Abstract
Objective
To test the hypothesis that the concentration of angiopoietin-2 relative to angiopoietin-1 (Ang-2/Ang-1) may be a useful biologic marker of mortality in acute lung injury (ALI) patients. We also tested the association of Ang-2/Ang-1 with physiologic and biologic markers of activated endothelium.
Design
Prospective observational cohort study.
Setting
Intensive care units in a tertiary care university hospital and a university-affiliated city hospital.
Patients
Fifty-six mechanically ventilated patients with ALI.
Interventions
Baseline plasma samples and pulmonary dead space fraction measurements were collected within 48 hours of ALI diagnosis.
Measurements and Main Results
Plasma levels of Ang-1 and Ang-2 and of biomarkers of endothelial activation were measured by ELISA. Baseline Ang-2/Ang-1 was significantly higher in patients who died [median 58 (IQR 17–117) vs. 14 (IQR 6–35), p=0.01]. In a multivariable analysis stratified by dead space fraction, Ang-2/Ang-1 was an independent predictor of death with an adjusted odds ratio of 4.3 (95% CI 1.3–13.5, p=0.01) in those with an elevated pulmonary dead space fraction (p=0.03 for interaction between pulmonary dead space fraction and Ang-2/Ang-1). Moderate to weak correlation was found with biologic markers of endothelial activation.
Conclusions
The ratio of Ang-2/Ang-1 may be a prognostic biomarker of endothelial activation in ALI patients and, along with pulmonary dead space fraction, may be useful for risk stratification of ALI patients, particularly in identifying subgroups for future research and therapeutic trials.
Keywords: acute lung injury, acute respiratory distress syndrome, angiopoietin, predictor, pulmonary dead space fraction, endothelial activation
Introduction
Acute lung injury (ALI) is a common cause of life threatening acute respiratory failure in children and adults. ALI is characterized by activation of the pulmonary endothelium, disruption of endothelial and alveolar epithelial barriers, and an increase in microvascular permeability, resulting in protein-rich pulmonary edema (1). While the exact pathogenetic mechanisms of this clinical disorder are unknown, an understanding of these mechanisms is critical for the development of novel therapies (2). Identification of cell-specific biologic markers whose levels are up or down-regulated in early ALI can provide such insight.
Vascular growth factors, such as angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2), have been proposed as biologic markers for ALI. Ang-1 and Ang-2 classically act in an agonist/antagonist manner on the endothelial tyrosine kinase receptor, Tie-2 (3). Ang-1 stabilizes the endothelium by inhibiting endothelial cell apoptosis and activation and decreasing inflammation (4–9). In contrast, Ang-2 is a context-dependent antagonist to the Tie-2 receptor that is pro-inflammatory and promotes endothelial and epithelial cell apoptosis, increases neutrophil adhesion, and causes cytoskeletal changes to increase interendothelial gaps (10–15). In concert, the two angiopoietins may act directly or indirectly to change the integrity of the vascular endothelium. Given the critical role of endothelial disruption in the formation of protein-rich pulmonary edema fluid, alterations in Ang-1 and Ang-2 may be associated with the pathogenesis of ALI.
While recent studies have demonstrated an association of plasma levels of Ang-1 and Ang-2 with poor clinical outcomes in critically ill patients (15–18), angiopoietin studies specific to ALI patients have been more limited. Higher levels of Ang-2 are found in the pulmonary edema fluid from ALI patients, compared to hydrostatic pulmonary edema fluid (19). Gallagher and colleagues reported elevated levels of Ang-2 in non-survivors of ALI and a rescue effect of Ang-1 on endothelial cells disrupted by plasma with a high concentration of Ang-2 (14). These results suggest that elevations in Ang-2 and decreases in Ang-1 may reflect a pro-inflammatory state best summarized by the ratio of Ang-2 to Ang-1. Given this prior evidence, we hypothesized that the concentration of Ang-2 relative to Ang-1 may be associated with the degree of endothelial injury in ALI. Our primary objective was to test the association between the Ang-2/Ang-1 ratio and clinical outcomes in ALI patients. The second objective was to test the association of the Ang-2/Ang-1 ratio with physiologic and biologic markers of activated endothelium.
Material and Methods
Subjects and patient samples
Fifty-six patients who met the American-European Consensus definition for ALI or acute respiratory distress syndrome (ARDS) (20) were prospectively enrolled from a tertiary care university hospital (Moffitt-Long University Hospital) and a university-affiliated city hospital (San Francisco General Hospital) from 2003 to 2007. Patients were enrolled within 48 hours of ALI/ARDS diagnosis. Eligible patients were mechanically ventilated and had an arterial line in place. Exclusion criteria were age <18 years, prisoners, pregnancy, patients involuntarily admitted for psychiatric reasons, a history of severe COPD or asthma, chronic interstitial lung disease or pulmonary vascular disease, recent pulmonary embolism, acute myocardial infarction within 30 days, history of congestive heart failure, lack of surrogate available for consent, or not committed to aggressive care. The study was approved by the institutional review boards of both hospitals and written informed consent was obtained for all subjects.
Clinical data and outcomes
Clinical data was abstracted from the medical record. Acute Physiology and Chronic Health Evaluation (APACHE) II scores (21) were calculated at baseline. Ventilator data, including measurement of pulmonary dead space fraction (22, 23), were recorded for each subject at the time of baseline plasma sample collection. Pulmonary dead space fraction measurements could not be obtained on three patients. The primary outcome was 28-day in-hospital mortality.
Plasma biomarker measurements
Baseline plasma samples were collected at the time of enrollment in heparinized tubes. After centrifugation, the plasma aliquots were stored at −70°C until the time of analysis. Levels of Ang-1, Ang-2, and receptor for advanced glycation end products (RAGE) were measured in duplicate using commercially available ELISA Kits (R&D Systems, Minneapolis, MN). Plasma levels of biomarkers of inflammation and disordered coagulation and fibrinolysis, specifically interleukin-6 (IL-6), interleukin-8 (IL-8), thrombomodulin, von Willebrand factor antigen (vWF), intercellular adhesion molecule-1 (ICAM-1), protein C, and plasminogen activator inhibitor-1 (PAI-1) have previously been reported in a subset of patients in this cohort (24), and these values were used for some of the analyses.
Statistical analyses
Analysis was conducted with STATA 10.0 (College Station, TX). A two-sided p-value of less than 0.05 was considered significant unless otherwise specified. Plasma Ang-1, Ang-2, and Ang-2/Ang-1 levels were not normally distributed, so data were compared between survivors and non-survivors using a non-parametric test (Mann Whitney rank sum). To test for a nonlinear effect of Ang-2/Ang-1 on mortality, we divided Ang-2/Ang-1 into tertiles and used a nonparametric test to test for trends (Cuzick trend test). To test the association of the Ang-2/Ang-1 ratio with mortality, we applied a natural log-transformation to the Ang-2/Ang-1 ratio and initially created an unadjusted logistic regression model.
For multivariable analysis, we considered baseline clinical features that may have affected outcomes including age, gender, partial pressure of arterial oxygen to fraction of inspired oxygen content (PaO2/FiO2), tidal volume, plateau pressure, and severity of illness, as measured by the APACHE II score (21). Predictors were eliminated from the model using backwards, stepwise regression; all predictors with a p-value less than 0.3 were retained in the final model, as well as age, gender, and APACHE II score for face validity. Because of an observed interaction with Ang-2/Ang-1 ratio and pulmonary dead space fraction, analyses were stratified by pulmonary dead space fraction. This was the only interaction tested and sought as pulmonary dead space fraction may be in part a physiologic marker of endothelial activation (25). P-value for potentially significant interactions was less than 0.1. Model specification was evaluated using the link test (26) and model fit was evaluated using the Hosmer-Lemeshow goodness-of-fit test (27).
Spearman rank correlation coefficients were used to correlate the ratio of Ang-2/Ang-1 with known biomarkers of ALI. Biomarkers levels were not normally distributed and a natural log transformation was applied prior to assessment in a backwards, step-wise logistic regression model. Likelihood ratio testing was used to test the contribution of Ang-2/Ang-1 to the area under the receiver operating characteristic (ROC) curve for the logistic regression model. (28, 29).
Results
Baseline clinical characteristics and angiopoietin levels
Baseline characteristics of the 56 study subjects are summarized in Table 1. The overall in-hospital mortality in the cohort was 34%. Baseline plasma Ang-1 and Ang-2 levels were compared in survivors (n=37) and non-survivors (n=19). There was a trend towards lower baseline Ang-1 levels in non-survivors compared to survivors, although this did not reach statistical significance [median 98 pg/mL (IQR 74–156 pg/mL) v. 140 pg/mL (IQR 93–282 pg/mL), p=0.09]. Baseline Ang-2 levels were higher in non-survivors compared to survivors [median 6300 pg/mL (IQR 2653–10200 pg/mL) v. 2900 pg/mL (IQR 1406–4732 pg/mL), p=0.01]. Baseline Ang-2/Ang-1 ratio was significantly higher in non-survivors compared to survivors [median 58 (IQR 17–117) v. 14 (IQR 6–35), p=0.01, Figure 1].
Table 1.
Baseline characteristics between survivors and non-survivors with acute lung injury*
| Characteristic | Survivors (n=37) | Non-survivors (n=19) |
|---|---|---|
| Age (years) | 51 ± 21 | 51 ± 14 |
| Male | 56% | 47% |
| APACHE II** | 20 ± 6 | 24 ± 5 |
| PaO2/FiO2 | 156 ± 64 | 170 ± 94 |
| Tidal Volume (ml/kg)*** | 6.4 ± 0.9 | 6.7 ± 1.5 |
| Pulmonary Dead Space Fraction | 0.58 ± 0.11 | 0.58 ± 0.12 |
| Sepsis as clinical risk factor for ALI | 22% | 63% |
| Trauma as a clinical risk factor for ALI | 6% | 5% |
Data represented as Mean ± SD or n(%)
Acute physiology and chronic health evaluation II (APACHE II).
Predicted body weight.
Figure 1.
Baseline plasma Ang-1 (a), Ang-2 (b), and Ang-2/Ang-1 (c) comparing survivors (n=37) with non-survivors (n=19). Ang-2/Ang-1 was significantly higher in non-survivors as tested by Mann-Whitney rank sum (p= 0.01). The horizontal line represents the median, box encompasses the 25th through 75th percentiles, and whiskers encompass the 10th through 90th percentiles.
Higher Ang-2/Ang-1 ratio is associated with mortality in ALI patients
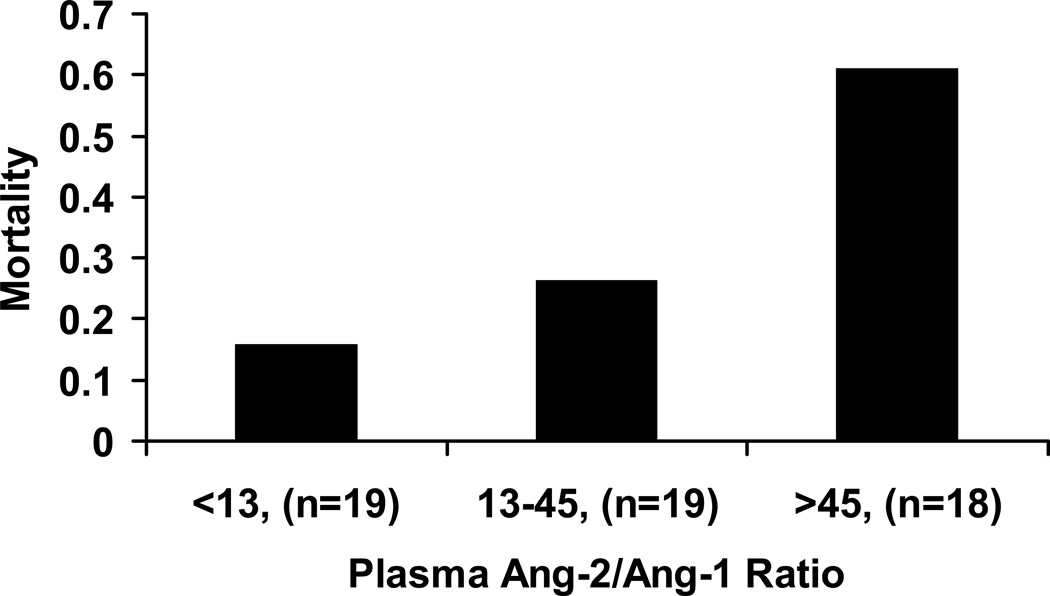
In univariate analysis, baseline plasma Ang-2/Ang-1 ratio was significantly associated with death (OR 1.9, 95% CI 1.2–3.2, p=0.01). Figure 2 summarizes the observed mortality by tertile of baseline Ang-2/Ang-1 ratio. In comparison to the overall mortality of 34%, a categorical increase in death was observed from 15% in the lowest tertile to 60% in the highest tertile, with a significant test for trend across tertiles (p=0.004). On further analysis of the association between baseline Ang-2/Ang-1 ratio and death, we detected a significant interaction between Ang-2/Ang-1 and pulmonary dead space fraction, a physiologic marker of endothelial activation (p=0.03). Therefore, we divided patients into groups with high and low pulmonary dead space fractions based on the mean pulmonary dead space fraction (0.58) from a large prior study that demonstrated that increased dead space fraction is associated with higher mortality in patients with ALI/ARDS (25). Baseline plasma Ang-2/Ang-1 ratio was strongly associated with death only in patients who had a high level of pulmonary dead space fraction (OR 4.7, 95% CI 1.5–14.7, p=0.009, Figure 3). Ang-2/Ang-1 ratio was not predictive of death in patients with a lower level of pulmonary dead space fraction (OR 1.1, 95% CI 0.5–2.1, p=0.89). In addition, pulmonary dead space fraction independent of Ang-2/Ang-1 was not associated with an increased odds of death (OR 0.7, 95% CI 0.1–9.5, p=0.8).
Figure 2.
Observed mortality according to tertile of baseline plasma Ang-2/Ang-1 (n=56). Test for trend across tertiles, p=0.004.
Figure 3.
Box plots of baseline plasma Ang-2/Ang-1 ratio by survival status stratified by level of pulmonary dead space fraction (n=53). Baseline Ang-2/Ang-1 was associated with in-hospital mortality only in the high pulmonary dead space fraction group. Comparisons were made with the Mann-Whitney rank sum test. The horizontal line represents the median, box encompasses the 25th through 75th percentiles, and whiskers encompass the 10th through 90th percentiles.
Multivariable models
The association between plasma Ang-2/Ang-1 ratio and death persisted in multivariable models. In the initial analysis, the predictors included in the model were baseline Ang-2/Ang-1 ratio, age, gender, APACHE II score, PaO2/FiO2 ratio, tidal volume, and plateau pressure. Predictors for retention in the final model were selected using a backwards, stepwise approach in the overall cohort. Apart from the baseline Ang-2/Ang-1 ratio, the APACHE II score and the PaO2/FiO2 ratio also were selected as independent predictors for death in the overall cohort (data not shown). Given the significant interaction of pulmonary dead space fraction with baseline Ang-2/Ang-1 ratio, we subsequently stratified our multivariable analysis of the association of Ang-2/Ang-1 ratio with death by pulmonary dead space fraction. Higher baseline plasma Ang-2/Ang-1 was the only independent predictor of death with an adjusted odds ratio of 5.5 (95% CI 1.2–24.9, p=0.03, Table 2) in those with a higher pulmonary dead space fraction. Misspecification of the model was not present as assessed by link test (p=0.81). The Hosmer-Lemeshow test indicated that the model was not over-fit (p=0.31). To test for potential clinical utility of Ang-2/Ang-1 in this model, we constructed ROC curves. The predictive accuracy of the model of clinical and physiologic variables alone (APACHE II score, age, gender, PaO2/FiO2 ratio, tidal volume, and plateau pressure) was modest as evidenced by area under the ROC curve (0.79). With Ang-2/Ang-1 added to the model, the area under the ROC curve increased to 0.92 with a significant contribution by likelihood ratio testing (p=0.003). We specifically chose not to adjust for sepsis in our multivariable model because one of the pathogenic mechanisms of sepsis-related ALI may be through disruption of the vascular endothelium mediated by levels of angiopoietins; thus if Ang-2/Ang-1 mediates the impact of sepsis on outcomes, adjusting for sepsis in the model would eliminate the opportunity to test Ang-2/Ang-1 as a prognostic predictor. There was no significant association between baseline Ang-2/Ang-1 ratio and mortality in patients with low pulmonary dead space fraction.
Table 2.
Multivariable logistic regression model for predictors of mortality in 24 patients with high pulmonary dead space fraction (>0.58)
| Predictor | Adjusted* Odds Ratio for Mortality (95% CI) | P-value |
|---|---|---|
| Ang-2/Ang-1 | 5.52 (1.22–24.9) | 0.03 |
| Age | 1.80 (0.59–5.54) | 0.30 |
| Male gender | 0.11 (0.002–8.3) | 0.32 |
| APACHE II | 1.24 (0.75–2.05) | 0.39 |
| PaO2/FiO2 | 0.71 (0.22–2.28) | 0.56 |
| Tidal volume | 2.20 (0.16–30.4) | 0.56 |
| Plateau pressure | 1.13 (0.82–1.54) | 0.46 |
Adjusted for log Ang-2/Ang-1, per 10-year increase in age, male gender, per point increase APACHE II score, and per 50 unit decrease PaO2/FiO2.
The Ang-2/Ang-1 ratio is associated with biologic markers of endothelial activation
Higher pulmonary dead space fraction in ALI may be due to both endothelial activation and microvascular thrombosis. Given the significant interaction of elevated Ang-2/Ang-1 ratio and high pulmonary dead space fraction, we tested the association between the Ang-2/Ang-1 ratio and known biomarkers associated with poor clinical outcomes and endothelial activation as well as other specific pathways of lung injury. A moderate and highly significant correlation was found with thrombomodulin, a marker of activated endothelium (rs = 0.64, p<0.0001). Significant, but weaker correlation was found with other markers of activated endothelium, namely vWF, ICAM-1, and lower levels of protein C (Table 3). Moderate to weak correlation was also found with markers of systemic inflammation, IL-8 and IL-6, but there was no significant correlation with the alveolar epithelial injury marker, RAGE.
Table 3.
Spearman rank correlation coefficients of Ang-2/Ang-1 with known biologic markers of acute lung injury.
| Marker | Correlation with Ang-2 /Ang-1 | P-value |
|---|---|---|
| Activated Endothelium | ||
| Thrombomodulin | 0.64 | <0.0001 |
| von Willebrand Factor Antigen | 0.40 | 0.01 |
| Intercellular Adhesion Molecule-1 | 0.38 | 0.02 |
| Protein C | −0.32 | 0.05 |
| Plasminogen Activator Inhibitor-1 | 0.16 | 0.34 |
| Epithelial Injury | ||
| Receptor for Advanced Glycation End Products | 0.12 | 0.38 |
| Systemic Inflammation | ||
| Interleukin-8 | 0.51 | 0.0008 |
| Interleukin-6 | 0.45 | 0.004 |
We used backwards, step-wise logistic regression to test whether the Ang-2/Ang-1 ratio was an independent predictor of mortality when included in a model with these other biologic markers. Ang-2/Ang-1 remained in the model as an independent predictor of mortality although the confidence interval widened (OR 10, 95%CI 1.3–80, p=0.04). Other independent predictors that remained in the model included markers of disordered coagulation (PAI-1, protein C), alveolar epithelial injury (RAGE), and systemic inflammation (IL-6). To examine the contribution of the Ang-2/Ang-1 ratio in relation to these other biologic markers, we tested the model with and without Ang-2/Ang-1. The area under the ROC curve increased to 0.97 from 0.91 when Ang-2/Ang-1 was included in the model. The contribution of the Ang-2/Ang-1 ratio to the logistic model was significant by likelihood ratio testing (p=0.0008), and the model was not over-fit by the Hosmer-Lemeshow test (p=0.99).
Discussion
In this study, higher Ang-2/Ang-1 ratio was an independent predictor of mortality in ALI patients even after adjustment for known clinical and physiologic variables associated with poor outcomes, including age and severity of illness. There was a significant interaction between the pulmonary dead space fraction and the Ang-2/Ang-1 ratio; that is, the predictive value of the Ang-2/Ang-1 ratio for mortality differed by level of the pulmonary dead space fraction. In patients with high pulmonary dead space fraction, the Ang-2/Ang-1 ratio was significantly elevated in non-survivors and the only predictor of mortality in a multivariate model. Ang-2/Ang-1 made a significant contribution to the predictive accuracy of this model of clinical parameters with an increase in the area under the ROC curve from 0.79 to 0.92. Furthermore, Ang-2/Ang-1 was also associated with biologic markers of endothelial injury and activation, but not epithelial injury, and remained an independent predictor of mortality in a multivariable model controlling for these biologic markers. In aggregate, these results indicate that the ratio of Ang-2/Ang-1 may be a useful prognostic marker of endothelial activation in ALI patients with higher dead space fraction, linking plasma biomarker measurements of endothelial activation with a physiologic measure of ALI severity.
The association of the Ang-2/Ang-1 ratio with endothelial activation and injury is reinforced by a growing body of evidence in both animal and in vitro models (10, 11, 30–32). Stimulation of microvascular endothelial cells in the lung has been shown to release Ang-2; incubation with Ang-1 attenuates the release of Ang-2 (32). Ang-2 also has been associated with vascular leakage in inflammatory settings (10). Despite this evidence, the cellular source of Ang-1 and Ang-2 remains controversial. Bhandari et al reported epithelial production of Ang-2 in a mouse model of hyperoxic lung injury while others have suggested an endothelial source (19, 31). We thus measured Ang-2/Ang-1 in association with several biomarkers to assess cellular compartments of ALI, including RAGE as a marker of epithelial injury. Our results suggest that Ang-2/Ang-1 reflects endothelial, not epithelial injury. Although the source of Ang-1 and Ang-2 in the plasma cannot be determined from the current study, the association between lower levels of Ang-1 and higher levels of Ang-2 with severity of ALI/ARDS suggests that the relative levels of these two mediators may be important determinants of the severity of endothelial injury.
Our results are consistent with and expand upon prior human studies. Plasma levels of Ang-2 were higher in trauma patients with ALI/ARDS in comparison to controls (34) and Ang-2 was the only biomarker of endothelial injury identified in a diagnostic panel of biomarkers that differentiated ALI from non-ALI. Also, serum Ang-2 levels were significantly increased in severe sepsis compared to controls (35). Ang-2 levels were higher in patients with septic shock compared to septic patients without shock and Ang-2 levels were associated with adverse clinical outcomes (33). In patients with ALI/ARDS, higher Ang-2 levels were associated with increased pulmonary leak of 67Gallium-transferrin, and Ang-2 levels correlated with a marker of endothelial injury, plasma von Willebrand factor (16, 35). In another study, the same group reported that plasma levels of the soluble angiopoietin-binding Tie-2 receptor were elevated in sepsis but did not affect the relationship between Ang-1, Ang-2 and pulmonary leak (36).
The significant interaction of the pulmonary dead space fraction and the ratio of Ang-2/Ang-1 in this study suggests a potential biologic impact of Ang-2/Ang-1 in the setting of greater endothelial activation in patients with ALI. Endothelial injury of the pulmonary microcirculation and resulting thrombosis have been well described in ALI pathophysiology and may partly account for an elevation in the dead space fraction in ALI patients (25, 37). Although other mechanisms including intrapulmonary shunt and low cardiac output have been reported to contribute to an increase in the dead space fraction (38–41), Dixon et al reported an association of increased dead space fraction with prothrombin fragment production in the pulmonary circulation in cardiac surgery patients (42). This group also reported a decrease in pulmonary dead space fraction with infusion of preoperative heparin, supporting dead space fraction as a physiologic marker of activated endothelium and microvascular thrombosis. The current study demonstrates a significant correlation of the ratio of Ang-2/Ang-1 with plasma levels of soluble thrombomodulin, suggesting that an elevated ratio of Ang-2/Ang-1 in patients with a high pulmonary dead space fraction may reflect severe endothelial injury with prognostic implications.
This study has some limitations. Ang-2 and Ang-1 production may not be exclusively derived from the pulmonary endothelium. Bhandari et al demonstrated Ang-2 expression in mouse epithelial cells (19). Ang-2 has also been shown to be context-dependent as an agonist or antagonist of the Tie-2 receptor, and its role may depend on other growth factors, such as VEGF-A (3). We did not measure VEGF-A in our cohort and cannot confirm its role in association with Ang-2. However we considered the ratio of Ang-2/Ang-1, which can be considered a measure of relative antagonism and agonism to the Tie-2 receptor. Finally, our cohort is modest in size, which limits us from making broad conclusions from our data. There are several clinical disorders that occur in association with acute lung injury that disrupt the vascular endothelium and may alter angiopoietin levels. However, this is a clinical study and cannot establish causality. We did find an independent association of Ang-2/Ang-1 with mortality after adjustment for known clinical and physiologic variables and recommend validation of the Ang-2/Ang-1 ratio as a prognostic factor in patients with ALI in a larger cohort to confirm and extend these findings. Future studies should test the association of Ang-2/Ang-1 with other markers of endothelial permeability, including airspace to plasma protein ratios and measurements of extravascular lung water.
This study demonstrates that the ratio of Ang-2 to Ang-1 is an independent predictor of mortality in patients with acute lung injury and markedly elevated pulmonary dead space fraction, after adjustment for multiple clinical and physiologic predictors of poor outcomes in this disease. The ratio of Ang-2 to Ang-1 may be a useful prognostic biomarker of endothelial activation in ALI patients, particularly in combination with measurements of the pulmonary dead space. Higher Ang-2/Ang-1 may be useful for risk stratification of ALI patients, particularly in identifying subgroups for future research and therapeutic trials.
Acknowledgments
Supported, in part, by contracts NHLBI HL51856, HL081332, and HR046059 and NCRR KL2RR024130.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Levitt JE, Gould MK, Ware LB, et al. Analytic review: the pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med. 2009;24(3):151–167. doi: 10.1177/0885066609332603. [DOI] [PubMed] [Google Scholar]
- 3.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312(5):630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Kwak HJ, Lee SJ, Lee YH, et al. Angiopoietin-1 inhibits irradiation- and mannitol-induced apoptosis in endothelial cells. Circulation. 2000;101(19):2317–2324. doi: 10.1161/01.cir.101.19.2317. [DOI] [PubMed] [Google Scholar]
- 5.Witzenbichler B, Westermann D, Knueppel S, et al. Protective role of angiopoietin-1 in endotoxic shock. Circulation. 2005;111(1):97–105. doi: 10.1161/01.CIR.0000151287.08202.8E. [DOI] [PubMed] [Google Scholar]
- 6.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6(4):460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 7.Kim I, Kim HG, So JN, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res. 2000;86(1):24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 8.Kim I, Moon SO, Park SK, et al. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89(6):477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 9.Jeon BH, Khanday F, Deshpande S, et al. Tie-ing the antiinflammatory effect of angiopoietin-1 to inhibition of NF-kappaB. Circ Res. 2003;92(6):586–588. doi: 10.1161/01.RES.0000066881.04116.45. [DOI] [PubMed] [Google Scholar]
- 10.Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314(2):738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 11.Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 12.Lemieux C, Maliba R, Favier J, et al. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105(4):1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 13.Scharpfenecker M, Fiedler U, Reiss Y, et al. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118(Pt 4):771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29(6):656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3(3):e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63(10):903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 17.Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247(2):320–326. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 18.Kumpers P, Lukasz A, David S, et al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care. 2008;12(6):R147. doi: 10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhandari V, Choo-Wing R, Lee CG, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12(11):1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J RespirCrit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 22.Romero PV, Lucangelo U, Lopez Aguilar J, et al. Physiologically based indices of volumetric capnography in patients receiving mechanical ventilation. Eur Respir J. 1997;10(6):1309–1315. doi: 10.1183/09031936.97.10061309. [DOI] [PubMed] [Google Scholar]
- 23.Kallet RH, Daniel BM, Garcia O, et al. Accuracy of physiologic dead space measurements in patients with acute respiratory distress syndrome using volumetric capnography: comparison with the metabolic monitor method. Respir Care. 2005;50(4):462–467. [PubMed] [Google Scholar]
- 24.McClintock D, Zhuo H, Wickersham N, et al. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12(2):R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 26.Vittinghoff E. Regression methods in biostatistics : linear, logistic, survival, and repeated measures models. New York: Springer; 2005. [Google Scholar]
- 27.Hosmer DW, Lemeshow S, May S. Applied survival analysis : regression modeling of time-to-event data. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- 28.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 29.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 30.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27(12):552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 32.Tsigkos S, Zhou Z, Kotanidou A, et al. Regulation of Ang2 release by PTEN/PI3-kinase/Akt in lung microvascular endothelial cells. J Cell Physiol. 2006;207(2):506–511. doi: 10.1002/jcp.20592. [DOI] [PubMed] [Google Scholar]
- 33.Kranidioti H, Orfanos SE, Vaki I, et al. Angiopoietin-2 is increased in septic shock: evidence for the existence of a circulating factor stimulating its release from human monocytes. Immunol Lett. 2009;125(1):65–71. doi: 10.1016/j.imlet.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Fremont RD, Koyama T, Calfee CS, et al. Acute lung injury in patients with traumatic injuries: Utility of a panel of biomarkers for diagnosis and pathogenesis. Journal of Trauma. 2009 doi: 10.1097/TA.0b013e3181c40728. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orfanos SE, Kotanidou A, Glynos C, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35(1):199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 36.van der Heijden M, van Nieuw Amerongen GP, van Hinsbergh VW, et al. The interaction of soluble Tie2 with angiopoietins and pulmonary vascular permeability in septic and non-septic critically ill patients. Shock. 2009 doi: 10.1097/SHK.0b013e3181b2f978. [DOI] [PubMed] [Google Scholar]
- 37.Tomashefski JF, Jr, Davies P, Boggis C, et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112(1):112–126. [PMC free article] [PubMed] [Google Scholar]
- 38.Greene R, Zapol WM, Snider MT, et al. Early bedside detection of pulmonary vascular occlusion during acute respiratory failure. Am Rev Respir Dis. 1981;124(5):593–601. doi: 10.1164/arrd.1981.124.5.593. [DOI] [PubMed] [Google Scholar]
- 39.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116(4):589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- 40.Ralph DD, Robertson HT, Weaver LJ, et al. Distribution of ventilation and perfusion during positive end-expiratory pressure in the adult respiratory distress syndrome. Am Rev Respir Dis. 1985;131(1):54–60. doi: 10.1164/arrd.1985.131.1.54. [DOI] [PubMed] [Google Scholar]
- 41.Dantzker DR, Brook CJ, Dehart P, et al. Ventilation-perfusion distributions in the adult respiratory distress syndrome. Am Rev Respir Dis. 1979;120(5):1039–1052. doi: 10.1164/arrd.1979.120.5.1039. [DOI] [PubMed] [Google Scholar]
- 42.Dixon B, Campbell DJ, Santamaria JD. Elevated pulmonary dead space and coagulation abnormalities suggest lung microvascular thrombosis in patients undergoing cardiac surgery. Intensive Care Med. 2008;34(7):1216–1223. doi: 10.1007/s00134-008-1042-7. [DOI] [PubMed] [Google Scholar]