Abstract
This review describes the historical emergence of the concept of bone marrow Mesenchymal Stem Cells (MSCs), summarizing data on Wolf and Trentin’s hematopoietic inductive microenvironment, Dexter’s hematopoiesis supportive stromal cells, Friedenstein’s osteogenic cells, Pittenger’s trilineal osteoblastic, chondrocytic and adipocytic precursors, to finally introduce the specific bone marrow mesenchymal stem cells with differentiation potential to four lineages (mesenchymal and vascular smooth muscle lineages), and stromal and immunomodulatory capacities. Two points are the object of detailed discussion. The first point envisions the stem cell attributes (multipotentiality, self-renewal, tissue regeneration, population heterogeneity, plasticity, lineage priming) compared to that of the paradigmatic hematopoietic stem cell. In the second point, we discuss the possible existence of bone marrow cells with larger differentiation potential, eventually pluripotential cells. The latter point raises the issues of cell fusion, reprogrammation, or selection under non standardized conditions of rare populations of neuroectodermal origin, or of cells that had undergone mesenchymal-to-epithelial transition. In the last section, we review data on MSC senescence and possible malignant transformation secondary to extensive culture, gene transfer of telomerase, or mutations such as leading to Ewing’s sarcoma. The set of data leads to the conclusion that bone marrow MSCs constitute a specific adult tissue stem cell population. The multiple characteristics of this stem cell type accounts for the versatility of the mechanisms of injured tissue repair. Although MSC administration may be extremely useful in a number of clinical applications, their transplantation is not without risks that must not be overlooked when developing cell therapy protocols.
Keywords: Bone Marrow Cells; Cell Aging; Hematopoiesis; History, 20th Century; Humans; Immunomodulation; Mesenchymal Stem Cells; Multipotent Stem Cells
1. The hematopoietic supportive stromal cell
In the 1970s Wolf and Trentin (review in (Trentin, 1989)) described a model of hematopoietic inductive microenvironment, whereby the differentiation of Hematopoietic Stem Cells (HSCs) would be driven by the spleen or bone marrow microenvironments seeded by HSCs (Fig 1A).
Figure 1. Historical overview.
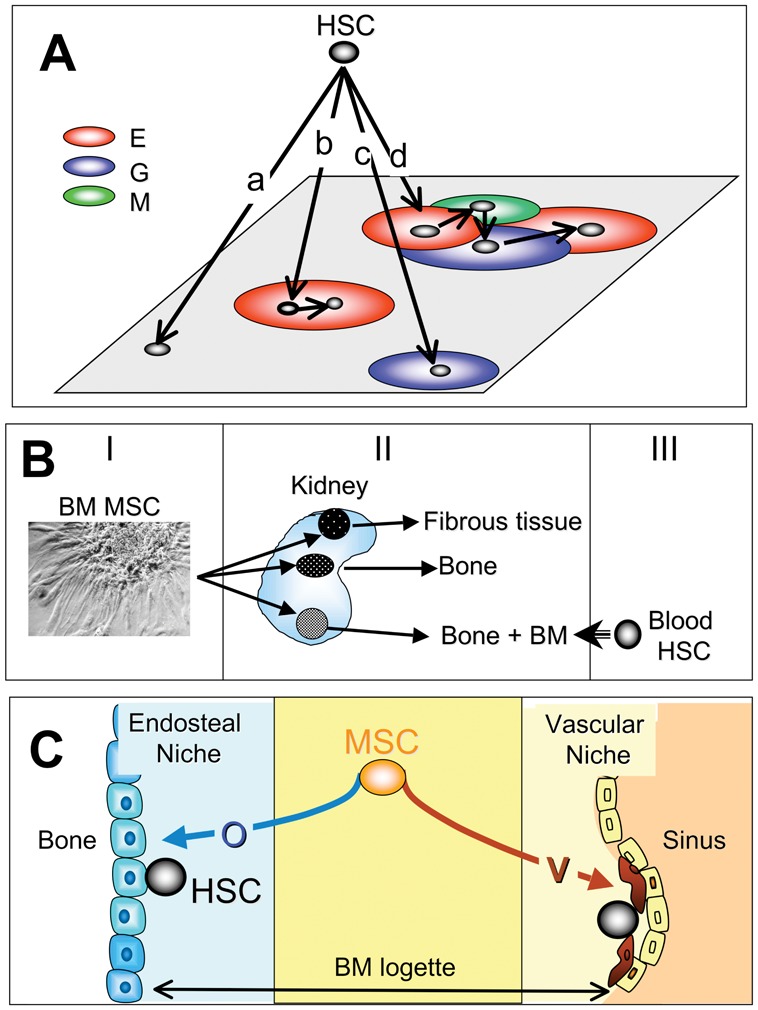
A: The hematopoietic inductive microenvironment of Wolf and Trentin
The authors hypothesized that the differentiation pathway of the HSC depends on the territory seeded by HSCs. HSCs seeding non-determined territories do not give rise to colonies (a). HSCs seeding erythroblastic territories (E) give rise to colonies made of maturing red cells; in the shown case the daughter stem cell remains in the same territory, leading to colony size increase (b). HSCs seeding granulocytic territories (G) gives rise to colonies made of maturing granulocytes (c). Large mixed colonies consisting in a mixture of erythroblasts (E), granulocytes (G) and megacaryocytes (M) result from the primary homing of HSC to erythroblastic territory, then migration of daughter HSCs to megakaryocytic and granulocytic territories (d).
B) The work of Alexander Friedenstein
It can be summarized as follows. I: bone marrow MSC clones (CFU-f) were developed. II: each clone was implanted under the kidney capsule of semi-syngeneic animals; a few weeks after implantation, clones were retrieved and analyzed. III: studies using chimeras revealed that in clones giving rise to bone and marrow, bone cells were of donor origin, while hematopoietic cells were of recipients; the latter probably resulted from homing of circulating blood HSCs and subsequent generation of hematopoietic cells.
C) Present view of the HSC niches
We hypothesize that the stromal component in osteoblastic niches results from the differentiation of MSCs into osteoblasts, while the stromal component in vascular niches results from the vascular smooth muscle differentiation of the MSCs.
In 1977, Dexter et al described bone marrow long-term cultures (Dexter et al., 1977). In this system, the maintenance over time of mouse HSCs was strictly dependent on the generation of an adherent layer of stromal cells. Different investigators adapted the system to human bone marrow (Gartner and Kaplan, 1980). In the following years, sytems were made more analytical since different populations of hematopoietic precursors (including HSCs) were seeded on preformed layers of stromal cells. Stromal cells were isolated according to their membrane antigen expression pattern (Simmons and Torok-Storb, 1991). Alternatively, continuous lines generated from bone marrow of mice or humans served as feeders (review in (Zipori, 1989)).
These systems demonstrated the requirement of stromal cells for HSC maintenance. The model of the stem cell niche illustrated this requirement (Schofield, 1978). In this model the HSC has to be in physical contact with the stromal cell, or with its associated extracellular matrix (ECM), to maintain the adequate balance (“stemness”) between self-renewal and commitment to the different blood lineages (Moore and Lemischka, 2006).
The model of the niche has been generalized to almost all types of stem cells (Spradling et al., 2001). In the drosophila ovariole, the physical association between a “hub” stromal cell and a germinal stem cell could be visualized. Upon division, the daughter cell that lost its contact with the hub cell became committed to the germinal lineage, while the daughter cell that kept its contact remained a stem cell. Recently, several publications have described two HSC niche models (reviews in (Adams and Scadden, 2006; Kopp et al., 2005)). In the osteoblastic niche model the stromal component is close to the endosteal lining or may be a subtype of osteoblasts. In the vascular niche model the stromal component is a cell constituting the vascular sinus.
A large number of regulatory pathways are operative in the molecular control of HSC by stromal cells (reviews in (Charbord, 2001; Charbord, 2004)). Adhesive pathways are essential, be it homophilic adhesion such as cadherin/cadherin, heterophilic adhesion such as VLA4/VCAM1 or cell-ECM adhesion such as integrins to fibronectins or collagens. Cytokine or morphogen pathways are also crucial, stromal cells expressing a wide range of early acting cytokines or morphogens such as hedgehog, notch or wnt families members. A point to be emphasized is the overlap of, and redundancy in the pathways, accounting for fine-tuning and robustness, features characteristic of network systems (Kitano, 2002).
A critical issue for this review is that of the differentiation program and origin of the stromal cells. At the time when long-term marrow cultures were developed two major contending hypotheses were raised.
The most challenging was that stromal cells were the progeny of HSCs, as suggested by simultaneous growth of HSCs and stromal cells in long-term marrow cultures. However, the identification of stromal/hematopoietic precursor has proven difficult. One study showed that rigorously cloned stromal cells generated, after infection with entire SV-40 virus, both CD45+/CD34+ round cells and vimentin+/SM-actin+ elongated cells (Singer et al., 1987). However, in the absence of colony-forming hematopoietic progenitors, conclusion on the hematopoietic nature of the round cells remained uncertain. Another study claimed to have purified to homogeneity a population of CD34+/HLA-DR+/CD38− cells with stromal and hematopoietic potential (Huang and Terstappen, 1994). However, data were subsequently retracted since colonies proved to be generated by more than one clonogenic cell. Is the hypothesis of a common stromal/hematopoietic precursor completely disqualified? There are still a few reports that support it (Menendez et al., 2009; Ogawa et al., 2006). In particular, mouse Multipotent Adult Progenitor Cells (MAPCs) generated by Catherine Verfaillie’s team (Jiang et al., 2002) were shown to give rise to HSCs with multilineage hematopoietic engraftment of immunodeficient mice (Serafini et al., 2007). MAPCs have been difficult to reproduce in other laboratories. However, taken together the data leave open the possibility that exists within stromal layers a very minor bipotential population that could be selected under specific culture conditions.
The mesenchymal origin of the stromal cells has been advocated early on. The capacity of the mesenchyme to synthesize and assemble the ECM accounts for the inclusion as mesenchymal cells of connective tissue-forming cells such as dermal and interstitial tissue fibroblasts, osteoblasts, chondrocytes, adipocytes and smooth muscle cells. These cells are for most, but not exclusively (as discussed below), of mesodermal origin. Many stromal lines were described as pre-adipocytic cells filled with fat-laden vesicles (Zipori, 1989). We have described the vascular smooth muscle differentiation of human and murine stromal cells/lines acquiring with time in culture vascular smooth muscle cytoskeletal markers, up to the most specific alternatively spliced isoforms of caldesmon (h-caldesmon) and of smooth muscle myosin heavy chains (SM1 and SM2) (Galmiche et al., 1993; Remy-Martin et al., 1999). Other reports have shown the presence of osteoblasts within stromal layers (Long et al., 1990). Collectively, these data allow to define stromal cells as mesenchymal cells, which does not specify the stem cell type of origin.
2. The tripotential adipocytic, osteoblastic and chondrocytic precursor cell
Definitive evidence that bone marrow includes cells that can generate connective tissue-forming cells was originally provided by the pioneering work of Alexander Friedenstein summarized in Fig 1B (review in (Friedenstein et al., 1970)). In the 1960s, this investigator made a number of experiments pointing to the existence within the bone marrow of different species of a precursor for osteoblasts and fibrous tissue. One to 2 weeks after seeding bone marrow cells at low density in liquid cultures containing serum, he observed discrete colonies consisting in plastic-adherent, nonphagocytic, elongated cells of fibroblastic appearance. The clonogenic cell at the origin of each colony was called colony-forming unit-fibroblasts (CFU-f). CFU-fs were shown, by suicide technique and cell cycle analysis, to be quiescent in vivo and at culture inception. Each colony seeded under the renal capsule of semi-syngeneic animals gave rise, a few weeks after transplantation, to fibrous tissue, to bone and to bone containing bone marrow. Using chimeric animals, Friedenstein further showed that marrow hematopoietic cells within the bony spaces were of recipient origin, contrarily to bone cells or fibrous tissue that were from the donor. These latter data suggested that some of the transplanted colonies constituted an adequate microenvironment for HSC homing and subsequent hematopoiesis, reinforcing therefore the hypotheses of hematopoietic inductive microenvironment and HSC niche.
In the 1980s and 1990s, other works extended these observations and made clear that the cells identified by Friedenstein were multipotent and could give rise to osteoblasts, chondrocytes and adipocytes (review in (Prockop, 1997)). In 1999, Mark Pittenger showed that trilineal adipocytic, osteoblastic and chondrocytic clones were present in human bone marrow and provided a membrane antigen analysis of the cells indicating that few endothelial or hematopoietic specific markers were expressed (Pittenger et al., 1999). Later studies asserted that the MSC population was clearly distinct from that of endothelial or hematopoietic cells (Delorme et al., 2008).
Since their initial description, these multipotent bone marrow cells have been given different names. Friedenstein used the term osteogenic stem cells, while Maureen Owen called them marrow stromal stem cells to underline that they generate stromal cells in long-term cultures (Owen et al., 1987). Arnold Caplan was the first to introduce the term Mesenchymal Stem Cells (Caplan, 1991), which has become popular in the current literature. However, Paolo Bianco and Pamela Robey have suggested the alternative term Skeletal Stem Cell to underscore their potential to give rise to the cellular components of the skeleton (Bianco et al., 2008), and James Dennis has suggested the name mesenchymal progenitor cells, arguing that these cells were not bona fide stem cells (Dennis et al., 1999). Following a similar reasoning, the International Society for Cellular Therapy has proposed the term multipotent Mesenchymal Stromal Cell (Horwitz et al., 2005).
Taken together, the data indicate that mesenchymal precursors are present in the bone marrow of multiple species. These cells can be extensively amplified in vitro, which allows their use in cell therapy applications.
3. The mesenchymal stem cell
The minimal criteria to qualify adult tissue stem cells are that they constitute an immature population of heterogeous, self-renewing cells able to regenerate, after injury, their tissue of origin (they are multipotential if the tissue consists in several cell types). An additional criterium is the flexibility of the stem cell attributes, e.g. that they can shift from quiescent to proliferative state or that differentiation can be reversed at least up to a certain stage (Loeffler and Potten, 1997; Loeffler and Roeder, 2002).
May the trilineal mesenchymal precursor be qualified as stem cell?
Multipotentiality is one of the hallmarks of these cells. Clones can be differentiated under appropriate conditions not only into adipocytes, chondrocytes and osteoblasts, but also into vascular smooth muscle cells (Delorme et al., 2009; Jeon et al., 2006; Kashiwakura et al., 2003; Kim et al., 2008; Kobayashi et al., 2004; Kurpinski et al., 2010). Moreover, these cells may differentiate into tenocytes as inferred from in vivo studies (Hoffmann et al., 2006), although evidence in vitro at the clonal level is at present lacking.
In 2007, Paolo Bianco’s team has shown that clonogenic human CD146+/CD90+ CFU-fs were able to self-renew (Sacchetti et al., 2007). One colony of such phenotype was implanted subcutaneously into an immunodeficient mouse. After 8 weeks the implant was retrieved and the CD146+/CD90+ minor population was sorted and cultured at cloning density. Two colonies of the same phenotype were obtained. Since it is generally agreed that the ability of a stem cell population to in vivo regenerate cells with characteristics identical to the initially explanted fraction practically reflects its self renewal capacity, these data indicate that MSCs are able to self-renew. More extended self-renewal capacity, requiring sequential passages to secondary and even tertiary recipients, has yet to be evidenced. However, there is no data supporting the notion that the in vivo serial repopulation assay is either feasible or entirely applicable in the case of MSCs, and it might not therefore represent the most appropriate in vivo model in determining their functional potential. Indeed, MSCs need to be cultured extensively before transplantation to attain sufficient numbers to regenerate a bone or cartilage defect. In addition, the turn-over rate of the mesenchymal tissue is much lower than that of hematopoiesis, which complicates the experimental setting (Bianco and Gehron Robey, 2000). Self-renewing mouse MSCs appear to be of smaller size and express a series of surface antigens not expressed by other culture-amplified cells (Colter et al., 2001). Sca-1/Ly-6A, another membrane murine MSC marker, has proven essential for the maintenance of the self-renewal capacity (Bonyadi et al., 2003).
Numerous in vivo experiments in the animal and a few clinical trials in humans have shown that MSCs were able to regenerate bone and cartilage. Particularly demonstrative were experiments in the mouse model of osteogenesis imperfecta (OI). One to 4 months after systemic injection in normal irradiated mice of MSCs expressing a human mini-gene for collagen I, cells with the characteristic collagen were found in bone, bone marrow and cartilage, and infusion of wild-type MSCs in OI mice improved bone mineralisation and collagen synthesis (Li et al., 2007; Pereira et al., 1995; Pereira et al., 1998). MSCs also contribute to the formation of vessels due to their capacity to differentiate into vascular smooth muscle cells (Au et al., 2008; Dufourcq et al., 2008).
MSCs constitute an heterogeneous population of immature cells as suggested by the multimodal expression (in flow cytometry studies) of membrane antigens specific for non differentiated cells, such as CD146 or CD200 (Delorme et al., 2008). However, heterogeneous expression of certain markers can also be observed in cells from clones, which may be due to gene or network noise (Kaern et al., 2005). This is why heterogeneity is optimally assessed in functional studies that have revealed great clonal heterogeneity in terms of proliferative capacity and differentiation potential (Muraglia et al., 2000; Pittenger et al., 1999). Moreover, the generation of bone-like tissue in vitro does not appear to reflect the capacity of forming bone in vivo, and only a fraction of MSC clones are able to generate bone in vivo (Bianco et al., 2008).
Flexibility of MSC attributes is exemplified by their differentiation plasticity. It is well-known that mesenchymal cells can shift from one differentiation pathway to another under modified external conditions. Chondrocytes in the growth plate undergo hypertrophy before turning into osteoblasts. Rocky Tuan’s team has shown that cloned osteoblasts can turn into chondrocytes or adipocytes (Song and Tuan, 2004; Song et al., 2006), and we have shown that cloned MSCs differentiated into vascular smooth muscle cells can turn into adipocytes, osteoblasts or chondrocytes (Delorme et al., 2009). Lineage conversion may proceed via a de-differentiation process (Song et al., 2006). To some extent, plasticity and self-renewal are two opposing attributes since the potential for a cell in the progeny of a stem cell to re-acquire the full differentiation potential negates the need for indefinite self-renewal capacity to maintain the stem cell pool. Indeed, pedigree hierarchical models of stem cell differentiation where stem cells are preordained entities differentiating into discrete populations of progenitors and differentiated cells are not compatible with plasticity (Loeffler and Roeder, 2004). Other models which account for the capacity of de-differentiation have to apply, such as the phase space model (Kirkland, 2004; Morad et al., 2008) or a model based on the concept of noise-driven stem cell differentiation (Krinner et al., 2010).
Lineage priming, another property of stem cells shared by MSCs (Delorme et al., 2009), is a molecular model for stem cell differentiation in which self-renewing stem cells, not induced to differentiate, express a subset of genes associated to the differentiation pathways to which they can commit (Hu et al., 1997). Of major interest is the expression of key transcription factors for differentiation, such as, for HSCs, PU1 and GATA1 for myeloid and erythroid differentiation, respectively (Huang, 2009; Huang et al., 2007). Analysis of individual HSCs has revealed variable expression of these transcription factors from one cell to another, which might reflect the high level of noise characteristic of stem cells. Induction of differentiation is marked by the decline of one factor at the expense of the other (e.g. downregulation of PU1 and upregulation of GATA1 as HSC undergo erythroid differentiation). Sui Huang has posited that similar process occurs in MSCs (e.g. RUNX2 downregulation and PPARG upregulation in adipogenic condition), which remains to be tested (Huang, 2009).
Taken together, the data indicate that MSCs are bona fide stem cells. However, HSCs and MSCs may constitute two extremes among adult tissue stem cells: in HSCs self-renewal appears to be the cardinal property while in MSCs plasticity would be the predominant feature (Zipori, 2005).
4. A unifying concept: MSC as multipotential stem cells with stromal and immuno-modulatory capacities
That MSC are hematopoietic supportive and implicated in the regulation of the immune system are additional properties not implied by stemness.
As already alluded to, in vivo HSC niches may be of two kinds: osteoblastic and vascular. Transplantation of human MSCs in immunodeficient mice have recently shown that the vascular component is the pericyte located on the abluminal side of the endothelial cell (Sacchetti et al., 2007). Four weeks after transplantation of human CD146+ bone marrow CFU-Fs in immunodeficient mice, the few human cells that retained the expression of CD146 were located on the abluminal side of mouse-derived endothelial cells forming incipient sinuses. By week 8, foci of hematopoietic cells were clearly associated to the CD146+ parasinusal cells. These cells are anatomically and morphologically similar to alkaline phosphatase+ adventitial reticular cells (ARCs) described in the 1960s (Lichtman, 1981; Westen and Bainton, 1979) and to parasinusal “myoid” cells expressing alpha-SM actin (Schmitt-Graff et al., 1989) found in fetal and adult human bone marrow (Charbord et al., 1996; Galmiche et al., 1993). As observed with ARCs and pericytes, parasinusal myoid cells extend cytoplasmic processes deep into the marrow space, making contact with numerous hematopoietic cells. Remarkably, CD146+ cells are alpha-SM actin+ and express additional smooth muscle markers such as calponin, desmin and leiomodin. However, direct and regular overlap in situ between alkaline phosphatase+ cells and alpha-SM actin+ cells has not been demonstrated. Pericytes from sites other than bone marrow may also give rise to MSC-like cells (Crisan et al., 2008).
HSC niches can be easily conceived within the framework of MSC differentiation (Fig 1C) since MSCs give rise to both osteoblasts and pericytes that belong to the family of vascular smooth muscle cells (reviews in (da Silva Meirelles et al., 2008; Hirschi and D’Amore, 1996; Sims, 1986)). Another evidence of the role of MSCs in HSC niche formation has been provided by Muguruma et al who reported observations made after intra-bone injection of human labelled culture-amplified bone marrow MSCs in immunodeficient mice (Muguruma et al., 2006). Ten weeks after transplantation 60% of the labelled cells were alpha-SM actin+ and located in the vicinity of sinuses, and 30% were alkaline phosphatase+ and located foremost in the endosteal region. Transplantation of human cord blood cells after that of MSCs revealed the frequent interaction of MSC progenies and CD34+/CD38− hematopoietic precursors.
The immunomodulatory property is likewise a property unrelated to stemness. Different reports have shown that dermal fibroblasts were able to suppress the mixed lymphocyte reaction similarly to bone marrow MSCs, leading to the hypothesis that immunosuppression is a property of the mesenchyme whatever its location (Haniffa et al., 2009; Haniffa et al., 2007; Jones et al., 2007).
Taken together, the data suggest that MSCs represent a specific type of adult tissue stem cells that, in addition to stem cell attributes, have the capacity to support hematopoiesis and to be immunosuppressive (Fig 2). The stromal property might be specific for bone marrow MSCs, while the immunomodulatory capacity might be shared by mesenchymal cells distributed throughout the body.
Figure 2. The MSC in its context.
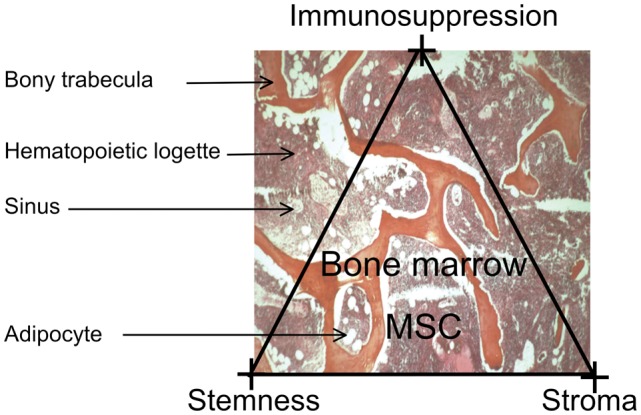
There is no precise information on the location of the MSCs in the marrow logettes; what is known is their progeny: osteoblasts, abluminal cells in the marrow sinuses and adipocytes. The 3 major properties of the MSC are emphasized: stemness, stromal capacity (stroma) and immunosuppressive potential (immunosuppression).
5. A step further: are MSCs pluripotent cells?
In addition to the mesenchymal lineages described above, MSCs have been reported to give rise to endothelial cells (Oswald et al., 2004), skeletal and cardiac muscle cells (Makino et al., 1999; Rose et al., 2008; Schulze et al., 2005; Umezawa et al., 1992; Wakitani et al., 1995), neural cells (Cho et al., 2005; Tondreau et al., 2008; Tropel et al., 2006; Trzaska et al., 2007; Wislet-Gendebien et al., 2005), hepatocytes (reviews in (Prindull and Zipori, 2004; Snykers et al., 2009)), and epithelial cells (Phinney and Prockop, 2007; Spees et al., 2003).
These reports raise a number of critical issues: fusion in vivo, criteria for differentiation, reprogrammation, selection by non-standardized culture conditions of rare cell populations.
Some of the differentiations observed in vivo may be due to fusion with cells in contact with MSCs, which is the case, in one report, for hepatocytes, cardiomyocytes and Purkinje neurons (Alvarez-Dolado et al., 2003). Similar cases of fusion have been reported with other types of adult tissue stem cells, primarily HSCs.
Criteria for differentiation need to be rigorously defined. It appears difficult to conclude as to a differentiation process from the detection of a number of markers without expression of the key transcription factors. For example, we have found that MSCs expressed cytoskeletal proteins usually expressed in neural stem cells (nestin), hepatocytes (cytokeratin 8 and 18), biliary cells (cytokeratin 19), sarcomeric muscle (troponins, alpha-C-actin), without expression of pro-neural or neuronal, pro-hepatocytic or myogenic key transcription factors (Delorme et al., 2009). Although cytoskeletal markers remain adequate indicators of a differentiation pathway, there are numerous exception to the rule, such as the expression of cytokeratin 18 in vascular smooth muscle cells in the synthetic phase. Similar misleading expression of factors has also been reported by other investigators (Montzka et al., 2009).
Some of the observed differentiations may result from reprogrammation. Dezawa et al have shown that rodent and human bone marrow MSCs can be reprogrammed into cells with skeletal muscle potential following a specific treatment comprising first cytokines and then gene transfer of the notch intracellular domain (Dezawa et al., 2005). The cells thus treated expressed the master skeletal transcription factors and were capable to regenerate muscle after transplantation in animal models of muscle injury. Remarkably, the cells could not be reprogrammed if the treatment sequence was reversed (gene transfer then cytokines administration). Similar reprogramming may take place when treating cells with DNA-demethylating agents such as 5-azacytidine, a procedure often used to induce sarcomeric muscle differentiation (Makino et al., 1999; Wakitani et al., 1995), and delivery of key transcription factors has also been used to reprogram MSCs into neural cells, pancreatic beta cells and endothelial cells (review in (Barzilay et al., 2009)).
When using standardized culture conditions set up within the European FP6 research program “Genostem”, we have generated human bone marrow MSCs whose differentiation lineages were restricted to the four lineages usually characterizing MSCs (Charbord et al.). This does not preclude the obtention by other culture methods of rarer population of cells with larger differentiation potential. Many factors may contribute: extensive screening of the serum batch, strict control of the degree of confluency, oxygen tension, stress such long-term trypsin incubation…
Some of the amplification protocols may select for MSCs of neuroectodermal origin that can differentiate into neural cells. There are many arguments indicating that some MSCs may derive from the neuroectodermal layer. Cell tracking studies have shown a long while ago that the connective tissue above the aortic arch derives from the neuroectoderm. Neural crest stem cells generated from embryonic stem cells can give rise under appropriate conditions to vascular smooth muscle cells, adipocytes, osteoblasts and chondrocytes (Lee et al., 2007). The neuroepithelium supplies the earliest wave of MSC differentiation in the mouse embryo (Takashima et al., 2007). Finally, few mouse bone marrow MSCs were proven to be neuroectodermal in origin (Morikawa et al., 2009; Nagoshi et al., 2008). Whether anti-NGFR/CD271 or anti-GD2 antibodies select for these neuroectodermal precursors in human bone marrow remains an open question (Martinez et al., 2007; Quirici et al., 2002).
Another population that may be selected under special conditions is that of pluripotent cells akin to embryonic stem cells with regard to their differentiation potential and even gene expression (Beltrami et al., 2007; Greco et al., 2007; Jiang et al., 2002; Kuroda et al., 2010). This is the case for MAPCs giving rise to mesodermal (including mesenchymal), neuroectodermal and endodermal lineages. Some strains express the telomerase and OCT4/POU5F1, a transcription factor essential for maintenance of pluripotency in embryonic stem cells. Other investigators have reported the expression in bone marrow MSCs of the pluripotency core transcription factors OCT4, NANOG and SOX2, and have found similar regulatory circuitries for OCT4 in MSCs and embryonic stem cells (Greco et al., 2007).
Taken together, the data indicate that the culture procedure may select for cells with large differentiation potential. Some of the differentiations are compatible with the mesodermal origin of the MSCs while others fit with the well-evidenced neuroectodermal origin of a MSC subpopulation. Differentiation into cells of endodermal origin might indicate that MSCs have undergone a mesenchymal-to-epithelial transition (MET), an instance common during development, but less frequent in the adult (Prindull and Zipori, 2004). Indeed, such MET have been described in MSC-like cells generated from the fetal liver (Chagraoui et al., 2003; Dan et al., 2006; Inada et al., 2008). Finally, the generation of pluripotent cells might result from the selection of rare vestigial embryonic stem cells having homed to the bone marrow. Alternatively, this population might result from reprogrammation, similarly to what occurs for induced Pluripotent Cells from dermal fibroblasts (Takahashi and Yamanaka, 2006).
6. Senescence and transformation of MSCs
MSCs present a high capacity of proliferation in vitro. However, many data suggest that MSCs cannot proliferate beyond the Hayflick limit of approximately 50 population doublings (PDs). This limit usually takes into account the total number of cell divisions that had occurred in vivo and after culture initiation. This might explain why cells in culture become senescent and why senescence may occur earlier in cultures of older individuals.
Senescent MSCs are characterized by flat hypertrophic phenotype with accumulation of β-galactosidase+ cells. Several studies have pointed out a number of mechanisms responsible for senescence: karyotypic abnormabilities (Izadpanah et al., 2008), reduction in telomere length (Banfi et al., 2002; Baxter et al., 2004), induction of expression of the cyclin inhibitor p16INK4A (Lee et al., 2009; Shibata et al., 2007), activation of progerin, a mutant form of lamin A, and subsequent activation of the notch pathway (Scaffidi and Misteli, 2008), induction of microRNAs that may impinge on cell cycle factors (Wagner et al., 2008), hypermethylation of promoters of the polycomb complex target genes (Teschendorff et al., 2010), reduction of antioxidant production and decrease in actin microfilament formation dynamics (Kasper et al., 2009; Lee et al., 2009).
Telomerase is usually not detected in MSCs (Banfi et al., 2002; Guillot et al., 2007; Serakinci et al., 2008). Expression of telomerase has been shown to slow down or abrogate the process of senescence. Remarkably, while extending the lifespan beyond 50 PDs, it also maintains or increases the osteogenic and stromal potential of the cells (Kawano et al., 2003; Shi et al., 2002; Simonsen et al., 2002). Telomerase proved therefore capable to immortalize MSCs without the chromosomic instability and crises characteristic of transfection with oncogenes such as H-ras, T of SV-40 or E6/E7 of papilloma virus (Kawano et al., 2003).
However, gene transfer with telomerase may also lead to malignant transformation. This has been shown by Moustapha Kassem’s team, which observed in extensively passaged telomerase+ lines the deletion of p16INK4A/ARF locus, an activating mutation of KRAS, and expression of cancer germline antigens (Gjerstorff et al., 2009; Serakinci et al., 2004). These data indicate that cells transferred with telomerase are adequate model to study transformation and some of the differentiation pathways, but do not constitute an appropriate cell source for cell therapy.
Ex vivo amplification of MSCs may lead to clonal selection and subsequent malignant transformation. The frequency of this process appears to be high in mouse MSCs where extensive passaging leads to cytogenetic aberrations and sarcoma development (Aguilar et al., 2007; Miura et al., 2006; Tolar et al., 2007). Spontaneous transformation has also been reported in human MSCs even after the cells had reached the post-senescent phase (Rosland et al., 2009; Rubio et al., 2005). However, a recent report suggests that the transformation reported in (Rubio et al., 2005) was an artifact resulting from the contamination with another tumor epithelial cell line (Garcia et al., 2010). Moreover, karyotypic abnormabilities are not consistently observed in transformed cells and, on the contrary, aneuploidy does not implicate that a malignant clone is generated (Tarte et al., 2010).
This latter conclusion does not rule out concerns on potential transformation. Strong arguments indicate that the fusion gene EWS-FLI1, resulting from the translocation of the EWS DNA-binding domain with the ets gene FLI1, may induce the transformation of MSCs into Ewing’s sarcoma cells (Burns et al., 2008; Kauer et al., 2009; Tirode et al., 2007). Tirode et al have shown that the profile of Ewing’s sarcoma lines converged to that of normal MSCs after EWS-FLI1 abrogation. Moreover, silenced lines could recover part of their differention potential, primarily toward adipocytes and osteoblasts. A population of CD133+ tumor cells appears to constitute the Ewing’s sarcoma stem cells since able to generate the tumor in serial transplantations in immunodeficient mice (Suva et al., 2009). Remarkably, the CD133+ cell population retains the mesenchymal differentiation potential and expresses higher levels of OCT4/POU5F1 and NANOG than the CD133− subset. Other data have indicated that Ewing’s sarcoma cells express neural markers, which is compatible with the transformation of neuroectodermally-derived MSCs or might result from EWS-FLI1-driven neuroectodermal differentiation in transformed MSCs (Riggi et al., 2009). Finally, transformed MSCs, either spontaneously or after transfer of the telomerase gene, express the CD99 antigen characteristic of Ewing’s sarcoma cells (Burns et al., 2008; Rosland et al., 2009).
Ewing’s sarcomas are not the only cancer type that may be generated by MSCs (Tang et al., 2008). In particular, the fusion gene SYT-SSX1 induces in transduced MSCs a transcriptomic profile similar to that of synovial sarcoma (Cironi et al., 2009). It has also been reported that some gastric cancers may originate from MSCs (Houghton et al., 2004); in these cases MSCs might have fused with gastric mucosa cells infected with helicobacter pilori.
As for senescence, the mechanisms of transformation appear to be diverse: induction of telomerase, deletion of CDKN2A/p16INK4A and activating mutation of KRAS genes (Burns et al., 2008; Miura et al., 2006; Rosland et al., 2009; Serakinci et al., 2004), loss of p53 in the absence or not of the cyclin inhibitor CDKN1A/p21CIP1/WAF1 (Armesilla-Diaz et al., 2009; Rodriguez et al., 2009). Remarkably, similar mechanisms are implicated in the induction of pluripotent cells, undescoring the relationship between reprogrammation, aging and cancer (Krizhanovsky and Lowe, 2009).
Taken together, the data suggest that MSCs are able to transform into malignant cells. Sarcomas of diverse types may represent for mesenchyme what leukemias represent for hematopoiesis. Target cells in each system may range from most immature cells (MSCs or HSCs) to mesenchymal or hematopoietic cells at different stages of differentiation.
7. Future considerations
A systems biology approach need to be taken to understand the dynamics of the stem cell attributes in MSCs. In particular, the molecular basis for plasticity remains poorly understood. Plasticity may be related to lineage priming, since differentiation in the primed lineages would not entail the set up of whole molecular program but the upregulation of only a few of the program components. Moreover, lineage priming indicate that promoters of the key transcription factors are in an open configuration, and therefore accessible to certain chromatin remodellers/transcriptional coactivators such as SETDB1 (Takada et al., 2007) or TAZ (Hong et al., 2005) that modify the balance in favor of one or the other pathway. Further studies on such molecules, studies of the epigenetic status of the promoters of key transcription factors before and during differentiation (such as reported in (Noer et al., 2009) for adipose-derived stem cells), and development of adequate biomathematical models are required at this step.
It is essential to understand in each clinical setting the precise mechanism of repair in order to optimize the procedure of administration (route, schedule, dose, pretreatment with cytokines of chemokines…). It is also critical to standardize amplification procedures such that cells with similar properties are delivered to each patient, and to make possible the comparison of clinical results. The multiple characteristics of the MSCs accounts for the versatility of the mechanisms of injured tissue repair (Phinney and Prockop, 2007). Unlike HSCs, it is only in a few situations that regeneration by repopulation and differentiation is taking place, e.g. MSCs implanted in bone or cartilage lesions may repair by direct differentiation into osteoblasts or chondrocytes. In other disease models, including vascular diseases, cardiac infarcts or immune diseases, benefit from MSC injection might result from the secretion of cytokines that would induce the proliferation and/or differentiation of nearby differentiated cells or resident stem cells. Alternatively, because of the known immunomodulatory properties of MSCs, MSC-dependent repair might be secondary to impairment of migration and proliferation or increased apoptosis of inflammatory cells. Finally, it remains possible that reprogrammation occurs in certain situations, following fusion with local cells, or due to a specific cytokinic context of the diseased/injured tissue.
Finally, one must not overlook the potential risks of MSC administration. The phenotypic instability related to plasticity raises concerns on the outcome of transplanted cells that may undergo unwanted and deleterious differentiation after implantation/engraftment at specific sites (Breitbach et al., 2007). The transformation potential is also a major concern. The mechanisms of transformation need to be understood (Richter et al., 2009; Riggi et al., 2010) so as to define strict release criteria for culture-amplified cells with minimal risk of transformation.
In conclusion, bone marrow MSCs constitute a specific adult tissue stem cell population. Although MSC administration may be extremely useful in a number of clinical applications, their transplantation is not without risks that must not be overlooked when developing cell therapy protocols.
Acknowledgments
Work supported by the European Community (Key action 1.2.4-3 Integrated Project “Genostem”, contract N° 503161 and by a grant from Institut National du Cancer (INCA project “Bortes”).
References
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, Stamp G, Bonnet D, Janes SM. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25:1586–1594. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Armesilla-Diaz A, Elvira G, Silva A. p53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp Cell Res. 2009;315:3598–3610. doi: 10.1016/j.yexcr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi A, Bianchi G, Notaro R, Luzzatto L, Cancedda R, Quarto R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 2002;8:901–910. doi: 10.1089/107632702320934001. [DOI] [PubMed] [Google Scholar]
- Barzilay R, Melamed E, Offen D. Introducing transcription factors to multipotent mesenchymal stem cells: making transdifferentiation possible. Stem Cells. 2009;27:2509–2515. doi: 10.1002/stem.172. [DOI] [PubMed] [Google Scholar]
- Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci U S A. 2003;100:5840–5845. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- Burns JS, Abdallah BM, Schroder HD, Kassem M. The histopathology of a human mesenchymal stem cell experimental tumor model: support for an hMSC origin for Ewing’s sarcoma? Histol Histopathol. 2008;23:1229–1240. doi: 10.14670/HH-23.1229. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Chagraoui J, Lepage-Noll A, Anjo A, Uzan G, Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003;101:2973–2982. doi: 10.1182/blood-2002-05-1341. [DOI] [PubMed] [Google Scholar]
- Charbord P. Mediators involved in the control of hematopoiesis by the microenvironment. In: Zon L, editor. Hematopoiesis: a developmental approach. Oxford University Press; 2001. pp. 702–717. [Google Scholar]
- Charbord P. Stromal support of hematopoiesis. In: Sell S, editor. Stem Cells Handbook. Totowa, NJ: Humana Press; 2004. pp. 143–154. [Google Scholar]
- Charbord P, Livne E, Gross G, Haupl T, Neves NM, Marie P, Bianco P, Jorgensen C. Human Bone Marrow Mesenchymal Stem Cells: A Systematic Reappraisal Via the Genostem Experience. Stem Cell Rev. 2010 doi: 10.1007/s12015-010-9125-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbord P, Tavian M, Humeau L, Peault B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood. 1996;87:4109–4119. [PubMed] [Google Scholar]
- Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye JH, Rameshwar P. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1 alpha. Stem Cells. 2005;23:383–391. doi: 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- Cironi L, Provero P, Riggi N, Janiszewska M, Suva D, Suva ML, Kindler V, Stamenkovic I. Epigenetic features of human mesenchymal stem cells determine their permissiveness for induction of relevant transcriptional changes by SYT-SSX1. PLoS One. 2009;4:e7904. doi: 10.1371/journal.pone.0007904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, Fausto N. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci U S A. 2006;103:9912–9917. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, Rosset P, Sensebe L, Layrolle P, Haupl T, Charbord P. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- Delorme B, Ringe J, Pontikoglou C, Gaillard J, Langonne A, Sensebe L, Noel D, Jorgensen C, Haupl T, Charbord P. Specific Lineage-Priming of Bone Marrow Mesenchymal Stem Cells Provides the Molecular Framework for Their Plasticity. Stem Cells. 2009;27:1142–1151. doi: 10.1002/stem.34. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamaziere JM, Couffinhal T, Duplaa C. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells. 2008;26:2991–3001. doi: 10.1634/stemcells.2008-0372. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82:66–76. [PubMed] [Google Scholar]
- Garcia S, Bernad A, Martin MC, Cigudosa JC, Garcia-Castro J, de la Fuente R. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res. 2010;316:1648–1650. doi: 10.1016/j.yexcr.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Gartner S, Kaplan HS. Long-term culture of human bone marrow cells. Proc Natl Acad Sci U S A. 1980;77:4756–4759. doi: 10.1073/pnas.77.8.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstorff M, Burns JS, Nielsen O, Kassem M, Ditzel H. Epigenetic modulation of cancer-germline antigen gene expression in tumorigenic human mesenchymal stem cells: implications for cancer therapy. Am J Pathol. 2009;175:314–323. doi: 10.2353/ajpath.2009.080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94:258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, Gross G, Gazit D. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940–952. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, Enver T. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 1997;11:774–785. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- Huang S. Reprogramming cell fates: reconciling rarity with robustness. Bioessays. 2009;31:546–560. doi: 10.1002/bies.200800189. [DOI] [PubMed] [Google Scholar]
- Huang S, Guo YP, May G, Enver T. Bifurcation dynamics in lineage-commitment in bipotent progenitor cells. Dev Biol. 2007;305:695–713. doi: 10.1016/j.ydbio.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Huang S, Terstappen LW. Formation of haematopoietic microenvironment and haematopoietic stem cells from single human bone marrow stem cells. Nature. 1994;368:664. doi: 10.1038/368664a0. [DOI] [PubMed] [Google Scholar]
- Inada M, Follenzi A, Cheng K, Surana M, Joseph B, Benten D, Bandi S, Qian H, Gupta S. Phenotype reversion in fetal human liver epithelial cells identifies the role of an intermediate meso-endodermal stage before hepatic maturation. J Cell Sci. 2008;121:1002–1013. doi: 10.1242/jcs.019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–4238. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Bae YC, Jung JS, Kim JH. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci. 2006;119:4994–5005. doi: 10.1242/jcs.03281. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- Kaern M, Elston TC, Blake WJ, Collins JJ. Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet. 2005;6:451–464. doi: 10.1038/nrg1615. [DOI] [PubMed] [Google Scholar]
- Kashiwakura Y, Katoh Y, Tamayose K, Konishi H, Takaya N, Yuhara S, Yamada M, Sugimoto K, Daida H. Isolation of bone marrow stromal cell-derived smooth muscle cells by a human SM22alpha promoter: in vitro differentiation of putative smooth muscle progenitor cells of bone marrow. Circulation. 2003;107:2078–2081. doi: 10.1161/01.CIR.0000070082.64414.B5. [DOI] [PubMed] [Google Scholar]
- Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kuhnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27:1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- Kauer M, Ban J, Kofler R, Walker B, Davis S, Meltzer P, Kovar H. A molecular function map of Ewing’s sarcoma. PLoS One. 2009;4:e5415. doi: 10.1371/journal.pone.0005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kobune M, Yamaguchi M, Nakamura K, Ito Y, Sasaki K, Takahashi S, Nakamura T, Chiba H, Sato T, Matsunaga T, Azuma H, Ikebuchi K, Ikeda H, Kato J, Niitsu Y, Hamada H. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood. 2003;101:532–540. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- Kim MR, Jeon ES, Kim YM, Lee JS, Kim JH. Thromboxane A2 Induces Differentiation of Human Mesenchymal Stem Cells to Smooth Muscle-Like Cells. Stem Cells. 2008;27:191–199. doi: 10.1634/stemcells.2008-0363. [DOI] [PubMed] [Google Scholar]
- Kirkland MA. A phase space model of hemopoiesis and the concept of stem cell renewal. Exp Hematol. 2004;32:511–519. doi: 10.1016/j.exphem.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Yasu T, Ueba H, Sata M, Hashimoto S, Kuroki M, Saito M, Kawakami M. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol. 2004;32:1238–1245. doi: 10.1016/j.exphem.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- Krinner A, Hoffmann M, Loeffler M, Drasdo D, Galle J. Individual fates of mesenchymal stem cells in vitro. BMC Syst Biol. 2010;4:73. doi: 10.1186/1752-0509-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Lowe SW. Stem cells: The promises and perils of p53. Nature. 2009;460:1085–1086. doi: 10.1038/4601085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Nabeshima Y, Fujiyoshi Y, Dezawa M. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer DV, Li S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28:734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee MO, Moon BH, Shim SH, Fornace AJ, Jr, Cha HJ. Senescent growth arrest in mesenchymal stem cells is bypassed by Wip1-mediated downregulation of intrinsic stress signaling pathways. Stem Cells. 2009;27:1963–1975. doi: 10.1002/stem.121. [DOI] [PubMed] [Google Scholar]
- Li F, Wang X, Niyibizi C. Distribution of single-cell expanded marrow derived progenitors in a developing mouse model of osteogenesis imperfecta following systemic transplantation. Stem Cells. 2007;25:3183–3193. doi: 10.1634/stemcells.2007-0466. [DOI] [PubMed] [Google Scholar]
- Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp Hematol. 1981;9:391–410. [PubMed] [Google Scholar]
- Loeffler M, Potten C. Stem cells and cellular pedigrees- a conceptual introduction. In: Potten C, editor. Stem Cells. San Diego: Academic Press; 1997. pp. 1–28. [Google Scholar]
- Loeffler M, Roeder I. Tissue stem cells: definition, plasticity, heterogeneity, self-organization and models--a conceptual approach. Cells Tissues Organs. 2002;171:8–26. doi: 10.1159/000057688. [DOI] [PubMed] [Google Scholar]
- Loeffler M, Roeder I. Conceptual models to understand tissue stem cell organization. Curr Opin Hematol. 2004;11:81–87. doi: 10.1097/01.moh.0000133648.83991.af. [DOI] [PubMed] [Google Scholar]
- Long MW, Williams JL, Mann KG. Expression of human bone-related proteins in the hematopoietic microenvironment. J Clin Invest. 1990;86:1387–1395. doi: 10.1172/JCI114852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez P, Catalina P, Rodriguez R, Melen GJ, Bueno C, Arriero M, Garcia-Sanchez F, Lassaletta A, Garcia-Sanz R, Garcia-Castro J. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J Exp Med. 2009;206:3131–3141. doi: 10.1084/jem.20091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- Montzka K, Lassonczyk N, Tschoke B, Neuss S, Fuhrmann T, Franzen R, Smeets R, Brook GA, Woltje M. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: misleading marker gene expression. BMC Neurosci. 2009;10:16. doi: 10.1186/1471-2202-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Morad V, Pevsner-Fischer M, Barnees S, Samokovlisky A, Rousso-Noori L, Rosenfeld R, Zipori D. The myelopoietic supportive capacity of mesenchymal stromal cells is uncoupled from multipotency and is influenced by lineage determination and interference with glycosylation. Stem Cells. 2008;26:2275–2286. doi: 10.1634/stemcells.2007-0518. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, Matsuzaki Y. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun. 2009;379:1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, Kato S, Ito M, Hotta T, Ando K. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Noer A, Lindeman LC, Collas P. Histone H3 modifications associated with differentiation and long-term culture of mesenchymal adipose stem cells. Stem Cells Dev. 2009;18:725–736. doi: 10.1089/scd.2008.0189. [DOI] [PubMed] [Google Scholar]
- Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108:2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- Owen ME, Cave J, Joyner CJ. Clonal analysis in vitro of osteogenic differentiation of marrow CFU-F. J Cell Sci. 1987;87(Pt 5):731–738. doi: 10.1242/jcs.87.5.731. [DOI] [PubMed] [Google Scholar]
- Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prindull G, Zipori D. Environmental guidance of normal and tumor cell plasticity: epithelial mesenchymal transitions as a paradigm. Blood. 2004;103:2892–2899. doi: 10.1182/blood-2003-08-2807. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- Remy-Martin JP, Marandin A, Challier B, Bernard G, Deschaseaux M, Herve P, Wei Y, Tsuji T, Auerbach R, Dennis JE, Moore KA, Greenberger JS, Charbord P. Vascular smooth muscle differentiation of murine stroma: a sequential model. Exp Hematol. 1999;27:1782–1795. doi: 10.1016/s0301-472x(99)00122-8. [DOI] [PubMed] [Google Scholar]
- Richter GH, Plehm S, Fasan A, Rossler S, Unland R, Bennani-Baiti IM, Hotfilder M, Lowel D, von Luettichau I, Mossbrugger I, Quintanilla-Martinez L, Kovar H, Staege MS, Muller-Tidow C, Burdach S. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci U S A. 2009;106:5324–5329. doi: 10.1073/pnas.0810759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggi N, Suva ML, De Vito C, Provero P, Stehle JC, Baumer K, Cironi L, Janiszewska M, Petricevic T, Suva D, Tercier S, Joseph JM, Guillou L, Stamenkovic I. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24:916–932. doi: 10.1101/gad.1899710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggi N, Suva ML, Stamenkovic I. Ewing’s sarcoma origin: from duel to duality. Expert Rev Anticancer Ther. 2009;9:1025–1030. doi: 10.1586/era.09.81. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Rubio R, Masip M, Catalina P, Nieto A, de la Cueva T, Arriero M, San Martin N, de la Cueva E, Balomenos D, Menendez P, Garcia-Castro J. Loss of p53 induces tumorigenesis in p21-deficient mesenchymal stem cells. Neoplasia. 2009;11:397–407. doi: 10.1593/neo.81620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, Keating SC, Parker TG, Backx PH, Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lonning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Graff A, Skalli O, Gabbiani G. Alpha-smooth muscle actin is expressed in a subset of bone marrow stromal cells in normal and pathological conditions. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57:291–302. doi: 10.1007/BF02899094. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schulze M, Belema-Bedada F, Technau A, Braun T. Mesenchymal stem cells are recruited to striated muscle by NFAT/IL-4-mediated cell fusion. Genes Dev. 2005;19:1787–1798. doi: 10.1101/gad.339305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini M, Dylla SJ, Oki M, Heremans Y, Tolar J, Jiang Y, Buckley SM, Pelacho B, Burns TC, Frommer S, Rossi DJ, Bryder D, Panoskaltsis-Mortari A, O’Shaughnessy MJ, Nelson-Holte M, Fine GC, Weissman IL, Blazar BR, Verfaillie CM. Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells. J Exp Med. 2007;204:129–139. doi: 10.1084/jem.20061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serakinci N, Graakjaer J, Kolvraa S. Telomere stability and telomerase in mesenchymal stem cells. Biochimie. 2008;90:33–40. doi: 10.1016/j.biochi.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T, Kassem M. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23:5095–5098. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- Shibata KR, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M, Kohno Y, Ito K, Fujibayashi S, Neo M, Nakayama T, Nakamura T, Toguchida J. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007;25:2371–2382. doi: 10.1634/stemcells.2007-0225. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG, Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- Sims DE. The pericyte--a review. Tissue Cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Singer JW, Charbord P, Keating A, Nemunaitis J, Raugi G, Wight TN, Lopez JA, Roth GJ, Dow LW, Fialkow PJ. Simian virus 40-transformed adherent cells from human long-term marrow cultures: cloned cell lines produce cells with stromal and hematopoietic characteristics. Blood. 1987;70:464–474. [PubMed] [Google Scholar]
- Snykers S, De Kock J, Rogiers V, Vanhaecke T. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577–605. doi: 10.1634/stemcells.2008-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. Faseb J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- Song L, Webb NE, Song Y, Tuan RS. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells. 2006;24:1707–1718. doi: 10.1634/stemcells.2005-0604. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suva D, Clement V, Provero P, Cironi L, Osterheld MC, Guillou L, Stamenkovic I. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69:1776–1781. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]
- Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9:1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M, Dulong J, Monnier D, Gourmelon P, Gorin NC, Sensebe L. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549–1553. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- Teschendorff AE, Menon U, Gentry-Maharaj A, Ramus SJ, Weisenberger DJ, Shen H, Campan M, Noushmehr H, Bell CG, Maxwell AP, Savage DA, Mueller-Holzner E, Marth C, Kocjan G, Gayther SA, Jones A, Beck S, Wagner W, Laird PW, Jacobs IJ, Widschwendter M. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 2010;20:440–446. doi: 10.1101/gr.103606.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- Tondreau T, Dejeneffe M, Meuleman N, Stamatopoulos B, Delforge A, Martiat P, Bron D, Lagneaux L. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics. 2008;9:166. doi: 10.1186/1471-2164-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentin J. Hematopoietic microenironments. Historical perspectives, status and projections. In: Tavassoli M, editor. Handbook of the Hemopoietic Microenvironment. Clifton, NJ: Humana Press; 1989. pp. 1–87. [Google Scholar]
- Tropel P, Platet N, Platel JC, Noel D, Albrieux M, Benabid AL, Berger F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- Trzaska KA, Kuzhikandathil EV, Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007;25:2797–2808. doi: 10.1634/stemcells.2007-0212. [DOI] [PubMed] [Google Scholar]
- Umezawa A, Maruyama T, Segawa K, Shadduck RK, Waheed A, Hata J. Multipotent marrow stromal cell line is able to induce hematopoiesis in vivo. J Cell Physiol. 1992;151:197–205. doi: 10.1002/jcp.1041510125. [DOI] [PubMed] [Google Scholar]
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Westen H, Bainton DF. Association of alkaline-phosphatase-positive reticulum cells in bone marrow with granulocytic precursors. J Exp Med. 1979;150:919–937. doi: 10.1084/jem.150.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- Zipori D. Cultured stromal cell lines from hemopoietic tissues. In: Tavassoli M, editor. Handbook of the Hemopoietic Microenvironment. Clifton, NJ: Humana Press; 1989. pp. 287–333. [Google Scholar]
- Zipori D. The stem state: plasticity is essential, whereas self-renewal and hierarchy are optional. Stem Cells. 2005;23:719–726. doi: 10.1634/stemcells.2005-0030. [DOI] [PubMed] [Google Scholar]


