosis and fibrosis. Int J Clin Pharmacol Ther. 2007;45:55–62. doi: 10.5414/cpp45055. [DOI] [PubMed] [Google Scholar]
- Richardson TA, Morgan ET. Hepatic cytochrome P450 gene regulation during endotoxin-induced inflammation in nuclear receptor knockout mice. J Pharmacol Exp Ther. 2005;314:703–709. doi: 10.1124/jpet.105.085456. [DOI] [PubMed] [Google Scholar]
- Robertson GR, Liddle C, Clarke SJ. Inflammation and altered drug clearance in cancer: transcriptional repression of a human CYP3A4 transgene in tumor-bearing mice. Clin Pharmacol Ther. 2008;83:894–897. doi: 10.1038/clpt.2008.55. [DOI] [PubMed] [Google Scholar]
- Sachdeva K, Yan B, Chichester C. Lipopolysaccharide and cecal ligation/puncture differentially affect the subcellular distribution of the pregnane X receptor but consistently cause suppression of its target genes CYP3A. Shock. 2003;19:469–474. doi: 10.1097/01.shk.0000048903.46342.ec. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Coward P, Schwarz M, Lehmann JM. Orphan nuclear receptors: from new ligand discovery technologies to novel signaling pathways. Curr Opin Drug Discov Devel. 2001;4:575–590. [PubMed] [Google Scholar]
- Shibuya M, Echizen H, Kubo S, Tamura N, Suzuki K, Ushiama H. Reduced urinary 6beta-hydroxycortisol to cortisol ratios in patients with liver cirrhosis. Hepatol Res. 2003;26:28–33. doi: 10.1016/s1386-6346(03)00005-6. [DOI] [PubMed] [Google Scholar]
- Shou M, Havashi M, Pan Y, Xu Y, Morrisev K, Xu L, Skiles GL. Modeling, Prediction, and In Vitro In Vivo Correlation of CYP3A4 Induction. Drug Metab Dispos. 2008;36:2355–2370. doi: 10.1124/dmd.108.020602. [DOI] [PubMed] [Google Scholar]
- Song X, Li Y, Liu J, Mukundan M, Yan B. Simultaneous substitution of phenylatine-305 and aspartate-318 of rat pregnane X receptor with the corresponding human residues abolishes the ability to transactivate the CYP3A23 promoter. J Pharmacol Exp Ther. 2005;312:517–528. doi: 10.1124/jpet.104.074971. [DOI] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7(5):584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- Thom M, Finnstrom N, Lundgren S, Rane A, Loof L. Expression of cytochrome P450 and MDR1 in patients with proctitis. Ups J Med Sci. 2007;112:303–312. doi: 10.3109/2000-1967-203. [DOI] [PubMed] [Google Scholar]
- Weiss RE, Ramos HE. Thyroid hormone receptor subtypes and their interaction with steroid receptor coactivators. Vitam Horm. 2004;68:185–207. doi: 10.1016/S0083-6729(04)68006-X. [DOI] [PubMed] [Google Scholar]
- Wilkinson GR. The effects of diet, aging and disease-states on presystemic elimination and oral drug bioavailability in humans. Adv Drug Deliv Rev. 1997;27:129–159. doi: 10.1016/s0169-409x(97)00040-9. [DOI] [PubMed] [Google Scholar]
- Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681–1692. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- Yang J, Yan B. Photochemotherapeutic agent 8-methoxypsoralen induces Cytochrome P450 3A4 and Carboxylesterase HCE2: Evidence on an Involvement of the Pregnane X Receptor. Toxicol Sci. 2007;95:13–22. doi: 10.1093/toxsci/kfl120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yue B, Wang GQ, Lu SL. Serum and ascites levels of macrophage migration inhibitory factor, TNF-alpha and IL-6 in patients with chronic virus hepatitis B and hepatitis cirrhosis. Hepatobiliary Pancreat Dis Int. 2002;1:577–580. [PubMed] [Google Scholar]