Abstract
Age-related synaptic change is associated with the functional decline of the nervous system. It is unknown whether this synaptic change is the cause or the consequence of neuronal cell loss. We have addressed this question by examining mice genetically engineered to over- or under-express neuregulin-1 (NRG1), a direct modulator of synaptic transmission. Transgenic mice over-expressing NRG1 in spiral ganglion neurons (SGNs) showed improvements in hearing thresholds, whereas NRG1 −/+ mice show a complementary worsening of thresholds. However, no significant change in age-related loss of SGNs in either NRG1 −/+ mice or mice over-expressing NRG1 was observed, while a negative association between NRG1 expression level and survival of inner hair cells during aging was observed. Subsequent studies provided evidence that modulating NRG1 levels changes synaptic transmission between SGNs and hair cells. One of the most dramatic examples of this was the reversal of lower hearing thresholds by “turning-off” NRG1 over-expression. These data demonstrate for the first time that synaptic modulation is unable to prevent age-related neuronal loss in the cochlea.
Keywords: Aging, Presbycusis, Neuregulin-1, Spiral ganglion neuron, Cochlea
1. Introduction
Functional decline of the nervous system is a cardinal feature of normal aging (Yankner et al., 2008). In the central nervous system (CNS), current views suggest that loss of neuronal connections rather than loss of neurons may be the major cause of age-related functional decline (Rapp and Gallagher, 1996; Scheff and Price, 2003; Rattner and Nathans, 2006; Morrison and Hof, 2007). In the peripheral nervous system (PNS), age-related loss of both synapses and neurons contribute to this functional decline (Coggan et al., 2004; Ohlemiller, 2006; Thrasivoulou et al., 2006). It is unclear, however, whether age-related synaptic change is the cause or simply an associated manifestation of neuronal loss in the PNS. One way to address this issue directly is to examine whether modulations of synaptic transmission at the adult stage can change age-related neuronal loss.
Neuregulin-1 (NRG1) is known to be important for synaptic transmission (Talmage and Role, 2004; Corfas et al., 2005; Buonanno et al., 2008; Mei and Xiong, 2008). The NRG1 gene encodes over 15 transmembrane and secreted isoforms (Fischbach and Rosen; 1997; Falls, 2003). Based on different amino-termini, NRG1 isoforms are classified into three types: type-I NRG1 has an immunoglobulin-like (Ig-like) domain, followed by a region of high glycosylation; type-II has a kringle-like domain plus an Ig-like domain; type-III has a cysteine-rich domain. Most isoforms are transmembrane proteins, which contain an epidermal growth factor- (EGF-) like extracellular domain, a transmembrane region, and an intracellular cytoplasmic domain. Extensive work has shown that NRG1 plays a critical role for synaptic transmission by both its forward and backward signaling pathways (for reviews, Bao, 2007; Buonanno et al., 2008; Mei and Xiong, 2008). NRG1 forward signaling pathways, via binding of its extracellular domains to erbB receptors, are able to regulate the expression of synaptic proteins such as neurotransmitter receptors and ion channels (Okazi et al., 1997; Liu et al., 2001; Okada and Corfas, 2004; Li et al., 2007; Zhong et al., 2008). The forward signaling pathways also contribute to the development and maturation of glial cells (Adlkofer and Lai, 2000; Taveggia et al., 2005; Birchmeier and Nave, 2008). NRG1 backward signaling pathways, via nuclear translocation of its cytoplasmic domain, are able to up-regulate apoptotic and synaptic gene expression (Bao et al., 2003; 2004). In the cochlea, type-III NRG1 is highly expressed in postsynaptic spiral ganglion neurons (SGNs). Its erbB receptors are present in presynaptic hair cells, Schwann glial cells, and supporting cells of the organ of Corti (Morley, 1998; Zhang et al., 2002; Bao et al., 2003; Hume et al., 2003; Stankovic et al., 2004). Therefore, NRG1 signaling is critical for synaptic transmission between hair cells and SGNs.
Hearing loss (presbycusis) is the third most prevalent complaint of the elderly (Gates and Mills, 2005; Ohlemiller and Frisina, 2008). In human presbycusis, a pattern of progressive hearing loss, typically starting at the high frequencies, corresponds to a loss of hair cells and SGNs in the basal region of the cochlea. This pattern is observed in C57BL/6J inbred mice, a well-studied animal model for presbycusis (Ohlemiller, 2006). In this model, age-related functional changes in synaptic transmission between inner hair cells and SGNs can be indirectly assessed using the amplitude of Wave I of the auditory brainstem response (ABR) (Melcher and Kiang, 1996). To test whether improvement of synaptic transmission between hair cells and SGNs in adult mice could delay age-related losses of hair cells and SGNs, we established a conditional tissue-specific transgenic model to express NRG1 in mouse SGNs after two months of age, a time after which the development of the auditory system is complete (Rubel and Fritzsch, 2002). This approach is based on the tetracycline-regulated system used successfully for the conditional expression of a variety of genes in transgenic mice (Mayford et al., 1996; Mansuy et al., 1998; Yamamoto et al., 2000). Regulation of the system is achieved through the tetracycline-regulated transactivator (tTA), an artificial fusion protein between the tet-repressor binding domain and a VP16 activation domain. This protein binds specifically to the tetO operator and induces transcription from an adjacent CMV minimal promoter. The combination of both the tTA and tetO elements allows for the continuous expression of a given transgene after induction. Tetracycline or its analog, doxycycline (dox), can bind to tTA and prevent its binding to tetO, thereby inhibiting transcription (Gossen and Bujard, 1992). Tissue specific expression is achieved by controlling the expression of tTA under a tissue specific promoter (Mayford et al., 1996). The advantage of this system is the ability to inhibit transgenic expression at any desired time point, which allows us to directly test our hypothesis without causing developmental complications due to transgene expression.
2. Methods
2.1 Generating NRG1 transgenic mice and animal care
The NRG1 transgenic lines were generated by cloning a mouse DNA fragment of whole NRG1 III-β1a fused with GFP into a pBI-3 plasmid. The new plasmid contained a bi-directional tetO sequence flanked by CMV minimal promoters with lacZ reporter sequences on one side and NRG1 fused with a 4 × lysine sequence followed with GFP and valine at the end on the opposite side. The valine was added at the end of GFP because it affects the maturation (including routing and cleavage) of type 1 membrane proteins (Bosenberg et al., 1992; Briley et al., 1997). Based on our previous studies (Bao et al., 2003; 2004), the cellular distribution and cleavage of this transgene are similar to endogenous NRG1. The whole 7.1 kb PstII-SalI fragment, containing both transgenes, was cut out, gene-cleaned, and microinjected into single-cell CBA×C57BL/6J embryos. The founder was crossed with wild-type CBAxC57BL/6J females. Southern analysis of the founders was used to determine the copy numbers and the integration sites. Positive F1 lines were crossed to the CamKIIa-tTA B line to generate the complete conditional transgenic mouse. Eight of these mice were examined and two expressing NRG1 in SGNs were crossed back to C57BL/6J (10 times). Mice were housed five per cage with food and water available. They were maintained in a noise-controlled environment on a 12 hr light/dark cycle, with light onset at 6:00 a.m. Mice on dox treatment were given 2 mg/ml dox in a 5% sucrose solution. Dox solution was maintained in dark bottles and changed once a week.
2.2. LacZ Staining
The LacZ reporter gene in all eight transgenic lines has the advantage of permitting highly sensitive visualization of β–galactosidase activity in all tissue expressing the transgene (a blue reaction product in the cell). After fixation in 4% paraformaldehyde, tissue was washed three times in PBS solution for 30 minutes each and then incubated with X-gal staining (Melford Laboratories) for 2–12 hours in the dark.
2.3. Real-time RT-PCR Assay for mRNA
Total RNA from individual mice (two cochleae) at each age group was extracted using RNAqueous (Ambion). To avoid any DNA contamination at the final step of RNA extraction, DNase I (1 µl) was added to 49 µl elution buffer in the RNA extraction column. The solution was incubated at 37 °C for 15 minutes and then heated to 100 °C for 5 minutes to denature the DNase I. RNA was quantified with RiboGreen RNA quantification reagent (Molecular Probes, Eugene, OR). Prior to cDNA generation by reverse-transcription, the quality of RNA was determined by examining ribosomal RNA integrity on a 3% denatured agarose gel. One fifth of the total RNA (50 µl) was reverse transcribed in 20 µl reaction mixtures using random hexamers and Superscript II reverse transcriptase (Invitrogen, CA). Standard concentration curves for GAPDH and tested genes were made using their respective cDNA plasmids at five dilutions. Primers used for real-time RT-PCR of these mouse genes were:
GAPDH: 5-CCTGGCCAAGGTCATCCATGACAAC-3 and 5-TGTCATACCAGGAAATGAGCTTGAC-3;
EGFP: 5-CGCACCATCTTCTTCAAGGACGAC-3 and 5-AACTCCAGCAGGACCATGTGATCG-3;
Type-III NRG1: 5-TCTAGTAAGCCTCTGCCTCTGCAT-3 and 5-GCGGTGGAGTGGAGTGTAAGGGA-3;
Type-II NRG1: 5-GAAGAAGGACTCGCTACTCACC-3 and 5-GGCTGACCTCCTTCTTGAGG-3;
Type-I NRG1: 5-ATGTCTGAGCGCAAAGAAGGCAGAGGCAA-3 and 5-CTGTATCTTGACGTTTTGTGGTTTATT-3;
ErbB2: 5-TCTGCCTGACATCCACAGTG-3 and 5-CAGGGATCTCCCGAGCTGGG-3;
ErbB3: 5-CTTACGGGACACAATGCTGA-3 and 5-GGCAAACTTCCCATCGTAGA-3;
ErbB4: 5-TGAACAATGTGATGGCAGGT-3 and 5-TGAAGTTCATGCAGGCAAAG-3;
PSD-95: 5-AGACTCGGTTCTGAGCTATG-3 and 5-TCTTTGGTAGGCCCAAGGAT-3;
SNAP-25: 5-CCTGGGGCAATAATCAGGATGGAG-3 and 5-CGTTGGTTGGCTTCATCAATTCTGG-3;
The expression change of each gene was measured using the LightCycler System 1.5 (Roche). PCR protocols of the LightCycler System for GAPDH and each tested gene were optimized with the LightCycler FastStart DNA MasterPLUS SYBR Green I kit (Roche). One tenth of each 20 µl cDNA was added to a 20 µl PCR reaction mixture containing 1 × PCR buffer, 0.5 µM of each primer, and Master Mix from the kit. The number of completed PCR cycles when the fluorescence intensity exceeded a predetermined threshold was measured automatically during PCR. The second derivative maximum method by the LightCycler Software 3.5.3 (Roche) was used to set this predetermined threshold for each sample, thus, potential human errors were avoided across different age groups. Quantification of the amount of template molecules was achieved by first establishing the standard curve for each gene (including GAPDH), then determining the concentration of each in the sample based on its standard curve. The difference in the initial amount of total RNA among the samples was normalized in every assay by dividing the concentration of each gene by the concentration of the house keeping gene, GAPDH. Besides using melting curve analysis to ensure the right PCR product, each PCR product was examined on a 3% agarose gel. The initial PCR products were cloned and sequenced to ensure correct identity.
2.3. Functional Assays
Mice were anesthetized (80 mg/kg ketamine, 15 mg/kg xylazine, i.p.) and positioned dorsal side up in a custom headholder. Core temperature was maintained at 37°C using a thermostatically controlled heating pad in conjunction with a rectal probe (Yellow Springs Instruments Model 73A). Platinum needle electrodes (Grass) were inserted subcutaneously just behind the right ear (active), at the vertex (reference), and in the back (ground). Electrodes were led to a Grass P15 differential amplifier (0.1–10 kHz, X100), to a custom broadband amplifier (0.1–10 kHz, X1000), then digitized at 30 kHz using a Cambridge Electronic Design micro1401, in conjunction with SIGNAL and custom signal averaging software, operating on a 120 MHz Pentium PC. Sine wave stimuli generated by a Hewlett Packard 3325a digital oscillator were shaped by a custom electronic switch to 5 ms total duration, including 1 ms rise/fall times. The stimuli were amplified by a Crown D150A power amplifier and led to an Alpine SPS-OEOA coaxial speaker located 10 cm directly lateral to the right external auditory meatus. Stimuli were presented freefield and calibrated using a B&K 4135 ¼ inch microphone placed where the pinna would normally be. Toneburst stimuli at each frequency and level were presented 1000 times at 20/s. The minimum sound pressure level required for a response (short-latency negative wave) was determined at 5.0, 10.0, 14.2, 20.0, 28.3, 40.0, and 56.6 kHz, using a 5 dB minimum step size. To establish the input and output curves of ABR Wave I at 10 kHz, we determined the ABR threshold at 10 kHz for each animal (around 16 dB for 2 months old, and 41 dB for 12 months old), and then measured the amplitude of Wave I from peak to baseline. The sound level was increased in 5 dB steps and terminated at 101 dB.
2.4. Quantification of SGNs, missing IHCs, and OHCs
Mice were administered a lethal dose of sodium pentabarbitol and when completely unresponsive, were perfused transcardially with cold 2% paraformaldehyde and 2% glutaraldehyde in PBS. Each cochlea was rapidly isolated, immersed in the same fixative, and the stapes immediately removed. Complete infiltration of the cochlea by fixative was ensured by making a small hole at the apex of the cochlear capsule and gently circulating the fixative over the cochlea using a transfer pipet. After overnight decalcification in sodium EDTA, cochleae were post-fixed in buffered 1% osmium tetroxide, dehydrated in an ascending acetone series, and embedded in Epon. Cochleae were sectioned parallel to the modiolar axis at a thickness of 50 µm. A Nikon microscope equipped with a motorized stage controlled by Stereo Investigator software was used for precise, well-defined movements along the x, y, and z three-dimensional axes. High-resolution images and a thin focal plane were obtained using a 60x oil immersion objective lens from Nikon with a numerical aperture of 1.40. The optical fractionator method was used to sample the SGN number in a fraction of the spiral ganglion as defined by the optical dissector. A clear nucleolus, a large nucleus, and a clearly defined, oval body were used to distinguish SGNs from glial cells. SGN counts were made based on visualization of their nucleolus. For the counts of missing IHCs and OHCs, the sections were reconstructed to include entire length of mouse cochlear basilar membrane with the Stereo Investigator software by focusing on the pillar heads between IHCs and OHCs. We focused the counts at 40% – 70% from the cochlear apex using the 60x oil immersion objective lens. The criterion for identification of missing hair cells was their replacement with a phalangeal scar. The percent loss of IHCs and OHCs was then divided into three segments that represented 10% of the organ of Corti length. All procedures followed NIH guidelines and were approved by the Institutional animal care and use committee.
3. Results
3.1. Characterization of transgenic mice
NRG1 transgenic mice conditionally expressing mouse type-III NRG1 in SGNs were established based on the pBI-3 vector, which contains a bi-directional tetO responsive promoter. This promoter controls the expression of the transgene NRG1-EGFP in one direction and the reporter gene LacZ in another direction (Fig. 1A). The major advantage of using this bi-directional tetO promoter is the relative ease in monitoring the expression of the LacZ reporter gene by simple but sensitive histological staining for the enzymatic activity of β-galactosidase. Eight transgenic lines were obtained and crossed to one transgenic line with the tTA transgene under the control of a calcium/calmodulin kinase II promoter (CamKIIα-tTA). In two transgenic lines, expression of the marker gene, LacZ, was found in SGNs of the cochlea (Fig. 1B), and this expression could be controlled by the administration of doxycycline (dox) in the drinking water (Fig. 1C). Since the expression pattern of both lines in the cochlea was identical, we focused on the one with greater SGN LacZ staining for further studies.
Fig. 1.
Validation of NRG1 conditionally tissue-specific transgenic mice. (A) Schematic diagram of the conditionally tissue-specific transgenic model, which resulted from a crossing of two transgenic mouse lines. One line had the tTA expression under the CamKIIα promoter, and another line we made contained the biTet-O promoter controlling the expression of both the reporter gene LacZ and the transgene NRG1-EGFP. (B) The LacZ staining showed that SGNs were the only cell type expressing the LacZ. (C) The SGN-specific LacZ expression could be turned-on or off by dox. (D) and (E) Real-time RT-PCR quantifications of the transgene expression (with EGFP primers) or the total expression of NRG1 in the cochlea (n=4, t-test, p < 0.05).
The level of transgene expression was quantified by real-time RT-PCR in the cochlea. As expected, the EGFP expression level was significantly higher in 4-month-old animals without dox treatment (Turned-On). The average concentration ratio between EGFP and GAPDH was 1.30 ± 0.4 (10−4) for the “Turned-On” group, and 0.28 ± 0.2 (10−4) for the non-induced control group with dox in their drinking water from pregnancy to 4 months of age (“Turned-Off”). Therefore, there was about 4.6-fold induction in our transgenic model (Fig. 1D). Similarly, a moderate but significant increase of total type-III NRG1 was found in the “Turned-On” group (Fig. 1E). To examine whether over-expression of type-III NRG1 altered expression of other NRG1 isoforms or their receptors, we examined the expression level of NRG1 type-I and type-II, erbB2, erbB3, and erbB4 in both “Turned-On” and their siblings without the transgene construct (WT). There was no significant difference between the “Turned-On” and control groups for the expression of these five genes, while there was a significant difference for type-III NRG1 between the same two groups (Fig. 2). The expression level of type-III NRG1 was 0.43 ± 0.1(10−4) for the control, 0.56 ± 0.1 (10−4) for the “Turned-Off”, and 0.91 ± 0.1(10−4) for the “Turned-On”.
Fig. 2.
No changes in the expression of NRG1 isoforms or their receptors after type-III NRG1 over-expression. (A) RT-PCR analysis of type-I, -II, -III NRG1, and ErbB 2, 3, 4 in cochleae from mice over-expressing type-III NRG1 or their sibling mice without NRG1 transgene at four months of age. No dramatic difference between the control and NRG1 over-expressing mice was observed. (B) Real-time RT-PCR quantification of the same groups of gene expression in the cochlea. No significant difference was found between the control and mice over-expressing NRG1 for NRG1 isoforms and their receptors except type-III NRG1 (n=4, t-test).
3.2. Nrg1 levels and age-related hearing loss
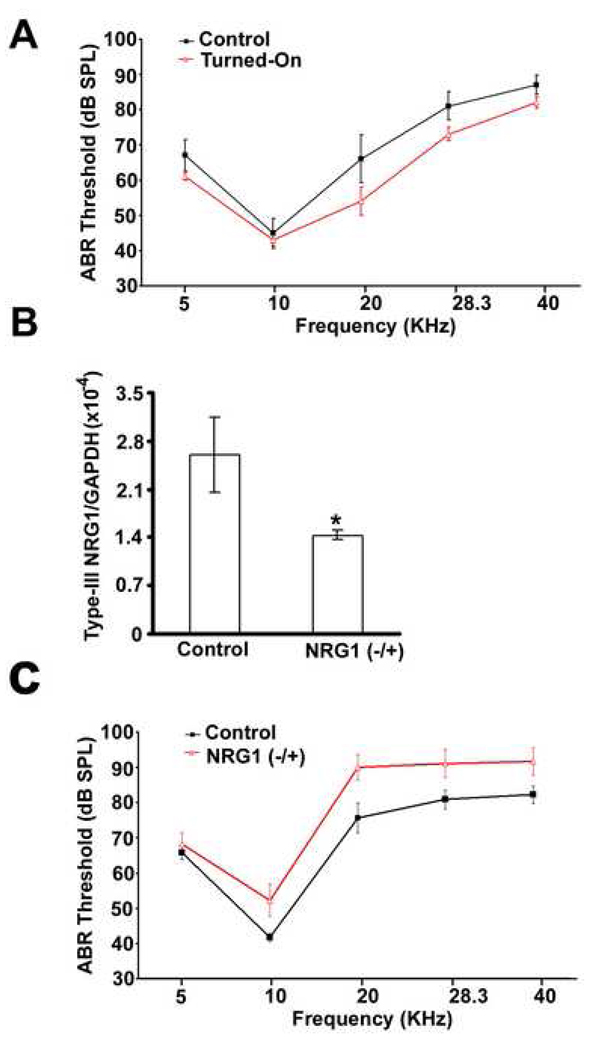
To test whether NRG1 over-expression in SGNs could delay age-related hearing loss, we compared the hearing threshold between the control and NRG-1 over-expression mice under the same C57/6J genetic background at 12 months of age. Dox was withdrawn at two months of age to induce NRG1 over-expression in the “Turned-On” group. ABR measurements showed a moderate but significant reduction of hearing loss for the “Turned-On” group (Fig. 3A). In order to further characterize the relationship between the level of NRG1 and hearing loss, we examined type-III NRG1 −/+ mice under the 129/Sv genetic background. NRG1 expression level in the cochleae of heterozygous mice at four months of age was significantly lower than in controls (Fig. 3B). The ratio between type-III NRG1 and GAPDH was about 1.4 × 10−4 for type-III NRG1 −/+ mice and 2.6 × 10−4 for their wild-type sibling controls. We next compared hearing thresholds between the heterozygous mice and their wild-type sibling controls. Opposite to the findings in NRG1 over-expression mice, at 12 months of age, NRG1 −/+ mice showed significantly worse hearing (Fig. 3C).
Fig. 3.
The NRG1 level modulates ABR thresholds. (A) ABR thresholds (Mean ±S.D) for mice over-expressing NRG1 (red) and their sibling mice without NRG1 transgene (Control; black) at 12 months of age (n=5 for each group, two females and three males; ANOVA, p < 0.05). (B) Real-time RT-PCR quantification of type-III NRG1 in mouse cochleae for the control and NRG1 −/+ mice at four months old (n=4; t-test, p < 0.05). (C) ABR thresholds (Mean ±S.D) for the control (n=8) and NRG1 −/+ mice (n=9) at 12 months old (ANOVA, p < 0.05).
To examine how NRG1 levels influence age-related hearing loss, we examined the total number of SGNs in the same animals, and also quantified outer hair cell (OHC) and inner hair cell (IHC) loss in the region extending 40% – 70% from the cochlear apex (subserving ~10–25 kHz; Ou et al., 2000). This region was selected for analysis because it was the site associated with the clearest preservation of hearing in NRG1 transgenic mice and clearest exacerbation of hearing loss in NRG1 −/+ mice (Fig. 3A and 3C). Surprisingly, no differences were found in age-related loss of SGNs between the control and NRG1 over-expression mice (Fig. 4A; left). For missing OHCs (Fig. 4B; left), two-way ANOVA analysis showed no differences in the mean values among genotypes (p = 0.412), locations (p = 0.125), or between genotypes and locations (p = 0.494). For missing IHCs (Fig. 4C; left), mice over-expressing NRG1 even showed significantly more missing cells (p = 0.006) while no differences were observed in locations (p = 0.252) or between genotypes and locations (p = 0.854). Thus, NRG1 over-expression during aging failed to protect SGNs and was toxic to IHCs.
Fig. 4.
Quantitative comparisons of SGNs, OHCs, and IHCs between the control and NRG1 over-expression mice, or the control and NRG1 −/+ Mice. (A). Quantitative comparison of total SGN number among the same four groups of animals tested in Figure 3. No significant difference was detected between the control and transgenic mice at 12 months old mice (t-test). (B) Quantitative comparison of missing OHCs at the 40–70% region from the apex (two-way ANOVA). (C) Quantitative comparison of missing IHCs in the same area (two-way ANOVA).
Similarly, NRG1 −/+ mice showed no significant difference versus controls in the survival of SGNs (Fig. 4A; right). For missing OHCs (Fig. 4B; right), two-way ANOVA analysis showed no differences in the mean values among genotypes (p = 0.439), locations (p = 0.349), or between genotypes and locations (p = 0.709). For missing IHCs (Fig. 4C; right), no difference was found among genotypes (p =0.761) or locations (p = 0.745). There was a difference between genotypes and locations (p = 0.011): the number of missing IHCs was less in NRG1 −/+ mice. In summary, neither improvements in hearing thresholds in the presence of NRG1 over-expression, nor worsening of hearing in the presence of reduced NRG1 levels (NRG1 −/+ mice), were associated with changes in SGN survival. However, the survival of IHCs was negatively correlated with the expression level of NRG1. To explain this enigmatic finding, we reasoned that NRG1 over-expression might increase only synaptic transmission between hair cells and SGNs, which reduces ABR thresholds. Thus, we further studied possible links between NRG1 levels and synaptic changes in the cochlea during aging.
3.3 Age-related changes in the expression of synaptic genes
We first examined whether the improved hearing thresholds after NRG1 over-expression were due to changes in the expression of synaptic proteins. Using real-time RT-PCR, we measured the expression level of genes encoding two synaptic proteins: (1) the postsynaptic marker postsynaptic density protein-95 (PSD-95); and (2) the presynaptic marker synaptosomal-associated protein-25 (SNAP-25). At four months of age, the level of PSD-95 in the cochlea was not significantly different between these two groups of mice (Fig. 5A). Similar to findings in the hippocampus (Nyffeler et al., 2007), the PSD-95 level dramatically increases in the cochlea in the 12-month-old control group. Interestingly, this age-related increase of PSD-95 was reduced after NRG1 over-expression. A similar observation was also made for the expression of SNAP-25: a significant increase of expression during aging in the control group, but not in the “Turned-On” group (Fig. 5B). These data clearly showed that age-related high expression levels for these two genes were reduced after NRG1 over-expression. However, decreased expression of two important synaptic genes could not explain an improved hearing threshold after NRG1 over-expression.
Fig. 5.
Quantitative comparisons of the expression of two synaptic genes during aging between the control and NRG1 over-expression mice. Age-related up-regulation of PSD-95 (A) and SNAP-25 (B) was found in the cochlea, and this significant up-regulation was diminished for these two genes after NRG1 over-expression (n=4, t-test).
3.4. Detailed temporal analysis of hearing thresholds during aging
We further analyzed the temporal changes of hearing thresholds in the control and “Turned-On” groups at 2, 4, 8, and 10 months of age (Fig. 6). Because the transgenic NRG1 gene was “turned on” at two months of age, there was no difference in the hearing thresholds between control and “Turned-On” groups at this age. At four months of age, however, a difference in the hearing thresholds at high frequency regions (10–40 kHz) was observed. A similar difference was observed at 8 and 10 months. The difference in the hearing threshold between these two groups was relatively constant from 4 to 10 months of age at about 5 dB on average.
Fig. 6.
Comparisons of ABR hearing thresholds between the control and NRG1 over-expression mice during aging. ABR threshold shifts (Mean ±S.D) for both the control and NRG1 over-expression mice were measured at 5, 10, 20, 28.3, and 40 kHz (n=9 for each group, five females and four males). The dotted line for each group represents the average threshold from these five measurement. The average thresholds increased during for both group, but the differences in average threshold between the two groups remained almost the same across ages, around 5 dB.
Similar to the function of NRG1 at the neuromuscular junction (Fischback and Rosen, 1997; Rimer, 2007), we reasoned that if the NRG1 level modulates the synaptic transmission between hair cells and SGNs, the amplitude of the ABR Wave I would be larger in NRG1 over-expression mice and smaller in the NRG1 −/+ mice. Because the elevated hearing thresholds during aging could make this method less sensitive, we focused on the 10 kHz cochlear region, which is the most sensitive hearing frequency region for mice. The amplitude of a 10 kHz tone was increased in 5 dB increments to establish the input-output relation for each mouse. At two months of age, there was no difference between control and NRG1 over-expressing mice (Fig. 7A). At 12 months of age, average response amplitudes were significantly higher in over-expressing mice (Fig. 7B), and significantly lower above 81 dB in NRG1−/+ mice (Fig. 7C). Although ABR thresholds (Fig. 6) and input-output curves for Wave I (Fig. 7) only indirectly reflect synaptic transmission between hair cells and SGNs, they support the argument that NRG1 levels modulate the synaptic connection between hair cells and SGNs. Thus, the improvement of hearing thresholds in 12-month-old mice over-expressing NRG1 could be due to an enhancement of synaptic transmission between SGNs and IHCs, which could even compensate an age-related loss of IHCs.
Fig. 7.
Comparisons of the input-output curves of Wave I between the control and NRG1 transgenic mice. (A) At two months old, there was no difference between these two groups under C57/6J genetic background in their input-out curves (n=5; two-way ANOVA). (B) At 12 months old, the input-output curve was much higher for NRG1 over-expression group. Please note that the amplitude at the same input intensity was much lower compared the amplitude from two months old mice (n=5; two-way ANOVA, p < 0.001). (C) At 12 months old, the input-out curve was lower at the high intensity range (over 81 dB) for the NRG1 −/+ mice, which expressed a lower amount of NRG1 (n=8; two-way ANOVA, p < 0.001).
3.5. NRG1 over-expression temporarily changes hearing thresholds
If the above suggestions were correct, a transient improvement of ABR hearing thresholds during aging would be predicted to disappear as soon as NRG1 over-expression was “turned-off”. Our conditional tissue-specific transgenic mice were ideal for this purpose (Yamamoto et al., 2000). In the control group, transgenic NRG1 expression was “turned-off” throughout life by continuous administration of dox. In another group, NRG1 over-expression was “turned-on” at two months of age, which led to an improvement of ABR thresholds at high frequencies at four months old. NRG1 over-expression was then “turned-off” by application of dox (Turned-On-Off). After two weeks of dox administration, the improvement of ABR thresholds dramatically disappeared (Fig. 8). The data were consistent with that NRG1 improved the hearing thresholds by enhancing synaptic transmission between hair cells and SGNs similar to its function at the neuromuscular junction (Fischback and Rosen, 1997; Rimer, 2007).
Fig. 8.
Reversibility of improved ABR thresholds. ABR thresholds (Mean ±S.D) were measured at four months old for the group with NRG1 over-expression always “turned-off” (n=7; solid black), or the group with NRG1 over-expression “turned-on” at two months old (n-9; solid gray). ABR thresholds (Mean ±S.D) were measured again for the late group after NRG1 over-expression was subsequently “turned-off” for two weeks.
4. Discussion
Our results showed that NRG1 could regulate hearing thresholds in mice. Conditional NRG1 over-expression in SGNs improved hearing thresholds, whereas hearing thresholds worsened in heterozygous mice (NRG1 −/+) having a sub-normal amount of NRG1. The decrease of hearing thresholds after NRG1 over-expression did not result from preservation of SGNs or hair cells during aging, but was associated with increased amplitude of ABR Wave I. Finally, improvement of hearing thresholds in NRG1 over-expression mice was reversed when NRG1 over-expression was “turned-off”. These observations suggest that the improvement in hearing sensitivity is due to the enhancement of synaptic transmission between SGNs and hair cells by NRG1. Thus, our novel finding suggests that enhancing synaptic transmission during aging does not delay age-related neuronal loss in the cochlea.
In the cochlea, previous studies have clearly demonstrated degeneration of SGN synaptic terminals and loss of SGNs and hair cells during aging (Stamataki et al., 2006; White et al., 2000; Zimmermann et al., 1995). It was unclear whether synaptic loss in the PNS was the cause or the consequence of age-related neuronal loss. We originally thought that loss of SGN synaptic terminals contributed to loss of SGNs and hair cells during aging. The conclusion based on current data suggested that high NRG1 levels could improve synaptic transmission in the cochlea without protecting SGNs (even harmful to IHCs) in vivo during aging. However, the amount of NRG1 expression is determined only at the mRNA level, which may not reflect its expression at the protein level. In addition, it is still impossible to directly measure synaptic transmission between hair cells and SGNs in vivo during aging, we did not have the direct evidence to prove that NRG1 could enhance synaptic transmission between single IHC and SGN. Nevertheless, the conclusion has been strongly supported by the following observations: (1) the difference in hearing threshold at different ages (from 2 to 12 months old) showed a consistent 4–5 dB improvement for mice moderately over-expressing NRG1 compared to controls mice; (2) the amplitude of Wave I at 10 kHz was related to the NRG1 level: a significant increase after NRG1 over-expression and a significant decrease in NRG1 −/+ mice respectively; (3) the improvement in hearing sensitivity offered by NRG1 over-expression was transient and disappeared as soon as it was “turned-off” by administration of doxycycline; and (4) there was an age-related change in the expression of synaptic proteins after cochlear NRG1 over-expression. The last piece of the argument needed further discussion because it was counterintuitive.
Synaptic loss is a hallmark of normal aging; however, studies have demonstrated an age-related increase in total synaptic contact area (Scheff and Price, 2003). Age-induced increases in protein expression of PSD-95, the gluR1 subunit of AMPA channels (Nyffeler et al., 2005), synapsin I, and the alpha subunit of the type II voltage-gated sodium channel (Burger et al., 2007) have been also found. It has been suggested that the increase in total synaptic contact area, along with increased synaptic protein expression, act as compensatory mechanisms to alleviate the age-related loss of synaptic transmissions (Nyffeler et al., 2005; Scheff and Price, 2003). In our study, NRG1 was expressed only by SGNs in the cochlea (Bao et al., 2003; Morley 1998). Any changes in synaptic marker expression due to NRG1 over-expression came mainly from the synapse between hair cells and SGNs. Because increasing NRG1 expression had no effect in the expression of genes encoding both pre- and post-synaptic proteins at four months of age, and we observed a less pronounced increase in expression of the same synaptic markers compared to controls at 12 months age, the data suggested a preservation of synaptic transmission between SGNs and hair cells after NRG1 over-expression. In addition, we observed a negative association between the expression level of NRG1 and IHC survival. In the CNS, alteration of NRG1 signaling can modulate glutamatergic synaptic transmission through α-amino-3-hydroxy-5-methyl-4 isoazolepropionic acid (AMPA) receptors (for reviews: Buonanno et al., 2008; Corfas et al., 2004; Mei and Xiong, 2008). In the cochlea, AMPA receptors are present both presynaptically (GluR4) and postsynaptically (GluR2 and 3; Chen et al., 2007; Luo et al., 1995; Matsubara et al., 1996). Therefore, the negative association between the expression level of NRG1 and IHC survival may be due to glutamate-induced excitotoxicity via AMPA receptors whose expression levels could be mediated by NRG1 signaling during aging. Further studies are needed to determine this possibility.
Extensive synaptic and neuronal loss contributes to the pathology of age-related neurodegenerative diseases such as Alzheimer’s disease (Morrison and Hof 2007; Scheff and Price 2003; Smith et al., 2000; Yankner et al., 2008). This raises a similar but clinically important question of whether synaptic loss in this case is the cause or the result of neuronal loss. Our data suggests a third possibility, a parallel independent biological process for age-related loss of neurons and synapses. Because neuronal aging is the common predisposing factor for neurodegenerative diseases, this possibility is worth exploring. In addition, our data suggests that the level of NRG1 can modulate synaptic transmission in the cochlea, similar to the neuromuscular junction. Modulating NRG1 function might be used clinically for tinnitus, by transiently diminishing synaptic transmission in the cochlea.
Acknowledgements
Special thanks to Barbara Bohne for her critical reading of the manuscript. This research has been funded in part by grants from the National Institute of Health R01AG024250, R21DC010489, DC004395, P30DC004665, and T32DC000022-22.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bao J. Signal transduction by the cytoplasmic domain of Neuregulin-1 and its roles in neuronal aging. Curr. Signal. Trans. Therapy. 2007;2:240–245. [Google Scholar]
- Bao J, Lei D, Du Y, Ohlemiller KK, Beaudet AL, Role LW. Requirement of nicotinic acetylcholine receptor subunit beta2 in the maintenance of spiral ganglion neurons during aging. J. Neurosci. 2005;25:3041–3045. doi: 10.1523/JNEUROSCI.5277-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat. Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J. Cell. Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56:1491–1497. doi: 10.1002/glia.20753. [DOI] [PubMed] [Google Scholar]
- Bosenberg MW, Pandiella A, Massagué J. The cytoplasmic carboxy-terminal amino acid specifies cleavage of membrane TGF alpha into soluble growth factor. Cell. 1992;71:1157–1165. doi: 10.1016/s0092-8674(05)80064-9. [DOI] [PubMed] [Google Scholar]
- Briley GP, Hissong MA, Chiu ML, Lee DC. The carboxyl-terminal valine residues of proTGF alpha are required for its efficient maturation and intracellular routing. Mol. Biol. Cell. 1997;8:1619–1631. doi: 10.1091/mbc.8.8.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Kwon OB, Yan L, Gonzalez C, Longart M, Hoffman D, Vullhorst D. Neuregulins and neuronal plasticity: possible relevance in schizophrenia. Novartis Found. Symp. 2008;289:165–177. doi: 10.1002/9780470751251.ch13. [DOI] [PubMed] [Google Scholar]
- Burger C, López MC, Feller JA, Baker HV, Muzyczka N, Mandel RJ. Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol. Learn. Mem. 2007;87:21–41. doi: 10.1016/j.nlm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kujawa SG, Sewell WF. Auditory sensitivity regulation via rapid changes in expression of surface AMPA receptors. Nat Neurosci. 2007;10:1238–1240. doi: 10.1038/nn1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan JS, Grutzendler J, Bishop DL, Cook MR, Gan W, Heym J, Lichtman JW. Age-associated synapse elimination in mouse parasympathetic ganglia. J. Neurobiol. 2004;60:214–226. doi: 10.1002/neu.20022. [DOI] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat. Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp. Cell. Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Fischbach GD, Rosen KM. ARIA: a neuromuscular junction neuregulin. Annu. Rev. Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. (USA) 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Kirkegaard M, Oesterle EC. ErbB expression: the mouse inner ear and maturation of the mitogenic response to heregulin. J. Assoc. Res. Otolaryngol. 2003;4:422–443. doi: 10.1007/s10162-002-3008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Woo R, Mei L, Manilow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ford B, Mann MA, Fischbach GD. Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J. Neurosci. 2001;21:5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Brumm D, Ryan AF. Distribution of non-NMDA glutamate receptor mRNAs in the developing rat cochlea. J Comp Neurol. 1995;361:372–382. doi: 10.1002/cne.903610303. [DOI] [PubMed] [Google Scholar]
- Mansuy IM, Winder DG, Moallem TM, Osman M, Mayford M, Hawkins RD, Kandel ER. Inducible and reversible gene expression with the rtTA system for the study of memory. Neuron. 1998;21:257–265. doi: 10.1016/s0896-6273(00)80533-4. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neuronal development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher JR, Kiang NY. Generators of the brainstem auditory evoked potential in cat. III: Identified cell populations. Hear. Res. 1996;93:52–71. doi: 10.1016/0378-5955(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Morley BJ. ARIA is heavily expressed in rat peripheral auditory and vestibular ganglia. Brain Res. Mol. Brain. Res. 1998;54:170–174. doi: 10.1016/s0169-328x(97)00355-0. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging cerebral cortex. Int. Rev. Neurobiol. 2007;81:41–57. doi: 10.1016/S0074-7742(06)81004-4. [DOI] [PubMed] [Google Scholar]
- Nyffeler M, Zhang WN, Feldon J, Knuesel I. Differential expression of PSD proteins in age-related spatial learning impairments. Neurobiol. Aging. 2007;28:143–155. doi: 10.1016/j.neurobiolaging.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Frisina RD. Clinical characterization of age-related hearing loss and its neural and molecular bases. In: Schacht J, Popper A, Fay R, editors. Auditory trauma, Protection and Treatment. New York: Springer-Verlag; 2008. pp. 45–194. (Chapter 6) [Google Scholar]
- Okada M, Corfas G. Neuregulin1 downregulates post-synaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus. 2004;14:337–344. doi: 10.1002/hipo.10185. [DOI] [PubMed] [Google Scholar]
- Okazi M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-beta induces expression of NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- Ou HC, Harding GW, Bohne BA. An anatomically based frequency-place map for the mouse cochlea. Hear. Res. 2000;145:123–129. doi: 10.1016/s0378-5955(00)00082-4. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. (USA) 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat. Rev. Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- Rimer M. Neuregulins at the neuromuscular synapse: past, present, and future. J. Neurosci. Res. 2007;85:1827–1833. doi: 10.1002/jnr.21237. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu. Rev. Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA. Synaptic pathology in Alzheimer’s disease: a review of ultrastructural findings. Neurobiol, Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in synaptophysin immunoreactivity predict spatial learning impairments in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear. Res. 2006;221:104–118. doi: 10.1016/j.heares.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J. Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmage DA, Role LW. Multiple personalities of neuregulin gene family members. J. Comp. Neurol. 2004;472:134–139. doi: 10.1002/cne.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear. Res. 2000;141:2–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The aging brain. Annu. Rev. Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ding D, Salvi R. Expression of heregulin and ErbB/Her receptors in adult chinchilla cochlear and vestibular sensory epithelium. Hear. Res. 2002;169:56–68. doi: 10.1016/s0378-5955(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Zhong C, Du C, Hancock M, Mertz M, Talmage DA, Role LW. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. J. Neurosci. 2008;28:9111–9116. doi: 10.1523/JNEUROSCI.0381-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann CE, Burgess BJ, Nadol JB. Patterns of degeneration in the human cochlear nerve. Hear. Res. 1995;90:192–201. doi: 10.1016/0378-5955(95)00165-1. [DOI] [PubMed] [Google Scholar]