Abstract
Sexually selected male ejaculate traits are expected to depend on the resource state of males. Theory predicts that males in good condition will produce larger ejaculates, but that ejaculate composition will depend on the relative production costs of ejaculate components and the risk of sperm competition experienced by low- and high-condition males. Under some conditions, when low condition leads to poorer performance in sperm competition, males in low condition may produce ejaculates with higher sperm content relative to their total ejaculate and may even transfer more sperm than high-condition males in an absolute sense. Previous studies in insects have shown that males in good condition transfer larger ejaculates or more sperm, but it has not been clear whether increased sperm content represents a shift in allocation or simply a larger ejaculate, and thus the condition dependence of ejaculate composition has been largely untested. We examined condition dependence in ejaculate by manipulating adult male condition in a ladybird beetle (Adalia bipunctata) in which males transfer three distinct ejaculate components during mating: sperm, non-sperm ejaculate retained within the female reproductive tract, and a spermatophore capsule that females eject and ingest following mating. We found that high condition males indeed transferred larger ejaculates, potentially achieved by an increased rate of ejaculate transfer, and allocated less to sperm compared with low-condition males. Low-condition males transferred ejaculates with absolutely and proportionally more sperm. This study provides the first experimental evidence for a condition-dependent shift in ejaculate composition.
Keywords: sexual selection, sperm, seminal proteins, condition dependence, ingested ejaculate, Adalia bipunctata
1. Introduction
A recurrent prediction for sexually selected traits is that they should exhibit heightened condition dependence relative to other traits (e.g. Alatalo et al. 1988; Rowe & Houle 1996; Cotton et al. 2004; Bonduriansky & Rowe 2005). This expectation is based on the assumption that individuals in good condition (i.e. with larger pools of resources available to allocate) have higher marginal benefits from increased investment in the expression and maintenance of sexually selected traits (Iwasa et al. 1991; Rowe & Houle 1996; Proulx et al. 2002; Getty 2006). The extent of condition dependence in sexually selected traits is a key issue for sexual selection research (Zahavi 1975; Iwasa & Pomiankowski 1994; Rowe & Houle 1996). Following a series of environmental manipulations of condition, there is now considerable empirical evidence for condition dependence in several types of sexually selected traits, including secondary sexual morphology, ornaments and pigmentation, and acoustic signals (Andersson 1994; Cotton et al. 2004 and references therein; Bonduriansky & Rowe 2005; Punzalan et al. 2008). Recent evidence suggests that male ejaculates are also sexually selected (Eberhard & Cordero 1995; Ramm et al. 2007; Simmons & Kotiaho 2007; Martin-Coello et al. 2009; Wigby et al. 2009) and costly (e.g. Dewsbury 1982). However, the extent of condition dependence in ejaculate traits is currently less well studied than these other sets of traits.
Whenever high-condition males have lower marginal costs of ejaculate production than low-condition males, they are expected to transfer larger ejaculates at mating (even as they invest less reproductive effort per ejaculation; Parker 1990; Tazzyman et al. 2009). Empirical studies involving experimental manipulations of condition generally support this prediction, reporting that high-condition males produce larger ejaculates (Gwynne 1990; Delisle & Bouchard 1995; Watanabe & Hirota 1999; Jia et al. 2000; Ferkau & Fisher 2006; Lewis & Wedell 2007; Blanco et al. 2009; but see Wedell 1993), transfer more sperm (Fedina & Lewis 2006; McGraw et al. 2007; Perez-Staples et al. 2008), and produce more ejaculate-derived nuptial gifts (Jia et al. 2000; but see Wedell 1993).
Although current theory does not directly address condition dependence in ejaculate composition (the relative allocation to its components), there are two mechanisms by which condition might affect composition. First, if production costs (or benefits) vary among ejaculate components, more costly components should be disproportionately condition-dependent and present in greater concentrations in the ejaculates of high-condition males. A second mechanism links condition dependence to sperm competition theory, which predicts that under some conditions, males that are disfavoured in sperm competition should invest more in sperm relative to their overall ejaculate expenditure, and in many cases should transfer more sperm (in an absolute sense) than favoured males (Cameron et al. 2007). One scenario in which this is expected is in those cases where investment in non-sperm components of the ejaculate tends to elevate short-term egg production. In some species, low-condition males may be consistently disfavoured in sperm competition (e.g. when female re-mating rate is elevated after matings with low-condition males, Chapman et al. 2003; Pitcher et al. 2003; or when females bias sperm storage towards high quality males, Vermeulen et al. 2008). Although previous studies have reported increased sperm transfer by high-condition males (referenced above), it is generally not clear whether this represents an increased allocation to sperm relative to non-sperm ejaculate components or an increase in total ejaculate, or even a decreased allocation to sperm if non-sperm components increase more than sperm. Consequently, the degree of condition dependence in ejaculate composition remains largely unknown.
Here we investigate the condition dependence of ejaculate size and composition in the two-spot ladybird beetle Adalia bipunctata. During copulation, male beetles transfer ejaculate via a spermatophore. Females eject and then ingest the emptied spermatophore capsule after copulation; notably, this ingestion causes an acceleration of egg production and a marked increase in female resistance to subsequent matings (Perry & Rowe 2008a). Thus, three distinct ejaculate components can be distinguished: the spermatophore capsule; sperm; and non-sperm seminal fluids that are retained within the female after mating (hereafter, ‘retained ejaculate’). We predict that (i) high-condition males will transfer a larger total ejaculate mass than low condition males (Parker 1990), and (ii) male condition will influence ejaculate composition. As discussed above, differences in composition could result from differential costliness of ejaculate components (unknown for these beetles) or from condition-dependent differences in the level of sperm competition. The latter may arise, for example, if production of the spermatophore capsule itself is condition-dependent: reduced capsule production should increase female re-mating (Perry & Rowe 2008a) and lead to increased sperm competition for low-condition males; if so, those low-condition males should increase the sperm content of their ejaculates (Cameron et al. 2007).
2. Material and Methods
(a). Experimental animals
Adalia bipunctata are aphid predators with a broad Holarctic distribution. Both males and females mate multiply (field estimates: 2–4 matings per female; Haddrill et al. 2008), with copulations lasting up to 6 h. We have never observed sperm transfer in copulations lasting less than 30 min, and in this study we excluded these shorter matings from analysis. Both mating and ejaculate production appear costly for male A. bipunctata: males are limited in their ability to produce sequential ejaculates and males that mate a single time have reduced survival compared with non-mating males (J. C. Perry & C. Tse 2008, unpublished data). The ladybirds used in these experiments were of the second and third generation reared in our laboratory from a founding population obtained from a biocontrol company (Natural Insect Control, Stevensville, Ontario, Canada). Ladybirds were maintained on pea aphids (Acyrthosiphon pisum, reared on broad bean, Vicia faba) and eggs of the Mediterranean flour moth (Ephestia kuehniella), a standard artificial diet (de Clerq et al. 2005).
(b). Experimental design
To investigate the effect of male condition on ejaculates, we randomly assigned adult males to a high- or low-food treatment for several days prior to assaying ejaculate size, composition and sperm content. Condition, or an organism's accumulated resources for allocation, is expected to have both genetic and environmental components (Rowe & Houle 1996), and food level treatments are commonly used to manipulate the environmental component (reviewed by Cotton et al. 2004). We expect that, on average, males assigned to the low-food treatment will have accumulated fewer resources for allocation to ejaculate traits than males assigned to high food.
Each male was mated once to a female from the stock population before the feeding treatment began. The experiment was replicated three times. In the first replicate, the feeding treatment consisted of adult pea aphids provided daily for 9 days at low (one aphid) or ad libitum levels, while in subsequent replicates males were fed flour moth eggs at low (15–25 eggs) or ad libitum levels for 10 days. We confirmed that the food treatments differentially affected a measure of condition (mass gain). Initial male mass did not differ between the high- and low-food groups (8.83 mg ± 0.20 versus 8.91 ± 0.21, respectively; mixed model with the fixed factor ‘feeding treatment’ and the random factor ‘replicate’: F1,159 = 0.1, p = 0.75). However, within each replicate low-food males lost more weight, or gained less weight, than high-food males (mean ± s.e., first replicate: low-food males: −0.671 mg ± 0.074, high-food males: −0.172 mg ± 0.076, t38 = 4.7, p < 0.0001; second replicate: low: −0.090 mg ± 0.187, high: 0.866 mg ± 0.130, t44 = 4.2, p < 0.0001; third replicate: low: −1.242 mg ± 0.089, high: −0.085 ± 0.100, t75 = 8.4, p < 0.0001). Ten of 96 high-food males and 29 of 108 low-food males died before the experiment began.
To begin the mating trial, female beetles were placed individually into Petri dishes for an hour to acclimate before a low- or high-food male was introduced. Virgin females were used to minimize the possibility that females would reject low-condition males, as we have never observed mating resistance by reproductively mature virgins from this population. In the first and second replicates, we recorded whether mating occurred within 2 h, copulation duration, the time until spermatophore capsule ejection, and spermatophore capsule mass immediately after ejection. In the third replicate, we expanded our measurements to include male mass immediately before and after copulation, dry spermatophore capsule mass, sperm transfer, and male post-mating survival after an imposed physiological stressor (described below).
To obtain an estimate of the mass of retained ejaculate, we used the difference between male mass before and after mating and corrected this difference for the per minute mass loss experienced by non-mating males (J. C. Perry 2008, unpublished data: 1.587 µg min−1 ± 0.264; n = 14); finally, we subtracted the mass of the ejected spermatophore capsule. This ‘retained ejaculate’ mass probably reflects the non-sperm component of ejaculate because sperm contributes little to ejaculate mass in many species (Eberhard & Cordero 1995; Simmons 2001; Owen & Katz 2005). We determined the water content of spermatophore capsules by re-weighing them after 65 h in a drying oven (60°C) and subtracting this mass from initial wet mass. Females do not appear to eject additional ejaculate apart from the spermatophore capsule.
(i). Quantifying sperm transfer
To assess sperm transfer, we froze the experimental females (−20°C) 1 h after mating and quantified sperm from the dissected female reproductive tracts following Arnaud et al. (2003). We transferred the bursa copulatrix and spermatheca (the sites of sperm deposition and storage, Ransford 1997) and ovarian tubes to a cavity slide containing 100 µl Ringer's solution. We ruptured the reproductive tract, crushed the spermatheca, and teased apart the tissues with fine forceps. We then washed the solution from the slide with 2 ml Ringer's solution into a microcentrifuge tube and vortexed the solution for 2 min. We pipetted two 20 µl samples from each solution on to a glass slide and allowed them to dry under a dust cover. Sperm were counted under a dark field phase contrast microscope at 400× magnification. We summed the number of sperm detected in both samples and multiplied by the dilution factor to estimate the total number of sperm transferred. The number of sperm was highly repeatable between the two samples (r = 0.86, p < 0.0001).
(ii). Male post-mating survival
Following the mating trial, we maintained the experimental males on the assigned low- or high-food treatment and assayed their survival after exposure to a physiological stress. On the 6th day after the mating trial, each male was placed in a 1.5 ml microcentrifuge tube containing a small air hole and placed in a 40°C water bath for 1 h (stressful conditions for these beetles, Acar et al. 2005). Males were returned to room temperature and provided with 75 µl distilled water daily. Male survival was monitored three times daily and mortality was recorded when we could no longer provoke a response by gentle prodding.
(iii). Statistical analyses
We analysed categorical responses by χ2-tests and continuous responses by fitting mixed models with the fixed factor ‘feeding treatment’ and the random factor ‘replicate’ or by a one-way ANOVA for the responses measured in the third replicate only. For two responses—the mass of retained ejaculate and sperm number—we tested for an effect of copulation duration by an ANCOVA with feeding treatment, copulation duration and their interaction. We tested for an effect of the feeding treatment on male survival using a proportional hazards model. Non-significant interactions were dropped from the final models. We transformed continuous responses to meet the assumptions of parametric tests and conducted non-parametric Wilcoxon tests if no transformation could make the data compatible.
To assess the correlation structure among aspects of ejaculate and mating, we conducted two principal components analyses (PCAs). The first analysis included three variables which represented the most complete dataset: copulation duration, the mass of retained ejaculate and number of sperm transferred. The second analysis included these variables and spermatophore capsule mass, which was available for a subset of the data. We then examined how the composite variables generated by the PCA related to male post-mating survival by testing for a correlation between survival and the principal component scores, using linear regression. Because the results from both PCAs were very similar, we present the second analysis here and the initial PCA in the appendix (electronic supplementary material).
3. Results
(a). Univariate analyses
Male condition, as determined by a low- or high-food diet, significantly impacted male mating behaviour. Compared with males provided with plentiful food, low-food males were less likely to mate (80% versus 95%) and copulated for shorter periods when they did mate (table 1). We next detail how male condition impacted ejaculate composition and the quantity of each ejaculate component.
Table 1.
Mating behaviour, ejaculate variables and pre-mating mass of males assigned to a low- or high-food treatment prior to mating. Means are given with s.e., or confidence intervals where means are back-transformed; an exception is that medians are given for male post-mating survival in the last row. (Mixed models included the fixed factor ‘feeding treatment’ and the random factor ‘replicate’. Means for copulation duration are back-transformed from square-root. ‘Retained ejaculate’ refers to ejaculate retained within the female, as opposed to the ejected spermatophore capsule. We were unable to obtain sperm data for four low-food males.) Significant p-values are given in italic.
| response | male food treatment |
model | test statistic | p-value | |
|---|---|---|---|---|---|
| low food | high food | ||||
| male pre-mating mass | 8.27 mg ± 0.28 | 9.03 mg ± 0.28 | mixed model | F1,161 = 12.2 | <0.001 |
| likelihood of mating | 66/83 | 82/86 | chi-square | χ12 = 9.7 | < 0.01 |
| copulation duration | 99 min (82, 116) |
137 min (120, 157) |
mixed model | F1,144 = 17.4 | < 0.0001 |
| retained ejaculate | |||||
| mass as a proportion of male mass before mating | 0.029 ± 0.003 | 0.045 ± 0.003 | ANOVA | F1,75 = 12.3 | < 0.001 |
| rate of ejaculate transfer | 2.23 µg min−1 (1.59, 2.97) | 3.37 µg min−1 (2.48, 4.41) | ANOVA | F1,75 = 3.7 | 0.06 |
| sperm transfer | |||||
| likelihood of sperm transfer | 22/39 | 22/34 | chi-square | χ12 = 0.5 | 0.47 |
| rate of transfer (number of sperm per minute) | 0.74 ± 0.13 | 0.36 ± 0.12 | ANOVA | F1,26 = 4.7 | 0.04 |
| ejected spermatophore capsules | |||||
| likelihood of ejection | 38/63 | 63/82 | chi-square | χ12 = 5.8 | 0.02 |
| latency until ejection | 13 ± 3 min | 12 ± 2 min | Wilcoxon test | Z = 0.8 | 0.40 |
| mass as a proportion of total ejaculate mass | 0.16 ± 0.02 | 0.14 ± 0.02 | ANOVA | F1,40 = 0.9 | 0.36 |
| water content | 10.8 ± 1.5 µg | 6.4 ± 1.3 µg | ANOVA | F1,26 = 5.0 | 0.03 |
| male post-mating survival | 1610 min (1217, 2637) |
6670 min (5931, 7799) |
proportional hazards | χ12 =46.5 | < 0.0001 |
(i). Retained ejaculate
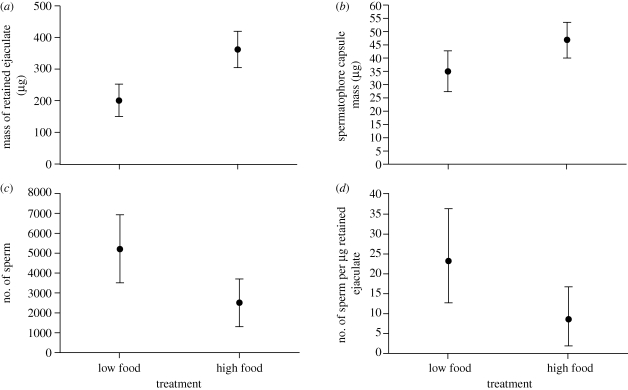
The portion of ejaculate retained within the female—i.e. the bulk of seminal fluids (see §2b)—was smaller following matings with low-food males compared with high-food males (F1,75 = 20.1, p < 0.0001, figure 1a). Furthermore, this retained ejaculate component represented a smaller fraction of the total body mass for low- compared with high-food males (table 1). However, retained ejaculate mass was not influenced by copulation duration (F1,73 = 0.1, p = 0.76) or by the interaction between copulation duration and the food treatment (F1,73 = 1.1, p = 0.30); these terms were therefore dropped from the final model. There was no significant difference in the rate of retained ejaculate transfer between low- and high-food males, though there was a trend of decreased transfer rate in low-food males (table 1).
Figure 1.
Mean effect of a low- or high-food treatment for males on allocation to ejaculate components. (a) mass of the ejaculate retained within the female after copulation; (b) mass of the spermatophore capsule ejected after mating; (c) number of sperm in the female reproductive tract after mating; (d) sperm concentration. 95% CI are indicated.
(ii). Sperm
Low- and high-food males were equally likely to transfer sperm, given a successful mating (table 1). However, when sperm transfer occurred, low-food males transferred over twice as much sperm as high-food males (F1,23 = 7.5, p = 0.01, figure 1c). Low-food males also transferred sperm at a higher rate, in contrast to the absence of a treatment effect on the transfer rate of the retained ejaculate component reported above (table 1).
There was little detectable effect of copulation duration on sperm transfer. Longer copulations did not increase the likelihood of sperm transfer; in fact, there was a weak negative relationship between copulation duration and the likelihood of sperm transfer (R2 =0.17, β = −0.02 ± 0.01, n = 73, χ12 = 16.9, p < 0.0001). Similarly, there was no correlation between copulation duration and sperm quantity (F1,22 = 0.9, p = 0.34), and no significant interaction effect on sperm quantity (F1,22 = 3.7, p = 0.07; no significant correlation between copulation duration and feeding treatment within either treatment group: low-food males: R2 = 0.14, β = 38.6 ± 32.0, p = 0.26; high-food males: R2 = 0.16, β = −12.6 ± 8.1, p = 0.15).
We controlled for a strong threshold relationship between spermatophore ejection latency and sperm transfer (electronic supplementary material, figure S1). When ejection latency exceeded 11 min, sperm transfer was very low or nil with only one exception; we therefore excluded cases where ejection latency exceeded this threshold.
(iii). The spermatophore capsule
Male condition significantly influenced both the size and composition of the spermatophore capsule. Females that mated with low-food males were less likely to eject a spermatophore capsule after mating (table 1); moreover, capsules ejected after matings with low-food males were smaller (F1,80 = 12.6, p < 0.001, figure 1b) and contained more water (31% versus 14%; table 1). However, both low- and high-food males transferred spermatophore capsules that represented a similar proportion of ejaculate mass (table 1).
Spermatophore capsule mass was not influenced by copulation duration (low-food males: R2 = 0, p = 0.81; high-food males: R2 = 0, p = 0.83) or by the latency until spermatophore ejection (low-food: R2 = 0.04, p = 0.30; high-food: R2 = 0, p = 0.89). Male condition did not influence the latency until spermatophore ejection (table 1). We excluded 17 spermatophores from analysis because they were partially consumed by females before we could remove them.
(iv). Ejaculate composition
There was a conspicuous shift in ejaculate composition between low- and high-food males. Specifically, low-food males transferred ejaculates that contained a higher concentration of sperm (a 2.5-fold increase, F1,23 = 4.5, p = 0.04; figure 1d). In contrast, high-food males transferred a higher concentration of non-sperm ejaculate components, indicated by their larger retained ejaculates (figure 1a); and as noted above, they transferred spermatophore capsules with decreased water content (table 1). However, the proportion of ejaculate made up of the spermatophore capsule was not sensitive to male condition (table 1).
(v). Male survival
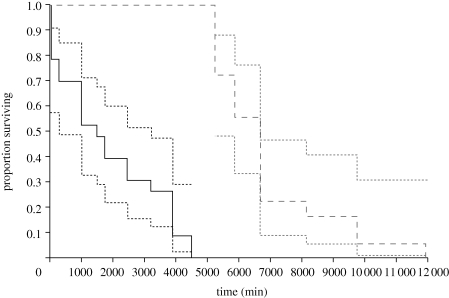
We obtained survival data for 22 low-condition males and 18 high-condition males. Improved male condition greatly increased male survival after exposure to a physiological stress (by over threefold; figure 2 and table 1), as would be expected if our low-food treatment stressed males.
Figure 2.
The effect of a low- or high-food treatment (solid and dashed lines, respectively) on male survival following exposure to a physiological stressor (40°C for 1 h), with confidence intervals indicated.
(b). Multivariate analyses
We used PCA to summarize the correlation structure among four variables: the mass of retained ejaculate, sperm transfer, spermatophore capsule mass and copulation duration. The first two principal components (PCs) from this analysis captured 65% of the variation (table 2). The first PC axis reflected positive correlations among all four aspects of responses, while the second axis captured a negative correlation between ejaculate mass and sperm transfer, indicating a change in the concentration of sperm that appears unrelated to copulation duration or spermatophore capsule mass (table 2).
Table 2.
Eigenvalues and eigenvectors for the principal components (PCs) from an analysis of male mating performance. Mass of retained ejaculate refers to ejaculate components retained by females after mating (e.g. seminal fluids).
| PC1 | PC2 | |
|---|---|---|
| eigenvalue | 1.6 | 1.0 |
| % variation explained | 38.9 | 26.1 |
| variable | ||
| mass of retained ejaculate | 0.355 | 0.785 |
| number of sperm transferred | 0.482 | −0.619 |
| spermatophore capsule mass | 0.590 | 0.001 |
| copulation duration | 0.541 | 0.035 |
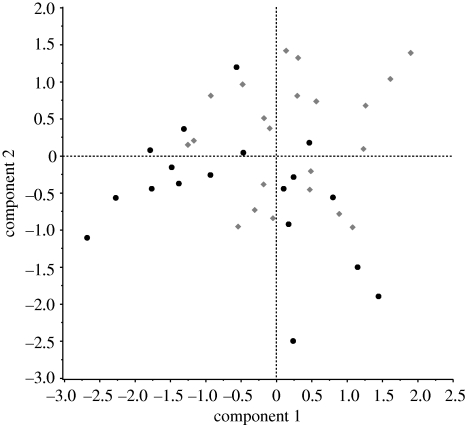
The male feeding treatment influenced both PC1 and PC2 (figure 3; PC1: F1,38 = 5.8, p = 0.02; PC2: F1,38 = 8.0, p < 0.01), but not PC3 or PC4 (PC3: F1,38 = 0.7, p = 0.40; PC4: F1,38 = 1.6, p = 0.21). Males in good condition had, on average, positive scores on PC1 (0.23 ± 0.22), indicating that they had high values for all four mating variables, while males in poor condition had negative PC1 scores on average (−0.55 ± 0.24), indicating decreased values for all variables. Similarly, males in good condition had on average positive scores on PC2 (0.24 ± 0.18) while poor condition males had negative scores (−0.50 ± 0.19); on this axis, though, these scores meant that high-condition males transferred ejaculates with a reduced concentration of sperm, and conversely poor condition males transferred ejaculates with a high sperm concentration (table 2). These results are in agreement with the univariate analyses above.
Figure 3.
Principal component scores for low- (circles) and high- (diamonds) food males, from an analysis summarizing variation among males in four mating variables: the mass of retained ejaculate, number of sperm transferred during mating, mass of the spermatophore capsule and copulation duration. Eigenvalues and eigenvectors are given in table 2.
We next investigated how male survival related to the composite of male mating responses described by PC1 and PC2. Male survival was positively correlated with PC1 scores (R2 = 0.28, n = 17, β = 1568 ± 647, p = 0.03), indicating an association between male survival and increased values for the four mating variables. In contrast, survival was not correlated with PC2 (R2 = 0.06, n = 17, β = 1052 ± 1114, p = 0.36).
4. Discussion
There is a growing appreciation for the potential influence of resource availability on ejaculate expenditure (Williams et al. 2005; Tazzyman et al. 2009). Our finding that ejaculate traits are differentially sensitive to male condition supports this emerging view. Males in good condition produced larger ejaculates, consistent with studies in other species (discussed below). However, males in poor condition strongly increased allocation to sperm despite decreased overall ejaculate size, representing a condition-dependent shift in ejaculate composition. Below, we discuss the evidence and implications for each of these findings, and examine two hypotheses for the mechanism underlying condition dependent ejaculate composition.
(a). Ejaculate size
Our finding that males in good condition produced larger non-sperm ejaculates than males in poor condition is consistent with both theory (Parker 1990) and previous results from two insect groups (Lepidoptera: Delisle & Bouchard 1995; Watanabe & Hirota 1999; Ferkau & Fisher 2006; Lewis & Wedell 2007; Blanco et al. 2009; Orthoptera: Jia et al. 2000, but see Wedell 1993). Although high-condition males had longer copulations than low-condition males, there was no evidence that longer copulations were the factor generating increased ejaculate transfer because copulation duration was not correlated with ejaculate mass. Instead, we found weak evidence that high-condition males transfer non-sperm ejaculate at a faster rate, although this difference was not statistically significant.
Relative to high-condition males, low-condition males decreased their investment in spermatophore capsules: their spermatophore capsules were smaller, and their mates were less likely to eject a capsule at all. Previous reports on the condition dependence of ingested ejaculate products are mixed, with high-condition males transferring larger ejaculate gifts in some insects (Jia et al. 2000; Lehmann & Lehmann 2009) but not others (Tuckerman et al. 1993; Wedell 1993; Simmons et al. 1999). For A. bipunctata, the reduction in spermatophore capsule ejection, and thus in capsule ingestion by females, probably did not represent a loss of nutrition for females because spermatophore capsules transfer little or no nutrition and do not improve female fitness (Perry & Rowe 2008a,b; Perry et al. 2009). Instead, ingesting spermatophores increases female re-mating resistance and accelerates short-term egg production (Perry & Rowe 2008a). The first factor will put low-condition males at a disadvantage in sperm competition through re-mating, and the second factor will lead to a reduced number of eggs that they are in competition for.
(b). Ejaculate composition
We found evidence for three condition-dependent changes in ejaculate composition. Most notably, low-condition males transferred ejaculates that were higher in absolute sperm content and in sperm concentration, compared with high-condition males. Low-condition males transferred more sperm despite their reduced copulation duration by transferring sperm at a faster rate than high-condition males, and this occurred despite the trend that high-condition males transferred retained (non-sperm) ejaculate at a faster rate (table 1). In contrast to our result, previous experimental studies in flies and flour beetles have found that improved male condition leads to increased absolute sperm transfer (Fedina & Lewis 2006; McGraw et al. 2007; Perez-Staples et al. 2008); however, these studies did not report sperm concentration and as a result it is not possible to determine whether and how ejaculate composition differed. An alternative explanation for our result is that females mating with low- or high-condition males differed in sperm storage or usage in the hour following mating. However, this is unlikely to have affected our measure of sperm transfer because we examined the entire female reproductive tract for sperm; furthermore, there is no reason to expect females to preferentially retain sperm from low-condition males.
In contrast to the result for sperm, high-condition males transferred larger retained ejaculates than low-condition males, an outcome that is consistent with increased allocation to seminal proteins because seminal proteins make up the bulk of ejaculate in many species (see §2b). Indeed, our results show that even greatly increased sperm numbers contribute little to ejaculate mass (figure 1).
We discussed earlier two mechanisms by which this sort of condition dependence in ejaculate composition might arise (see §1). First, more costly ejaculate component are expected to be more condition-dependent. Our finding of condition dependence in sperm and non-sperm ejaculate components may be consistent with this prediction if seminal proteins have greater production costs than sperm in A. bipunctata. There is currently no data available on production costs in this or indeed in many species, making it impossible to predict the direction of condition dependence by this mechanism a priori.
A second mechanism stems from a model of ejaculate composition with regard to sperm competition (Cameron et al. 2007). It predicts that if seminal proteins function to influence female fecundity or the outcome of sperm competition, then males with an advantage in sperm competition should allocate a larger portion of their ejaculate budget to seminal proteins whereas disfavoured males should allocate more to sperm. Moreover, the model describes a broad parameter space where disfavoured males should produce not only a proportionally greater allocation to sperm but an absolutely greater quantity than favoured males (Cameron et al. 2007). Our results fit these predictions if, as in many other animals (e.g. Chapman et al. 2003; Pitcher et al. 2003; Vermeulen et al. 2008), low-condition male A. bipunctata are disfavoured in sperm competition. There is no direct evidence available on this point, but our finding that low-condition males transfer fewer and smaller spermatophore capsules—which induce female re-mating resistance—makes it quite plausible that low-condition males are indeed disfavoured.
The composition of the spermatophore capsule itself varied with male condition: capsules produced by low-condition males had a higher water content relative to those of high-condition males. A possible explanation is that low-condition males that transfer small ejaculates attempt to maintain spermatophore capsule volume by increasing the water content, if doing so stimulates stretch receptors in the female reproductive tract (Ferkau & Fisher 2006). An additional and non-exclusive hypothesis is that females benefit from the water content of spermatophore capsules, based on recent evidence of such benefit in the seed beetle Callosobruchus maculatus (Edvardsson 2007; Ursprung et al. 2009). However, this hypothesis is unlikely to apply because A. bipunctata feeds on a water-rich prey: a single adult pea aphid contains over 25 times more water (289 µg ± 24; n = 3; J. C. Perry 2006, unpublished data) than a spermatophore capsule (table 1).
(c). Two aspects of condition dependence
Condition may influence mating behaviour in two distinct ways. First, reduced condition means a smaller pool of resources, and this may mean a decreased budget for the expression of sexual traits. Second, reduced condition may alter the optimal allocation of resources to traits and thus relative trait expression.
We suggest that the multivariate analysis presented here captures these two dimensions of condition dependence. In this sense, PC1 reflects a change in the size of the resource pool because (i) increasing PC1 scores indicate increasing values for copulation duration and three ejaculate variables (table 2) and (ii) PC1 is positively correlated with post-mating survival, which suggests that it reflects a male's resources available for somatic maintenance. By contrast, PC2 reflects a shift in allocation strategy: increasing scores here signify a shift in allocation to sperm and away from other ejaculate components, and PC2 shows no correlation with post-mating survival, indicating no relationship to overall male somatic quality.
(d). Conclusion
Characterizing condition dependence in ejaculate traits is an exciting challenge because these traits are important arenas for sperm competition, cryptic female choice and sexual conflict (Chapman & Davies 2004; Eberhard 2009). We have provided the first evidence, to our knowledge, supporting the prediction that condition influences allocation to distinct ejaculate components. Future work is needed to investigate the condition dependence of both ejaculate and sperm quality, traits that are highly plastic in other contexts (e.g. Cornwallis & O'Connor 2009). With increasing empirical evidence of condition- and context-dependent variation in ejaculates, there is also a need for theoretical work addressing adaptive ejaculate composition (e.g. Alonzo & Pizzari 2010).
Acknowledgements
We thank C. Tse for laboratory assistance and J. Biernaskie, A. Cutter, D. Gwynne, L. Kwan, T. Long, T. Pizzari and two anonymous reviewers for comments on the manuscript. This study was funded by grants to LR from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Research Chairs program, and by scholarships to JCP from NSERC, the Entomological Society of Canada, and the Government of Ontario (Dr F. M. Hill Scholarship in Science and Technology).
References
- Acar E. B., Mill D. D., Smith B. N., Hansen L. D., Booth G. M.2005Comparison of respiration in adult Harmonia axyridis Pallas and Hippodamia convergens Guerrin-Manaville (Coleoptera: Coccinellidae). Environ. Entomol. 34, 241–245 (doi:10.1603/0046-225X-34.2.241) [Google Scholar]
- Alatalo R. V., Höglund J., Lundberg A.1988Patterns of variation in tail ornament size in birds. Biol. J. Linn. Soc. 34, 363–374 (doi:10.1111/j.1095-8312.1988.tb01969.x) [Google Scholar]
- Alonzo S. H., Pizzari T.2010Male fecundity stimulation: conflict and cooperation within and between the sexes: model analyses and coevolutionary dynamics. Am. Nat. 175, 174–185 (doi:10.1086/649596) [DOI] [PubMed] [Google Scholar]
- Andersson M.1994Sexual selection. Monographs in behavior and ecology.Princeton, NJ: Princeton University Press [Google Scholar]
- Arnaud L., Spinneux Y., Haubruge E.2003Preliminary observations of sperm storage in Adalia bipunctata (Coleoptera: Coccinellidae): sperm size and number. Appl. Entomol. Zool. 38, 301–304 (doi:10.1303/aez.2003.301) [Google Scholar]
- Blanco C. A., Rojas M. G., Groot A. T., Morales-Ramos J., Abel C. A.2009Size and chemical composition of Heliothis virescens (Lepidoptera: Noctuidae) spermatophores. Ann. Entomol. Soc. Am. 102, 629–637 (doi:10.1603/008.102.0407) [Google Scholar]
- Bonduriansky R., Rowe L.2005Sexual selection, genetic architecture, and the condition dependence of body shape in the sexually dimorphic fly Prochyliza xanthostoma (Piophilidae). Evolution 59, 138–151 [PubMed] [Google Scholar]
- Cameron E., Day T., Rowe L.2007Sperm competition and the evolution of ejaculate composition. Am. Nat. 169, E158–E172 (doi:10.1086/516718) [DOI] [PubMed] [Google Scholar]
- Chapman T. W., Davies S. J.2004Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides 25, 1477–1490 (doi:10.1016/j.peptides.2003.10.023) [DOI] [PubMed] [Google Scholar]
- Chapman T., Bangham J., Vinti G., Seifried B., Lung O., Wolfner M. F., Smith H. K., Partridge L.2003The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl Acad. Sci. USA 100, 9923–9928 (doi:10.1073/pnas.1631635100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwallis C. K., O'Connor E. A.2009Sperm: seminal fluid interactions and the adjustment of sperm quality in relation to female attractiveness. Proc. R. Soc. B 276, 3467–3475 (doi:10.1098/rspb.2009.0807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton S., Fowler K., Pomiankowski A.2004Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. Lond. B 271, 771–783 (doi:10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Clerq P., Bonte M., Van Speybroeck K., Bolckmans K., Deforce K.2005Development and reproduction of Adalia bipunctata (Coleoptera: Coccinellidae) on eggs of Ephestia kuehniella (Lepidoptera: Phycitidae) and pollen. Pest Manage. Sci. 61, 1129–1132 [DOI] [PubMed] [Google Scholar]
- Delisle J., Bouchard A.1995Male larval nutrition in Choristoneura rosaceana (Lepidoptera: Tortricidae): an important factor in reproductive success. Oecologia 104, 508–517 (doi:10.1007/BF00341349) [DOI] [PubMed] [Google Scholar]
- Dewsbury D. A.1982Ejaculate cost and male choice. Am. Nat. 119, 601–610 (doi:10.1086/283938) [Google Scholar]
- Eberhard W. G.2009Postcopulatory sexual selection: Darwin's omission and its consequences. Proc. Natl Acad. Sci. USA 106, 10 025–10 032 (doi:10.1073/pnas.0901217106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard W. G., Cordero C.1995Sexual selection by cryptic female choice on male seminal products—a new bridge between sexual selection and reproductive physiology. Trends Ecol. Evol. 10, 493–496 (doi:10.1016/S0169-5347(00)89205-8) [DOI] [PubMed] [Google Scholar]
- Edvardsson M.2007Female Callosobruchus maculatus mate when they are thirsty: resource-rich ejaculates as mating effort in a beetle. Anim. Behav. 74, 183–188 (doi:10.1016/j.anbehav.2006.07.018) [Google Scholar]
- Fedina T. Y., Lewis S. M.2006Proximal traits and mechanisms for biasing paternity in the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae). Behav. Ecol. Sociobiol. 60, 844–853 (doi:10.1007/s00265-006-0228-7) [Google Scholar]
- Ferkau C., Fischer K.2006Costs of reproduction in male Bicyclus anynana and Pieris napi butterflies: effects of mating history and food limitation. Ethology 112, 1117–1127 (doi:10.1111/j.1439-0310.2006.01266.x) [Google Scholar]
- Getty T.2006Sexually selected signals are not similar to sports handicaps. Trends Ecol. Evol. 21, 83–88 (doi:10.1016/j.tree.2005.10.016) [DOI] [PubMed] [Google Scholar]
- Gwynne D. T.1990Testing parental investment and the control of sexual selection in katydids: the operational sex ratio. Am. Nat. 136, 474–484 (doi:10.1086/285108) [Google Scholar]
- Haddrill P. R., Shuker D. M., Amos W., Majerus M. E. N., Mayes S.2008Female multiple mating in wild and laboratory populations of the two-spot ladybird, Adalia bipunctata. Mol. Ecol. 17, 3189–3197 (doi:10.1111/j.1365-294X.2008.03812.x) [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Pomiankowski A.1994The evolution of mate preferences for multiple sexual ornaments. Evolution 48, 853–867 (doi:10.2307/2410492) [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Pomiankowski A., Nee S.1991The evolution of costly mate preferences. II. The ‘handicap’ principle. Evolution 45, 1431–1442 (doi:10.2307/2409890) [DOI] [PubMed] [Google Scholar]
- Jia Z., Jiang Z., Sakaluk S. K.2000Nutritional condition influences investment by male katydids in nuptial food gifts. Ecol. Entomol. 25, 115–118 (doi:10.1046/j.1365-2311.2000.00239.x) [Google Scholar]
- Lehmann G. U. C., Lehmann A. W.2009Condition-dependent spermatophore size is correlated with male's age in a bushcricket (Orthoptera: Phaneropteridae). Biol. J. Linn. Soc. 96, 354–360 (doi:10.1111/j.1095-8312.2008.01129.x) [Google Scholar]
- Lewis Z., Wedell N.2007Effect of adult feeding on male mating behaviour in the butterfly, Bicyclus anynana (Lepidoptera: Nymphalidae). J. Insect Behav. 20, 201–213 (doi:10.1007/s10905-007-9075-2) [Google Scholar]
- Martin-Coello J., Dopazo H., Arbiza L., Ausio J., Roldan E. R. S., Gomendio M.2009Sexual selection drives weak positive selection in protamine genes and high promoter divergence, enhancing sperm competitiveness. Proc. R. Soc. B 276, 2427–2436 (doi:10.1098/rspb.2009.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw L. A., Fiumera A. C., Ramakrishnan M., Madhavarapu S., Clark A. G., Wolfner M. F.2007Larval rearing environment affects several post-copulatory traits in Drosophila melanogaster. Biol. Lett. 3, 607–610 (doi:10.1098/rsbl.2007.0334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. H., Katz D. F.2005A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 26, 459–469 (doi:10.2164/jandrol.04104) [DOI] [PubMed] [Google Scholar]
- Parker G. A.1990Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126 (doi:10.1098/rspb.1990.0114) [Google Scholar]
- Perez-Staples D., Harmer A. M. T., Collins S. R., Taylor P. W.2008Potential for pre-release diet supplements to increase the sexual performance and longevity of male Queensland fruit flies. Agricultural and forest entomology 10, 255–262 (doi:10.1111/j.1461-9563.2008.00385.x) [Google Scholar]
- Perry J. C., Rowe L.2008aIngested spermatophores accelerate reproduction and increase mating resistance but are not a source of sexual conflict. Anim. Behav. 76, 993–1000 (doi:10.1016/j.anbehav.2008.05.017) [Google Scholar]
- Perry J. C., Rowe L.2008bNeither mating rate nor spermatophore feeding influences longevity in a ladybird beetle. Ethology 114, 504–511 (doi:10.1111/j.1439-0310.2008.01499.x) [Google Scholar]
- Perry J. C., Sharpe D. M. T., Rowe L.2009Condition-dependent female remating resistance generates sexual selection on male size in a ladybird beetle. Anim. Behav. 77, 743–748 (doi:10.1016/j.anbehav.2008.12.013) [Google Scholar]
- Pitcher T. E., Neff B. D., Rodd F. H., Rowe L.2003Multiple mating and sequential mate choice in guppies: females trade up. Proc. R. Soc. Lond. B 270, 1623–1629 (doi:10.1098/rspb.2002.2280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx S., Day T., Rowe L.2002Older males signal more reliably. Proc. R. Soc. Lond. B 269, 2291–2299 (doi:10.1098/rspb.2002.2129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzalan D., Cooray M., Rodd F. H., Rowe L.2008Condition dependence of sexually dimorphic colouration and longevity in the ambush bug Phymata americana. J. Evol. Biol. 21, 1297–1306 (doi:10.1111/j.1420-9101.2008.01571.x) [DOI] [PubMed] [Google Scholar]
- Ramm S. A., Oliver P. L., Ponting C. P., Stockley P., Emes R. D.2007Sexual selection and the adaptive evolution of mammalian ejaculate proteins. Mol. Biol. Evol. 25, 207–219 (doi:10.1093/molbev/msm242) [DOI] [PubMed] [Google Scholar]
- Ransford M. O.1997Sperm competition in the 2-spot ladybird. Adalia bipunctata. University of Cambridge, UK [Google Scholar]
- Rowe L., Houle D.1996The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421 (doi:10.1098/rspb.1996.0207) [Google Scholar]
- Simmons L. W.2001Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press [Google Scholar]
- Simmons L. W., Kotiaho J. S.2007Quantitative genetic correlation between trait and preference supports a sexually selected sperm process. Proc. Natl Acad. Sci. USA 104, 16 604–16 608 (doi:10.1073/pnas.0704871104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W., Beesley L., Lindhjem P., Newbound D., Norris J., Wayne A.1999Nuptial feeding by male bushcrickets: an indicator of male quality? Behav. Ecol. 10, 263–269 (doi:10.1093/beheco/10.3.263) [Google Scholar]
- Tazzyman S. J., Pizzari T., Seymour R. M., Pomiankowski A.2009The evolution of continuous variation in ejaculate expenditure strategy. Am. Nat. 174, E71–E82 (doi:10.1086/603612) [DOI] [PubMed] [Google Scholar]
- Tuckerman J. F., Gwynne D. T., Morris G. K.1993Reliable acoustic cues for female mate preference in a katydid (Scudderia curvicauda, Orthoptera: Tettigoniidae). Behav. Ecol. 4, 106–113 (doi:10.1093/beheco/4.2.106) [Google Scholar]
- Ursprung C., den Hollander M., Gwynne D. T.2009Female seed beetles, Callosobruchus maculatus, remate for male-supplied water rather than ejaculate nutrition. Behav. Ecol. Sociobiol. 63, 781–788 (doi:10.1007/s00265-009-0711-z) [Google Scholar]
- Vermeulen A., Engels S., Sauer K. P.2008Mating effort and cryptic sperm choice in scorpionflies: male investment strategy vs. female control. Ethology 114, 1166–1172 (doi:10.1111/j.1439-0310.2008.01569.x) [Google Scholar]
- Watanabe M., Hirota M.1999Effects of sucrose intake on spermatophore mass produced by male swallowtail butterfly Papilio xuthus L. Zool. Sci. 16, 55–61 (doi:10.2108/zsj.16.55) [Google Scholar]
- Wedell N.1993Mating effort or paternal investment? Incorporation rate and cost of male donations in the wartbiter. Behav. Ecol. Sociobiol. 32, 239–246 [Google Scholar]
- Wigby S., Sirot L. K., Linklater J. R., Buehner N., Calboli F. C. F., Bretman A., Wolfner M. F., Chapman T.2009Seminal fluid protein allocation and male reproductive success. Curr. Biol. 19, 751–757 (doi:10.1016/j.cub.2009.03.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. D., Day T., Cameron E.2005The evolution of sperm-allocation strategies and the degree of sperm competition. Evolution 59, 492–499 [PubMed] [Google Scholar]
- Zahavi A.1975Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]