Abstract
Pde1c is a calcium/calmodulin-regulated, dual-specificity cyclic nucleotide phosphodiesterase. We have used a transposon insertion line to investigate the physiological function of Pde1c in Drosophila melanogaster and to show that the insertion leads to male sterility and male mating behavior defects that include reduced copulation rates. Sterility appears to be primarily due to elimination of sperm from the female reproductive system. The male mating behavior defects were fully rescued by expression of exogenous Pde1c under the control of either a Pde1c or a pan-neuronal promoter, whereas the sterility could be only partially rescued by expression of exogenous Pde1c under the control of these promoters. We also show that Pde1c has a male-specific expression pattern in the CNS with an increased number of Pde1c-expressing neurons in the abdominal ganglion in males.
THE cyclic nucleotides, cyclic AMP (cAMP) and cyclic GMP (cGMP), have been known for many years to regulate a wide variety of physiological processes in all animals (e.g., Siegel et al. 1994). Similarly, there is a large body of research focused on an understanding of the structure and regulation of the enzymes that synthesize cAMP and cGMP, the adenylyl and guanylyl cyclases, respectively. Although the concentrations of cyclic nucleotides within a cell are regulated by both their synthesis and their degradation, less attention has been devoted to the function and regulation of the enzymes that break down cyclic nucleotides, the phosphodiesterases (PDEs).
Mammals have >20 genes that code for cyclic nucleotide PDEs, which have been subdivided into 11 families on the basis of their sequences, substrate specificities, and regulatory properties (Conti and Beavo 2007). Insects also have a wide variety of PDEs with Drosophila melanogaster containing 6 genes that code for cyclic nucleotide PDEs (Morton and Hudson 2002; Day et al. 2005). On the basis of their sequence similarity to the mammalian PDEs, these 6 genes have been classified into 6 of the 11 families: Pde1c, Pde4, Pde6, Pde8, Pde9, and Pde11 (Day et al. 2005). When their biochemical properties have been investigated, they match well with other members of the same family (Day et al. 2005).
Despite the importance of cyclic nucleotides in insect physiology, only one of the Drosophila PDEs has been associated with a mutant phenotype. This gene, Pde4, also known as dunce, was one of the first learning and memory mutants discovered (Byers et al. 1981; Davis et al. 1995). In this study, we have investigated the phenotypes associated with reduced expression of Pde1c, a PDE that has dual specificity for both cAMP and cGMP and that is stimulated in the presence of calcium and calmodulin (Day et al. 2005). Here we show that Pde1c is required for male fertility and male mating behavior. Male sterility appears to be primarily due to females rejecting sperm or failing to store the sperm from mutant males.
MATERIALS AND METHODS
Fly lines and maintenance:
Fly stocks were maintained at 25° on a 12 hr light/dark photo cycle using a standard cornmeal–agar–molasses diet (Greenspan 2004). The following fly lines were obtained from the Bloomington Stock Center: w1118, PBac{PB}Pde1cc04487 (Pde1cc04487), P{UAS-GFP.nls}, P{UAS-mCD8 GFP}, P{UAS-dsRED}, P{FRT(w+)Tub-PBac\T}2, and Df(2L)Exel6030, P{PZ}fru3.
Reverse transcriptase PCR:
Total RNA was extracted from homogenates of 20 whole flies using Trizol (Invitrogen, San Diego), and cDNA was synthesized using 2–3 μg of total RNA using superscript reverse transcriptase (Invitrogen). Nested PCR was then used to amplify Pde1c transcript using the following primer pairs: outer—5′ GCACGACATTGCCACAAA 3′ and 5′ AAAGATCTCCAGGTCCGTCA 3′; inner—5′ CCAGTTCATCAGACCCGTGTTG 3′ and 5′ CCTCCAGCTGAATTCCATGT 3′.
Generation of the Pde1c promoter-GAL4 and UAS-Pde1c fly lines:
PCR was used to amplify a 6.2-kb genomic fragment that includes the first exon of the Pde1c-RC transcript. The following primers were used: 5′ TGTAATTTCACATGTATATAAGCAACA 3′ and 5′ TCTTCTTCTCCTGCGTCTCC 3′, and amplification was carried out using the elongase polymerase (Invitrogen). The product was cloned into TOPO-XL (Invitrogen) and then subcloned onto pTGAL (https://dgrc.cgb.indiana.edu/) using the NotI and KpnI restriction sites. Flies were transformed using this construct by standard methods using a commercial service (BestGene, Chino Hills, CA). A plasmid containing the coding region of Pde1c-RC was obtained from the Drosophila Genomics Resource Center, and the region spanning the translational start and stop sites was amplified by PCR using Expand high fidelity polymerase (Roche Applied Science, Indianapolis) and the following primers: 5′ GCGCGGCCGCACATTGCCACAAAGTCAACCTC 3′ and 5′ GGTACCAGTGTAGCCATCAAAGTTTCCTCTT 3′. The PCR product was subcloned into pGEM (Invitrogen) and then into pUAST at the NotI and KpnI sites. The resulting plasmid was sequenced and used to transform flies as above.
Microscopy and immunocytochemistry:
To examine the expression pattern of Pde1c, we crossed the Pde1c promoter-GAL4 line with a UAS-green fluorescent protein (GFP) line with a nuclear localization signal. Nervous tissue from these progeny was mounted in aqueous mounting media (Gel Mount, Sigma, St. Louis) and viewed on a Nikon Eclipse E800 confocal microscope equipped with argon and helium/neon lasers (Radiance 2100, Bio-Rad, Hercules, CA) using 500- to 530- and 570- to 650-nm emission filters. Unless otherwise stated, a stack of 15 sections was taken (z-axis step size, 2.5 μm) using Laser Sharp 2000 software (Bio-Rad), and image processing was carried out using Image J (http://rsb.info.nih.gov/ij/). To count the number of cells in the abdominal ganglia, individual 5-μm sections were converted to binary images, and all particles with circularity >0.5 were counted and analyzed. The number of particles was converted into a cell number by correcting for diameter and section thickness as previously described (Truman and Booker 1986).
Immunofluorescence was carried out by first fixing tissue in 4% paraformaldehyde and 7.5% picric acid in phosphate buffered saline (PBS) for 1 hr on ice followed by washing in PBS for 45 min The tissue was then permeabilized in Tris-buffered saline (TBS; 0.1 m Tris–HCl, 0.3 m NaCl, pH 7.4) with 0.5% triton X-100 for 45 min and blocked in 4% normal goat serum (NGS) in TBS for 1 hr. To stain for GFP and 5-hydroxytryptamine immunofluorescence, tissue was incubated overnight in 1:200 mouse anti-GFP (Santa Cruz Biotechnology, Santa Cruz, CA) and 1:500 rabbit anti-5-HT (Sigma) in 4% NGS and TBS at 4°. After additional blocking in 4% NGS for 1 hr, the tissue was incubated in 1:1000 goat Cy2-labeled goat anti-mouse and Cy3-labeled goat anti-rabbit secondary antibodies (Jackson Labs, West Grove, PA) for 4 hr at room temperature, mounted in Gel Mount, and viewed as before.
Fertility and courtship assays:
To determine the fertility of male flies, individual males were placed in vials with individual control (w1118) virgin females. After 7 days, the number of pairs that had produced any offspring were logged. In some cases, the adults were removed, and the vial was kept to determine the number of adult offspring produced from that mating. To monitor courtship behavior, a custom acrylic apparatus that consisted of 14 chambers measuring ∼1 cm wide, 4 cm long, and 0.6 cm high was designed. Each chamber was divided in half with a removable paper barrier. Individual males and females were placed in each half and allowed to recover for at least 1 hr, and the paper barrier was removed. All 14 chambers were then recorded simultaneously on video (Canon Vixia HG21 camcorder) and subsequently analyzed for a variety of courtship parameters. These parameters included the courtship index, i.e., the fraction of time spent engaged in any part of courtship behavior (chasing, touching, wing extension, or copulation) within the first 10 min of the assay; the time taken before copulation; the length of time engaged in copulation; and the percentage of males that copulated within the first hour of the assay. In most cases, males and virgin females were used 3–5 days post eclosion. Males and virgin females were kept isolated in vials in groups of ≤10 before use.
Statistical analysis:
All data were analyzed for statistically significant differences using GraphPad Prism 3.0 (San Diego). Where the data were binary in format, the following formula was used to calculate the standard deviation: SD =  , where p = percentage of observed events (Le 2003).
, where p = percentage of observed events (Le 2003).
RESULTS
Reduced expression of Pde1c is associated with male infertility:
To examine the physiological function of Pde1c, we took advantage of a fly line that contained a piggybac transposon inserted within an intron of the Pde1c gene (Figure 1, A and B). Reverse transcriptase PCR (RT–PCR) of males homozygous for this insertion showed a significant reduction in the levels of Pde1c transcript (Figure 1C). Both male and female flies homozygous for this insertion were viable, and when heterozygous flies were crossed, the expected Mendelian ratios of heterozygous and homozygous adults were obtained (data not shown). Nevertheless, we were unable to propagate a homozygous line because the male flies were infertile. To quantify this, individual homozygous Pde1cc04487 males were crossed to individual control (w1118) females, and the presence or absence of larvae after 7 days was noted (Figure 2A). We rarely, but occasionally, obtained progeny(2 of 82 pairs). The number of adults that eclosed from these occasional successful matings was greatly reduced compared to controls (one and two adults from the two successful matings). To determine if this infertility was due to the presence of the transposon within Pde1c, we mobilized the transposon by crossing with flies that expressed a piggybac transposase under a tubulin promoter. We isolated two independent lines that lacked the transposon as determined by a reversion to the white-eyed phenotype, and both of these lines were viable and fertile (Figure 2A) and generated similar numbers of offspring compared to control crossings (43 ± 4, n = 28 for hopped c04487; 32 ± 4, n = 43 for w1118). In addition, we crossed the Pde1cc04487 line with a line containing a deletion that covered Pde1c (Figure 1A). The male offspring of this cross were also infertile (Figure 2A).
Figure 1.—
Genomic organization of Pde1c and the position of the transposon in Pde1cc04487. (A) Arrangement of intron and exons in Pde1c and their relationship to the deficiency Df(2L)Exel6030. (Top) The solid blocks represent the Pde1c exons with the alternative transcription start site for each transcript shown. The deficiency covers the entire Pde1c gene and includes an additional nine genes. The position of the piggybac transposon within an intron of Pde1c is also shown. (B) Alternative spliced transcripts of Pde1c. There are six predicted transcripts that result in five different predicted proteins. Also shown is the promoter-GAL4 construct for Pde1c (pPde1c-GAL4). The open box represents the position of the genomic DNA used as a promoter, and the solid box represents the GAL4 coding region. (C) Reduced expression of Pde1c in Pde1cc04487. RT–PCR was performed on total RNA extracted from whole flies of either w1118 or Pde1cc04487. For a loading control, the cDNA was also amplified with actin primers.
Figure 2.—
Pde1cc04487 mutants are male sterile and exhibit courtship deficits. (A) Fertility assay. Individual males of the genotypes shown were allowed to mate with virgin female w1118 flies for 1 week. The flies were removed, and the number of pairs that had any progeny were logged. Flies homozygous for Pde1cc04487 and trans-heterozygous to a deficiency covering Pde1c were significantly less fertile than w1118 controls or flies with an excised transposon (hopped c04487). Infertility was partially rescued by expression of Pde1c under the control of either Pde1c-GAL4 or ELAV-GAL4 (shaded bars). The number of pairs producing progeny in these two rescue lines was significantly less than w1118 controls (P < 0.001) and significantly greater than Pde1cc04487 mutants (P < 0.001). Shaded bars in all panels represent data from male flies expressing Pde1c under the control of either Pde1c-GAL4 or ELAV-GAL4 drivers in a Pde1cc04487 mutant background. (B–D) Courtship assays. Individual males of the genotypes shown were introduced to virgin female w1118 flies and recorded on video for 1 hr. (B) Copulation success. The number of pairs that copulated within the first hour was logged. Homozygous Pde1cc04487 flies copulated significantly less frequently than excised c04487 transposon and heterozygous Pde1cc04487 controls (P < 0.001). Both rescue experiments were not significantly different from either control and were significantly greater than the homozygous Pde1cc04487 flies (P < 0.001). (C) Copulation latency. For the pairs in B that successfully copulated, the time taken to initiate copulation was recorded. Homozygous Pde1cc04487 flies took significantly longer to initiate copulation compared to excised c04487 and heterozygous Pde1cc04487 siblings and both rescue genotypes (P < 0.05). (D) Courtship index. The fraction of time spent in any aspect of courtship during the first 10 min of the assay was recorded. No significant differences were seen between the different genotypes. All data shown are means ± SEMs for at least 20 pairs of flies (A and B), 7–35 pairs (C), and at least 16 pairs (D). The data were tested for significant differences between groups with a one-way ANOVA followed by Bonferroni's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001.
To confirm that the male sterility was caused by the loss of expression of Pde1c, we generated flies containing a Pde1c-RC cDNA under the control of a UAS promoter and also flies containing a 6.2-kb piece of genomic DNA that included the first exon and translational start site of the Pde1c-RC transcript (Figure 1B) driving expression of GAL4. We crossed these transgenes into the Pde1cc04487 background to yield flies that expressed Pde1c under the control of the Pde1c promoter in a Pde1c mutant background. Males from this line were partially fertile with >50% of pairs producing progeny (Figure 2A). The number of progeny resulting from these successful matings was similar to control matings (data not shown). As Pde1c is predominantly neuronal, we also used the neuronal driver, ELAV, to drive Pde1c expression only in neurons. This was less successful in rescuing male infertility with <25% of pairs producing progeny (Figure 2A). We also used a tubulin-GAL4 driver to express Pde1c ubiquitously; however, this was lethal (data not shown).
Courtship deficits:
To determine the cause of infertility, we removed the male reproductive system and examined it for the presence of mature sperm. The testis appeared normal with the presence of spermatocytes and spermatids. When we ruptured the seminal vesicle, normal, motile, mature sperm were released, suggesting that infertility was not caused by defects in meiosis or sperm maturation (Castrillon et al. 1993). To further explore the underlying cause of male infertility in the Pde1cc04487 flies, we examined the courtship behavior of individual males paired with control (w1118) females. We first logged the number of males that successfully copulated with the female within the first hour of their pairing and the length of time before copulation took place (Figure 2, B and C). These results show that homozygous Pde1cc04487 males copulated significantly less frequently than their heterozygous siblings and transposon mobilized controls (Figure 2B). Those males that did copulate also took significantly longer to begin copulation than either controls (Figure 2C). Both of these defects were completely rescued by driving Pde1c expression with either the Pde1c-GAL4 or the ELAV-GAL4 drivers (Figures 2, B and C). The defects in copulation suggest that either male Pde1cc04487 flies were more reluctant to mate or they were less attractive to females and hence rejected by females. To distinguish between these possibilities, we determined the courtship index, which measures how long a male is engaged in courtship behavior in the first 10 min of the presence of a receptive female (Figure 2D). These results showed that homozygous Pde1cc04487 males spent a similar fraction of time engaged in courtship behavior compared to either controls, suggesting that the reduced copulation in Pde1cc04487 males is more likely due to a rejection by the female rather than to reluctance on the part of the male.
Male infertility is likely due to elimination of sperm from females:
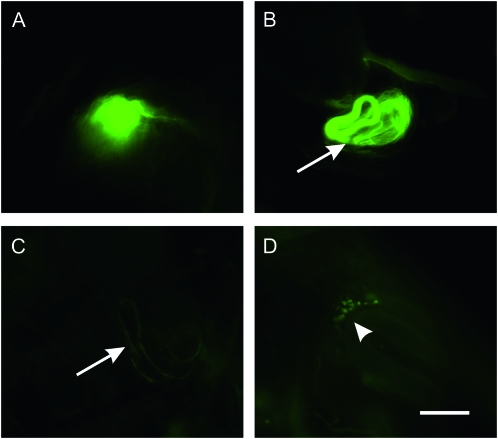
Because a significant fraction of male Pde1c mutant flies copulated with females, courtship defects alone are insufficient to explain the male sterility. We then examined whether sperm transfer took place when male homozygous Pde1cc04487 flies copulated with females by using flies that carry a transgene coding for a GFP-labeled don juan protein (DJ-GFP), which labels sperm with GFP (Santel et al. 1997). As this transgene is on the same chromosome as Pde1c, we recombined the Pde1cc04487 and DJ-GFP transgenes and selected lines that had males with GFP-labeled testes and that were sterile as homozygotes. We then mated these sterile males with wild-type females. After 4 hr, we examined the females with a fluorescent microscope and found several with GFP-labeled abdomens, suggesting that sperm had been transferred. When the reproductive system from these females was removed 24 hr after mating and examined, GFP-labeled sperm were clearly visible in the uterus and seminal receptacle (Figure 3A). This suggests that at least some matings do result in sperm being successfully transferred to the female. We then examined whether sperm could be stored by the female. Male Pde1cc04487-DJ-GFP flies were allowed to mate with wild-type females for 4 hr, and all the females that had GFP in their abdomens were isolated and kept separately for 72 hr. The reproductive system was then removed and examined by fluorescence microscopy. The seminal receptacle from females that had mated with control DJ-GFP males 72 hr earlier contained a large amount of GFP-labeled sperm (Figure 3B). By contrast, we rarely detected GFP-labeled sperm in the reproductive system of females that had mated with male Pde1cc04487-DJ-GFP flies 72 hr earlier. Occasionally, a small amount of GFP-labeled sperm was detected in the seminal receptacles (Figure 3C), and in some cases, small GFP-labeled particles were detected in the seminal receptacles (Figure 3D), suggesting that the sperm were either eliminated or degraded. These findings suggest that the cause of male infertility in Pde1cc04487 flies is either that insufficient sperm are transferred or that sperm are rejected and/or eliminated by the female.
Figure 3.—
Reduced storage of sperm in females mated with mutant Pde1c males. Homozygous Pde1cc04487 and control males, both carrying the DJ-GFP transgene, were mated with w1118 females for 4 hr. Females that had GFP visible in their abdomens were separated and kept for 24 and 72 hr, and then their reproductive systems were removed and examined. (A) Reproductive system from a female mated with a Pde1cc04487-DJ-GFP male showing abundant GFP-labeled sperm in the uterus 24 hr after mating. (B) Reproductive system from a female mated with a DJ-GFP male showing abundant GFP-labeled sperm in the seminal receptacle 72 hr after mating (arrow). (C and D) Reproductive system from a female mated with Pde1cc04487-DJ-GFP males showing greatly reduced levels of GFP-labeled sperm in the seminal receptacle 72 hr after mating (arrow in C). (D) Occasionally, the presence of GFP-labeled particles could be seen (arrowhead). Bar, 100 m.
Male-specific expression of Pde1c in the CNS:
The behavioral deficits seen in the Pde1cc04487 males suggest that a major function of Pde1c is within the nervous system. Microarray expression data show that Pde1c is primarily, but not exclusively, expressed in the nervous system (FlyAtlas; http://www.flyatlas.org). To examine the expression pattern of Pde1c, we generated a Pde1c promoter-GAL4 transgenic fly line. We amplified a 6.2-kb piece of genomic DNA that included the first exon and translational start site of the Pde1c-RC transcript (Figure 1B) with PCR and subcloned it into a GAL4 transformation vector. When this experiment was designed, the Pde1c-RC transcript was the only Pde1c transcript that was predicted on FlyBase. Subsequently, additional Pde1c transcripts were identified such that the promoter region that we isolated lies within an intron of the additional transcripts (Figure 1B). Nevertheless, when we crossed the Pde1c promoter-GAL4 line with a UAS-GFP line and examined the progeny for GFP expression, we observed clear expression. The majority of this expression was observed in the CNS with numerous neurons present in the brain and ventral ganglia (Figure 4). Little expression was seen in other tissues as suggested by the microarray data (FlyAtlas) although we also detected expression in peripheral putative chemosensory neurons in the antennae, proboscis, tarsi, and wings (A. Vermehren-Schmaedick and D. B. Morton, unpublished observations). When we examined the CNS from males and females, we saw clear differences in the expression patterns with notably more neurons in the abdominal regions of the ventral ganglia of males compared to females (Figure 4, A–D). We counted the number of GFP-expressing neurons in the most posterior region of the ventral ganglia and found that there were almost twice as many labeled neurons in this region in males compared to females (Figure 4E).
Figure 4.—
Expression of Pde1c in ventral ganglia. The ventral nerve cord of male (A) and female (B) flies expressing the Pde1c-GAL4 and UAS-GFP transgenes were imaged with confocal microscopy, revealing a larger number of neurons that express Pde1c in the abdominal regions of the ganglion. For A–G, the images represent a stack of confocal scans. The inset in A and B is enlarged and shown in C and D. (E) Cell counts for the region shown in C and D reveal that there are about twice as many neurons that express Pde1c in the male abdominal neuromeres compared to females. In C and D, the arrows indicate examples of cells that were counted. The data represent the means ± SEMs of six to seven determinations. P < 0.05 two-tailed t-test. (F and G) Double-labeled immunocytochemistry revealed that the Pde1c neurons do not express 5-HT. Ganglia were stained with anti-GFP (green, arrow) and anti-5-HT (red, arrowhead) antisera and show no colocalization of signal in the abdominal (F) or thoracic (G) portions of the ganglion. Bar, 50 m for all images.
Male-specific neurons in the abdominal ganglia were also observed when examining the expression pattern of the sex-determining gene, fruitless (Lee and Hall 2001). As many of these abdominal fruitless neurons also express the neurotransmitter 5-HT and these neurons were shown to be important for reproductive function and sperm transfer (Lee and Hall 2001), we used 5-HT immunoreactivity as a marker for these male-specific neurons and examined coexpression with Pde1c. Despite the similar location of the 5-HT neurons with the Pde1c neurons, we did not see any colocalization (Figure 4, F and G).
DISCUSSION
We provide evidence here that the calcium/calmodulin-regulated cyclic nucleotide PDE, Pde1c, is required for male fertility in Drosophila. A transposon insertion within the Pde1c gene caused reduced expression of Pde1c and male sterility. A deficiency that covers the entire Pde1c gene failed to complement Pde1cc04487 for male sterility. Excision of the transposon fully restored male fertility, and expression of a Pde1c-RC cDNA partially restored fertility when driven by the Pde1c promoter. The lack of complete rescue suggests that our Pde1c-GAL4 construct does not represent the complete expression pattern of Pde1c. Interestingly, driving Pde1c expression only in neurons using an ELAV-GAL4 driver was less effective at rescuing fertility. This could be either due to a requirement of Pde1c expression in non-neuronal cells for fertility or because ELAV is a weaker promoter than Pde1c. The identity of any non-neuronal cells is unknown, as we failed to consistently detect GFP expression in non-neuronal tissue when this was driven by the Pde1c promoter. Microarray data (FlyAtlas) do suggest that there is a low level of expression outside the nervous system. The fact that neuronal expression of Pde1c rescued the courtship defects as completely as expression driven by the Pde1c promoter suggests that a neuronal defect causes these phenotypes.
Previous studies have shown that the most common causes for male sterility are defects in spermatogenesis and sperm maturation (Castrillon et al. 1993; Wakimoto et al. 2004). Pde1c is unlikely to be required for these processes as the Pde1cc04487 males appeared to have normal, motile sperm. At least part of the cause of the male sterility in Pde1cc04487 males is likely due to courtship defects as they had significantly reduced copulation rates and took significantly longer to begin copulation. The courtship index value obtained for Pde1cc04487 males was not significantly reduced compared to control males, suggesting that these defects are not due to general behavioral inactivity or that they fail to recognize females. This suggests that females found Pde1cc04487 males unacceptable mates and that males' attempts to mate were rejected.
These behavioral defects, however, are unlikely to be the entire cause of male fertility. Within the 1-hr observation period, a significant number (15%) of males did copulate, and the length of time spent copulating was similar to wild-type animals (data not shown). Comparison of the behavioral defects of Pde1cc04487 males with the classical male courtship mutant, fruitless, supports our hypothesis that behavioral defects alone do not explain the male sterility of Pde1cc04487 flies. Male flies mutant for fru were never observed to copulate or exhibit any component of courtship behavior during the observation period (Gailey and Hall 1989; R. Clemens-Grisham and D. B. Morton, unpublished observations). Nevertheless, even when male Pde1cc04487 flies were left with control females for a week, they very rarely produced progeny (<3%), and the number of progeny produced was very low. This suggests that there were also defects in sperm transfer, in sperm storage in the female, and/or in the ability of the sperm to fertilize the eggs.
Experiments utilizing don juan-GFP labeled sperm show that Pde1cc04487 males did transfer sperm to females. Considering only fluorescence intensity, the quantity of sperm transferred appeared normal, but no efforts were made to quantify this accurately. In wild-type Drosophila, after copulation sperm move from the uterus to the seminal receptacle (Lefevre and Jonsson 1962) where they are stored for up to 2 weeks (Bloch Qazi et al. 2003). Although we detected substantial levels of sperm in the female reproductive system 24 hr after mating with Pde1cc04487 males, by 72 hr very few sperm were detected either in the seminal receptacle or in other parts of the female reproductive system. Occasionally, we detected GFP-labeled particles, suggesting that the sperm had been partially degraded. The successful transfer of sperm to the seminal receptacles and their storage depends on the components of the male accessory gland secretions (Bloch Qazi et al. 2003). It seems likely that some component of this ejaculate is defective in Pde1cc04487 males, leading to female rejection and elimination of the sperm. In wild-type females, sperm move rapidly from the uterus to the seminal receptacle (Bloch Qazi et al. 2003), and it is unusual to find sperm in the uterus 24 hr after mating as is the case with matings with Pde1c mutant males. This might indicate that there are defects in the disposal of unstored sperm or that the GFP is no longer associated with the sperm.
The observed male-specific expression pattern of Pde1c in neurons in the abdominal ganglia suggested that innervation of the male reproductive system was in some way defective. Male-specific serotonergic neurons that innervate the male reproductive system have been previously described, and this innervation is defective in fru mutants (Lee and Hall 2001; Lee et al. 2001). However, as we could detect no colocalization of 5-HT and Pde1c, it is unlikely that these neurons are defective in the Pde1cc04487 flies. The identities of the Pde1c neurons are unknown, and we were unable to consistently detect Pde1c-driven GFP in axons that innervated the male reproductive system.
In summary, we have shown that Pde1c is necessary for male fertility and that a component of this sterility is reduced completion of courtship behaviors. The likely major cause of sterility is that the female eliminates the sperm some time after copulation. The effects on male courtship behavior could be mediated by the male-specific Pde1c expression pattern in the CNS.
Acknowledgments
This work would not have been possible without the resources provided by FlyBase and the Bloomington Stock Center. The project was supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education and Extension Service, grant no. 2006-35607-16593 (D.B.M.) and by National Institute of Neurological Disorders and Stroke grant no. NS29740 (D.B.M.).
References
- Bloch Qazi, M. C., Y. Heifetz and M. F. Wolfner, 2003. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev. Biol. 256 195–211. [DOI] [PubMed] [Google Scholar]
- Byers, D., R. L. Davis and J. A. Kiger, 1981. Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature 289 79–81. [DOI] [PubMed] [Google Scholar]
- Castrillon, D. H., P. Gönczy, S. Alexander, R. Rawson, C. G. Eberhart et al., 1993. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics 135 489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, M., and J. Beavo, 2007. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components of cyclic nucleotide signaling. Annu. Rev. Biochem. 76 481–511. [DOI] [PubMed] [Google Scholar]
- Davis, R. L., J. Cherry, B. Dauwalder, P. L. Han and E. Skoulakis, 1995. The cyclic AMP and Drosophila learning. Mol. Cell. Biochem. 149 271–278. [DOI] [PubMed] [Google Scholar]
- Day, J. P., J. A. T. Dow, M. D. Houslay and S. A. Davies, 2005. Cyclic nucleotide phosphodiesterases in Drosophila melanogaster. Biochem. J. 388 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailey, D. A., and J. C. Hall, 1989. Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Genetics 121 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan, R. J., 2004. Fly Pushing, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Le, C. T., 2003. Introductory Biostatistics. John Wileyand Sons, New York.
- Lee, G., and J. C. Hall, 2001. Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila. J. Neurosci. 21 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, G., A. Villella, B. J. Taylor and J. C. Hall, 2001. New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J. Neurobiol. 47 121–149. [DOI] [PubMed] [Google Scholar]
- Lefevre, G., and U. B. Jonsson, 1962. Sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics 47 1719–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton, D. B., and M. L. Hudson, 2002. Cyclic GMP regulation and function in insects. Adv. Insect Physiol. 29 1–54. [Google Scholar]
- Santel, A., T. Winhauer, N. Blumer and R. Renkawitz-Pohl, 1997. The Drosophila don juan (dj) gene encodes a novel sperm specific protein component characterized by an unusual domain of a repetitive amino acid motif. Mech. Dev. 64 19–30. [DOI] [PubMed] [Google Scholar]
- Siegel, G. J., B. W. Agranoff, R. W. Albers and P. B. Molinoff, 1994. Basic Neurochemistry, Ed. 5. Raven Press, New York.
- Truman, J. W., and R. Booker, 1986. Adult-specific neurons in the nervous system of the moth, Manduca sexta: selective chemical ablation using hydroxyurea. J. Neurobiol. 17 613–625. [DOI] [PubMed] [Google Scholar]
- Wakimoto, B. T., D. L. Lindsley and C. Herrara, 2004. Toward a comprehensive genetic analysis of male fertility in Drosophila melanogaster. Genetics 167 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]