Abstract
Troponin T (TnT) and troponin I (TnI) are two evolutionarily and functionally linked subunits of the troponin complex that regulates striated muscle contraction. We previously reported a single amino acid substitution in the highly conserved TnT-binding helix of cardiac TnI (cTnI) in wild turkey hearts in concurrence with an abnormally spliced myopathic cardiac TnT (cTnT) (Biesiadecki, B. J., Schneider, K. L., Yu, Z. B., Chong, S. M., and Jin, J. P. (2004) J. Biol. Chem. 279, 13825–13832). To investigate the functional effect of this cTnI mutation and its potential value in compensating for the cTnT abnormality, we developed transgenic mice expressing the mutant cTnI (K118C) in the heart with or without the deletion of the endogenous cTnI gene to mimic the homozygote and heterozygote of wild turkeys. Double and triple transgenic mice were created by crossing the cTnI-K118C lines with transgenic mice overexpressing the myopathic cTnT (exon 7 deletion). Functional studies of ex vivo working hearts found that cTnI-K118C alone had a dominantly negative effect on diastolic function and blunted the inotropic responses of cardiac muscle to β-adrenergic stimuli without abolishing the protein kinase A-dependent phosphorylation of cTnI. When co-expressed with the cTnT mutation, cTnI-K118C corrected the significant depression of systolic function caused by cTnT exon 7 deletion, and the co-existence of exon 7-deleted cTnT minimized the diastolic abnormality of cTnI-K118C. Characterization of this naturally selected pair of mutually rescuing mutations demonstrated that TnI-TnT interaction is a critical link in the Ca2+ signaling and β-adrenergic regulation in cardiac muscle, suggesting a potential target for the treatment of troponin cardiomyopathies and heart failure.
Keywords: Cardiac Muscle, Contractile Protein, Evolution, Heart, Mutant, Protein-Protein Interactions, Troponin
Introduction
Troponin T (TnT)3 and troponin I (TnI) are two evolutionarily and functionally linked subunits of the troponin complex that regulates striated muscle contraction (1–3). We previously reported that domestic turkeys with inherited dilated cardiomyopathy (DCM) and heart failure had an abnormal low molecular weight cardiac TnT (cTnT) resulting from aberrant splice-out of an 11-amino acid segment encoded by exon 8 (4). Abnormal splice-out of the corresponding exon (exon 7) was also found in cTnT of mammalian species that have inherited DCM (5). The deletion of this N-terminal segment from cTnT altered binding affinities for TnI and tropomyosin and myofilament sensitivity to Ca2+ activation (4). Transgenic expression of the aberrant cTnT in mouse hearts resulted in decreased cardiomyocyte contractility, supporting a causal relationship to the development of DCM (5).
Wild turkey hearts showed the abnormally spliced cTnT at the same level as that in domestic turkey hearts (6). Therefore, the cTnT aberrant splicing preexisted prior to the domestication of turkeys. However, we found an interesting point mutation/polymorphism in cardiac TnI (cTnI) in wild but not domestic turkey hearts, which encodes a single amino acid substitution of Cys for Arg111 (6). Arg111 in avian cTnI corresponds to Lys117 in human cTnI and Lys118 in mouse cTnI. This amino acid resides in the TnT-contacting α-helix of cTnI in the I-T arm of the troponin complex (7, 8) and is highly conserved as Arg or Lys in all three muscle fiber type-specific (cardiac, slow, and fast skeletal muscle) TnI isoforms across vertebrate species (6, 9). Protein binding experiments demonstrated that the R111C substitution decreased the binding affinity of turkey cTnI for cTnT, which is potentially compensatory against the increased TnI binding affinity of the exon 8-deleted myopathic cTnT (6). These findings suggest that the presence of the cTnI-Cys111 allele in the wild turkey population may have a significant fitness value during natural selection.
In this study, we examined the functional effect of the cTnI-Cys111 mutation and its potential value in rescuing the myopathic phenotype of abnormally spliced cTnT. We developed transgenic mice expressing the mutant mouse cTnI (K118C) in the heart with or without a deletion of the endogenous cTnI gene to mimic the homozygote and heterozygote of cTnI-Cys111 wild turkeys. Double and triple transgenic mice were created by crossing the cTnI-K118C transgenic mice with the exon 7-deleted cTnT transgenic mouse line. Functional analysis in ex vivo working hearts found that cTnI-K118C decreased diastolic function and blunted the inotropic responses of cardiac muscle to β-adrenergic stimuli. However, cTnI-K118C corrected the severe depression in systolic function caused by cTnT exon 7 deletion, and the co-existence of exon 7-deleted cTnT minimized the diastolic abnormality of cTnI-K118C. These mutual rescues demonstrated that TnI-TnT interaction is a crucial link in Ca2+ signaling and β-adrenergic regulation of cardiac muscle function, suggesting a novel target for the treatment of troponin cardiomyopathies and heart failure.
MATERIALS AND METHODS
Genetically Modified Mouse Lines
The transgenic mice overexpressing exon 7-deleted cTnT in the adult heart under the control of α-myosin heavy chain (MHC) promoter were described previously (5).
Transgenic mouse lines overexpressing cTnI-K118C in the adult heart were developed by construction of a transgene driven by the α-MHC promoter (a gift from Dr. Jeffrey Robbins, University of Cincinnati) as described previously (5). The injection of transgene DNA into fertilized mouse eggs and embryo implantation were carried out by the Gene Targeting and Transgenic Core Facility at Northwestern University Feinberg School of Medicine. Genotyping of the transgenic founders and offspring was carried out using PCR on DNA samples extracted from tail biopsies.
A mouse line with a deletion of the endogenous cTnI gene (Tnni3) (cTnI-KO) was described previously (10). The cTnI-K118C transgene allele was used to rescue the postnatal lethality of cTnI-KO mice. Similar to the previously described rescue using another α-MHC promoter-directed cTnI transgene allele (11), double transgenic mice were generated by crossing the cTnI-K118C transgenic mice with heterozygotes of cTnI-KO mice. Double heterozygotes were interbred to generate cTnI-K118C-positive offspring that are homozygotes of cTnI-KO. The genotyping was done using PCR, and the expression of cTnI-K118C in the absence of endogenous cTnI in the adult hearts of double transgenic mice was examined using Western blotting as described below.
We then generated transgenic mice co-expressing cTnI-K118C and exon 7-deleted cTnT in adult hearts. Double transgenic mice were produced by crossing the cTnT exon 7 deletion line with a cTnI-K118C line. Triple transgenic mice were generated by crossing the cTnT exon 7 deletion line with the cTnI-K118C/cTnI-KO line to co-express exon 7-deleted cTnT and cTnI-K118C in the absence of endogenous cTnI. The genotyping was done using PCR, and the co-expression of exon 7-deleted cTnT and cTnI-K118C in the presence or absence of endogenous wild type cTnI was examined by Western blotting as described below.
Mice of both sexes were used in this study, and all protocols were approved by the Institutional Animal Care and Use Committee.
SDS-PAGE and Western Blotting
Cardiac muscle from left ventricular free wall was rapidly isolated post-mortem and homogenized in SDS-PAGE sample buffer containing 2% SDS and 1% β-mercaptoethanol, pH 8.8, using a high speed mechanical homogenizer to extract total proteins. The SDS-PAGE samples were heated at 80 °C for 5 min, centrifuged in a microcentrifuge to remove insoluble materials, and resolved on 14% SDS gel with an acrylamide:bisacrylamide ratio of 180:1 using a modified Laemmli buffer system in which both stacking and resolving gels were at pH 8.8. The protein bands resolved in the gel were stained with Coomassie Blue R-250. Total protein in each lane was quantified by ImageJ software for normalizing the amount of sample loading.
Copies of the SDS gels were transferred to nitrocellulose membrane using a Bio-Rad semidry electrotransfer device at constant current of 5 mA/cm2 for 15 min. The blotted membranes were blocked in 1% bovine serum albumin (BSA) in Tris-buffered saline (TBS, 150 mm NaCl, 50 mm Tris, pH 7.5) with shaking at room temperature for 30 min. The blocked membrane was probed with an anti-TnI monoclonal antibody (mAb) TnI-1 that recognizes a C-terminal epitope (12) or an anti-cardiac and slow TnT mAb CT3 (13), both diluted in TBS containing 0.1% BSA, with gentle rocking at 4 °C overnight. The membranes were then washed three times with TBS containing 0.5% Triton X-100 and 0.05% SDS, incubated with alkaline phosphatase-labeled goat anti-mouse IgG second antibody (Santa Cruz Biotechnology), washed again as above, and developed in 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate solution to visualize the cTnI and cTnT bands detected by the mAbs.
Glycerol-SDS-PAGE Examination of Cardiac MHC Isoforms
Glycerol-SDS-PAGE was employed to examine cardiac MHC isoforms. Total myocardial protein extract was resolved on 8% SDS gel with an acrylamide:bisacrylamide ratio of 50:1, containing 30% glycerol, 0.4% SDS, 200 mm Tris-HCl, and 100 mm glycine, pH 8.8. The upper running buffer was composed of 100 mm Tris base, 150 mm glycine, 0.1% SDS, and 10 mm β-mercaptoethanol. The lower running buffer was a 1:2 dilution of the upper running buffer without β-mercaptoethanol. The gel was run at 100 V at 4 °C for 24 h and stained with Coomassie Blue R-250 to visualize the protein bands.
Paraffin Sectioning and Hematoxylin and Eosin Staining
Hearts were isolated from transgenic and wild type mice and cannulated for retrograde Langendorff perfusion through the aorta at 37 °C with Krebs-Henseleit buffer containing 50 mm KCl to relax the cardiac muscle. The hearts were then fixed in 3.7% formalin in neutral sodium phosphate buffer at 4 °C for over 16 h. With standard paraffin embedding, cross-sections of 5 μm thickness were cut, stained with hematoxylin and eosin, examined under a Zeiss Axiovert 100 microscope, and photographed. The size of ventricular chambers and the thickness of left ventricular free wall were measured using NIH ImageJ software.
Isolated Working Heart Preparations and Base-line Cardiac Function Measurements
Mouse working heart study was carried out in a genotype-blinded manner as described previously (14, 15). Thirty min after intraperitoneal injection of 100 units of heparin, a mouse of 25–30 g body weight (2–3 months old) was anesthetized by intraperitoneal injection of pentobarbital sodium (100 mg·kg−1 bodyweight). The thoracic cavity was opened by transverse incision, and the heart was rapidly isolated. The heart was placed in a modified Krebs-Henseleit buffer (118 mm NaCl, 4.7 mm KCl, 2.25 mm CaCl2, 2.25 mm MgSO4, 1.2 mm KH2PO4, 0.32 mm EGTA, 25 mm NaHCO3, 15 mm d-glucose, and 2 mm sodium pyruvate, pH 7.4) bubbled with 95% oxygen and 5% carbon dioxide at 37 °C. The aorta was cannulated with a modified 18-gauge needle (6-mm path with thinned wall), and Langendorff retrograde perfusion was started within 3 min after the opening of the thoracic cavity.
A modified 16-gauge needle was then used to cannulate the pulmonary vein for antegrade perfusion through the left atrium. Care was taken to avoid air bubbles in the antegrade perfusion line. After ligating the venae cavae, a beveled PE-50 tubing was used to cannulate the pulmonary artery to collect the coronary outflow.
A 1.2 French pressure-volume (P-V) catheter (model 898B, Scisense, calibrated for pressure and volume at 37 °C in 95% oxygen and 5% carbon dioxide-bubbled Krebs-Henseleit buffer) was used to record the left ventricular pressure and volume. A 30-gauge needle was used to puncture a path through the apex for inserting the P-V catheter into the center of the left ventricular chamber. Aortic pressure was measured using an MLT844 pressure transducer (Capto, Horten, Norway). As described previously (16, 17), a 0.5-ml air bubble was placed in the left ventricular outflow path to mimic the aortic compliance. Left ventricular pressure (LVP) and volume signals and aortic pressure signal were recorded simultaneously using a Powerlab 16 SP digital data acquiring system and computer sampling software (Chart 5, AD Instruments).
After all the cannulations were established, the heart was switched from Langendorff perfusion mode to working mode. Cardiac output was recorded by counting drops of the aortic flow and pulmonary effluent (the later represents the coronary flow) using two pairs of copper electrodes with differentiated electrical potential via the PowerLab 16 SP digital data acquiring system and Chart 5 software. During the functional measurements, the heart rate was paced supraventricularly at 8 Hz (480 beats per min) using an isolated electrical stimulator (A365, World Precision Instruments) through two platinum electrodes attached to the right atrium.
Base-line heart function was measured at 10 mm Hg preload and 55 mm Hg afterload by recording LVPmax, LVPmin, ±dP/dtmax, stroke volume (μl/mg heart weight), and the pressure-volume loop. All hearts used for the functional studies were examined with Western blotting using mAbs TnI-1 and CT3 as above to confirm the wild type and transgenic genotypes.
Measuring Cardiac Function under Varied Preload or Afterload and upon Isoproterenol Treatment
After measuring the base-line heart function, preload was altered stepwise between 5 and 20 mm Hg to study the effects of cTnT-exon 7 deletion and/or cTnI-K118C on the responses of cardiac function to volume load at 55 mm Hg afterload. 10 min after returning the preload to 10 mm Hg, afterload was increased stepwise from 55 to 90 mm Hg to study the responses to pressure load. For each preload and afterload settings, the measurements were recorded when the cardiac function became stable.
Ten min after the afterload was switched back to 55 mm Hg, 10 nm isoproterenol (Sigma) was applied through antegrade perfusion to study the response of cardiac function to β-adrenergic stimulation (at 10 mm Hg preload).
Pro-Q Diamond Phosphoprotein Staining
To study the effect of cTnI-K118C on cTnI phosphorylation for its contribution to the β-adrenergic regulation of cardiac function, Pro-Q Diamond phosphoprotein staining (Invitrogen) was carried out according to the manufacturer's instruction. Total cardiac muscle proteins were resolved on 14% SDS-polyacrylamide gel as described above. The SDS-polyacrylamide gel was prefixed in 50% methanol and 10% acetic acid overnight with a change after the first 45 min. The fixed gel was washed in deionized water for three changes of 10 min each and stained with shaking in Pro-Q Diamond reagent in a dark box for 90 min. The gel was then destained in 20% acetonitrile, 50 mm sodium acetate, pH 4.0, in the dark for three changes of 30 min each and washed twice with deionized water for 5 min each in the dark. The destained gel was scanned on a Typhoon 9410 fluorescence scanner (GE Healthcare) with excitation at 532 nm and recording emission at 560 nm. The amount of phosphorylated cTnI was quantified by ImageJ software. The same gel was then stained with Coomassie Blue R-250, and the amount of total protein of each lane was quantified using ImageJ software for normalizing the level of cTnI phosphorylation.
Data Analysis
The SDS gel and Western blot images were scanned at 600 dpi for densitometry analysis. Statistical significance of the comparisons was determined using Student's t test.
RESULTS
cTnI-K118C Transgenic Mouse Lines
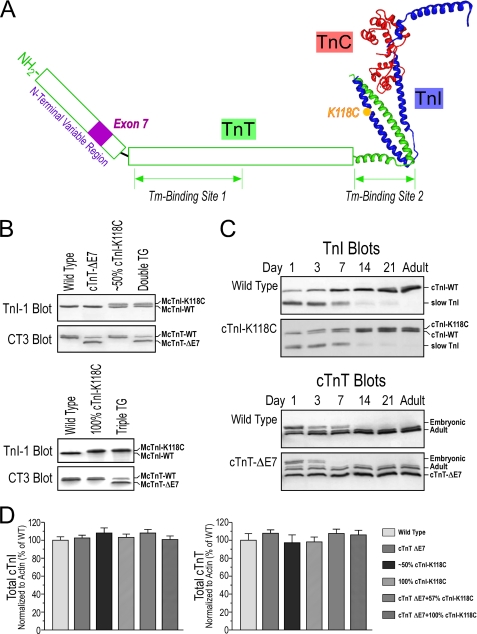
We successfully developed two transgenic mouse lines overexpressing cTnI-K118C in postnatal cardiac muscle using the α-MHC promoter. The K118C modification in cTnI is illustrated in Fig. 1A together with the deletion of the exon 7-encoded segment in the N-terminal variable region of cTnT.
FIGURE 1.
Transgenic mouse models expressing exon 7-deleted cTnT and/or K118C-cTnI in the heart. A, locations of exon 7-encoded segment in cTnT and the K118C mutation in cTnI are illustrated in this model structure of troponin complex. The high resolution portion was adapted from the crystal structure of partial human cardiac troponin complex (7). The two tropomyosin (Tm)-binding sites in TnT (27) are outlined. B, CT3 and TnI-1 Western blots showed the expression of exon 7-deleted (ΔE7) cTnT and/or cTnI-K118C in the transgenic (TG) mouse hearts in the presence (upper panel) or absence (lower panel) of endogenous wild type cTnI. C, Western blots using anti-cTnT mAb CT3 and anti-TnI mAb TnI-1 demonstrated the postnatally up-regulated expression of exon 7-deleted cTnT and/or cTnI-K118C in the transgenic mouse cardiac muscle along with wild type (WT) controls that showed the developmental switching of cTnT and TnI isoforms. D, densitometry analysis showed that the total levels of cTnT and cTnI were not different in the transgenic mouse hearts as compared with that in wild type controls. Error bars indicate S.D.
The Western blots in Fig. 1B showed that the single amino acid substitution of Cys for Lys118 in mouse cTnI resulted in slower mobility in SDS-PAGE. This effect is similar to that found in the natural substitution of Cys for Arg111 in wild turkey hearts (6). The replacement of a Lys or Arg with Cys did not increase but decreased the molecular mass of cTnI. Therefore, the slower gel mobility of the mutant cTnI was not due to a larger mass. A possibility is that the mutant cTnI bound less SDS than that of the wild type and thus migrated slower. More likely, this change reflected altered molecular conformation and/or flexibility in the mutant cTnI. This change is readily detectable even in the presence of a high concentration of SDS and thus indicates that this single amino acid substitution resulted in rather significant and stable conformational and/or flexibility changes in the cTnI molecule. Further studies following this lead may reveal mechanistic insight for the functional change resulting from this cTnI mutation.
Transgenic Expression of cTnI-K118C Rescued the Lethality of Endogenous cTnI Gene Deletion
By crossing the cTnI-KO mouse line with a cTnI-K118C transgenic mouse line, we successfully rescued the postnatal lethality of the cTnI-KO mice. The double transgenic mice had 100% cTnI-K118C at normal stoichiometry in the adult hearts (Fig. 1B). Independent Mendelian segregations were observed for the cTnI-KO allele and the cTnI-K118C transgene allele, indicating their locations in different autosomes.
The Western blots in Fig. 1C showed that the cTnI-K118C transgene allele used for rescuing the loss of endogenous cTnI was up-regulated postnatally to sufficiently complement for the developmental down-regulation of slow skeletal muscle TnI in the cardiac muscle. The data in Fig. 1C also showed that the developmental up-regulation of the cTnI-K118C transgene is temporally similar to that of endogenous cTnI gene, while the down-regulation of the embryonic expression of slow TnI remained the same as that in wild type mouse hearts (18).
Whereas a transgenic mouse line with cTnI-K118C replacing ∼50% (57%) of the endogenous cTnI was used in our study to mimic the expression of cTnI-R111C in the hearts of heterozygote wild turkeys (6), the cTnI-K118C/cTnI-KO double transgenic mice provided an experimental system mimicking the expression of only cTnI-R111C in the hearts of homozygote wild turkeys (Fig. 1B) (6). Densitometry analysis of the Western blots showed that the total levels of cTnI in the single and double transgenic mouse cardiac muscle remained similar to that in wild type controls (Fig. 1D). This outcome is consistent with our previous observation that myofilament incorporation determines the stoichiometry of TnI during heart development as well as in over- and underexpression conditions (11). The maintained normal level of total cTnI validated the use of the transgenic mouse hearts as physiological experimental models to study the effect of the cTnI-K118C mutation on cardiac function.
cTnI-K118C/cTnT-Exon 7 Deletion Double and Triple Transgenic Mice
By crossing the cTnI-K118C and the exon 7-deleted cTnT single transgenic mouse lines, we successfully generated a double transgenic mouse line co-expressing exon 7-deleted cTnT and cTnI-K118C together with nearly equal amounts of wild type cTnI (Fig. 1B). Independent Mendelian segregations were observed for the exon 7-deleted cTnT and the cTnI-K118C transgene alleles, indicating their locations in different autosomes. The Western blots in Fig. 1C showed that the exon 7-deleted cTnT transgene allele was up-regulated postnatally in the heart and replaced the embryonic cTnT similarly to the developmental up-regulation of endogenous normal adult cTnT. The co-expression of cTnI-K118C and the exon 7-deleted cTnT in adult mouse hearts was shown in the Western blot in Fig. 1B. This double transgenic mouse line provides an experimental model system mimicking the hearts of heterozygote cTnI-Cys111 wild turkeys (6).
By crossing the cTnI-K118C/cTnI-KO double transgenic mice with the exon 7-deleted cTnT transgenic mice, we successfully generated triple transgenic mice co-expressing exon 7-deleted cTnT and cTnI-K118C in the absence of endogenous wild type cTnI. Independent Mendelian segregations were seen for all of the three alleles involved, indicating their locations in different autosomes. The co-expression of 100% cTnI-K118C with exon 7-deleted cTnT in adult mouse hearts was shown in the Western blot in Fig. 1B. The triple transgenic mice provide a model system mimicking the hearts of homozygote cTnI-Cys111 wild turkeys (6).
Densitometry analysis of the SDS gel and Western blots showed that the total level of cTnT in the hearts of exon 7-deleted cTnT transgenic mice remained similar to that in wild type mouse hearts (Fig. 1D). The analysis also showed that the total levels of cTnI and cTnT in the double and triple transgenic mouse cardiac muscles remained similar to that in wild type controls (Fig. 1D). Therefore, these transgenic mouse hearts provide physiological experimental systems to study the combined functional effects of cTnI-K118C and exon 7-deleted cTnT in comparison with their separate effects.
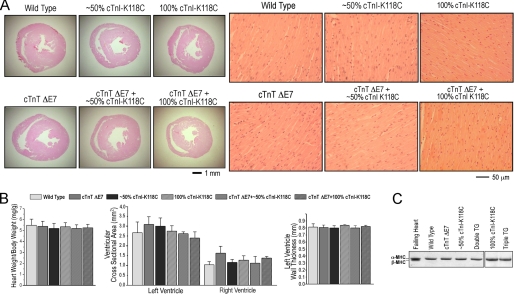
The double and triple transgenic mice showed normal base-line life activity and had no cardiac hypertrophy or dilation at 2–3 months of age when they were used for the functional studies (Fig. 2). Histology studies showed normal myocardial structure in the double and triple transgenic mouse hearts (Fig. 2A). Glycerol-SDS gel showed only α-MHC expression in the ventricular muscle same as that in wild type hearts (Fig. 2C), indicating no significant adaptive remodeling. These observations further validated the use of the young adult transgenic mouse hearts for investigating the functional effect of the cTnI and cTnT mutations prior to the development of clinical cardiomyopathy and heart failure.
FIGURE 2.
Unchanged morphology and myosin isoform expression in 3-month-old transgenic mouse hearts expressing exon 7-deleted cTnT and/or cTnI-K118C. A, low and high magnification images of hematoxylin and eosin-stained cross-sections of wild type and the single, double, and triple transgenic mouse hearts. B, heart weight:body weight ratio, the cross area of left and right ventricular chambers, and the thickness of left ventricular free wall did not show statistical differences. Error bars indicate S.D. C, glycerol-SDS-PAGE showed only α-MHC in wild type, exon 7-deleted (ΔE7) cTnT, and cTnI-K118C single and double transgenic (TG) mouse hearts. A failing ventricular muscle sample from Gsα knock-out mouse heart (15) was used as control for the adaptive expression of β-MHC.
Failing Phenotype of the cTnT Exon 7 Deletion Single Transgenic Mouse Hearts
The parameters for the base-line cardiac function of transgenic and wild type control mice measured in ex vivo working hearts are summarized in Table 1.
TABLE 1.
Summary of base-line cardiac function in ex vivo working hearts
Heart rate was paced at 8 Hz, and preload and afterload were maintained at 10 and 55 mm Hg, respectively.
| Wild type, n = 5 | E7-deleted cTnT, n = 9 | ∼50% cTnI-K118C, n = 8 | 100% cTnI-K118C, n = 6 | E7-deleted cTnT+∼50% cTnI-K118C, n = 5 | E7-deleted cTnT +100% cTnI-K118C, n = 4 | |
|---|---|---|---|---|---|---|
| Stroke volume (μl/mg) | 0.16 ± 0.003 | 0.13 ± 0.01a | 0.15 ± 0.005 | 0.15 ± 0.01 | 0.15 ± 0.004b | 0.16 ± 0.01b |
| LVPmax (mm Hg) | 79.03 ± 1.01 | 70.51 ± 0.67a | 75.88 ± 0.83 | 76.65 ± 0.79 | 76.26 ± 0.40b | 76.16 ± 3.76b |
| LVPmin (mm Hg) | 3.83 ± 0.28 | 3.56 ± 0.19 | 4.34 ± 0.27a | 5.04 ± 0.29a | 3.73 ± 0.20c | 3.83 ± 0.48c |
| LVPdev (mm Hg) | 75.20 ± 1.14 | 66.95 ± 0.70a | 71.55 ± 1.00 | 71.62 ± 1.02 | 72.53 ± 0.42b | 76.18 ± 2.52b |
| +dP/dt (mm Hg/s) | 4470.7 ± 118.9 | 3527.9 ± 123.5a | 4610.0 ± 121.0 | 4392.7 ± 172.2 | 4518.0 ± 50.0b | 4547.5 ± 114.0b |
| −dP/dt (mm Hg/s) | −3393.5 ± 76.6 | −2851.5 ± 70.5a | −2911.4 ± 43.0a | −2926.2 ± 76.2a | −3211.2 ± 107.4b,c | −3304.9 ± 141.5b,c |
| Stroke work (μl·mm Hg/mg) | 8.32 ± 0.13 | 6.64 ± 0.39a | 7.62 ± 0.24 | 7.58 ± 0.42 | 7.72 ± 0.18b | 7.86 ± 0.28b |
a p < 0.05 compared with wild type using two-tailed Student's t test.
b p < 0.05 compared with exon 7-deleted cTnT; using two-tailed Student's t test.
c p < 0.05 compared with ∼50% and 100% cTnI K118C; using two-tail Student's t test.
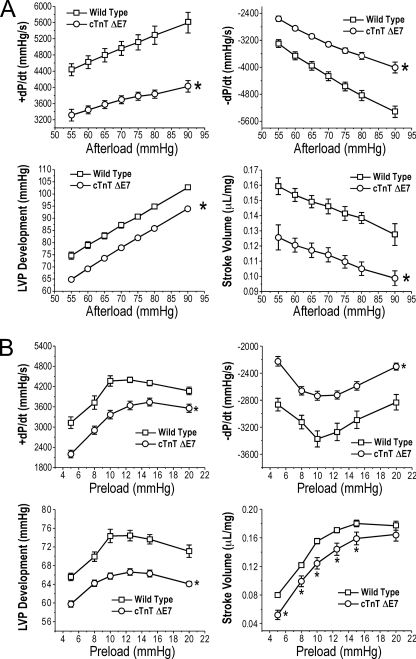
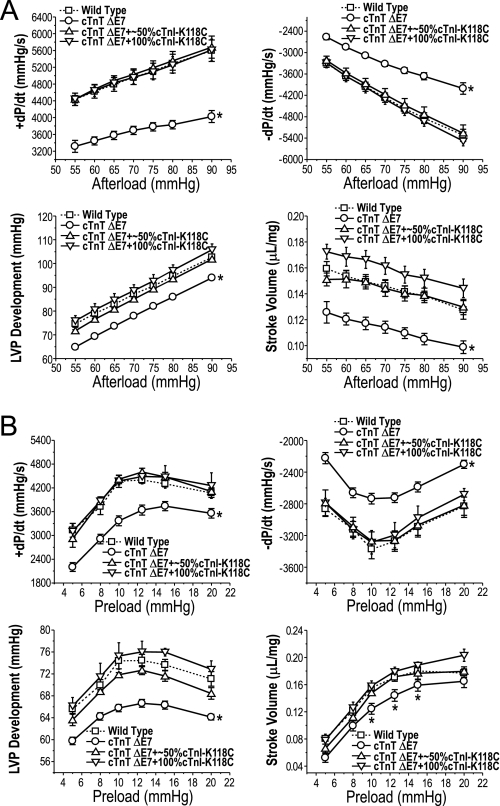
The transgenic mouse hearts overexpressing exon 7-deleted cTnT showed significantly lower contractile and relaxation velocities, LVP development, and stroke volume when compared with that of age-matched wild type control hearts (Fig. 3). At the base-line preload of 10 mm Hg, the decreases in cardiac function, especially the contractile and relaxation velocities, were more severe when afterload was increased (Fig. 3A). At the base-line afterload of 55 mm Hg, the depressed cardiac function was persistent in a wide range of preloads, although the Frank-Starling responses remained parallel with that of the wild type hearts (Fig. 3B). These results from ex vivo working heart studies are in agreement with the previous observation that the abnormal splicing of cTnT to aberrantly delete a segment in the N-terminal region of cTnT is a dominantly negative factor relating to the pathogenesis of DCM and heart failure in domestic turkeys (4).
FIGURE 3.
Function of the transgenic mouse hearts overexpressing exon 7-deleted cTnT. Contractile and relaxation velocities, LVP development, and stroke volume were measured in ex vivo working heart preparations under different afterloads with preload fixed at 10 mm Hg (A) or under different preloads with afterload fixed at 55 mm Hg (B). The results demonstrated that all of the parameters indicated significantly decreased function of the exon 7-deleted (ΔE7) cTnT transgenic mouse hearts as compared with wild type controls. *, p < 0.05 compared with wild type using two-tailed Student's t test. Error bars indicate S.D.
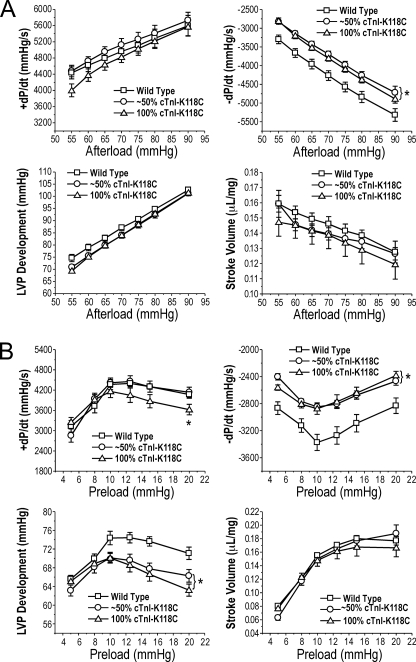
Decreased Function of cTnI-K118C Transgenic Mouse Hearts
The transgenic mouse hearts overexpressing cTnI-K118C to replace approximately half or all of the endogenous wild type cTnI both had decreased function (Fig. 4). At the base-line preload of 10 mm Hg and a wide range of afterloads, the cTnI-K118C transgenic mouse hearts showed slower relaxation velocity (−dP/dt) than that of wild type controls, whereas contractile velocity, LVP development, and stroke volume were apparently normal in comparison with that of the wild type hearts (Fig. 4A). This observation indicated a main dominant negative effect of cTnI-K118C on the diastolic function of the heart.
FIGURE 4.
Function of the transgenic mouse hearts expressing cTnI-K118C. Contractile and relaxation velocities, LVP development, and stroke volume were measured in ex vivo working heart preparations under different afterloads with preload fixed at 10 mm Hg (A) or under different preloads with afterload fixed at 55 mm Hg (B). The results demonstrated that cTnI-K118C decreased cardiac function, specifically the relaxation velocity and LVP development, in the transgenic mouse hearts as compared with wild type controls. The 100% cTnI-K118C hearts also exhibited decreased contractile velocity when preload was increased. *, p < 0.05 compared with wild type using two-tailed Student's t test. Error bars indicate S.D.
At the base-line afterload of 55 mm Hg, the reduction in the function of cTnI-K118C hearts was confirmed by the decreased relaxation velocity in a wide range of preloads (Fig. 4B). Furthermore, LVP development was decreased at high preloads. The transgenic mouse hearts expressing 100% cTnI-K118C, but not that expressing ∼50% cTnI-K118C, also showed decreased contractile velocity (+dP/dt) at high preloads (Fig. 4B). These changes suggest a possibly decreased Frank-Starling response.
Altogether, the most striking negative phenotype of the cTnI-K118C transgenic mouse hearts was the decreased diastolic function (relaxation velocity). On the other hand, the stroke volume did not show significant change as compared with the wild type controls in the wide range of afterloads and preloads examined (Fig. 4). Therefore, the 2–3-month-old cTnI-K118C hearts were in a functionally compensated state and provided a proper experimental system for testing the primary effects of cTnI-K118C on myocardial contractility.
The functional characterizations of cTnI-K118C transgenic mouse hearts demonstrated that the cTnI-R111C allele found in wild turkeys is not a neutral polymorphism but a dominantly negative mutation. This conclusion is consistent with the fact that this residue is a highly conserved position in cardiac, slow, and fast skeletal muscle TnI isoforms across vertebrate species (6, 9).
Mutual Rescues between cTnI-K118C and cTnT-Exon 7 Deletion Mutations in Transgenic Mouse Hearts
An intriguing finding was that the ex vivo functional parameters measured in the double and triple transgenic mouse hearts co-expressing exon 7-deleted cTnT and ∼50 or 100% cTnI-K118C were apparently normal as compared with the wild type control (Fig. 5). The severely impaired function of exon 7-deleted cTnT single transgenic mouse hearts was remarkably corrected even at high afterloads (Fig. 5A). It is worth noting that the triple transgenic mouse hearts co-expressing exon 7-deleted cTnT and 100% cTnI-K118C even showed trends of larger stroke volumes than the wild type control over a wide range of afterloads (Fig. 5A) and at high preload (Fig. 5B).
FIGURE 5.
Mutually rescued cardiac function when exon 7-deleted cTnT and cTnI-K118C were co-expressed in transgenic mouse hearts. Contractile and relaxation velocities, LVP development, and stroke volume were measured in ex vivo working heart preparations under different afterloads with preload fixed at 10 mm Hg (A) or under different preloads with afterload fixed at 55 mm Hg (B). The exon 7-deleted (ΔE7) cTnT/cTnI-K118C double (∼50% cTnI-K118C) and triple (100% cTnI-K118C) transgenic mouse hearts exhibited apparently normal function similar to that of the wild type controls (dashed curves). *, p < 0.05 compared with wild type using two-tailed Student's t test. Error bars indicate S.D.
The outcome that the combination of two dominantly negative mutations in cTnT and cTnI resulted in mutual rescues of cardiac function is an interesting novel finding. It supports a value of the cTnI-Cys111 allele in wild turkeys during natural selection, in which the myopathic effect of exon 7-deleted cTnT could have been suppressed (6). The double and triple transgenic mouse hearts containing ∼50 or 100% cTnI-K118C both had sufficient rescuing effects. Therefore, the rescue of myopathic defects of the exon 7-deleted cTnT by cTnI-K118 was a rather dominant effect. Demonstrating a similarly dominant rescuing effect, the negative phenotype of cTnI-K118C hearts disappeared when the exon 7-deleted cTnT was present even in the hearts containing 100% cTnI-K118C (Fig. 5).
The negative phenotypes of the cTnT and cTnI mutations as well as their mutual rescues were readily detectable at 2–3 months of age. However, pathophysiological phenotypes may develop in these double and triple transgenic mouse models in later adulthood, and this possibility remains to be investigated.
cTnI-K118C Blunted the β-Adrenergic Response in Transgenic Mouse Hearts
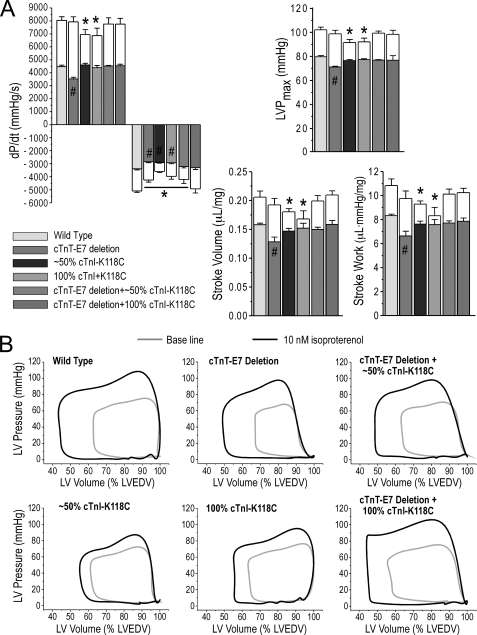
The effects of 10 nm isoproterenol on ex vivo working heart function are shown in Fig. 6. The results first revealed that although the transgenic mouse hearts expressing exon 7-deleted cTnT had depressed base-line function, they exhibited larger inotropic responses to β-adrenergic stimulation by larger increases in contractile velocity, LVP development, and stroke volume as compared with that of wild type hearts. As the outcome, the β-adrenergic potential was able to compensate for the intrinsic decrease in contractility in the 2–3-month-old cTnT exon 7 deletion transgenic mouse hearts (Fig. 6A).
FIGURE 6.
Responses of cardiac function to β-adrenergic stimulation. A, effects of 10 nm isoproterenol on contractile and relaxation velocities, LVP development, stroke volume, and stroke work were examined in ex vivo working hearts at 10 mm Hg preload and 55 mm Hg afterload. In the histograms, the open boxes indicate the extent of functional change upon isoproterenol treatment. The results showed that isoproterenol induced profound increases in cardiac function from the decreased base line in exon 7 (E7)-deleted cTnT hearts to reach the wild type level. In contrast, cTnI-K118C hearts had blunted responses to isoproterenol treatment as compared with the wild type controls. The double (∼50% cTnI-K118C) and triple (100% cTnI-K118C) transgenic mouse hearts co-expressing exon 7-deleted cTnT and cTnI-K118C reached wild type level of function in response to 10 nm isoproterenol treatment. Error bars indicate S.D. B, representative P-V loops outline the β-adrenergic responses of the wild type and single, double, and triple transgenic mouse hearts. LVEDV, left ventricular end diastolic volume. #, p < 0.05 comparing the base-line cardiac function with the wild type control; *, p < 0.05 after 10 nm isoproterenol treatment, using two-tailed Student's t test.
In contrast, the transgenic mouse hearts expressing cTnI-K118C had significantly decreased responses to β-adrenergic stimulation in comparison with wild type controls, especially the stroke volumes of the 100% cTnI-K118C hearts (Fig. 6A). Although the base-line function of cTnI-K118C hearts was normal except for slower relaxation velocity, their weaker β-adrenergic response suggested a restriction to stress responses.
The results then showed that the β-adrenergic responses of the double and triple transgenic mouse hearts co-expressing exon 7-deleted cTnT with ∼50 or 100% cTnI-K118C became similar to the wild type controls (Fig. 6A). Together with the near-normal base-line function (Fig. 5), the double and triple transgenic mouse hearts exhibited full potential in cardiac function upon β-adrenergic stimulation. These results further support the notion that these two dominantly negative mutations in cTnT and cTnI mutually rescue each other. The representative P-V loops in Fig. 6B outlined the base-line function and β-adrenergic responses of the transgenic and control mouse hearts. These observations demonstrated prominent roles of cTnT and cTnI structure-function modifications in β-adrenergic regulation of cardiac muscle contractility.
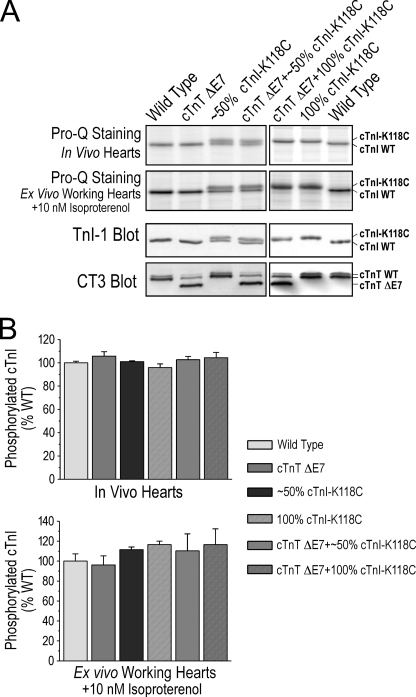
cTnI-K118C Did Not Affect Isoproterenol-induced Phosphorylation of cTnI
Pro-Q Diamond staining of SDS-polyacrylamide gels and densitometry quantification revealed that the level of phosphorylation in vivo had no difference between cTnI-K118C and wild type cTnI in the transgenic mouse hearts (Fig. 7A). The total level of cTnI phosphorylation in vivo showed no difference among wild type and the transgenic mouse lines (Fig. 7B).
FIGURE 7.
PKA-dependent phosphorylation of cTnI was preserved in the transgenic mouse hearts. A, Pro-Q Diamond phosphoprotein staining of SDS gels was used to measure phosphorylated cTnI in mouse cardiac muscle in vivo and after 10 nm isoproterenol treatment of ex vivo working hearts. The total levels of cTnI and cTnT were measured by Western blots using mAbs TnI-1 and CT3. B, densitometry quantification of the Pro-Q-stained gels and Western blots for the relative levels of the phosphorylated versus total proteins showed that the cTnI-K118C mutation did not reduce the level of cTnI phosphorylation in vivo or upon β-adrenergic treatment ex vivo as compared with the wild type (WT) controls. The presence of exon 7-deleted (ΔE7) cTnT alone or in combination with cTnI-K118C did not affect the level of cTnI phosphorylation. Error bars indicate S.D.
10 nm isoproterenol treatment of ex vivo working hearts further showed that the protein kinase A (PKA)-dependent phosphorylation was not different between cTnI and cTnI-K118C or among wild type and the transgenic mouse hearts (Fig. 7). The results indicated that although the K118C mutation in the TnT-binding helix of cTnI (Fig. 1A) resulted in potential conformational change in cTnI as shown by altered SDS gel mobility (Fig. 1B) and blunted the functional effect of β-adrenergic stimulation (Fig. 6), it did not abolish the PKA-dependent phosphorylation in the N-terminal extension.
DISCUSSION
TnI and TnT are two protein subunits in the troponin complex of striated muscle (2, 3). They function interactively in the Ca2+ regulation of myofilaments during contraction-relaxation cycles (19, 20). Diverged genes have evolved in vertebrates to encode cardiac, slow, and fast skeletal muscle fiber type-specific TnI and TnT isoforms (9). Consistent with the co-evolved functions of the two subunits of troponin, the TnI-TnT isoform genes are closely linked in three pairs in the vertebrate genomes with co-evolutionary relationships (21). Therefore, the structures and functions of TnI and TnT are very much inter-related. This study characterized a pair of naturally selected mutually rescuing mutations in cTnI and cTnT with the following major findings.
cTnI-K118C Alone Is a Dominantly Negative Mutation
When we previously found the cTnI-R111C mutation in wild turkey hearts, we reported it as a polymorphism while proposing that it might be a compensatory change to minimize the DCM phenotype of exon 7-deleted cTnT and improve the cardiac function of wild turkeys (6). By developing transgenic mouse models and characterizing heart function, we found in this study that the cTnI-K118C single amino acid substitution decreased the diastolic function and potentially Frank-Starling response of the ventricular muscle (Table 1 and Fig. 3B). cTnI-K118C also blunted the positive inotropic effect of β-adrenergic stimulation on cardiac function (Fig. 6A). These phenotypes indicate that the R111C substitution in wild turkey cTnI is actually a dominantly negative mutation other than a benign polymorphism. This conclusion is consistent with the fact that this residue is highly conserved as Arg or Lys in all TnI isoforms sequenced to date in a broad range of vertebrate species.
It is worth noting that although a large number of myopathic cTnI mutations have been identified to date in human and other animals, none of them resides in the I-T arm region (i.e. amino acids 90–136). Consistent with the high degree of evolutionary conservation in this segment of TnI (6, 9), the absence of any disease mutation fixed in this region implies that dominantly negative mutations in the TnT-binding structure would be reproductively devastating. Therefore, the unique case of cTnI-Cys111 mutation in wild turkeys may be based on a symbiotic relationship with the cTnT exon 8 deletion. On the other hand, the survival of cTnI-R118C transgenic mice under ample care indicates the evolutionary disadvantage cTnI-Cys111 might only show in a stringent natural environment.
Experimental Confirmation of Exon 7-deleted cTnT-caused Heart Failure
Although exon 7-deleted cTnT increased the calcium sensitivity of isolated cardiac myofilaments (5), the working heart study found that exon 7-deleted cTnT decreased the systolic function of the transgenic mouse hearts with significantly slower contractile velocity, lower LVP development, and less stroke volume when compared with the wild type mouse hearts in the absence of β-adrenergic stimuli (Table 1 and Fig. 4). These phenotypes confirmed the causal relationship of cTnT exon 7 or exon 8 deletions to canine (5) and turkey DCM (4).
In the meantime, the exon 7-deleted cTnT transgenic mouse hearts at 2–3 months of age prior to any signs of cardiomyopathy or clinical heart failure showed preserved responses to isoproterenol treatment, which were able to bring the cardiac function to a level similar to that of the wild type mouse hearts under the same dose of β-adrenergic stimulation (Fig. 6). The retained β-adrenergic potential suggests that the exon 7-deleted cTnT-expressing mouse hearts at 2–3 months of age were still sustained in a nonfailing state in vivo, although decreased function was detectable in ex vivo working hearts in the absence of β-adrenergic stimulation (Table 1 and Fig. 4).
We previously performed an initial study of the exon 7-deleted cTnT and found a negative isoproterenol response in the contractile and relaxation velocities of cardiomyocytes isolated from older (5–6 months of age) transgenic mouse hearts (5). Whereas age-related adaptation or more likely the restricted proteolytic removal of the N-terminal variable region of cTnT during the isolation of cardiomyocytes (22) might have contributed to the earlier observation, the contractility of isolated cardiomyocytes in the absence of external load was not as physiologically relevant as the comprehensive analyses of ex vivo working hearts in this study, which provided further understanding of the effects of exon 7-deleted cTnT on cardiac function.
Highly Effective Mutual Rescues between Two Dominantly Negative Mutations of cTnI and cTnT
It was very intriguing that the two dominantly negative mutations in cTnT and cTnI could mutually rescue each other when co-existing in the heart. One such example was that specific myosin heavy chain mutations suppressed a TnI mutation in Drosophila muscles (23). These cases demonstrated complex phenotype compensations in the highly integrative striated muscle sarcomere. The mechanism for the mutual rescues of cTnT exon 7 deletion and cTnI-K118C when these two dominantly negative mutations were combined merits further investigation.
Suggesting a compensatory function, we previously observed that cTnI-R111C countered the increased TnI binding affinity of exon 7-deleted cTnT (6). A novel finding in this study was the mutual rescues between the two dominantly negative mutations of cTnI and cTnT in a physiological setting. The rescues were highly effective and restored nearly normal cardiac function in transgenic mouse hearts (Table 1 and Fig. 5). The ∼50% replacement with cTnI-K118C and the ∼70% replacement with exon 7-deleted cTnT produced sufficient mutual rescues, indicating that the rescuing effects are dominant phenotypes. These mouse models provide a convincing support for the hypothesis that a specific fitness value of the cTnI-Cys111 allele during natural selection resulted in its unique fixation in wild turkey populations (6).
Although it has been well documented that mutations in cardiac myofilament proteins are presented with complex heart phenotypes (24), a remaining question is how the combination of two dominantly negative mutations resulted in a restoration of normal cardiac function. The molecular mechanism by which the effects of two dominantly negative structure-function alterations in TnI and TnT could cancel each other requires further investigation. The similarly altered mobility of turkey cTnI-R111C (6) and mouse cTnI-K118C in SDS-PAGE (Fig. 1B) indicates a significant change in overall molecular conformation or flexibility. However, structural modeling suggested that the substitution of Cys for Arg/Lys at this position would not disrupt the overall structure of TnI (6). Therefore, the slower gel mobility of turkey cTnI-R111C and mouse cTnI-K118C would more likely reflect a decreased molecular flexibility, which may be responsible for its functional effects. On the other hand, the deletion of the exon 8-encoded segment in turkey cTnT resulted in conformational changes in other regions and increased the binding affinity for wild type cTnI (4), and the latter was minimized by cTnI-Cys111 (6). Therefore, integrative changes in allosteric properties of cTnI and cTnT may be responsible for this mutual rescuing effect.
TnI-TnT Interface Is a Vital Link in the Thin Filament Regulatory System
The wild turkey cTnI-R111C mutation or the mouse cTnI-K118C modification resides in the coiled-coil interface between TnI and TnT in the troponin complex (the TnT-TnI double α-helical structure corresponding to amino acid residues Phe90–Arg136 in human cTnI and Glu226–Lys276 of human cTnT) (7, 8). The role of the cTnI-R111C point mutation in rescuing the abnormality of a myopathic cTnT demonstrated that the interaction between TnI and TnT is a vital link in the thin filament Ca2+ regulatory system.
The exon 7 deletion reflects an alteration in the N-terminal region of TnT, which is a variable domain with a role in modulating the molecular conformation and function of other regions of TnT (9). It has long been established that the N-terminal variable region of TnT is under the regulation of alternative mRNA splicing during heart development (9). However, the exon 7-encoded segment is conserved in cTnT, and its deletion presents a structural abnormality (4, 5). In the meantime, the core function of cTnT is preserved without the corresponding segment or even the entire N-terminal variable region (14, 22). Therefore, the pathological effect of cTnT exon 7 deletion involves long range conformational and functional modulations of other regions of cTnT. Our observation that the effect of this long range functional modulation can be countered by the K118C modification in the TnT-binding site of cTnI suggests that the TnI-TnT interface is a targeting structure for the regulatory function of the N-terminal variable region of TnT. The finding that exon 7-deleted cTnT corrected the diastolic dysfunction in cTnI-K118C hearts (Fig. 5) further suggests that the N-terminal variation of cTnT altered the function of TnI-TnT interface.
Another novel hypothesis suggested by the experimental data is that the interaction between cTnI and cTnT is a key link in the β-adrenergic regulation of myocardial contractility. Without abolishing the PKA-catalyzed phosphorylation of cTnI, cTnI-K118C blunted the inotropic effects of β-adrenergic stimulation when ventricular function was examined in intact ex vivo working hearts. This pattern indicates that the K118C substitution did not alter the accessibility of the N-terminal region to PKA, whereas the structural and functional consequences of phosphorylation at the N-terminal Ser23 and Ser24 were blocked/attenuated by this single amino acid substitution in a distal site that interacts with TnT. This hypothesis is of 2-fold significance. First it suggests a novel model that PKA phosphorylation of cTnI modulates myocardial contractility via the cTnI-cTnT interface; second, reducing the N-terminal phosphorylation of cTnI, for example with β-adrenergic blockage, may minimize the negative effect of cTnT N-terminal abnormality.
The highly cooperative nature of striated muscle thin filament (25, 26) indicates structural and functional interdependence among the troponin subunits. The characterization of cTnI-K118C mutation and the mutual rescues of cardiac function when it co-exists with the exon 7-deleted cTnT identified the TnI-TnT interaction as a cross-talk point in the signal transduction pathway of the thin filament Ca2+-regulatory system in striated muscle. In addition to better understanding the function of troponin complex in muscle regulation, the TnI-TnT interface is an attractive target for therapeutic intervention and merits further investigation.
Acknowledgments
We thank Hui Wang for technical assistance and Dr. Jeffrey Robbins for the mouse α-MHC promoter. B. W. thanks Dr. Guangju Ji for advice.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-078773 and AR-048816.
- TnT
- troponin T
- TnC
- troponin C
- BSA
- bovine serum albumin
- cTnI
- cardiac TnI
- cTnT
- cardiac TnT
- LVP
- left ventricular pressure
- TnI
- troponin I.
REFERENCES
- 1.Gordon A. M., Homsher E., Regnier M. (2000) Physiol. Rev. 80, 853–924 [DOI] [PubMed] [Google Scholar]
- 2.Perry S. V. (1998) J. Muscle Res. Cell Motil. 19, 575–602 [DOI] [PubMed] [Google Scholar]
- 3.Perry S. V. (1999) Mol. Cell. Biochem. 190, 9–32 [PubMed] [Google Scholar]
- 4.Biesiadecki B. J., Jin J. P. (2002) J. Biol. Chem. 277, 18459–18468 [DOI] [PubMed] [Google Scholar]
- 5.Biesiadecki B. J., Elder B. D., Yu Z. B., Jin J. P. (2002) J. Biol. Chem. 277, 50275–50285 [DOI] [PubMed] [Google Scholar]
- 6.Biesiadecki B. J., Schneider K. L., Yu Z. B., Chong S. M., Jin J. P. (2004) J. Biol. Chem. 279, 13825–13832 [DOI] [PubMed] [Google Scholar]
- 7.Takeda S., Yamashita A., Maeda K., Maéda Y. (2003) Nature 424, 35–41 [DOI] [PubMed] [Google Scholar]
- 8.Vinogradova M. V., Stone D. B., Malanina G. G., Karatzaferi C., Cooke R., Mendelson R. A., Fletterick R. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5038–5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin J. P., Zhang Z., Bautista J. A. (2008) Crit. Rev. Eukaryot. Gene Expr. 18, 93–124 [DOI] [PubMed] [Google Scholar]
- 10.Huang X., Pi Y., Lee K. J., Henkel A. S., Gregg R. G., Powers P. A., Walker J. W. (1999) Circ. Res. 84, 1–8 [DOI] [PubMed] [Google Scholar]
- 11.Feng H. Z., Hossain M. M., Huang X. P., Jin J. P. (2009) Arch. Biochem. Biophys. 487, 36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin J. P., Yang F. W., Yu Z. B., Ruse C. I., Bond M., Chen A. (2001) Biochemistry 40, 2623–2631 [DOI] [PubMed] [Google Scholar]
- 13.Jin J. P., Chen A., Ogut O., Huang Q. Q. (2000) Am. J. Physiol. Cell Physiol. 279, C1067–C1077 [DOI] [PubMed] [Google Scholar]
- 14.Feng H. Z., Biesiadecki B. J., Yu Z. B., Hossain M. M., Jin J. P. (2008) J. Physiol. 586, 3537–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng H. Z., Chen M., Weinstein L. S., Jin J. P. (2008) J. Biol. Chem. 283, 33384–33393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbato J. C., Huang Q. Q., Hossain M. M., Bond M., Jin J. P. (2005) J. Biol. Chem. 280, 6602–6609 [DOI] [PubMed] [Google Scholar]
- 17.How O. J., Aasum E., Kunnathu S., Severson D. L., Myhre E. S., Larsen T. S. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H2979–H2985 [DOI] [PubMed] [Google Scholar]
- 18.Jin J. P. (1996) Biochem. Biophys. Res. Commun. 225, 883–889 [DOI] [PubMed] [Google Scholar]
- 19.Tobacman L. S. (1996) Annu. Rev. Physiol. 58, 447–481 [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi T., Solaro R. J. (2005) Annu. Rev. Physiol. 67, 39–67 [DOI] [PubMed] [Google Scholar]
- 21.Chong S. M., Jin J. P. (2009) J. Mol. Evol. 68, 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Biesiadecki B. J., Jin J. P. (2006) Biochemistry 45, 11681–11694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronert W. A., Acebes A., Ferrús A., Bernstein S. I. (1999) J. Cell Biol. 144, 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tardiff J. C. (2005) Heart Fail. Rev. 10, 237–248 [DOI] [PubMed] [Google Scholar]
- 25.Boussouf S. E., Geeves M. A. (2007) Adv. Exp. Med. Biol. 592, 99–109 [DOI] [PubMed] [Google Scholar]
- 26.Moss R. L., Razumova M., Fitzsimons D. P. (2004) Circ. Res. 94, 1290–1300 [DOI] [PubMed] [Google Scholar]
- 27.Jin J. P., Chong S. M. (2010) Arch. Biochem. Biophys. 500, 144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]