Abstract
Background
T cell differentiation determines susceptibility and resistance to experimental cutaneous leishmaniasis, yet mixed T1/Th2 responses characterize the clinical spectrum of human infection with Leishmania Viannia species.
Materials and Methods
To discern the inter-relationship of T cell differentiation and outcome of human infection, we examined factors that regulate T cell differentiation and Th1/Th2 cytokine responses in asymptomatic infection, active and historical chronic and recurrent cutaneous leishmaniasis. T-bet, GATA-3, Foxp3 and cytokine gene expression were quantified by real time PCR, and correlated with IL-2, IFN-γ, TNF-α, IL-4, IL-13, IL-10 secretion during in vitro response to live L. panamensis.
Results
Higher GATA-3 than T-bet expression occurred throughout the 15 days of co-culture with promastigotes, however neither transcription nor secretion of IL-4 was detected. Sustained, inverse correlation between GATA-3 expression and secretion of proinflammatory cytokines IFN-γ and TNF-α was observed in asymptomatic infection. In contrast, higher T-bet expression and T-bet:GATA-3 ratio characterized active recurrent disease. Down-regulation of T-bet and GATA-3 expression and increased IL-2 secretion compared to control was directly correlated with Foxp3 expression and IL-13 secretion in chronic disease.
Conclusions
Regulation of the inflammatory response rather than biased Th1/Th2 response distinguished asymptomatic and recalcitrant outcomes of infection with Leishmnania Viannia species.
Keywords: Th1/Th2 differentiation, Human cutaneous leishmaniasis, T-bet, GATA-3, Foxp3, Cytokines, Leishmania (Viannia)
INTRODUCTION
Chronic disease with a propensity to reactivate, and persistent infection characterize human dermal leishmaniasis caused by species of the Viannia subgenus [1–5]. Subclinical or asymptomatic infection, manifest as cutaneous delayed type hypersensitivity (DTH) to Leishmania antigen, occurs with variable frequency in different epidemiological settings [6–8]. Exuberant DTH responses have long been noted in chronic disease, whether mucosal or cutaneous [9, 10, 11], and the inflammatory response has been clinically [12] and experimentally linked with activation of subclinical infection and pathogenesis [13,14]. Although the host response to infection is known to be a primary determinant of the outcome of infection, the immunological mechanisms that are conducive to non-healing dermal disease versus asymptomatic infection in the human host have yet to be deciphered.
Contrary to the dichotomy of protective Th1 and non-healing Th2 responses induced by L. major infection in mouse models of cutaneous leishmaniasis [15, 16], a mixed Th1/Th2 response is found across the spectrum of human infection with L. (Viannia) species [9, 17, 18]. In vitro and in situ analyses of cytokine responses during active human dermal Leishmaniasis including localized disease caused by L. mexicana have revealed diverse profiles of both Th1 and Th2 cytokines, however no clear or consistent bias towards one or the other [19, 20–22]. Marked cutaneous hypersensitivity generally characterizes mucosal disease [10, 11] and has been linked with a poorly regulated Th1- like proinflammatory response, yet occurs in the presence of Th2 cytokines [23]. Differentiation of the T cell response and its impact on pathogenesis or protection from disease in human dermal leishmaniasis caused by Leishmania of the Viannia subgenus, or indeed any species of Leishmania, has not been discernable based on cytokine production alone.
Transcription factors T-bet, GATA-3 and Foxp3 are master regulators of Th1, Th2 and T regulatory cell development respectively. These factors cross regulate one another and are selectively expressed in the corresponding cell populations [24–27]. T-bet modulates GATA-3 function and Th2 cytokines block Th1 differentiation [28]. Additionally, GATA-3 has recently been shown to inhibit Foxp3 transcription by binding to the Foxp3 gene promoter [29]. The interrelationship of these molecules and the effector responses that they regulate define the host response and could therefore, determine the clinical outcome of infection.
This study evaluated the interplay of transcription factors regulating T cell differentiation and Th1/Th2 cytokines in the outcome of human infection with Leishmania (Viannia) species.
MATERIALS AND METHODS
Study Description
T-bet and GATA-3, and Foxp3 expression were examined in recurrent and chronic cutaneous leishmaniasis representing “non-healing” clinical outcomes and asymptomatic infection as “clinical resistance” (figure 1). Transcription and secretion of Th1 and Th2 associated cytokines were concomitantly evaluated. Delayed type hypersensitivity (DTH) elicited by intradermal inoculation or in vitro lymphocyte proliferation in response to Leishmania antigens are indicative of infection. Specific DTH response is detected in a proportion endemically exposed individuals in the absence of clinical history or physical evidence of either healed or active leishmaniasis, providing a marker of asymptomatic infection. Historic “non-healing” disease based on documented prior parasitologically confirmed chronic or recurrent lesions, was included in the study in order to determine whether the pattern of cytokines and regulatory mechanisms operating during active disease would be re-elicited after resolution of disease.
Figure 1.
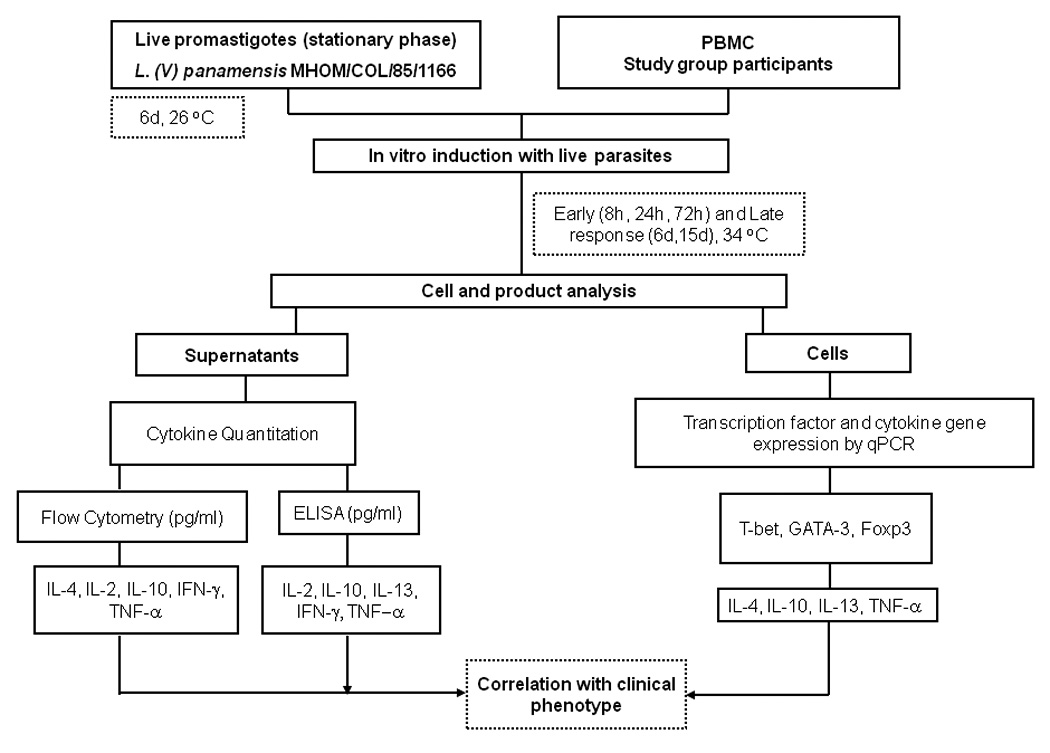
Schematic summary of the research strategy to evaluate transcription factor expression and cytokine response profile in relation with outcome of infection with Leishmania (Viannia) species.
The rationale of our approach was that an integrated analysis of transcription factors regulating T cell differentiation at the level of gene expression and Th1/Th2 cytokine production in “non-healing” and “resistant” clinical phenotypes could elucidate the immunological mechanisms that drive the pathogenesis of recalcitrant disease or lead to clinical resistance.
Study population
Thirty-nine individuals, 22 males, 17 females, from 18 to 67 years of age participated in the study. Six study groups, each constituted by 6–7 participants > 18 years of age of either sex, were defined as follows:1) Non immune Controls, individuals without history of exposure to transmission and negative leishmanin skin test or in vitro proliferative response to Leishmania antigen; 2) Asymptomatic Infection, residents of endemic areas of transmission of cutaneous leishmaniasis without active or scared lesions attributable to leishmaniasis having a positive Montenegro skin test or in vitro proliferative response; 3) Active Chronic Disease, patients with active parasitologically confirmed lesions > 3 months duration who had not initiated treatment; 4) Active Recurrent Disease, patients with active lesions who had documented prior parasitologically proven lesions that healed, and had not initiated treatment for their current active lesions; 5) Historical Chronic Disease, individuals currently without active lesions who had documented clinical history of parasitologically confirmed lesions of > 3 months duration; 6) Historical Recurrent Disease, individuals currently without active lesions who had documented clinical history of parasitologically confirmed recurrent disease..
Exclusion criteria for all groups were: age below 18 years, immunosuppressive disease, immunosuppressive pharmacotherapy, pregnancy, unwillingness to participate, or unwillingness to have HIV testing.
Eighteen Leishmania strains isolated from active and historical patients at the time of diagnosis were identified as L (V) panamensis.
This study was approved and monitored by CIDEIM and Yale University institutional review boards for the ethical conduct of research involving human subjects in accordance with national and international guidelines. Blood samples of up to 40ml were obtained by venipuncture. All patients with active lesions were referred for treatment following guidelines of the Colombian Ministry of Social Protection.
Parasites
Leishmania (Viannia) panamensis strain MHOM/COL/85/1166 was harvested during stationary phase on day 6 of culture for in vitro evaluation of the response of peripheral blood mononuclear cells (PBMCs).
Isolation and culture of mononuclear cells
PBMCs were isolated by centrifugation over Ficoll-hypaque 1.077 (Sigma-Aldrich, St Louis, MO), and resuspended at 2 × 106/mL in RPMI 1640 [Sigma-Aldrich] containing 10% fetal calf serum (FCS; GIBCO, Grand Island, NY). Livepromastigotes were opsonized for 1 hour at 34°C in RPMI 1640 medium containing 10% heat inactivated AB+ serum in a humidified 5% CO2 atmosphere, washed and resuspended in complete RPMI. Opsonization was conducted to optimize infection and approximate in vivo conditions. Based on dose response experiments, PBMCs were co-cultured with 2.5 × 105/mL promastigotes (approximate parasite to monocyte ratio 1:1) or medium alone.
Supernatants were harvested at 24 and 72 hours for cytokine quantification. Based on preliminary studies and published reports, six time intervals from 8 hours to 15 days were included in the analysis of the kinetics of transcription factor expression. On day 6 and 10, complete medium plus recombinant IL-2 (rIL-2) (0.5 ng/ml) (R&D Systems, Minneapolis, MN) was added to sustain the responding cell population through day 15.
Th1/Th2 Differentiation Controls
Th1 or Th2 biased PBMCs provided controls for expression of T-bet and GATA-3 and selection of the housekeeping gene for normalization of gene expression. PBMCs were activated via T cell receptor stimulation with monoclonal antibodies in the presence of polarizing cytokines [30, 31]. Two million PBMCs/mL complete RPMI 1640 were cultured in 24mm wells with α CD3 (1 µg/mL) and α CD28 (1 µg/ml) monoclonal antibodies for 15 days at 34°C. Human rIL-12 (30 ng/mL) (R&D Systems) and anti IL-4 (2.5 µg/ml) (R&D Systems) monoclonal antibody were added on day 1 to obtain Th1 biased PBMCs. Medium containing rIL-2 (0.5 ng/ml) was added on days 6 and 10. Human rIL-4 (20 ng/ml) (R&D Systems) and neutralizing anti IFN-γ (2.5 µg/ml) (R&D Systems) were used to obtain a Th2 biased control response. Up-regulation of T-bet and GATA-3 expression was confirmed by qRT-PCR.
RNA extraction and cDNA synthesis
RNA was extracted from PBMCs using RNeasy Micro kit (QIAGEN, Valencia, CA), resuspended in 18 µL RNAse free water and stored at −80°C. RNA quality was verified by agarose gel electrophoresis of each sample before preparing cDNA, which was generated within 24 hours of RNA extraction using random hexamers and M-MLV RT (Super ScriptTM III First-Strand Synthesis System for reverse transcriptase-PCR, Invitrogen).
Transcription factor and Th1/Th2 cytokine cDNA cloning
cDNAs encoding human T-bet, GATA-3, Foxp3, β-actin, GAPDH, 18S rRNA, IFN-γ, TNF-α, IL-10 and IL-13 were cloned into E. coli to generate standard curves for qRT-PCR. Total mRNA from Th1 and Th2 polarized PBMCs was used to generate cDNA by amplification with Taqman® primer/probe gene expression assays (Applied Biosystems, Foster City, CA). Purified PCR products were cloned into PCR 2.1-TOPO vector (Invitrogen), and transformed into competent Escherichia coli DH5-α (Invitrogen). Positive colonies were selected by colony PCR using T7/M13 primers. Plasmids were purified using the Wizard Plus Minipreps DNA Purification System (Promega Corporation, Madison, WI) and concentrations estimated by absorbance at 260 nm. The miniprep product was serially diluted from 102 to 108 copies and amplified by qRT-PCR.
Validation and selection of Housekeeping Gene
Three housekeeping genes, 18S rRNA, GAPDH and β-actin were evaluated for normalization of transcription factor and cytokine gene expression. The efficiency of amplification of 18S rRNA, GAPDH and β-actin ranged between 1.89 and 1.95. β-actin was selected for normalization based on the level of expression relative to the genes of interest and low variability under the study conditions, (cycle threshold: mean= 24.6, SE= 0.8).
Quantitative PCR
Gene expression was determined using a LightCycler® FastStart DNA MasterPLUS (Roche Diagnostics) and primers/probes of the Taqman Gene Expression Assay described above and Fast Start DNA Master Hybridization Probes (Roche Diagnostics). mRNA copy number was calculated using the LightCycler integrated Quantitative Analysis Program software (version 4.0, Roche Diagnostics). Each experiment was internally controlled using at least one standard curve and calibrant of known concentration (104 copies /µL). Results were expressed as normalized values of cytokine/transcription factor and β-actin cDNA ratios. T-bet/β-actin / GATA-3/β-actin ratio was also determined as an indicator of Th1/Th2 differentiation [26, 27, 32, 33].
Cytokine Detection
IL-4, IL-10, TNF-α and IFN-γ were quantified in 72 hour culture supernatants using the BDTM CBA human Th1/Th2 cytokine kit II, (BD Pharmingen, San Diego, CA) and BD FACSCalibur cytometer. The sensitivity of the kit was 2.6 pg/mL for IL-4, 2.8 for TNF-α and 7.1 pg/mL for IFN-γ. Because concentrations frequently exceeded the upper limit of the CBA kit, IFN-γ, IL-10 and IL-13 were also measured by ELISA [34]. IL-2 was determined in 24 and 72 hour culture supernatants using BD Pharmingen human ELISA set.
Data Management and Statistical Analyses
Analyses of expression of T-bet, GATA-3, Foxp3 and cytokine production among the different clinical groups were conducted using non-parametric Kruskal-Wallis test for independent samples. Multiple comparisons were performed using the Dunn procedure. Correlation analysis between the ratios T-bet/β-actin/GATA-3/β-actin, Foxp3/β-actin and cytokine production were conducted using the Spearman coefficient. P < .05 was considered statistically significant. SPSS version 7.0 was used for all analyses.
RESULTS
T-bet and Gata-3 expression
Differences in expression of T-bet and GATA-3 among the study groups were significant by 8 hours and sustained at 6 days [P < .01], (figure 2). Both T-bet and GATA-3 were down regulated in PBMCs from patients with history of chronic or recurrent leishmaniasis. Differences among study groups were evident in the ratio of expression of T-bet/GATA-3 at 24 hours [P = .019], day 6 [P < .003] and 15 [P < .006] (data not shown).
Figure 2.
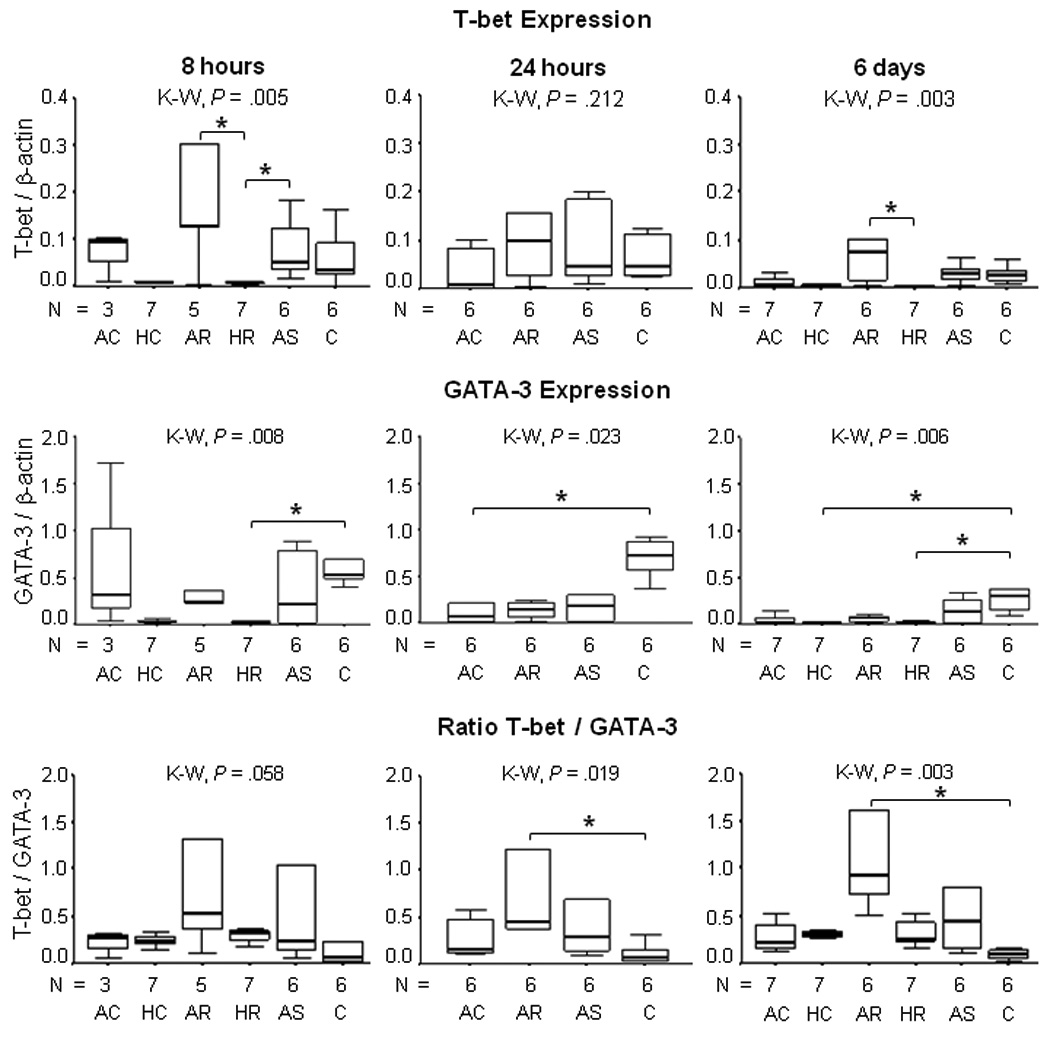
T-bet and GATA-3 expression in PBMCs from patients with active and historic cutaneous leishmaniasis, asymptomatically infected residents of endemic areas and healthy controls co-cultured during 8 hours, 24 hours, and 6 days with live promastigotes of L. (V) panamensis. All data were normalized to b-actin. P values were calculated using Kruskal Wallis (K-W) Test and Dunn procedure, *P < .05. Active Chronic (AC); Historic Chronic (HC); Active Recurrent (AR); Historic Recurrent (HR); Asymptomatic (AS); Healthy Control (C).
Overall, GATA-3 expression was higher than T-bet expression and varied significantly among the study groups at 8, 24 and 72 hours and 6 days, (figure 2). Healthy control donors presented the highest GATA-3 expression over the 15 days of observation following exposure to promastigotes.
Foxp3 gene expression
Based on the kinetics of Foxp3 expression under the experimental conditions, transcription was evaluated at 24 hours. Foxp3 gene expression was low (order of magnitude lower than GATA3 expression) yet varied significantly among the clinical and control groups post exposure to L. panamensis [P = .006] being highest in the healthy control group, (figure 3A). Foxp3 was down regulated in historic chronic and recurrent disease compared with other study groups.
Figure 3.
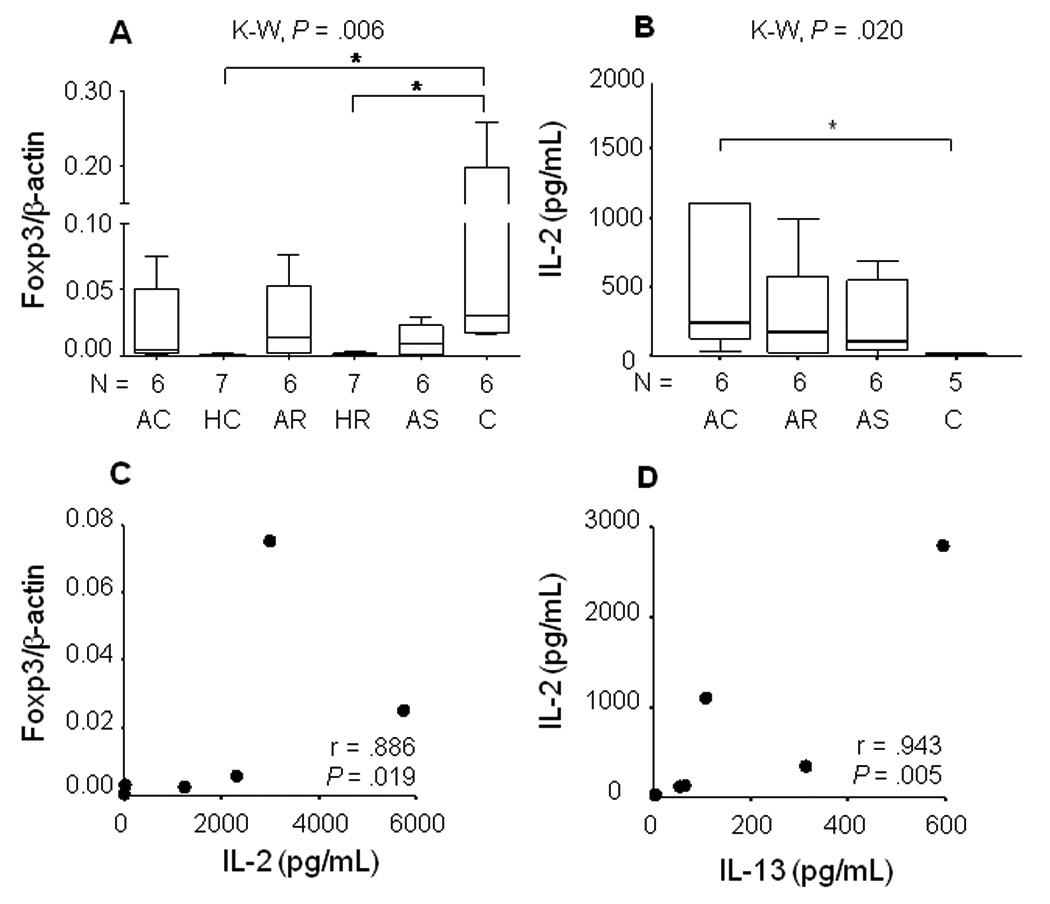
Inter-relation of Foxp3 expression, IL-2 and IL-13 production by PBMCs in cutaneous Leishmaniasis. A) Foxp3 expression at 24 hours in PBMCs from patients with Active Chronic (AC), Active Recurrent (AR) disease, asymptomatically infected individuals (AS) and Healthy Controls (C) co-cultured with live promastigotes of L.(V) panamensis; Kruskal-Wallis Tests, Dunn *P < .05. Data were normalized to β-actin. B) Net IL-2 secretion C) Correlation between IL-2 secretion and Foxp3 expression, D) Correlation between IL-2 at 24 hours and IL-13 secretion at 72 hours.
Cytokine secretion
Exposure of PBMCs from patients with active or historical disease and asymptomatic infection, to promastigotes induced the secretion of both Th1 and Th2 cytokines, (figure 4). However, no secreted IL-4 was detected. When cytokine production was analyzed without distinction of clinical outcome, IL-2, TNF-α, IFN-γ, and IL-13 were all positively correlated [r = .527–.885, P = .001 ] whereas IL-10 secretion correlated only with TNF-α [r = .543, P = .001] and IFN-γ [r = .620, P = .001].
Figure 4.
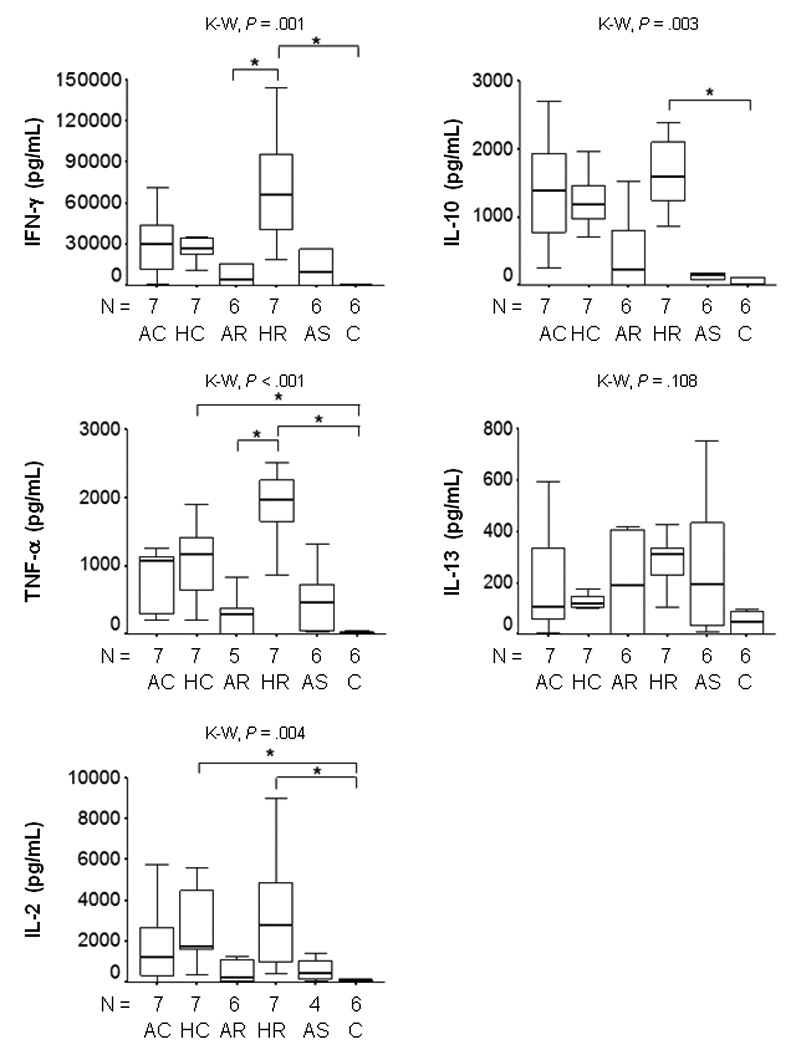
Net Cytokine secretion by PBMCs from patients with Active and Historic Cutaneous Leishmaniasis, Asymptomatically infected residents of endemic area and Healthy controls co-cultured during 72 hours with live promastigotes of L. V. panamensis. P values were calculated using Kruskal Wallis Test and Dunn procedure, *P < .05. Net production was calculated by subtraction of background (PBMC plus medium) from cytokine production in the presence of live promastigotes. Active Chronic (AC); Historic Chronic (HC); Active Recurrent (AR); Historic Recurrent (HR); Asymptomatic (AS); Healthy Control (C).
Cytokine gene expression
Transcription of IL-4 was 3 to 4 orders of magnitude lower than IFN-γ, TNF-α, IL-13 and IL-10. When all participants were included in the analysis, the transcription and secretion of IFN-γ [r = .572, P = .001], TNF-α, [r = .328, P = .048] and IL-13 [r = .370, P = .022] were positively correlated. In contrast, transcription and production of IL-10 were inversely correlated [r = −.350, P =.031] indicating distinct regulatory control of this cytokine.
Transcription factor expression and Th1/Th2 cytokine transcription and secretion in infection and disease
Asymptomatic Infection
A consistently high and significant negative correlation was observed between GATA-3 expression and pro-inflammatory cytokines TNF-α and IFN-γ during recall response of PBMCs from asymptomatically infected individuals. This relationship prevailed over the entire period of observation from 8 hours to 15 days post exposure to L. panamensis, (figure 5A, B). Conversely, the T-bet/β-actin: GATA-3/β-actin ratio was positively correlated with TNF-α production at all time points, [r = .886, P = .019 at 8 and 24 hours; r = .829, P = .042 at 6 days and r = .943, P = .005 at 15 days], data not shown, and with IFN-γ at 6 and 15 days [r = .812, P = .050]. Secretion of these proinflammatory cytokines was also positively correlated during asymptomatic infection [r = .899, P = .015]. The coordinate production of these two cytokines and correlation with the T-bet/β-actin: GATA-3/β-actin ratio is consistent with a regulated proinflammatory response.
Figure 5.
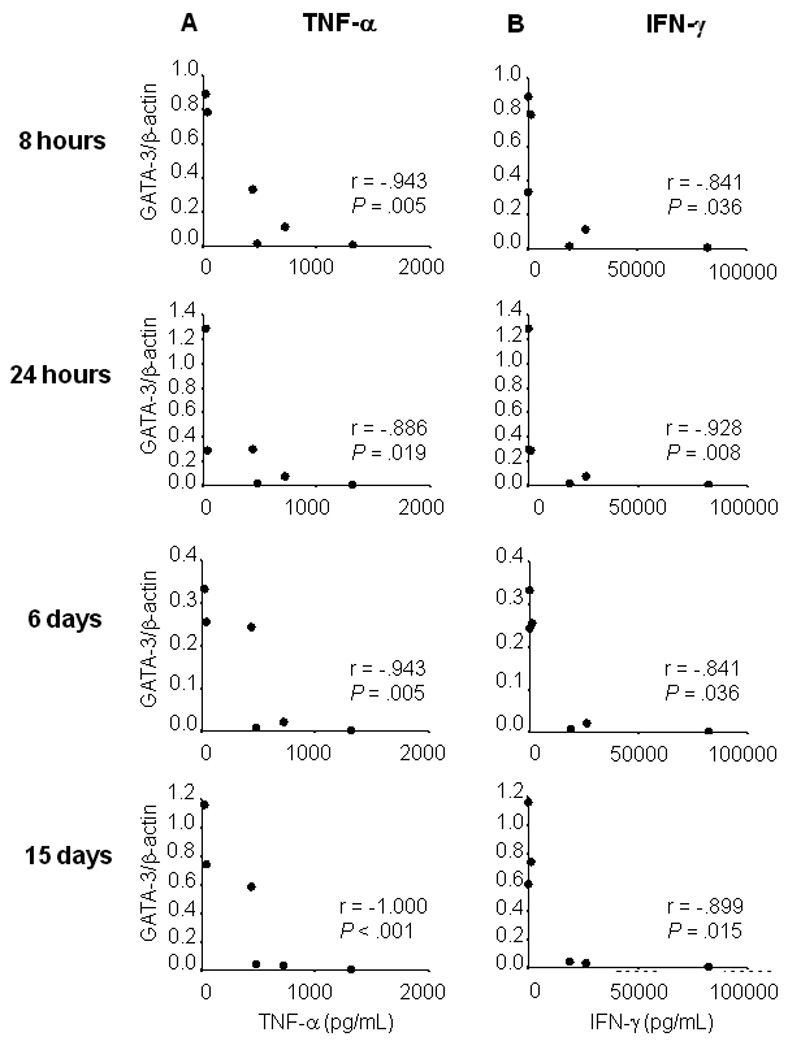
Inverse correlation between ratio GATA-3/B-actin expression with TNF-α and IFN-γ in Asymptomatic infection with Leishmania (Viannia) species. A) Regression analysis of TNF-α secretion (pg/mL), vs GATA-3 expression at 8 hours, 24 hours, 6 and 15 days of co-culture of PBMCs with live promastigotes of L. (V) panamensis, B) Regression analysis of IFN-γ secretion (pg/mL) at 8 hours, 24 hours, 6 and 15 days of co-culture of PBMCs with live promastigotes of L. (V) panamensis.
Active Chronic disease
Although T-bet expression was low, the T-bet/β-actin ratio was highly and significantly correlated with IL-2 secretion from 24 hours to 15 days in active chronic disease, (figure 6). IL-2 secretion was further distinguished in this patient group by being significantly higher than the control group at 24 hours, (figure 3B) and positively correlated with Foxp3 expression (figure 3C) and IL-13 secretion, (figure 3D),which are associated with the development of T regulatory cells.
Figure 6.
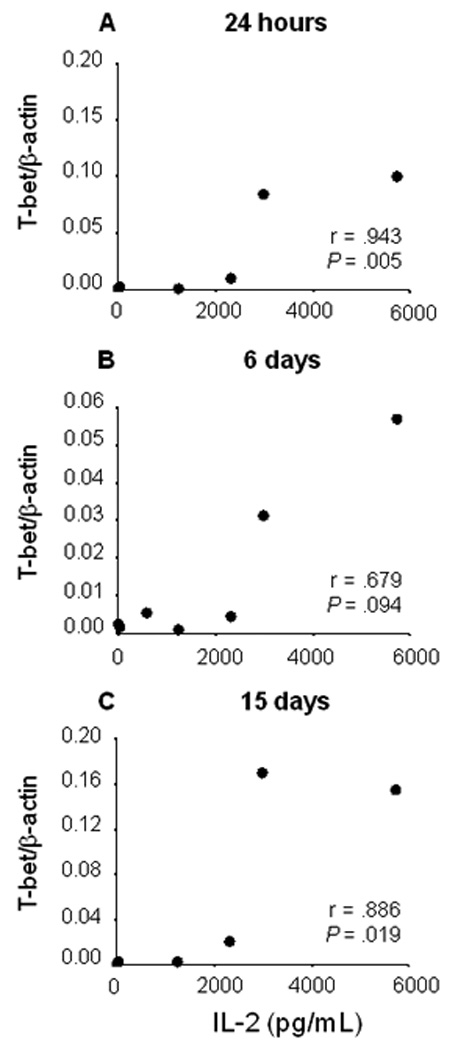
Correlation between IL-2 (pg/mL) secretion at 24 hours and T-bet expression at 24 hours, 6 days, and 15 days of co-culture of PBMCs from patients with active chronic cutaneous leishmaniasis
Active Recurrent Disease
This patient group presented the highest T-bet expression throughout the period of observation and the highest T-bet/β-actin : GATA-3/β-actin ratio, which was significantly higher than that of healthy controls at 24 hours, 6 and 15 days (data not shown) of co-culture with L. panamensis [P < .05], (figure 2). Prominent T-bet transcription was not accompanied by a Th1 biased cytokine response, rather a mixed Th1/Th2 response including correlation TNF-α and IL-13 transcription [r = .829, P = .042]. Transcription factor expression was not correlated with cytokine secretion in this group of patients, suggesting dysregulation of the cytokine response.
Historic Recurrent and Chronic Disease
Transcription factor expression was remarkably down regulated in these patient groups. PBMCs from patients who had long recovered from chronic or recurrent disease presented very low transcription factor expression. Both GATA-3 (figure 2) and Foxp3 expression (figure 3A) were significantly lower in historic disease than the healthy control group. Transcription of the housekeeping gene β-actin confirmed that gene expression was not impaired. Cytokine gene expression was also very low, except IFN-γ which was significantly higher for historic recurrent disease than healthy controls at 24 hours of co-culture, P < .05 [data not shown]. Despite the low level of gene transcription, historic “non healing” disease groups presented the highest secretion of Th1 and Th2 cytokines, (figure 4).
Discussion
This study revealed distinctive interrelationships between transcription factors that regulate T cell differentiation, and cytokine gene expression and secretion during asymptomatic infection, active chronic and recurrent disease, and following clinical resolution of the latter disease presentations. Differences in T cell differentiation in relation with clinical outcomes were not discernable by cytokine gene transcription or secretion alone. The results also established that IL-4 was not induced during in vitro recall in human infection with L. panamensis, rather IL-13.
Asymptomatic infection was distinguished by a controlled pro-inflammatory response that was inversely correlated with GATA-3 transcription, and positively correlated with the T-bet/β-actin to GATA-3/β-actin ratio. This finding concurs with the report of lower pro-inflammatory cytokines in asymptomatic infection with L. braziliensis [18]. Validation of the inverse relationship between GATA-3 expression and secretion of TNF-α and IFN-γ as a marker of clinical resistance could provide a correlate of protective response.
Although both active chronic and recurrent disease presented mixed Th1/Th2 cytokine responses, concomitant analysis of transcription factor expression yielded evidence of ongoing negative regulation of the pro-inflammatory response in chronic disease, and positive regulation in recurrent disease. High IL-2 secretion was elicited in PBMCs during active chronic disease, and IL-2 correlated with Foxp3 expression. In contrast, T-bet transcription and the T-bet/β-actin to GATA-3/β-actin ratio were highest in active recurrent disease. These findings indicate involvement of distinct immunoregulatory mechanisms in these two “non-healing” manifestations of human cutaneous leishmaniasis caused by Leishmania (Viannia) species. Mucosal leishmaniasis, which is also a non-healing manifestation if untreated, has been associated with defective regulation of a Th1 type CD4 mediated response [23].
IL-2 has recently been shown to participate in the induction of the negative transcriptional regulator Foxp3, Treg cell development, expansion and maintenance [35–37]. The positive correlation between IL-2 and Foxp3 in active chronic cutaneous leishmaniasis in this study is consistent with this finding and encourages the investigation of the participation of Treg cells during chronic cutaneous leishmaniasis. In experimental infection of BALB/c mice with L. major, IFN-γ producing CD4+CD25− effector lymphocytes and Treg cells (Foxp3+, CD4+CD25+) producing IL-10 accumulated in the pro-inflammatory site of infection [38]. IL-10-producing CD4+CD25−Foxp3−Th1 cells are also an important source of anti-inflammatory IL-10 and contribute to the persistence of Leishmania [39]. Although Treg cells have been detected in acute cutaneous leishmaniasis lesions [40] their role in the resolution or perpetuation of human leishmaniasis is unknown.
The prominent expression of T-bet and high T-bet/β-actin to GATA-3/β-actin ratio elicited during active recurrent cutaneous disease suggests that reactivation of disease may involve a T-bet regulated shift in response from the immunologic homeostasis achieved during asymptomatic infection or following resolution of lesions. Significantly lower cutaneous DTH responses to leishmanin were found in patients with recurrent disease than chronic disease [3] and immunosuppressive disease can lead to reactivation of cutaneous and mucosal leishmaniasis [41]. However since inflammatory stimuli also induce activation of leishmaniasis, in asymptomatically infected humans [12] and experimental models [13, 14], the observed upregulation of T-bet expression during recurrence could signal an ongoing proinflammatory response.
The profuse secretion of Th1 and Th2 cytokines and low cytokine gene and transcription factor expression elicited by restimulation of PBMCs from donors who had resolved prior chronic or recurrent disease, likely corresponds with a long term memory response. In contrast with primary activation of T helper cells, which requires T cell receptor (TCR) signaling, co-stimulation and differentiation signaling, antigen experienced cells re-express cytokines with only TCR stimulation and cytokine memory [42]. Supporting this interpretation, transcription and secretion of cytokines were not correlated in the groups of patients with a prior history of disease.
We did not detect IL-4, the principal cytokine driving the differentiation of specific CD4+T cells toward Th2 development, at either transcriptional or protein levels in PBMCs from the different clinical groups. In contrast, IL-13 was detected in all clinical groups at both levels of analysis, perhaps due in part to upregulation of IL-13 production by GATA-3 [43]. IL-4 has been detected with low frequency or been absent in most reports on cytokine response in human cutaneous leishmaniasis [20, 21, 44]. Infecting species of Leishmania could conceivably influence the repertoire of cytokines elicited and corresponding regulatory mechanisms, however this has not been evaluated. Because IL-13 shares many functions with IL-4 [21, 45, 46] it may effectively replace IL-4 in human cutaneous leishmaniasis caused by L. (Viannia) species.
IL-4 and IL-13 negatively regulate pro-inflammatory responses, yet IL-13 together with IL-2 enhances IFN-γ production by NK cells [47], which produce IL-12 and IFN-γ on exposure to live Leishmania promastigotes [48]. Concomitantly high levels of IFN-γ, IL-2 and IL-13 observed during the recall response of human PBMCs to promastigotes might reflect such a synergistic effect. The importance of IL-13 in pathogenesis is substantiated by the conversion of resistant C57BL/6 mice to susceptible to L. major by over-expression of IL-13 [49]. Evidence that IL-13 contributes to pathogenesis has also been reported for human cutaneous leishmaniasis caused by L. guyanensis [21]. In the current study, IL-13 was secreted by PBMCs of participants in all study groups but correlation of expression and secretion of IL-13 was found only in active chronic and recurrent disease. This finding does not allow conclusions to be drawn regarding the role of IL-13 in these non-healing presentations. Nevertheless, the relationship of this anti-inflammatory cytokine with T regulatory cells merits investigation since IL-13 as well as IL-4, can induce CD25+CD4+ regulatory T cells from CD25-CD4+ precursors in an Ag-dependent manner [48] and T regulatory cells produce IL-13 [50].
This study provides evidence of regulatory differences in the T cell mediated response in humans during chronic and recurrent disease and asymptomatic infection with Leishmania Viannia species. Therapeutic interventions targeting regulatory mechanisms may allow more effective management of clinically susceptible individuals.
Acknowlegdements
The Assistance of Maria Consuelo Miranda, Martin Prager, Michelle Talbert, Robinson Pacheco, Wilson Cortez, the Regional Hospital San Andres and Department of Valle del Cauca Vector Borne Disease Program in the enrollment, treatment and clinical management of the participants in this study is gratefully acknowledged. We thank James Becerra for data management support, Neal Alexander and Mauricio Perez for their advice and assistance in data analysis and Diane McMahon Pratt and Maria Adelaida Gómez for critically reviewing the manuscript.
This study was supported by the NIAID ICIDR Program grant 1 U19AIO65866 Fogarty Global Infectious Diseases Research Training Program grant D43 TW006589, COLCIENCIAS grant # 2229-34319213 and UBS Optimus Foundation.
Footnotes
None of the authors have a commercial or other association that might pose a conflict of interest in the conduct of this study or the publication of the results
Preliminary results of this study were presented in the American Society of Tropical Medicine and Hygiene 54th Annual Meeting, December 11–15, 2005. Washington DC, USA.
Reprints or correspondence: Dr. Nancy Gore Saravia, Centro Internacional de Entrenamiento e Investigaciones Medicas (CIDEIM), Carrera 125 # 19-225, AA 5390, Cali, Colombia. Tel: (+ 57 2) 555 2164 Fax: (+ 57 2) 555 2638 nsaravia@cideim.org.co.
References
- 1.Marsden PD. Clinical presentations of Leishmania braziliensis braziliensis. Parasitol Today. 1985;1:129–133. doi: 10.1016/0169-4758(85)90057-2. [DOI] [PubMed] [Google Scholar]
- 2.Weigle KA, Saravia NG. Natural History, Clinical Evolution, and the Host-Parasite Interaction in New World Cutaneous Leishmaniasis. Clin Dermatol. 1996;14:433–450. doi: 10.1016/0738-081x(96)00036-3. [DOI] [PubMed] [Google Scholar]
- 3.Saravia NG, Weigle KA, Segura I, et al. Recurrent lesions in human Leishmania braziliensis infection: reactivation or reinfection? Lancet. 1990;336:398–402. doi: 10.1016/0140-6736(90)91945-7. [DOI] [PubMed] [Google Scholar]
- 4.Ramírez JL, Guevara P. Persistent infections by Leishmania (Viannia) braziliensis. Mem Inst Oswaldo Cruz. 1997;92:333–338. doi: 10.1590/s0074-02761997000300006. Review. [DOI] [PubMed] [Google Scholar]
- 5.Schubach A, Marzochi MC, Cuzzi-Maya T, et al. Cutaneous scars in American tegumentary leishmaniasis patients: a site of Leishmania (Viannia) braziliensis persistence and viability eleven years after antimonial therapy and clinical cure. Am J Trop Med Hyg. 1998;58:824–827. doi: 10.4269/ajtmh.1998.58.824. [DOI] [PubMed] [Google Scholar]
- 6.Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: a longitudinal study of the natural history, prevalence, and incidence of infection and clinical manifestations. J Infect Dis. 1993;168:699–708. doi: 10.1093/infdis/168.3.699. [DOI] [PubMed] [Google Scholar]
- 7.Davies CR, Llanos-Cuentas EA, Pyke SD, Dye C. Cutaneous leishmaniasis in the Peruvian Andes: an epidemiological study of infection and immunity. Epidemiol Infect. 1995;114:297–318. doi: 10.1017/s0950268800057964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz G, Davies CR. Leishmania panamensis transmission in the domestic environment: the results of a prospective epidemiological survey in Santander, Colombia. Biomédica. 2006;s1:131–144. [PubMed] [Google Scholar]
- 9.Saravia NG, Valderrama L, Labrada M, et al. The relationship of Leishmania braziliensis subspecies and immune response to disease expression in New World leishmaniasis. J Infect Dis. 1989;159:725. doi: 10.1093/infdis/159.4.725. [DOI] [PubMed] [Google Scholar]
- 10.Walton BC, Valverde L. Racial differences in espundia. Ann Trop Med Parasitol. 1979;73(1):23–29. doi: 10.1080/00034983.1979.11687222. [DOI] [PubMed] [Google Scholar]
- 11.Llanos-Cuentas EA, Cuba CC, Barreto A, Marsden P. Clinical characteristics of human Leishmania braziliensis braziliensis infections. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1984;78:845–846. doi: 10.1016/0035-9203(84)90043-9. [DOI] [PubMed] [Google Scholar]
- 12.Wortmann GW, Aronson NE, Miller RS, Blazes D, Oster CN. Cutaneous leishmaniasis following local trauma: a clinical pearl. Clin Infect Dis. 2000;31:199–201. doi: 10.1086/313924. [DOI] [PubMed] [Google Scholar]
- 13.Travi BL, Osorio Y, Saravia NG. The inflammatory response promotes cutaneous metastasis in hamsters infected with Leishmania (Viannia) panamensis. J Parasitol. 1996;82:454–457. [PubMed] [Google Scholar]
- 14.Bertho AL, Santiago MA, Coutinho SG. An experimental model of the production of metastases in murine cutaneous leishmaniasis. J Parasitol. 1994;80:93–99. [PubMed] [Google Scholar]
- 15.McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or visceral disease? Immunol Rev. 2004;1:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 16.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 17.Convit J, Ulrico M, Fernández CT, et al. The clinical and immunological spectrum of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1993;87:444–448. doi: 10.1016/0035-9203(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 18.Follador I, Araújo C, Bacellar O, et al. Epidemiologic and immunologic findings for the subclinical form of Leishmania braziliensis infection. Clin Infect Dis. 2002;34:E54–E58. doi: 10.1086/340261. [DOI] [PubMed] [Google Scholar]
- 19.Melby PC, Andrade-Narvaez FJ, Darnell BJ, Valencia-Pacheco G, Tryon VV, Palomo-Cetina A. Increased proinflamatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect Immun. 1994;62:837–842. doi: 10.1128/iai.62.3.837-842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceição-Silva F, Modlin RL. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin Inves. 1993;91:1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourreau E, Prévot G, Pradinaud R, Launois P. Interleukin (IL)–13 Is the Predominant Th2 Cytokine in Localized Cutaneous Leishmaniasis Lesions and Renders Specific CD4+ T Cells Unresponsive to IL-12. J Infect Dis. 2001;183:953–959. doi: 10.1086/319249. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho EM, Johnson WD, Barreto E, et al. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J. Immunol. 1985;135:4144–4148. [PubMed] [Google Scholar]
- 23.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70(12):6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agnello D, Lankford CS, Bream J, et al. Cytokines and Transcription Factors That Regulate T Helper Cell Differentiation: New Players and New Insights. J Clin Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 25.Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human Th1 and Th2 lymphocytes. Nat Immunol. 2003;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 26.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A Novel Transcription Factor, T-bet, Directs Th1 Lineage Commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 27.Zheng W, Flavell R. The Transcription Factor GATA-3 Is Necessary and Sufficient for Th2 Cytokine Gene Expression in CD4 T Cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 28.Hwang ES, Szabo S, Schwartzberg P, Glimcher LH. T Helper Cell Fate Specified by Kinase-Mediated Interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 29.Mantel PY, Kuipers H, Boyman O, et al. GATA-3-driven Th2 responses inhibit TGF-β1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:2847–2861. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund R, Aittokallio T, Nevalainen O, Lahesmaa R. Identification of novel genes regulated by IL-12, IL-4, or TGF-β during the early polarization of CD4+ lymphocytes. J Immunol. 2003;171:5328–5336. doi: 10.4049/jimmunol.171.10.5328. [DOI] [PubMed] [Google Scholar]
- 31.Wu CY, Kirman JR, Rotte MJ, et al. Distinct lineages of TH1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 32.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 33.Farrar J, Ouyang W, Löhning M, et al. An Instructive Component in T Helper Cell Type 2 [Th2] Development Mediated by GATA-3. J. Exp. Med. 2001;193:643–649. doi: 10.1084/jem.193.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosque F, Saravia NG, Valderrama L, Milon G. Distinct Innate and Acquired Immune Responses to Leishmania in Putative Susceptible and Resistant Human Populations Endemically Exposed to L. (Viannia) panamensis Infection. Scand. J. Immunol. 2000;51:533–541. doi: 10.1046/j.1365-3083.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 35.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Zorn E, Nelson EA, Mohseni A, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popmihajlov Z, Smith KA. Negative feedback regulation of T cells via interleukin-2 and FOXP3 reciprocity. PLoS ONE. 2008;3:e1581. doi: 10.1371/journal.pone.0001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 39.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4[+]CD25[−] Foxp3[−] Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campanelli AP, Roselino AM, Cavassani KA, et al. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis. 2006;193:1313–1322. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]
- 41.Escobar MA, Saravia NG, Weigle K. Concurrent mucosal leishmaniasis and pulmonary tuberculosis: two case reports and review. Clin Infect Dis. 1996;23:836–837. doi: 10.1093/clinids/23.4.836. [DOI] [PubMed] [Google Scholar]
- 42.Löhning M, Richter A, Radbruch A. Cytokine memory of T helper lymphocytes. Adv Immunol. 2002;80:115–181. doi: 10.1016/s0065-2776(02)80014-1. Review. [DOI] [PubMed] [Google Scholar]
- 43.Lavenu-Bombled C, Trainor CD, Makeh I, Romeo PH, Max-Audit I. Interleukin-13 gene expression is regulated by GATA-3 in T cells: role of a critical association of a GATA and two GATG motifs. J Biol Chem. 2002;277:18313–18321. doi: 10.1074/jbc.M110013200. [DOI] [PubMed] [Google Scholar]
- 44.Cáceres-Dittmar G, Tapia FJ, Sánchez MA, et al. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin Exp Immunol. 1993;91:500–505. doi: 10.1111/j.1365-2249.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 46.Chomarat P, Banchereau J. Interleukin-4 and lnterleukin-13: Their Similarities and Discrepancies. Intern. Rev. Immunol. 1998;17:1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 47.Minty A, Chalon P, Derocq JM, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 48.Nylén S, Maasho K, Söderstrom K, Ilg T, Akuffo H. Live Leishmania promastigotes can directly activate primary human natural killer cells to produce interferon-gamma. Clin Exp Immunol. 2003;131:457–467. doi: 10.1046/j.1365-2249.2003.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthews D, Emson C, McKenzie G, Jolin H, Blackwell J, McKenzie A. IL-13 Is a Susceptibility Factor for Leishmania major Infection. J Immunol. 2000;164:1458–1462. doi: 10.4049/jimmunol.164.3.1458. [DOI] [PubMed] [Google Scholar]
- 50.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]


