Abstract
Hashimoto’s thyroiditis, a common autoimmune disease, is associated with autoantibodies to thyroglobulin (Tg) and thyroid peroxidase (TPO). TPO, unlike abundant and easily purified Tg, is rarely investigated as an autoantigen in animals. We asked whether antibodies (Abs) develop to both TPO and Tg in thyroiditis that is induced (C57BL/6 and DBA/1 mice) or arises spontaneously (NOD.H-2h4 mice). Screening for TPOAbs was performed by flow cytometry using mouse TPO-expressing eukaryotic cells. Sera were also tested for binding to purified mouse Tg and human TPO. The antibody data were compared with the extent of thyroiditis. Immunization with mouse TPO adenovirus broke self-tolerance to this protein in C57BL/6 mice, but thyroiditis was minimal and TgAbs were absent. In DBA/1 mice with extensive granulomatous thyroiditis induced by Tg immunization, TPOAbs were virtually absent despite high levels of TgAbs. In contrast, antibodies to mouse TPO, with minimal cross-reactivity with human TPO, arose spontaneously in older (7–12 months) NOD.H-2h4 mice. Unexpectedly, TgAbs preceded TPOAbs, a time course paralleled in relatives of probands with juvenile Hashimoto’s thyroiditis. These findings demonstrate a novel aspect of murine and human thyroid autoimmunity, namely breaking B cell self-tolerance occurs first for Tg and subsequently for TPO.
Thyroid peroxidase antibodies arise spontaneously in NOD.H-2h4 mice (but not in experimentally induced thyroiditis) and appear after thyroglobulin antibodies.
Hashimoto’s thyroiditis is associated with antibodies (Abs) to two thyroid-specific glycosylated proteins, thyroglobulin (Tg) and thyroid peroxidase (TPO). Tg, a soluble protein comprising a large homodimer (∼300 kDa monomers), constitutes the colloid and is the predominant component of the thyroid gland. TPO is a much less abundant membrane-bound protein located at the apical surface of thyroid epithelial cells; it is also a homodimer (∼100 kDa monomers) with a heme prosthetic group. Differences in the location and abundance of Tg and TPO may significantly influence self-tolerance to and recognition of these two thyroid autoantigens by the immune system.
TgAbs were recognized in Hashimoto’s disease in 1956 (1), and at the same time, thyroiditis was induced in rabbits by immunization with Tg and adjuvant (2). Two years later, antibody reactivity was reported to the thyroid microsomal antigen (3). The identity of this protein remained elusive for nearly 30 yr until immunological evidence (4) and molecular cloning demonstrated that the microsomal antigen was the enzyme TPO (5,6). Because of its abundance and ease of purification, Tg was the thyroid antigen of choice for induction of experimental autoimmune thyroiditis in animals, particularly mice (for example Refs. 7 and 8). Tg was also found to be an immune target in spontaneous thyroiditis in obese strain chickens (9), BioBreeding rats (10), and NOD mice (11). In the diabetes-resistant NOD.H-2h4 strain, increased dietary iodide accelerates the development of thyroiditis and TgAbs (12,13,14).
In Hashimoto patients, the prevalence of TgAbs and TPOAbs is similar (for example Ref. 15). Unlike in humans, there are no previous reports that TPOAbs arise spontaneously in animals, including NOD mice that develop thyroiditis (16). Also, in contrast to Tg, relatively few studies have used TPO (protein or cDNA) to immunize mice (17,18,19,20,21). Remarkably, even though the transgenic expression in mice of a pathogenic human TPO-specific T-cell receptor induces thyroiditis and hypothyroidism (22,23), there is no evidence that TPOAbs arise in these animals (23).
The present concept therefore is that antibody responses associated with autoimmune thyroiditis involve both Tg and TPO in humans but are biased toward Tg in mice. On the other hand, it is possible that TPOAbs in mice have not been detected because TPOAbs cross-react poorly with TPO from different species. It is known that human-TPOAbs bind minimally to nonprimate thyroid microsomes (24). Therefore, murine TPOAbs may not cross-react with human TPO. Indeed, a previous study failed to detect TPOAbs in NOD mice in an assay using human TPO (16). Moreover, thyroiditis and hypothyroidism were induced using murine (not human) TPO (of bacterial origin) or a murine TPO peptide (20,21). Furthermore, TPO generated in bacteria is not well recognized by human Abs and, for full immunoreactivity, recombinant TPO needs to be expressed in eukaryotic cells (reviewed in Ref. 25). In the present study, we used mouse TPO generated in mammalian cells to address the question of whether TPOAbs arise in Tg-induced thyroiditis and especially in mice that develop spontaneous thyroiditis.
Materials and Methods
Adenovirus-encoding mouse TPO
The cDNA for full-length mouse TPO (26) was generously provided by Dr. S. Ohtaki (Miyazaki Medical College, Miyazaki, Japan). Adenovirus encoding mouse TPO (mTPO-Ad) was generated by excising this cDNA from pUC19 and inserting it into pAdHM4, as described for the human TSH receptor (TSHR) A-subunit (27). As a control (Con-Ad), we used adenovirus lacking an insert (28). Adenoviruses were amplified in 293 human embryonic kidney cells, purified by CsCl density gradient centrifugation, and viral particle concentration determined by measuring the absorbance at 260 nm, as described previously (27). To test for expression, COS-7 cell monolayers were infected with mTPO-Ad or, as a positive control, with human TPO (hTPO)-Ad (29) After 2 d, cells were resuspended and incubated with human recombinant TPO-specific Fab (that recognize the TPO A domain) or with TR1.8 or TR1.9 (that recognize the TPO B domain) (30). Antibody binding was detected using phycoerythrin-conjugated antihuman kappa (Caltag, Burlingame, CA), and fluorescence was analyzed using the Becton Dickinson FACScan-CELLQuest system (San Jose, CA).
Immunization with TPO adenovirus (C57BL/6)
Female C57BL/6 mice (6–7 wk old; The Jackson Laboratory, Bar Harbor, ME) were injected in the thigh muscle with mTPO-Ad or Con-Ad (∼5 × 1010 viral particles in 50 μl PBS per injection) on three occasions at 3-wk intervals. In approaches to deplete T regulatory cells (Treg), some mice were pretreated with anti-CD25 (or anti-CD122), as described (31,32,33). The antibodies were generated by injecting rat hybridoma PC61 (or TMbeta1) into nude mice and harvesting the ascites. The efficacy of Protein G affinity-purified anti-CD25 (or anti-CD122) was tested in BALB/c mice (Charles River Laboratory Inc., Yokohama, Japan). Mice received 500 μg anti-CD25 (or 250 μg anti-CD122) ip 4 d before each adenovirus injection. Mice were euthanized 4 wk after the third immunization. We also tested sera available from BALB/c mice immunized with hTPO-Ad (109 or 107 viral particles) (29).
All animal studies (described above and reported below) were carried out according to the Guideline for the Care and Use of Laboratory Animals (Nagasaki University) and the Institutional Animal Care and Use Committees (Cedars-Sinai Medical Center and University of Missouri) and performed with the highest standards of care in pathogen-free facilities.
Granulomatous experimental autoimmune thyroiditis (DBA/1)
Wild-type (WT) DBA/1 mice and CD2-FLIP transgenic mice on the DBA/1 background (34) were bred in the animal facility at the University of Missouri. Mice (male and female) aged 6–10 wk were induced to develop granulomatous experimental thyroiditis as previously described (35,36). Briefly, donor mice were immunized twice at 10-d intervals with 150 μg mouse Tg and 15 μg lipopolysaccharide (Escherichia coli O11:B4; Sigma Chemical Co., St. Louis, MO). Seven days later, donor spleen cells were restimulated in vitro with 25 μg/ml mouse Tg and 5 ng/ml IL-12 (35,36). After 72 h, cells were harvested and washed twice, and 3.5 × 107 cells were transferred iv to 500-Rad irradiated syngeneic recipients. Mice were euthanized to obtain blood and thyroids at the following times: 18–20 d (just before and at the peak of disease) and at 40 or 55 d (resolution phase).
Spontaneous thyroiditis or thyrocyte hyperplasia (NOD.H-2h4)
The following NOD.H-2h4 mouse strains were bred (University of Missouri): WT; interferon (IFN)-γ- and IL-4-deficient (IFN-γ−/−, IL-4−/−) (37); IFN-γ receptor deficient (IFN-γR−/−), CD28 deficient (CD28−/−) (38), TGFβ transgenics (39), and NOD.H-2h4 mice with severe combined immunodeficiency (SCID).
Mice were maintained on regular water or water supplemented with 0.05% NaI for 2–12 months (specified in the text) before euthanasia and obtaining blood and thyroids. Thyroiditis is the outcome in WT animals, IL-4−/− and CD28−/− mice (13). Thyrocyte hyperplasia develops in the following strains exposed to iodized water: IFN-γ−/−, IFN-γR−/−, TGFβ transgenics, and NOD.H-2h4.SCID recipients of splenocytes from IFN-γ−/− donors (37).
Thyroid histology
Thyroids were fixed in formalin and paraffin embedded, and sections were stained with hematoxylin and eosin (Research Animal Diagnostic Laboratory, University of Missouri, Columbia, MO). Thyroid sections were scored for the extent of lymphocytic thyroiditis and graded on a scale from 1 to 5 as previously reported (13). Briefly, 0 indicates infiltration by few or no lymphocytes; 1+ thyroiditis is defined as an infiltrate of at least 125 cells in one or several foci; 2+ represents 10–20 foci of cellular infiltration, each the size of several follicles and involving up to one quarter of the gland; 3+ indicates that a quarter to half the gland is infiltrated by lymphocytes; and 4+ indicates that greater than half the gland is destroyed. The extent of thyrocyte hyperplasia was assessed and graded as described (37).
Flow cytometry for antibodies to mouse TPO
Chinese hamster ovary (CHO) cells stably expressing mouse TPO were established as follows: mouse TPO cDNA was excised from the pUC19 vector using XbaI and NheI and transferred to the same sites in pcDNA5/FRT (Invitrogen, Carlsbad, CA). This mTPO-pcDNA5/FRT plasmid was used to transfect Flp-In(CHO) cells (Invitrogen) using Fugene HD (Roche, Indianapolis, IN) and selection with hygromycin B (100 μg/ml). We obtained a clonal cell line expressing mouse TPO as detected by the binding of the human Fab WR1.7 (not shown).
For mouse sera, test samples (1:50 dilution) were incubated with mTPO-CHO cells, and binding was detected using fluorescein isothiocyanate-conjugated affinity purified goat antimouse IgG (Caltag Laboratories). Cells staining with propidium iodide (1 μg/ml) were excluded from analysis. Assays for IgG class antibody binding to mTPO included sera from unimmunized or Con-Ad immunized BALB/c or C57BL/6 mice (negative controls). Positive controls included mouse monoclonal TPOAb no. 24 (40) (generously provided to us many years ago by Drs. Pierre Carayon and Jean Ruf (U555 Institut National de la Santé et de la Recherche Médicale-URA, Faculté de Médecine Timone, Marseille, France) and, for analysis of DBA/1 and NOD.H-2h4 mice, sera from mTPO-Ad immunized mice. Fluorescence was analyzed as described above and TPO binding data are expressed as geometric means.
ELISA for antibodies to mouse Tg and human TPO
Tg was isolated from murine thyroid glands (Pel-Freez Biologicals, Rogers, AR) homogenized in PBS, precipitated with 45% saturated (NH4)SO4, followed by dialysis and fractionation on a Sephadex G-200 column (41). Human TPO ectodomain produced by CHO cells was affinity purified from culture supernatants as previously described (42). ELISA wells were coated with mouse Tg (1.5 μg/ml) or human TPO (1 μg/ml) and incubated with test sera (duplicate aliquots, 1:100 dilution for most strains; 1:3000 for DBA/1 mice). Antibody binding was detected with horseradish peroxidase-conjugated goat antimouse IgG (Sigma Chemical Co., St. Louis, MO), the signal developed with o-phenylenediamine and the reaction stopped using 20% H2SO4. The data are presented as the OD at 490 nm for TgAbs or human TPOAbs.
Human studies
To study the prevalence TgAbs or TPOAbs in human populations, we used data accessible on-line from the National Health and Nutrition Examination Survey (NHANES) III study (43). The NHANES surveys were designed to give national normative estimates of the health and nutritional status of the U.S. civilian, noninstitutionalized population. Youths and the elderly were oversampled to provide sufficient data for studies of these groups. Given the disparities of thyroid disease in some minority groups, only Caucasian data were included in our analysis. Therefore, our analysis is restricted to data from 7,094 subjects older than 12 yr of age who had thyroid assessments, representing 144,483,300 people. (In the NHANES III survey, each person is mathematically weighted to represent a specific number and proportion of people in the population and during analysis weighting is used to adjust for the over sampling and nonresponse.) TgAb and TPOAb assessments were performed on serum samples as previously described (43,44). Subjects were considered disease free with TgAbs less than 1 IU/ml or TPOAbs less than 0.5 IU/ml.
In a second approach, we compared TgAbs and TPOAbs in the sera of probands, parents, and siblings from four families with juvenile Hashimoto’s thyroiditis. These sera were obtained from Dr. C. L. Burek (Johns Hopkins University, Baltimore, MD) for a collaborative study on TPOAb epitopic fingerprints (45). In the present study, we measured TgAbs and TPOAbs in these sera using standardized RIA kits (Kronus Inc., Star, ID) following the protocol of the manufacturer with a minor modification. Many samples were available to us diluted 1:10 in saline + 1% BSA. Therefore, we diluted all neat sera in the same way and performed the assays on 25-μl aliquots of diluted serum + 25 μl of assay diluent, yielding the same final serum concentration (1:50) as required by the kit. TgAb and TPOAb values are reported as units per milliliter based on the calibrators provided in the kits.
Statistical analysis
Differences between groups (mice and Hashimoto’s thyroiditis family members) were tested by Student’s t test or rank sum test as appropriate; Fishers’ exact test was used to compare proportions of Ab-positive and -negative individuals (SigmaStat; Jandel Scientific Software, San Rafael, CA). NHANES III data were analyzed with SAS software (version 9.1.3; SAS Institute, Cary, NC) using survey sampling and analysis procedures to account for the complex survey sampling design using the weights assigned to the individual samples to represent the U.S. population.
The prevalence of TgAbs (>1 IU/ml) or TPOAbs (>0.5 IU/ml) was computed by age using PROC SURVEYFREQ program within the SAS software (see above). The nonparametric Kolmogorav-Smirnov two-sample test was then used to compare the two distributions by age. Prevalence proportion estimates are presented with 95% confidence intervals.
Results
Expression of mouse TPO
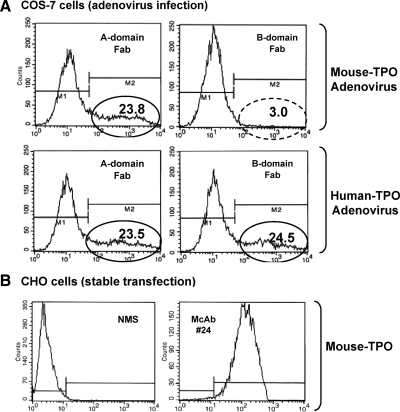
To confirm adenovirus expression of mouse TPO, COS-7 cells were infected with mTPO-Ad or human TPO (29) and tested 2 d later. We observed that mouse TPO is recognized by TPO A domain-specific Fab but not B domain-specific Fab (Fig. 1A, upper panels). In contrast, COS-7 cells expressing human TPO are recognized by both A and B domain TPO-specific Fab (Fig. 1A, lower panels). To test for TPOAbs in mouse sera, we generated CHO cells stably expressing mouse TPO. This clonal cell line was recognized by TPO A domain-specific Fab WR1.7 (not shown) and some human TPO-specific monoclonals (for example no. 24) derived from BALB/c mice immunized with human TPO protein plus adjuvant (40) (Fig. 1B, right panel).
Figure 1.
Expression of mouse TPO measured by flow cytometry. A, COS-7 cells transiently infected with mTPO-Ad (upper panels) and hTPO-Ad (lower panels) and tested with human TPO recombinant antibody Fab that recognize the A-domain (Fab WR1.7) or the B-domain (Fab TR1.8). The oval indicates the percentage of positive cells in the M2 fraction. B, CHO cells stably expressing mouse TPO: normal mouse serum (NMS, left panel) and mouse monoclonal antibody no. 24 (right panel) generated against human TPO (40).
These reagents enabled us to test for TPOAbs in mice immunized with TPO (mouse or human), Tg (mouse), and also mice that develop thyroiditis spontaneously. Previously, in transgenic mice expressing the human TSHR A-subunit in their thyroids, we found that Treg depletion before immunization with human TSHR adenovirus induced extensive thyroid lymphocytic infiltration and thyroid damage. Remarkably we also observed antibody spreading from the TSHR to Tg and TPO (33). Based on these observations, in the present study, we hypothesized that extensive thyroiditis would be associated with TPOAbs.
Abs induced by TPO adenovirus immunization (C57BL/6 and BALB/c)
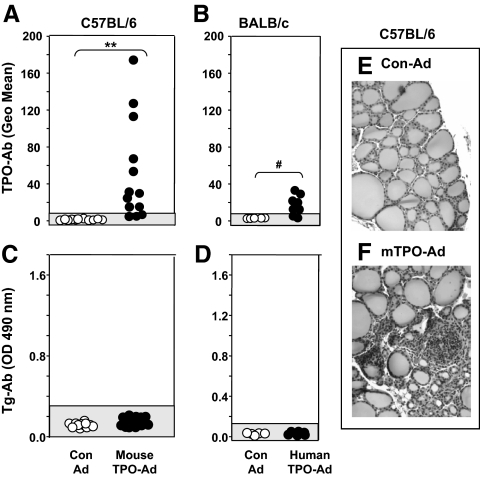
C57BL/6 mice immunized with mouse TPO adenovirus (Ad) developed antibodies to mouse TPO detectable by flow cytometry (d 70; 4 wk after the third and final immunization) (Fig. 2A). Mice treated with anti-CD25 (but not with anti-CD122), approaches aimed at depleting Treg, developed moderately enhanced TPOAb levels vs. untreated animals immunized in parallel (Supplemental Fig. 1A, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). However, TPOAb levels in anti-CD25-treated mice did not exceed those observed in some untreated mice. Overall, the development of TPOAbs in 10 of 13 mice indicates that immunization with adenovirus alone is sufficient to break self-tolerance to mouse-TPO, at least in the C57BL/6 strain. The variability in TPOAb levels may arise because, unlike conventional immunization with purified protein and adjuvant, adenovirus immunization uses a biological reagent.
Figure 2.
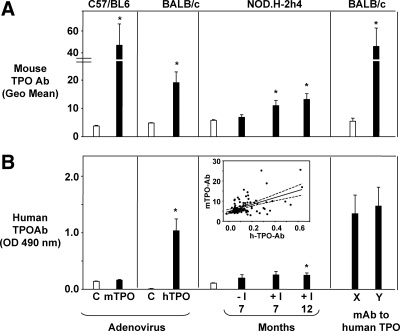
TPOAbs, but not TgAbs, develop in C57BL/6 mice immunized with mTPO-Ad. BALB/c mice immunized with hTPO-Ad (29) develop Abs that cross-react with mouse TPO but not TgAbs. Sera were tested from mice euthanized 4 wk after the third immunization with mTPO-Ad, hTPO-Ad, or control (Con)-Ad. Data are shown for individual mice. Shaded areas represent the mean ± 2 sd for Con-Ad-immunized mice. A and B, TPOAbs measured by flow cytometry with mouse TPO-CHO cells (sera 1:50) in C57BL/6 (A) and BALB/c (B) mice; data are expressed as the geometric mean (Geo Mean). **, P < 0.001, Statistically significant differences; #, P < 0.002, (rank sum tests). C and D, TgAbs measured by ELISA (sera 1:100) in C57BL/6 (C) and BALB/c (D); values are reported as the OD 490 nm. Positive control values (OD 1.33 ± 0.12) were obtained for sera from A-subunit transgenic mice that developed TgAbs after Treg depletion and immunization with TSHR adenovirus (33). E and F, Normal thyroid histology in mice immunized with Con-Ad (E) and lymphocytic infiltrate in a few animals immunized with mTPO-Ad (F). Magnification, ×100.
We also examined sera from BALB/c mice immunized with hTPO-Ad (29) for binding to mouse TPO cells. Six of eight mice had Abs that cross-reacted with mouse TPO, although the highest values were lower than those attained by mTPO-Ad immunized mice (Fig. 2B vs. 2A). Neither C57BL/6 nor BALB/c mice immunized with mouse or human TPO developed detectable TgAbs (Fig. 2, C and D). Minimal thyroiditis developed in a small proportion of mTPO-Ad-immunized C57BL/6 mice (Fig. 2, F vs. E). Previously we found no thyroid lymphocytic infiltration in BALB/c animals immunized with hTPO-Ad (29).
Granulomatous experimental thyroiditis (DBA/1 mice)
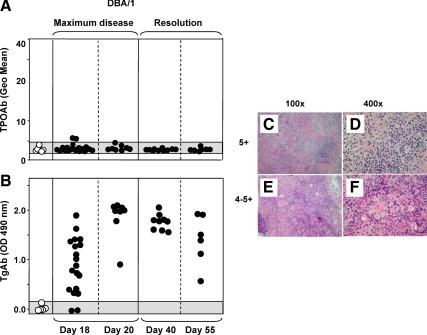
In DBA/1 recipients of splenocytes primed in vivo and restimulated in vitro with mouse Tg, granulomatous experimental thyroiditis is maximal at d 20 and is followed by a resolution phase (d 40–55) (46). Only two of 49 sera obtained at 18 d, but no sera obtained at later times (d 20, 40, and 55), had marginally positive TPOAb levels (Fig. 3A). As expected, virtually all mice were positive for TgAbs, even in sera diluted 1:3000 (Fig. 3B). We elected to study granulomatous thyroiditis (for which sera were available) because extensive lymphocytic infiltration develops in this model (scores 4+ or higher; Fig. 3, C–F). However, our findings in this adoptive transfer model with severe induced disease are contrary to our hypothesis that thyroiditis in itself would be associated with the development of TPOAbs.
Figure 3.
Granulomatous thyroiditis induced in DBA/1 mice is not associated with TPOAbs despite high levels of TgAbs and extensive thyroiditis. Thyroiditis was induced by transferring primed splenocytes activated in vitro with mouse Tg + IL-12 (35). Observations were pooled for WT and Flip-2 transgenic DBA/1 mice because they were similar (Supplemental Fig. 1B). Sera from mice without thyroiditis (grade 0) provided negative controls. Mice were tested at the time of maximum disease (d 18 and 20) and in the resolution phase (d 40 and 55). Thyroiditis scores were high (4–5+) at all time intervals in affected animals. Data are shown for individual mice; shaded areas represent the mean ± 2 sd for mice lacking thyroiditis. A, TPOAbs measured by flow cytometry (sera 1:50). Positive control: Geo mean 54.1 (serum from C57BL/6 mouse immunized with mTPO-Ad). B, TgAbs measured by ELISA (sera diluted 1:3000). C–F, Thyroid histology depicting lymphocytic thyroiditis grade 5+ (C and D) and 4–5+ (E and F). Magnifications, ×100 (C and E) and ×400 (E and F).
Spontaneous thyroiditis (NOD.H-2h4 mice)
NOD.H-2h4 mouse strains develop two distinct forms of disease accentuated by excess iodide ingestion, namely lymphocytic thyroiditis vs. thyrocyte hyperplasia. The first form of disease, thyroiditis, occurs in WT NOD.H-2h4 mice, although to a moderate extent (grade 2+). Thyroiditis is also moderate in most IL-4 knockouts (37) but a small proportion develop more severe thyroiditis (grade 5+) as in CD28 knockouts (both strains on the NOD.H-2h4 background). Unlike the rapid onset and resolution by 2 months of granulomatous thyroiditis in DBA/1 mice, most NOD.H-2h4 mice exhibit thyroiditis 1.5–2 months after NaI administration and inflammation remains chronic over 3–4 months (13). The second form of disease, thyrocyte hyperplasia, develops in several other NOD.H-2h4 strains (knockouts for IFN-γ or the IFNγR, TGFβ transgenics, and SCID recipients of splenocytes from IFN-γ−/− mice) (47).
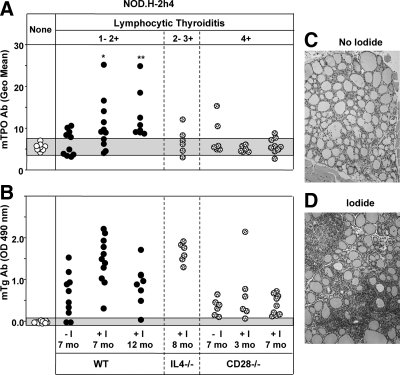
Baseline TPOAb values were obtained for NOD.H-2h4 mice (WT and non-WT) that did not develop thyroiditis or thyrocyte hyperplasia. Relative to these levels, 50% of WT mice 7 months of age had detectable TPOAbs; animals exposed to iodide for 7 and 12 months had higher TPOAb titers, and all 12-month-old mice were TPOAb positive (Fig. 4A). Contrary to our expectation, fewer mice with severe thyroiditis (CD28−/− and a subgroup of IL-4−/−) had detectable TPOAbs compared with WT animals, which had mild or moderate thyroiditis (Fig. 4A). Of 21 mice with thyrocyte hyperplasia, only two had low levels of TPOAbs (Supplemental Fig. 1C). Turning to TgAbs, the majority of NOD.H-2h4 mice were positive (Fig. 4B). Representative examples of minimal and moderate thyroiditis (without or with iodide, respectively) are shown in Fig. 4, C and D.
Figure 4.
Development of TPOAbs in NOD.H-2h4 mice is associated with increasing age. Sera were tested from WT mice (solid circles) and IL-4−/− and CD28−/− mice (stippled circles) all on the NOD.H-2h4 background. Mice received regular water (−I) or water supplemented with 0.05% NaI (+I) for times ranging from 3 to 12 months. Shaded areas represent mean ± 2 sd for mice without thyroid lesions. It should be noted that the IL-4−/− mice tested were a subgroup with severe thyroiditis; most IL-4−/− animals develop mild thyroiditis as in WT mice (37). A, TPOAbs measured by flow cytometry with mouse TPO-CHO cells (sera 1:50). *, P < 0.02, **, P < 0.001, values significantly different from mice without thyroid lesions (rank sum test). B, TgAbs measured by ELISA (sera 1:100). C and D, Thyroid pathology in NOD.H-2h4 mice not exposed to NaI (C) or after exposure to NaI (0.05% NaI in drinking water). [Reproduced with permission from S. M. McLachlan, et al.: Endocrinology 46:294–300, 2005. ©The Endocrine Society.]
The foregoing data taken together indicate that some animals spontaneously developed TPOAbs and TgAbs, even without excess iodide ingestion. Regardless of iodide ingestion, many mice that developed TgAbs lacked TPOAbs (e.g. IL-4−/− mice) (Fig. 4, A and B) but all animals with TPOAbs also had TgAbs (data not shown).
TPOAbs in mice cross-react poorly with human TPO
Having observed antibody reactivity with mouse TPO in different mouse strains, whether after immunization or arising spontaneously, we examined reactivity of these sera with purified, recombinant human TPO of mammalian cell origin. To compare antibody reactivity with mouse and human TPO, the data shown previously for individual mice (Figs. 2A, 3A, and 4A) are depicted in bar graphs (Fig. 5A). Binding to mouse TPO was significantly higher (compared with their respective controls) for all groups except 7-month-old NOD.H-2h4 (Fig. 5A). In addition to the foregoing polyclonal sera, we observed that four of eight monoclonal Abs to human TPO generated in BALB/c mice (40) also recognized mouse TPO (Fig. 5A, group Y).
Figure 5.
TPOAbs that develop in mice immunized with mTPO-Ad (C57BL/6) or that arise spontaneously (NOD.H-2h4) cross-react poorly with human TPO. In contrast, TPOAbs in mice immunized with hTPO-Ad (BALB/c), as well as some monoclonals from mice immunized with human TPO (40), bind to mouse TPO. Binding data are compared for mouse vs. human TPO (black bars) for the following strains: C57BL/6 immunized with mTPO-Ad; BALB/c immunized with hTPO-Ad; NOD.H2h4 not exposed (−I) or exposed (+I) to NaI water for 7 or 12 months; monoclonals from BALB/c immunized with human TPO and adjuvant (40). Control values (white bars): Control (C) adenovirus immunized C57BL/6 or BALB/c; mice without thyroid lesions (NOD.H2h4); group × BALB/c monoclonals. [Note: our subdivision of the monoclonals (40) into groups X and Y based on their recognition of mouse TPO: nonbinders, group X (monoclonals no. 40, 47, 53, and 64) and binders, group Y (no. 18, 24, 30, and 59)]. A, Antibody binding to mouse TPO CHO cells; data are shown as the mean (+sem) of values for individual mice in Figs. 2, 3, and 4. B, Antibody binding in ELISA to human TPO (mean + sem). Inset, Correlation between TPOAbs measured using mouse or human TPO for NOD.H2h4 mice; note the high proportion of values outside the 95% confidence limits.
When tested for recognition of human TPO, only hTPO-Ad-immunized mice, 12-month-old NOD.H-2h4 and, of course, the monoclonal Abs to human TPO were positive (Fig. 5B). Regression analysis of the relationship between Ab reactivity to mouse and human TPO in NOD.H-2h4 mice revealed many values outside the 95% confidence limits (inset, Fig. 5B). Consequently, without a mouse-specific TPO assay, we would not have detected TPOAb in 7-month-old NOD.H-2h4 animals (Fig. 5A). Moreover, our findings for this group of NOD.H-2h4 mice provided the impetus for expanding our screen to include older NOD.H-2h4 mice (Fig. 4A).
Age-related development of TPOAbs and TgAbs in mice
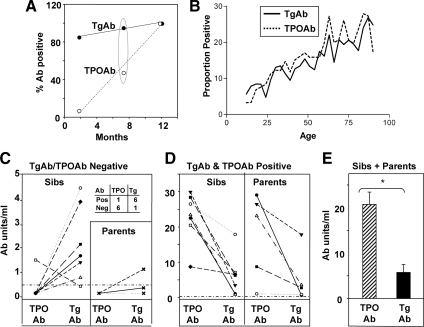
Because advanced age was important for TPOAb development (Figs. 4A and 5A), we compared the prevalence of TPOAbs and TgAbs in NOD.H-2h4 mice (WT as well as other strains on the same background) in the following age groups: 2–3 months, approximately 7 and 12 months. The prevalence of TgAbs was greater than 80% in young mice, and virtually all animals were TgAb positive by 7 months. In contrast, the prevalence of TPOAbs rose from 7% in young mice and reached 100% only in 12-month-old animals (Fig. 6A). For 7-month-old mice, the proportion of TPOAb-positive mice was significantly lower than that of mice positive for TgAbs [Fisher’s exact test (P < 0.001)]. These studies indicate that antibody reactivity develops earlier to Tg than to TPO in the spontaneous NOD.H-2h4 thyroiditis model.
Figure 6.
Relationship between age and development of TgAbs or TPOAbs. A, Prevalence of TgAbs and TPOAbs in NOD.H2h4 mice at 2, 7, and 12 months. B, Proportion of Caucasians aged 12–80 yr positive for TgAbs and TPOAbs (plotted from the on-line data from Ref. 43). C–E, TPOAbs and/or TgAbs in siblings (sibs) or parents of juvenile Hashimoto’s thyroiditis probands. Horizontal dashed line (C and D, negative cutoff, <0.5 U/ml). Inset (C), Proportions of siblings positive or negative for TgAbs and TPOAbs. E, mean + sem. #, P = 0.003, TPOAb or TgAb levels significantly different (rank sum test); *, P < 0.001, TPOAb or TgAb levels significantly different (t test).
Relationship between TPOAbs and TgAbs over time in humans
The foregoing data in mice raised the important question of whether Tg or TPO was the primary autoantigen in human autoimmune thyroiditis. We used two approaches to address this question. First, we examined data from the NHANES III survey conducted between 1988 and 1994 (43). Because Caucasians comprised the largest number of subjects, we focused on this group. Among individuals aged 12–90 yr, the prevalence of TgAbs and TPOAbs was 12.9 and 14.3% (95% confidence intervals of 11.8–14.1 and 13.5–27.9%), respectively.
Rates increased with age for both Abs (Fig. 6B), as previously reported (43), although there was no overall difference in the prevalence rates by age between TgAbs and TPOAbs (P = 0.998). Based on the NOD.H-2h4 mouse data, we hypothesized that analysis of younger individuals would shed light on the temporal emergence of TgAbs vs. TPOAbs in humans. In individuals aged 12–19 yr, 7.5% (3.6–7.9%) were positive for TgAbs vs. only 4.6% (1.5–4.3%) positive for TPOAbs. However, this difference was not statistically significant.
Next, we examined TgAbs and TPOAbs in family members of four probands with juvenile Hashimoto’s thyroiditis. Blood samples were obtained from probands and family members at time 0; sampling was repeated after 2 and/or 15 yr and many sera were available for the present investigation. TgAbs and TPOAbs were measured with the same RIA kits used for the NHANES III survey. When sera were tested for an individual at different time points, antibody data were usually consistent, although two siblings lost TgAb and TPOAb activity at follow-up, and some individuals gained low levels of one antibody (Supplemental Table 1). For the following analysis (Fig. 6, C–E), for each individual family member, we chose the serum with the highest TgAb or TPOAb level.
Sera from three of the four probands (no serum was available from the fourth proband) were positive for both TgAbs and TPOAbs (Supplemental Table 1). Among family members, seven siblings and three parents were negative for one or both Abs (Fig. 6C). Intriguingly, significantly more siblings were positive for TgAbs than for TPOAbs (Fisher’s exact test, P = 0.029). Seven siblings and five parents had readily detectable levels of both TgAbs and TPOAbs (Fig. 6D). Individuals with both TPOAbs and TgAbs had much higher antibody levels than individuals positive for only one thyroid autoantibody (note the different y-axes in Fig. 6, C and D). Surprisingly, TPOAb levels were higher than TgAb levels in siblings and parents with both TgAbs and TPOAbs (Fig. 6E). These observations suggest that, at least in families with juvenile Hashimoto’s thyroiditis, TgAbs arise first and are followed by TPOAbs. However, once the antibody response emerges to both thyroid autoantigens, the TPO autoantibodies become dominant.
Discussion
TPOAbs are a major characteristic of human Hashimoto’s disease. In contrast, mouse models of thyroiditis, whether induced or spontaneous, almost invariably involve Tg as the key autoantigen. Compared with the abundant rodent literature for Tg as an autoantigen, TPO has only rarely been used to immunize mice to induce TPO autoantibodies and/or thyroiditis or hypothyroidism (17,18,19,20,21). Moreover, despite one report of antibodies to mouse thyroid membrane antigens in NOD mice (11), TPOAbs have not been shown to arise spontaneously in these mice (16). Unexpectedly, we recently found that TSHR A-subunit transgenic mice depleted of regulatory T cells before immunization with TSHR adenovirus developed extensive thyroid lymphocytic infiltration, hypothyroidism, and antibodies to mouse TPO as well as to mouse Tg (33). These observations raised the possibility that thyroid damage plays a role in the initiation of antibody responses to TPO, an example of a process termed autoimmune escalation (48).
In the present investigation, we asked whether antibodies to TPO are present in several mouse models. Human autoantibodies cross-react poorly with thyroid microsomes from nonprimates (24), and conversely, thyroiditis and hypothyroidism are induced only by conventional immunization with mouse TPO ectodomain (of bacterial origin) or a mouse TPO peptide (20,21). Moreover, for optimal recognition by human antibodies, recombinant TPO needs to be generated in eukaryotic (not prokaryotic) cells (reviewed in Ref. 25). Therefore, to establish unequivocally that TPOAbs develop in mice, we developed an assay based on antibody binding to CHO cells stably expressing full-length murine TPO.
We began by generating adenovirus encoding mouse TPO to investigate whether immunization with membrane-associated TPO would break self-tolerance. C57BL/6 mice were selected because this strain developed Abs and thyroiditis after immunization with bacterial mouse TPO ectodomain protein and conventional adjuvant (20). We anticipated that breaking self-tolerance might not be achieved by the mild stimulatory properties of adenovirus. In addition, because Treg control tolerance to some antigens (49), for some experiments mice were pretreated with anti-CD25 (or anti-CD122) as approaches to deplete Treg. However, most C57BL/6 mice developed TPOAbs, showing that mTPO-Ad immunization alone is sufficient to break self-tolerance. Only a few mice developed minimal thyroiditis (and none had TgAbs).
These findings were unexpected because thyroiditis developed in C57BL/6 mice after Treg depletion and immunization with TSHR adenovirus (31) and, as mentioned above, after immunization with mouse TPO protein and Complete Freund’s Adjuvant (20). Clearly, the induction of extensive thyroiditis by immunization with mouse TPO requires a more potent adjuvant than adenovirus.
We next screened for TPOAbs in another induced model, namely DBA/1 mice in which extensive granulomatous thyroiditis is induced by transferring splenocytes primed and restimulated in vitro with mouse Tg and IL-12 (35,36). Despite high TgAb levels, less than 10% of mice developed marginally positive TPOAbs in the early disease phase and TPOAbs were absent during in the resolution phase. Consequently, granulomatous thyroiditis does not break B cell tolerance to TPO. It should be noted that transgenic mice expressing the chemokine CCL21 in the thyroid develop massive lymphocytic thyroid infiltration in the absence of thyroid antibodies and thyroid function remains normal (50).
Our finding that TPOAbs arise spontaneously in NOD.H-2h4 mice was unexpected and intriguing. Development of TPOAbs was unrelated to the magnitude of thyroiditis, thyrocyte hyperplasia, or iodide supplementation. Instead, the critical factor was the age of the mice, TPOAbs being rare in young mice (2–3 months), more common in 7-month-old animals, and present in all 12-month-old mice. These findings are reminiscent of early studies describing a delay of up to 100 d for antibody spreading from Tg to thyroid microsomal antigen in two rhesus monkeys immunized with purified Tg (51). Returning to NOD.H-2h4 mice, it should be noted that even the highest TPOAb levels were much lower than those attained in C57BL/6 animals immunized with mTPO-Ad.
Incidentally, the use of mouse (not human) TPO was critical for the novel detection of TPOAb in aging NOD.H-2h4 mice. The importance of using mouse TPO is supported by our observation that even very high titer sera from mTPO-Ad-immunized C57BL/6 mice did not cross-react with human TPO. Our findings for lack of cross-reactivity with human TPO differ from studies on C57BL/6 mice more vigorously immunized with mouse TPO protein and Complete Freund’s Adjuvant (20). Of interest, BALB/c mice immunized with human TPO adenovirus had detectable mouse TPOAbs. The amino acid homology between human (52) and mouse (26) TPO is only 73.4%. It is likely that these human-mouse TPO differences, in combination with the particular mouse strain, permitted induction of sufficiently high levels of human-specific TPOAb levels for cross-reactivity with mouse TPO. Similarly, the potent antibody responses to human TPO in BALB/c mice immunized with human TPO and adjuvant, as well as in patients with autoimmune thyroid disease, enabled the isolation of some mouse monoclonal Abs (40) and some recombinant human Fab (30) that recognize mouse TPO.
Based on our unexpected finding in NOD.H-2h4 mice that antibodies to Tg precede those to TPO, we asked whether a similar TgAb/TPOAb time difference occurs in humans. Analysis of the population data from the large NHANES III study (43) revealed no difference in the prevalence of TgAbs vs. TPOAbs, even in the youngest age group (12–19 yr of age). Similarly, Prentice et al. (15) found no difference in the prevalence of TgAbs and TPOAbs in 18 yr olds although, as in the NOD.H-2h4 mice, the prevalence of both thyroiditis autoantibodies increased with age in both human studies (15,43).
In a different analysis of thyroid autoantibody precedence in humans, we examined sera from families with juvenile Hashimoto’s thyroiditis. Some of these families had been previously investigated for thyroid Abs (53) and by ourselves for TPOAb epitopic fingerprints (45). In the present study, we used standardized TgAb and/or TPOAb kits to analyze sera for family members, in some cases at different time intervals for up to 15 yr. Remarkably, among individuals negative for one thyroid antibody, a significantly higher proportion was positive for TgAbs than TPOAbs. These observations parallel our findings in NOD.H-2h4 mice. However, in family members positive for both thyroid Abs, TPOAb levels were significantly higher than for TgAbs. These data suggest that in humans there is a reversal over time in the antibody response from Tg to TPO.
Our evidence that TgAbs arise before TPOAbs in both mice and humans is consistent with some, but not all, biochemical, genetic, and immunological data for these two thyroid antigens. On the one hand, peptides processed from Tg, the most abundant thyroid protein, can be expected to dominate the binding sites of major histocompatibility complex molecules for presentation to T cells. Indeed, Tg peptides were the only identifiable thyroid-specific peptides eluted from major histocompatibility complex class II protein purified from human thyroids (54). Moreover, polymorphisms in Tg (but not TPO), together with HLA (human leukocyte antigen)-DR, confer susceptibility to development of autoimmune thyroid disease (reviewed in Ref. 55) (56). On the other hand, central tolerance at the T cell level should theoretically be greater for Tg than TPO because thymic expression of Tg is higher than TPO in BALB/c and C57BL/6 mice (57). Moreover, B cell tolerance to Tg might be greater than to TPO because Tg (58), unlike TPO (59), is detectable in serum.
Our data indicate that, at least for Abs, these expectations for self-tolerance are not borne out. Nevertheless, other findings support the relevance of the immune response to TPO in humans. First, autopsy studies provide strong evidence that TPOAbs are associated with thyroid lymphocytic infiltration in the absence of clinical disease (60). Second, TPOAbs (but not TgAbs) are predictive of hypothyroidism in population studies (for example Ref. 43). Consequently, TPOAbs represent the major marker of subclinical or ongoing human disease.
In conclusion, our question, do autoantibodies develop to both TPO and Tg in mice as in humans, is answered positively by the unequivocal presence of TPOAbs in spontaneous thyroiditis (NOD.H-2h4 mice) but not in granulomatous thyroiditis induced with Tg (DBA/1 mice). In the NOD.H-2h4 strain, TgAbs and TPOAbs develop in association with lymphocytic infiltration but without the need for thyroid destruction or iodide supplementation. Unexpectedly, TgAbs arise first, followed later by TPOAbs, and this time course in mice parallels that in humans, at least in the relatives of probands with juvenile Hashimoto’s thyroiditis. These findings demonstrate a previously unknown aspect of murine and human thyroid autoimmunity, namely that breaking B cell self-tolerance occurs first for Tg and subsequently for TPO.
Supplementary Material
Acknowledgments
We appreciate the assistance of Kronus Inc., Star ID in providing TgAbs and TPOAbs RIA kits at reduced cost. We are also grateful for contributions by Dr. Boris Catz (Los Angeles, CA).
Footnotes
This work was supported by National Institutes of Health Grants DK54684 (to S.M.M.), DK36182 (to B.R.), and DK19289 (to B.R).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 23, 2010
Abbreviations: Ab, Antibody; CHO, Chinese hamster ovary; Con-Ad, control using adenovirus lacking an insert; hTPO-Ad, adenovirus encoding human TPO; IFN, interferon; IFN-γR, IFN-γ receptor; mTPO-Ad, adenovirus encoding mouse TPO; NHANES, National Health and Nutrition Examination Survey; SCID, severe combined immunodeficiency; Tg, thyroglobulin; TPO, thyroid peroxidase; Treg, T regulatory cells; TSHR, TSH receptor; WT, wild type.
References
- Roitt IM, Doniach D, Campbell PN, Hudson RV 1956 Autoantibodies in Hashimoto’s disease (lymphadenoid goitre). Lancet ii:820–822 [DOI] [PubMed] [Google Scholar]
- Rose NR, Witebsky E 1956 Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immun 76:417–427 [PubMed] [Google Scholar]
- Belyavin G, Trotter WR 1959 Investigations of thyroid antigens reacting with Hashimoto sera. Evidence for an antigen other than thyroglobulin. Lancet i:648–652 [DOI] [PubMed] [Google Scholar]
- Czarnocka B, Ruf J, Ferrand M, Carayon P, Lissitzky S 1985 Purification of the human thyroid peroxidase and identification as the microsomal antigen in thyroid diseases. FEBS Lett 190:147–152 [DOI] [PubMed] [Google Scholar]
- Seto P, Hirayu H, Magnusson RP, Gestautas J, Portmann L, DeGroot LJ, Rapoport B 1987 Isolation of a cDNA clone for the thyroid microsomal antigen: homology with the gene for thyroid peroxidase. J Clin Invest 80:1205–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F, Ruel J, Ludgate M, Swillens S, Alexander N, Vassart G, Dinsart C 1987 Thyroperoxidase, an auto-antigen with a mosaic structure made of nuclear and mitochondrial gene modules. EMBO J 6:4193–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel PS, Rose NR, Kong YC 1977 Induction of autoimmunity in good and poor responder mice with mouse thyroglobulin and lipopolysaccharide. J Exp Med 145:1250–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H, Johnson M, Sharp GC, Kyriakos M 1985 Induction of experimental autoimmune thyroiditis in mice with in vitro activated splenic T cells. Cell Immunol 93:132–143 [DOI] [PubMed] [Google Scholar]
- Wick G, Most J, Schauenstein K, Kromer G, Dietrich H, Ziemicki A, Fassler R, Schwarz S, Neu N, Hala K 1985 Spontaneous autoimmune thyroiditis—a bird’s eye view. Immunol Today 6:359–365 [DOI] [PubMed] [Google Scholar]
- Allen EM, Appel MC, Braverman LE 1986 The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology 118:1977–1981 [DOI] [PubMed] [Google Scholar]
- Bernard NF, Ertug F, Margolese H 1992 High incidence of thyroiditis and anti-thyroid autoantibodies in NOD mice. Diabetes 41:40–46 [DOI] [PubMed] [Google Scholar]
- Rasooly L, Burek CL, Rose NR 1996 Iodine-induced autoimmune thyroiditis in NOD-H2h4 mice. Clin Immunol Immunopathol 81:287–292 [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H, Sharp GC, Medling B, Tang H 1999 Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun 12:157–165 [DOI] [PubMed] [Google Scholar]
- Hutchings PR, Verma S, Phillips JM, Harach SZ, Howlett S, Cooke A 1999 Both CD4(+) T cells and CD8(+) T cells are required for iodine accelerated thyroiditis in NOD mice. Cell Immunol 192:113–121 [DOI] [PubMed] [Google Scholar]
- Prentice LM, Phillips DI, Sarsero D, Beever K, McLachlan SM, Smith BR 1990 Geographical distribution of subclinical autoimmune thyroid disease in Britain: a study using highly sensitive direct assays for autoantibodies to thyroglobulin and thyroid peroxidase. Acta Endocrinol (Copenh) 123:493–498 [DOI] [PubMed] [Google Scholar]
- Verma S, Hutchings P, Guo J, McLachlan S, Rapoport B, Cooke A 2000 Role of MHC class I expression and CD8(+) T cells in the evolution of iodine-induced thyroiditis in NOD-H2(h4) and NOD mice. Eur J Immunol 30:1191–1202 [DOI] [PubMed] [Google Scholar]
- Kotani T, Umeki K, Hirai K, Ohtaki S 1990 Experimental murine thyroiditis induced by porcine thyroid peroxidase and its transfer by the antigen-specific T cell line. Clin Exp Immunol 80:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan SM, Atherton MC, Nakajima Y, Napier J, Jordan RK, Clark F, Rees Smith B 1990 Thyroid peroxidase and the induction of autoimmune thyroid disease. Clin Exp Immunol 79:182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JC, Gardas A, Wan Q, Gora M, Alsharabi G, Wei WZ, Giraldo AA, David CS, Kong YM, Banga JP 2004 Superiority of thyroid peroxidase DNA over protein immunization in replicating human thyroid autoimmunity in HLA-DRB1*0301 (DR3) transgenic mice. Clin Exp Immunol 137:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HP, Banga JP, Kung AW 2004 Development of a murine model of autoimmune thyroiditis induced with homologous mouse thyroid peroxidase. Endocrinology 145:809–816 [DOI] [PubMed] [Google Scholar]
- Ng HP, Kung AWC 2006 Induction of autoimmune thyroiditis and hypothyroidism by immunization of immunoactive T cell epitope of thyroid peroxidase. Endocrinology 147:3085–3092 [DOI] [PubMed] [Google Scholar]
- Quaratino S, Badami E, Pang YY, Bartok I, Dyson J, Kioussis D, Londei M, Maiuri L 2004 Degenerate self-reactive human T-cell receptor causes spontaneous autoimmune disease in mice. Nat Med 10:920–926 [DOI] [PubMed] [Google Scholar]
- Badami E, Maiuri L, Quaratino S 2005 High incidence of spontaneous autoimmune thyroiditis in immunocompetent self-reactive human T cell receptor transgenic mice. J Autoimmun 24:85–91 [DOI] [PubMed] [Google Scholar]
- Doniach D 1975 Humoral and genetic aspects of thyroid autoimmunity. Clin Endocrinol Metab 4:267–285 [Google Scholar]
- McLachlan SM, Rapoport B 1992 The molecular biology of thyroid peroxidase: cloning, expression and role as autoantigen in autoimmune thyroid disease. Endocr Rev 13:192–206 [DOI] [PubMed] [Google Scholar]
- Kotani T, Umeki K, Yamamoto I, Takeuchi M, Takechi S, Nakayama T, Ohtaki S 1993 Nucleotide sequence of the cDNA encoding mouse thyroid peroxidase. Gene 123:289–290 [DOI] [PubMed] [Google Scholar]
- Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM 2003 The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim. J Clin Invest 111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, Aliesky HA, Guo J, Rapoport B, McLachlan SM 2006 Blockade of costimulation between T cells and antigen-presenting cells: an approach to suppress murine Graves’ disease induced using thyrotropin receptor-expressing adenovirus. Thyroid 16:427–434 [DOI] [PubMed] [Google Scholar]
- Guo J, McLachlan SM, Pichurin PN, Chen CR, Pham N, Aliesky HA, David CS, Rapoport B 2005 Relationship between thyroid peroxidase T cell epitope restriction and antibody recognition of the autoantibody immunodominant region in HLA DR3 transgenic mice. Endocrinology 146:4961–4967 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Portolano S, Russo D, Hutchison JS, Rapoport B, McLachlan SM 1993 Human organ-specific autoimmune disease: molecular cloning and expression of an autoantibody gene repertoire for a major autoantigen reveals an antigenic dominant region and restricted immunoglobulin gene usage in the target organ. J Clin Invest 92:62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh O, Nagayama Y 2006 Regulation of Graves’ hyperthyroidism with naturally occurring CD4+CD25+ regulatory T cells in a mouse model. Endocrinology 147:2417–2422 [DOI] [PubMed] [Google Scholar]
- Saitoh O, Abiru N, Nakahara M, Nagayama Y 2007 CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology 148:6040–6046 [DOI] [PubMed] [Google Scholar]
- McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A, Aliesky HA, Rapoport B 2007 The link between Graves’ disease and Hashimoto’s thyroiditis: A role for regulatory T cells. Endocrinology 148:5724–5733 [DOI] [PubMed] [Google Scholar]
- Fang Y, Wei Y, Demarco V, Chen K, Sharp GC, Braley-Mullen H 2007 Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis in DBA/1 mice. Am J Pathol 170:875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H, Sharp GC, Tang H, Chen K, Kyriakos M, Bickel JT 1998 Interleukin-12 promotes activation of effector cells that induce a severe destructive granulomatous form of murine experimental autoimmune thyroiditis. Am J Pathol 152:1347–1358 [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H, Sharp GC 2000 Adoptive transfer murine model of granulomatous experimental autoimmune thyroiditis. Int Rev Immunol 19:535–555 [DOI] [PubMed] [Google Scholar]
- Yu S, Sharp GC, Braley-Mullen H 2002 Dual roles for IFN-γ, but not for IL-4, in spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol 169:3999–4007 [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA 2000 B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:431–440 [DOI] [PubMed] [Google Scholar]
- Yu S, Sharp GC, Braley-Mullen H 2008 TGF-β promotes thyroid epithelial cell hyperplasia and fibrosis in IFN-γ-deficient NOD.H-2h4 mice. J Immunol 181:2238–2245 [DOI] [PubMed] [Google Scholar]
- Ruf J, Toubert ME, Czarnocka B, Durand-Gorde JM, Ferrand M, Carayon P 1989 Relationship between immunological structure and biochemical properties of human thyroid peroxidase. Endocrinology 125:1211–1218 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Rapoport B 1978 Studies on the binding of radiolabeled thyrotropin to cultured human thyroid cells. Endocrinology 103:2011–2019 [DOI] [PubMed] [Google Scholar]
- Guo J, McLachlan SM, Hutchison S, Rapoport B 1998 The greater glycan content of recombinant human thyroid peroxidase of mammalian than on insect cell origin facilitates purification to homogeneity of enzymatically active protein remaining soluble at high concentration. Endocrinology 139:999–1005 [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE 2002 Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- Gunter EW, Lewis BG, Koncikowski SM 1996 Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Atlanta: U.S. Department of Health and Human Services; Public Health Service Centers for Disease Control and Prevention, National Center for Environmental Health, and Hyattsville, MD: National Center for Health Statistics [Google Scholar]
- Jaume JC, Burek CL, Hoffman WH, Rose NR, McLachlan SM, Rapoport B 1996 Thyroid peroxidase autoantibody epitopic ‘fingerprints’ in juvenile Hashimoto’s thyroiditis: evidence for conservation over time and in families. Clin Exp Immunol 104:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Wei Y, Sharp GC, Braley-Mullen H 2003 Mechanisms of spontaneous resolution versus fibrosis in granulomatous experimental autoimmune thyroiditis. J Immunol 171:6236–6243 [DOI] [PubMed] [Google Scholar]
- Yu S, Sharp GC, Braley-Mullen H 2006 Thyroid epithelial cell hyperplasia in IFN-γ deficient NOD.H-2h4 mice. Clin Immunol 118:92–100 [DOI] [PubMed] [Google Scholar]
- Brent L, Cohen IR, Doherty PC, Feldmann M, Matzinger P, Ghost Lab, Holgate ST, Lachmann P, Mitchison NA, Nossal G, Rose NR, Zinkernagel R 2007 Crystal-ball gazing—the future of immunological research viewed from the cutting edge. Clin Exp Immunol 147:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M, Sakaguchi S 2007 Natural regulatory T cells: mechanisms of suppression. Trends Mol Med 13:108–116 [DOI] [PubMed] [Google Scholar]
- Martin AP, Coronel EC, Sano G, Chen SC, Vassileva G, Canasto-Chibuque C, Sedgwick JD, Frenette PS, Lipp M, Furtado GC, Lira SA 2004 A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J Immunol 173:4791–4798 [DOI] [PubMed] [Google Scholar]
- Rose NR, Skelton FR, Kite Jr JH, Witebsky E 1966 Experimental thyroiditis in the rhesus monkey. 3. Course of the disease. Clin Exp Immunol 1:171–188 [PMC free article] [PubMed] [Google Scholar]
- Magnusson RP, Chazenbalk GD, Gestautas J, Seto P, Filetti S, DeGroot LJ, Rapoport B 1987 Sequences of interest: molecular cloning of the complementary deoxyribonucleic acid for human thyroid peroxidase. Mol Endocrinol 1:856–861 [DOI] [PubMed] [Google Scholar]
- Burek CL, Hoffman WH, Rose NR 1982 The presence of thyroid autoantibodies in children and adolescents with autoimmune thyroid disease and in their siblings and parents. Clin Immunol Immunopathol 25:395–404 [DOI] [PubMed] [Google Scholar]
- Muixí L, Carrascal M, Alvarez I, Daura X, Martí M, Armengol MP, Pinilla C, Abian J, Pujol-Borrell R, Jaraquemada D 2008 Thyroglobulin peptides associate in vivo to HLA-DR in autoimmune thyroid glands. J Immunol 181:795–807 [DOI] [PubMed] [Google Scholar]
- Jacobson EM, Tomer Y 2007 The genetic basis of thyroid autoimmunity. Thyroid 17:949–961 [DOI] [PubMed] [Google Scholar]
- Jacobson EM, Yang H, Menconi F, Wang R, Osman R, Skrabanek L, Li CW, Fadlalla M, Gandhi A, Chaturvedi V, Smith EP, Schwemberger S, Osterburg A, Babcock GF, Tomer Y 2009 Employing a recombinant HLA-DR3 expression system to dissect major histocompatibility complex II-thyroglobulin peptide dynamism: a genetic, biochemical, and reverse immunological perspective. J Biol Chem 284:34231–34243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misharin AV, Rapoport B, McLachlan SM 2009 Thyroid antigens, not central tolerance, control responses to immunization in BALB/c versus C57BL/6 mice. Thyroid 19:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt-Rasmussen U, Hyltoft Petersen P, Date J 1979 Sex and age correlated reference values of serum thyroglobulin measured by a modified radioimmunoassay. Acta Endocrinol (Copenh) 90:440–450 [DOI] [PubMed] [Google Scholar]
- Premawardhana LD, Kiso Y, Phillips DI, Morteo C, Furmaniak J, Rees Smith B 1993 Is TPO detectable in the circulation? Thyroid 3:225–228 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Amino N, Yagawa K, Uemura K, Satoh M, Miyai K, Kumahara Y 1978 Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab 46:859–862 [DOI] [PubMed] [Google Scholar]
- McLachlan SM, Braley-Mullen H, Chen CR, Aliesky H, Pichurin PN, Rapoport B 2005 Dissociation between iodide-induced thyroiditis and antibody-mediated hyperthyroidism in NOD.H-2h4 mice. Endocrinology 146:294–300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.