Summary
We report our experience in the treatment of thoracic and lumbosacral spinal pain due to vertebral bone fractures. This pathology can be related to osteoporosis but also to metastatic disease and less frequently vertebral haemangioma.
From April 2001 through December 2004 we treated 238 patients for a total of 455 vertebral bodies. 175 patients had osteoporosis, 70 had metastasis and 13 had vertebral haemangioma. Sacroplasty was performed in six patients to obtain a cement filling of sacral metastasis.
The procedures were mostly performed under fluoroscopy and only in cases of metastasis or sacroplasty was CT/fluoroscopy guidance preferred for optimal filling of the area of osteolysis.
We evaluated the results at six and 18 months follow-up and analysed the incidence of new vertebral fractures, vascular and disk leakage and the incidence of major and minor complications. Biopsy was performed only in doubtful cases. We obtained different results considering the etiology of the disease. We obtained a 92% success rate at six months follow-up and 89% success at 18 months follow-up in osteoporosis, a 77% and 72% success rate at six and 18 months follow-up in metastastic patients, and no change at six and 18 months follow-up in patients with vertebral haemangioma in which the success rate was of 95%.
We noted extravertebral leakage in 41% of vertebral bodies of which 31 % were treated at the level of the vascular space and only 10% at the level of the disk space, and symptomatic in only two cases (acute compressive radiculitis, medically treated and resolved within a month).
Six patients presented new fractures in the adjacent vertebral body and 30% had a partial recovery in the height of the vertebral body with kyphosis curve reduction.
Vertebroplasty is a good technique to obtain spine pain relief and has a low incidence of side effects. Good quality equipment is important to obtain these results.
Key words: vertebral fractures, vertebroplasty, cement
Introduction
Vertebral collapse can be traumatic, pathological or osteoporotic and can alter the static load of the spinal column, inducing pain over spinal biomechanic and biodynamic modifications.
Osteoporosis is the most frequent cause of vertebral collapse and traditional therapy consists in bed rest, the use of orthopedic corsets and medical therapy such as vitamin D and drugs to reduce reabsorption of the osteoid matrix to prevent further fractures Oestrogen therapy can be used with caution in women in menopausal age.
Other causes of vertebral collapse include bony haemangiomas, metastasis and multiple myeloma determining pathological fracture. A common finding shared by all these conditions is spinal pain which varies widely in intensity, with or without spinal cord and nerve root compression.
Conditions that are not relieved by medical therapy and do not require surgical stabilization are indicated for structural consolidation with injection into the vertebral body of an acrylic cement polymethylmethacrylate (PMMA) using a percutaneous approach, a procedure called vertebroplasty (VP).
VP was first proposed in France in 1987 by Galimbert and Deramond and was initially used for the treatment of vertebral aggressive haemangiomas 17. They verified the utility of the procedure (both for analgesic and stabilization effect) and decided to extend the indication to other types of pathology not always treatable with conservative therapy such as osteoporotic fractures, vertebral metastasis and osteolytic lesions from myeloma, lymphoma and leukemia11,16,18.
The prolonged average length of human life has determined a progressive increase in geriatric pathology in which osteoporosis is the most common and invalidating due to the elevated frequency of metameric fractures.
In the USA it has been calculated that about 700.000 vertebral fractures a year are induced by osteoporotic damage with about 115.000 hospital admissions correlated to the pathology. This results in a considerable increase in cost related to the fact that most of these patients are still of working age, rehabilitative therapies are particularly long and expensive, and there is also a marked reduction of the quality of life.
It has been calculated that in the course of the lives of each individual of white race, one woman in two and one man in eight will have a vertebral porotic collapse. This risk is increased in the Asian population, while it is reduced in the subjects of black race2,4.
In addition, the risk of new fractures increases exponentially in relation to the number of vertebral collapses: when a patient has a vertebral fracture the risk of a second fractures is increased fivefold and when this happens the following risk is increased 20 times4,33,36.
The prolonged therapeutic use of steroids determines an increase in the risk of iatrogenic vertebral fractures 12,39,41.
For all these reasons, VP is currently applied in the treatment of vertebral collapse due to osteoporosis, metastasis and vertebral haemangioma due to the high analgesic and stiffness effect and the low cost and safety of the procedure 14,31,37,38,40.
Material and Methods
From April 2001 to December 2004 we treated with VP 238 patients distributed as follows:
- 155 patients treated for osteoporosis (figure 1)
Figure 1.
Vertebroplasty in a patient with porotic bone fracture of T12. Sagittal T1 wi (A) and T2 wi (B) ahow evidence of an abnormal morphology of T12 that appears hypointense in T1 and moderately hyperintense in T2 wi. CT axial scan (C) disclosed microfractures and mild sclerotic reaction. The patient had pain for three months before treatment. Vertebroplasty was performed with a good clinical outcome with a small amount of cement injected seen in AP (D) and LL (E) views.
- 70 patients treated for vertebral metastasis
- 13 patients treated for angiomas (figure 2) for a total of 455 vertebral bodies treated.
Figure 2.
Aggressive vertebral haemangioma. Sagittal T1 wi (A) and T2 wi (B) show partial L3 collapse with isointensity in T1 wi and hyperintensity in T2 wi. CT axial scan (C) confirms the typical findings. VP was performed obtaining a good filling with extravertebral leakage visible in AP (D) and LL (E) views.
A bipedicular approach was performed in 265 vertebrae and a monopedicular approach in 190 vertebral bodies, mostly at thoracic level. We did not perform phlebography before the treatment due to the fact that the cement and the contrast have different characteristics, such as density and different flow, for which such technique does not represents a reliable criterion to reduce vascular or endocanalicular leakage of cement. Biopsy was undertaken in doubtful cases in 55 patients or in osteolysis in patients with unknown primary lesions (26 cases).
Table 1 shows the diagnostic algorithm used to evaluate patients for possible treatment (figure 3). It may be useful to perform X-ray evaluation in a prone position, supine or in orthostasis to verify changes vertebral collapse leading to metameric instability justifying an immediate treatment with VP.
Table 1.
| DIAGNOSTIC ALGORITHM | |||
| Patients with vertebral pain | |||
| X-ray | |||
| MR | |||
|
Alteration of bone marrow for oedema or angioma |
Neg |
suspicious metastasis NM and CT |
|
| NO VP | VP | ||
| 1 VP | up to 3 VP | ||
Figure 3.
Diagnostic approach in patients with spine pain. After AP and LL x-ray views, MR represent the first approach in T1 (A),T2 (B) and STIR sequences (C) that can clarify the presence of an acute bone marrow oedema that is more evident with STIR sequences. CT scan (D) focuses on evaluation of the posterior wall of the vertebral body to identify microfractures.
Selected patients were all symptomatic from at least one up to six months before treatment, with spine pain resistant to common analgesic therapies.
Treatment was performed at two levels in 185 patients, three levels in two patients and five levels in four patients in double stages. Patients were always positioned in the pronetion, lying down with a pillow under the chest to allow good ventilation. All patients received local anaesthesia using neuroleptoanalgesia in 65% of cases. General anaesthesia was never required.
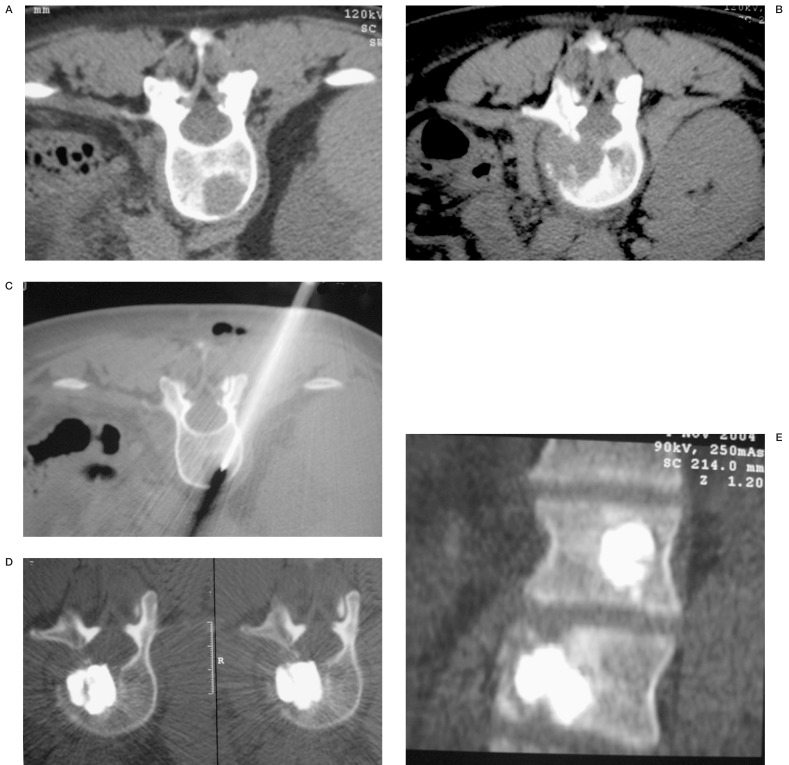
A combined approach using CT plus fluoroscopy was adopted in patients with metastasis for an easier approach to the osteolytic area (figure 4) while only a fluoroscopic approach was used in all other cases.
Figure 4.
Vertebroplasty in a patient with osteolytic metastasis. CT scan shows two lytic lesions at T12 (A) and L1 (B) levels. Under CT guidance a 13 G needle is positioned within the lytic area (C). Post VP axial scan (D) shows a good filling of the lytic lesion, better visible in MPR (E).
The quantity of injected cement varied from a minimum of four ml for thoracic vertebrae with a monopedicular approach up to 5-6 ml in the thoracolumbar region and 12 ml at lumbar level.
CT control was limited to cases with persistent, even partial pain. The type of filling achieved, homogeneous or inhomogeneous, was considered in relation to the remission of pain. A blood test (with PT, PTT and fibrinogen) was performed in all patients as routine pre-operative examination and to exclude coagulopathies.
Execution technique
The first step was the correct positioning of the vertebra to treat under fluroscopy, taking care to find the correct incidence of the x ray beam to allow the alignment of the somatic thresholds and the central position of the spinous process.
The needle used for VP was usually caliber 11G or 13G, varying in length from 10 cm to 15 cm with a bevelled shape and fortified lateral small wings to facilitate rotation. We currently use the Optimed Chiba needle but many other good products are commercially available. The needle for paravertebral anaesthesia is instead a simple spinal needle (from 18G to 22G), 1012 cm long.
In the case of execution under CT, axial scans were an excellent guide to reach the vertebral body easily.
The polymer used was a low density cement with a reduced polymerization temperature, constituted by a methacrylate powder and a solvent (Ospeopal VBiomet). This cement does not include any antibiotic in the powder.
In sterile conditions, under fluoroscopic control and PA view, one peduncle of the vertebra to treat was taken as a reference point. When the tip of the needle touched the bone, the C arm was rotated in a LL position.
At this stage, once the needle had been passed through the posterior wall, it was positioned in the III anterior part of the vertebral body using a rotatory motion or an orthopaedic hammer previously sterilized. No difficulty was encountered in porotic patients in passing through the cortex and positioning the needle in the centre.
In 298 cases we used a 1 ml syringe to inject the cement and guarantee the operator the correct injection pressure. As an alternative we used available commercial kits with spin rotation systems that ensure satisfactory injection pressure18,24. After the needle was C prepared the cement that consists in the addition of a solvent (polymethlmethacrylate) to the powder cement (methacrylate) to obtain low density and viscosity cement with a lower polymerization temperature. The polymerization process occurs when the cement mixture is ready (powder and solvent are mixed) and can be slowed down preserving the prepared cement in syringes to dip in iced sterile physiological solution7.
This allows more time available for low density cement and to perform the procedure without the need to use additional an cement box. This can be particularly useful in the case of monometameric bipedicular approaches or in case of a monopedicular polymetameric approach with the needle already in the correct position1,28.41,49.
After the vertebra was sufficiently filled, the needle was filled with mandrine before being removed to avoid the release of cement in the space created by the needle. This must be done scopically due to the fact that the residual cement could otherwise go into an undesiderable space.
After treatment, patients were positioned lying down in prone decubitus for about one/two hours, and discharged under antibiotic therapy i.m. for three days. Under CT guidance six patients underwent sacroplasty for osteolysis areas from breast Ca or pathological fractures (figure 5,6).
Figure 5.
Sacroplasty in a patient with lytic lesion of the left sacrum wing. Axial T1 wi (A) shows a hypointense area of the left sacral wing that is very well filled with cement injection (B) as also confirmed by MPR (C).
Figure 6.
Sacroplasty in a patient with pathologic fractures. CT scan (A) shows pathologic fractures of both sacral wings, more evident on the left side. Under CT guidance (B) two 13 G needles are positioned and the post-operative CT axial scan (C) shows a good filling of the fracture lines, visible also in MPR (D).
We also assessed possible metameric recovery in height of the vertebral fracture after injection of endovertebral cement. The injection must always be done slowly and steadily under fluoroscopic control to verify cement leakage through the walls.
The only absolute contraindication is the presence of systemic or local infection. The relative contraindications to the procedure consist in epidural extension of secondary tumours, the presence of clinical signs like nerve root or spinal cord compression, complete collapse of the vertebra, certain type of lesions such as osteoblastic metastasis, the origin of diffuse doral pain not consistent with clinical examination and negative MR without signal alterations in the bone marrow, and all coagulation disorders not amenable to medical therapy.
Results
We obtained satisfactory results on the basis of a modified McNab method and on the VAS scale. Tables 2 and 3 show the results of the 238 patients divided according to pathology accountable for symptoms at three and 18 months.
Table 2.
| Osteoporosis | Angiomas | Secondary lesions |
|---|---|---|
| 92% success | Success 90% | Success 77% |
| 8% failure | Failure 10% | Failure 23% |
Table 3.
| Osteoporosis | Angiomas | Secondary lesions |
|---|---|---|
| 89% success | Success 90% | Success 72% |
| 11% failure | Failure 10% | Failure 28% |
The patients had follow-up examinations at three, six and up to 18 months with minimal changes in the success or failure noted (Table 3).
We observed extravertebral cement leakage in 180 cases (figure 7), but only in two cases did we encounter clinical complications such as radiculitis from iatrogenic radicular compression treated and solved with medical therapy within a month. During follow-up we found fractures of adjacent vertebrae in six patients at a short distance from the first treatment and the new fractures were subsequently treated with VP (figure 8).
Figure 7.
VP of a patient with metastasis and left paravertebral leakage. AP (A) and LL (B) views show evidence of a good filling of the R half vertebral body while the filling in the left half is inhomogeneous with visible left paravertebral leakage.
Figure 8.
VP of T12 in a patient with a previous fracture of L1 already treated with vertebroplasty. The sagittal T1 wi (A) and T2 (B) show an area hypointense in T1 and T2 wi of L1 due to the presence of the cement and an area hypointense in T1 wi and hyperintense in T2 wi of T12 due to bone marrow oedema (new fracture a week after the previous one). The patient was treated with a new VP at T12 level with good recovery.
In one case of compressive osteoangioma VP was useful to stabilize the vertebra without yet managing to avoid decompressive laminectomy. In neoplastic cases, VP always preceded radiotherapy which often determines a sclerotic reaction of the vertebral bone marrow hampering injection of the cement15. The porotic patient did not require an orthopaedic corset after treatment.
There was no correlation between the amount of cement injected and remission of symptoms which depended on the degree of vertebral erosion and the level of the vertebra treated with a smaller quantity of injected cement at thoracic level with a wedge shape (even only four ml) in comparison with the lumbar vertebrae not completely wedged (up to 12 ml).
A recovery in height of the vertebra with a reduction of the kyphosis was found in 135 vertebrae treated (30%). The recovery ranged from 2 mm up to 3 mm (figure 9). The measurement was obtained on the basis of Cobb's angle and on the recovery in mm before and after the treatment at the centre of the vertebral body.
Figure 9.
Recovery of the height of the vertebral body after VP. The patient presents a vertebral porotic bone fracture treated with a monopedicular approach (A). The filling of the vertebral body is homogeneous as seen in AP (B) and LL (C) views. Comparing the vertebral height before (D) and after (E) treatment, a recovery of more than 30% is seen.
Diffusion and filling of the vertebrae was much more homogeneous and regular in the cases of osteoporosis or angiomas in comparison with vertebrae affected by secondary lesions. However, the success of the treatment was not related to homogeneous or inhomogeneous diffusion of the cement in the vertebra.
We had no major complications like pulmonary embolisms or spinal cord compression.
Discussion
The diagnostic-therapeutic protocol (table 1) of a subject with vertebral pain begins with a standard radiologic study that must show the fracture centers. The spinal interventional neuroradiologist must make a careful clinical evaluation of the patient to recognize and differentiate radicular syndromes, facet syndrome, myalgic syndromes and obviously spinal pain from vertebral collapse.
Magnetic Resonance (MR) with T1WI, T2WI and STIR sequences is the key method of choice to differentiate acute from chronic metameric collapse, due to the possibility to disclose signal changes in the spinal cord that is substantially iso/hypointense in chronic lesions in all sequences (due to sclerosis), and the presence of hypointensity in T1 wi, hyperintensity in T2 wi and STIR sequences in acute lesions related to bone marrow oedema. Nevertheless, MR will also disclose conditions of metameric oedema without collapse due to microfracture, thereby demonstrating a pathological condition in the initial phase responsible for pain, to be treated with VP2,36. In the case of spinal pain in the absence of bone marrow oedema visible at MR or if the patient cannot perform a MR, bone nuclear medicine scan may be useful to differentiate subsequently chronic from acute fractures18.
CT generally is performed to verify and better characterize the integrity of the posterior wall of the collapsed metastatic vertebra (that when interrupted increases the risk of cement leakage into the spinal canal). CT is also the only radiologic examination that can be performed after standard x-ray in patients with contraindications to magnetic field exposure.
Generally the clinical sign of metameric focal pain at finger pressure associated with exacerbated pain in the bending manoeuvre of the spine is highly indicative of the centre level of the acute process. In these cases patients have high level pain in an erect position whereas pain is reduced in a clinostatic position.
Before starting treatment it is important to find a correspondence between clinical findings and the abnormal MR signal.
Given its incidence, there is an indication for the treatment with VP of the intermediate vertebra between the ones affected by the acute pathological process (e.g. the treatment even of L2 in a patient with acute fracture of L1 and L3). This is the only case in which a prophylactic vertebroplasty is performed in our experience. In those cases, it is suggested VP be performed in the same session, even if the vertebra is not yet fractured. This choice is dictated by the biomechanic and biodynamic changes related to the process of fracture, and subsequently by VP.
The peripheral neurologic signs will verify the presence of cord or nerve root compression.
The radiation dose in VP is critical and operators should minimize fluoroscopy in order to reduce exposure34.
As regards the quantity of cement to inject, in our experience there is no exact correspondence between the quantity of cement injected and a disappearance of symptoms14,22,28.
A problem to which much attention has been paid is the possible leakage of cement into extrametameric areas, vascular, discal or endocanalicular30. Endocanalicular leakage appears be the main complication to avoid. In our experience, injecting the cement slowly and attempting to observe its in the vertebra seem to be the most reliable precautions to prevent such complication or to reduce the ensuing damage. If vascular or endocanalicular leakage is noted during the procedure, the injected is stopped, at least temporarily, even if neurologic damage is not inevitable46,56.
The procedure is also interrupted in the case of leakage of even a small quantity of cement into the disk29. It is often enough to interrupt the injection momentarily to allow focal polymerization of the cement in the leakage point, then to resume the injection to allow the cement to undertake a new route of diffusion. Only if there is still leakage in an undesired area is the procedure abandoned. In our experience repositioning the needle is not advisable as it potentially facilitates leakage of cement cortical microinterruption.
We do not perform a preventive phlebography before treatment as it does not appear to be decisive in predicting venous leakage 13,52,55 due to different density and viscosity between contrast media and cement.
There are no doubts that the risk of venous leakage of cement is increased in patients with tumours due to the presence of anarchic vascularization and intraneoplastic anastomosis, and in those with aggressive angiomas due to multiple hematic lakes. However, if the procedure is done with a high quality machine (CT and/or angio suite) this complication is unlikely to arise and can be easily managed16,17,44,47,54.
In our experience the risk of emboli in the lungs or other anatomic locations is minimal even in consideration of the fact that pulmonary microemboli do not represent ct23,25,45,53.
In the case of multiple lesions such as that from metastasis, lymphoma, leukaemia and multiple myeloma, the treatment levels will be chosen by careful analysis of the clinical and diagnostic-instrumental parameters (systemic oncologic evaluation).The final indication for VP in all cases will be the result of a risk-benefit assessment by the operator.
In the case of secondary lesions, VP is one of the additional techniques available for pain relief and antineoplastic action (chemotoxicity). The VP must be effected in oncologic cases before or just after radiotherapeutic treatment when a sclerotic post-attinic reaction is possible and the injection of cement would be more difficult. Among the vertebral organic diseases the most common metastases are osteolytic, (breast, lung, kidney and colon represent the most frequent primary tumours), less frequently osteoblastic and mixed.
VP is not indicated in osteoblastic lesions secondary to prostate cancer, whereas it may be taken into consideration in mixed lesions but entails a greater degree of difficulty.
In osteolytic lesions, when the bony lesion is small and well defined, in the absence of direct involvement of the posterior walls, the CT approach is preferred to ensure the exact position of the tip of the needle within the central lytic area to enhance cement release. Even in the case of posterior wall involvement by a metastatic lesion, it is important to balance the treatment-benefit of the VP and perform the procedure under fluoroscopic control to avoid undesirable complications. Before VP an easy biopsy is useful in cases of unknown primary lesions as VP can also be followed by radiofrequency treatment35,44
Treatment of secondary lesions is also indicated at sacral level called sacroplasty where a CT guided approach is essential for correct positioning of the needle in the area of osteolysis.
The frequent and even occasional finding of metameric haemangiomas requires a classification of lesions susceptible to VP. There are four subtypes of haemangioma:
1) asymptomatic without aggressive signs
2) symptomatic but without aggressive signs
3) asymptomatic with aggressive signs
4) symptomatic with aggressive signs
The radiologic signs of aggressiveness are abnormal MR findings with hypointensity in T1 wi, hyperintensity in T2 wi, enhancement after contrast media injection, and the presence of an epidural solid component and cortical erosion. VP is not indicated in patients belonging to group 1. VP can be entertained by virtue of substantial pain in group two patients. Patients belonging to group three need to follow-up with MR because VP may be suggested in case of an objective increase in the signs of aggressiveness even in the absence of symptoms. VP is indicated in all group four patients.
The only current absolute contraindication to the treatment of VP is the presence of local inflammatory-infectious or systemic infection. Relative contraindications are given by the clinical conditions of the patient such as coagulopathy, radicular compression, epidural extension of metastatic lesions or aggressive angiomas, marked vertebra plana, patients with old fractures, spinal cord or radicular syndromes and the presence of osteoblastic metastasis.
The acrylic cement (PMMA) needs to be mixed with a sterilized powder with an elevated atomic number (barium sulphate, tantalium, tungsten) and its density increased to improve the radiopacity27. Currently it is possible to buy many cements already mixed with radiopaque powders up to 25% of the mixture, with antibiotics too, and the polymerization can be controlled and slowed down by storing it at room temperature or in iced physiological solution.
Many authors 5 have shown that to obtain optimal recovery of the metameric resistance it is not necessary for cement to overload the vertebra. Studies by Belkoff and Tohmeh6 emphasised that it is enough to inject 2.5 ml of cement into the vertebra at thoracic level, 3 ml in the thoracolumbar region and 4 ml into the lumbar region to have a recovery of resistance.
The bilateral transpedicular approach guarantees optimal homogeneous distribution of the cement in all cases. Nevertheless the monopedicular approach is sufficient for the purposes of optimal filling and clinical outcome if the tip of the needle is located in the centre of the vertebra.
The type of cement distribution will differ depending on the type of pathology. In osteoporotic collapse being the result of a marked reduction of mineralization, the cement will find reduced resistance and hence the injection pressure will be easily modulated to obtain a homogeneous filling. In secondary lesions the presence of a solid component opposed to the injection of cement will increase the risk of extravertebral leakage, due also to the pathological neovascularity and for this reason we often obtain an inhomogeneous filling in those cases.
Limited cement leakage into the disk centre does not determine an immediate change in spine biodynamics 5. Large cement leakage into the disk space may increase the risk of fractures to vertebrae adjacent to the one treated.
The mechanism by which VP determines a reduction of pain remains unsettled. Three theories have been proposed to explain the clinical results: chemical reaction, the thermal theory and the mechanical theory31.
Chemical - Cytotoxic hypothesis
When not polymerized, methylmethacrylate shows an intrinsic high cytotoxicity that has a destructive effect on neoplastic cells, such as to prevent new proliferation in the vertebrae. The cement in practice would only have an adjuvant action in the resolution of pain because studies on cellular cultures have shown that to induce a complete cytotoxic action a greater concentration of the monomer would be needed.
Thermal hypothesis
The polymerization process of the cement generates heat. VP determines the disappearance of the pain after a period that varies from 24-48 hours after treatment, up to 30 days, with an average of seven days. The thermal effect has been postulated by the necrosis induction of the nerve near the periostium in contact with the cement. But this theory can only be taken into consideration if the temperature reaches and overcomes 50° for longer than a minute whereas the temperature of the mass cementing agent in the surface will be lower. Therefore the thermal effect only partially explains the analgesic effect of VP.
Mechanical hypothesis
The introduction of cement in the vertebra determines an increase in resistance and this is probably the principal reason for the success of the teaching method. After a variable mean interval from three to six minutes after injection, the cement polymerizes reaching about 90% of its definitive strength with a consequent recovery of metameric resistance. This strict correlation between cause and effect is not comparable with the processes of physiological sclerosis after radiotherapy or embolization of vertebral tumours that needs some months to optimize.
Conclusions
French and US scientists have approached the same technique with rigorous methodology, identifying the possible mechanisms of VP action and diffusing the method worldwide.
The over 35.000 treatments performed in the United States in 2002 in a population of about 260 million citizens 10 may indicate an abuse of the method, but also testifies its reliability and therapeutic efficacy.
In epidemiological terms metameric collapse due to osteoporosis represents the pathology mostly treated with VP. The most common fracture region is the thoracolumbar and lumbar spine and sometimes this structural abnormality occurs even in the absence of trauma.
In spite of the use of rehabilitative and pharmacological therapies, the evolution of the metameric collapse leads to a progressive wedge and deformity of the vertebra, with consequent hyperkyphotic conditions that involve reduced lung function with - pulmonary overinfection with a change in spine biomechanics.
The limits of standard therapies and the known failures of surgical therapy in the elderly, in addition to the high social cost for the chronically disabled patient, account for the enormous interest surrounding the VP procedure that introduces less risk, lower cost, and elevated patient satisfaction after prompt pain resolution.
Some authors42, as in our experience, found that the injection of cement into the vertebra determined a height recovery of the vertebra to different degrees. In this connection a recent work by Hiwataschi et Al.21 examined a series of 37 patients and 85 vertebrae treated with VP for porotic pathology. They verified metameric recovery after VP on three lines of somatic measurement, comparing the measurements with MR images before intervention and post-CT.
Images after the intervention with measurements of the non pathological adjacent metamer. Their results showed that following VP there is an average 2.2 mm recovery of height of the vertebra, more evident in the intermediate segment of the body and mostly visible in porotic pathology with a consequent reduction of the degree of kyphosis. These findings were obtained without any overload of the metamer (average injection of cement of about 7 ml).
To avoid endocanalicular diffusion of the cement, it has been suggested to add intrathecal contrast media to better visualize controlled epidural diffusion43. Some authors3,19,51 have postulated a greater risk of fractures in vertebrae adjacent to the site of cement injection. In our experience we have only noted this correspondence in a few cases and there is no contraindication to treat even a new vertebra fracture with VP.
Histological evaluation of the vertebral body after VP shows a mild granulation tissue without inflammatory degenerative changes48. Experimental studies have also shown that during VP there is only a minimal increase in endovertebral pressure, not causing an increased risk of extravertebral cement leakage 50.
We did not compare VP with other percutaneous vertebral procedure such as kyphoplasty that in our opinion is indicated in selected cases like acute post-traumatic vertebral fractures and not in all cases of vertebral porotic ormetastatic fractures 9,20,26,32.
Reviewing the international literature and our experience, VP is currently a safe and reliable percutaneous therapeutic procedure that has a twofold action of stabilization and analgesia on the vertebra.
As in all medical practice, execution of VP has a learning curve for the operator who be supervised by an experienced tutor to reduce complications that are possible due to inexperience and useless attempts at metameric overload to satisfy an iconographic gratification but which are useless and potentially harmful for the patient.
VP must be performed with a high quality machine and by skilled interventional neuroradiologists.
References
- 1.Amar AP, Larsen DW, et al. Percutaneous transpedicular polymethylmethacrylate vertebroplasty for the treatment of spinal compression fractures. Neurosurgery. 2001;49:1105–1114. [PubMed] [Google Scholar]
- 2.Andreula C, Muto M, Leonardi M. Interventional spinal procedures. Eur J Radiol. 2004;50:112–119. doi: 10.1016/j.ejrad.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Anita A, Uppin MD, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj S, Saag KG. Osteoporosis: evaluation and treatment. Curr Womens Health Rep. 2003;3:418–124. [PubMed] [Google Scholar]
- 5.Baroud G, Nemes J, Heini P. Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. Eur Spine J. 2003:1. doi: 10.1007/s00586-002-0512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkoff SM, Mathis JM, et al. An ex vivo biomechanical evaluation of a hydroxyapatite cement for use with vertebroplasty. Spine. 2001;26:1542–1546. doi: 10.1097/00007632-200107150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Chavali R, Resijek R, et al. Extending polymerization time of polymethylmethacrylate cement in percutaneous vertebroplasty with ice bath cooling. Am J Neuroradiol. 2003;24:545–546. [PMC free article] [PubMed] [Google Scholar]
- 8.Cotten A, Boutry N, et al. Percutaneous vertebroplasty: state of the art. Radiographics. 1998;18:311–320. doi: 10.1148/radiographics.18.2.9536480. [DOI] [PubMed] [Google Scholar]
- 9.Crandall D, Slaughter MD, et al. Acute versus chronic vertebral compression fractures treated with kyphoplasty: early results. The spine J. 2004;4:418–424. doi: 10.1016/j.spinee.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 10.David A, Nussbaum MS, et al. A review of complications associated with vertebroplasty and kyphoplasty as reported to the food and drug administration medical device related web site. J Vasc Interv Radiol. 2004;15:1185–1192. doi: 10.1097/01.RVI.0000144757.14780.E0. [DOI] [PubMed] [Google Scholar]
- 11.Deramond H, Depriester C, et al. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36:533–546. doi: 10.1016/s0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 12.Deramond H, Mathis JM. Vertebroplasty in osteoporosis. Semin Musculoskelet Radiol. 2002;6:263–268. doi: 10.1055/s-2002-36724. [DOI] [PubMed] [Google Scholar]
- 13.Do HM. Intraosseous venography during percutaneous vertebroplasty: is it needed? Am J Neuroradiol. 2002;23:508–509. [PMC free article] [PubMed] [Google Scholar]
- 14.Evans AJ, Jensen ME, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty: retrospective report of 245 cases. Radiology. 2003;226:366–372. doi: 10.1148/radiol.2262010906. [DOI] [PubMed] [Google Scholar]
- 15.Fourney DR, Schomer DF, et al. Percutaneus vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg Spine. 2003;98:2130. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 16.Galimbert P, Deramond H. Percutaneous acrylic vertebroplasty as a treatment of a vertebral angioma as well as painful and debilitating diseases. Chirurgie. 1990;116:326–334. [PubMed] [Google Scholar]
- 17.Galimbert P, Deramond H, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 18.Gangi A, Guth S, et al. Percutaneous vertebroplasty: indications, technique, and results. Radiographics. 2003;23:10–20. doi: 10.1148/rg.e10. [DOI] [PubMed] [Google Scholar]
- 19.Gaughen JR, Jr, Jensen ME, et al. The therapeutic benefit of repeat percutaneous vertebroplasty at previously treated vertebral levels. Am J Neuroradiol. 2002;23:1657–1661. [PMC free article] [PubMed] [Google Scholar]
- 20.Heini PF, Orler R. Kyphoplasty for treatment of osteoporotic vertebral fracture. Eur Spine J. 2004;13:184–192. doi: 10.1007/s00586-003-0654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiwatashi A, Moritani T, et al. Increase in vertebral body height after vertebroplasty. Am J Neuroradiol. 2003;24:185–189. [PMC free article] [PubMed] [Google Scholar]
- 22.Hodler J, Peck D, Gilula LA. Midterm Outcome after Vertebroplasty: Predictive Value of Technical and Patient-related Factors. Radiology. 2003;227:662–668. doi: 10.1148/radiol.2273011930. [DOI] [PubMed] [Google Scholar]
- 23.Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine. 2002;27:416–418. doi: 10.1097/00007632-200210010-00021. [DOI] [PubMed] [Google Scholar]
- 24.Kallmes DF, Jensen ME. Percutaneous Vertebroplasty: how I do it. Radiology. 2003;229:27–36. doi: 10.1148/radiol.2291020222. [DOI] [PubMed] [Google Scholar]
- 25.Kirby BS, Doyle A, Gilula LA. Acute bronchospasm due to exposure to polymethylmethacrylate vapors during percutaneous vertebroplasty. Am J Roentgenol. 2003;180:543–544. doi: 10.2214/ajr.180.2.1800543. [DOI] [PubMed] [Google Scholar]
- 26.Leiberman I, Reinhardt MK. Vertebroplasty and Kyphoplasty for osteolytic vertebral collapse. Clin Orthop S. 2003:176–186. doi: 10.1097/01.blo.0000093841.72468.a8. [DOI] [PubMed] [Google Scholar]
- 27.Leibold RA, Gilula LA. Sterilization of barium for vertebroplasty: an effective, reliable and inexpensive method to sterilize powders for surgical procedures. Am J Roentgenol. 2002;179:198–200. doi: 10.2214/ajr.179.1.1790198. [DOI] [PubMed] [Google Scholar]
- 28.Liebschner MA, Rosenberg WS, Keaveny TM. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine. 2001;26:1547–1554. doi: 10.1097/00007632-200107150-00009. [DOI] [PubMed] [Google Scholar]
- 29.Lin EP, Ekholm S, et al. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. Am J Neuroradiol. 2004;25:175–180. [PMC free article] [PubMed] [Google Scholar]
- 30.Mathis JM. Percutaneous Vertebroplasty: complication avoidance and technique optimisation. Am J Neuroradiol. 2003;24:1697–1706. [PMC free article] [PubMed] [Google Scholar]
- 31.Mathis JM, Barr JD, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. Am J Neuroradiol. 2001;22:373–381. [PMC free article] [PubMed] [Google Scholar]
- 32.Mathis JM, Orlando O, Zoarski GH. Vertebroplasty versus Kyphoplasty: a comparison and contrast. Am J Neuroradiol. 2004;25:840–845. [PMC free article] [PubMed] [Google Scholar]
- 33.Mehbod A, Aunoble S, Le Huec JC. Vertebroplasty for osteoporotic spine fracture: prevention and treatment. Eur Spine J. 2003;12:155–162. doi: 10.1007/s00586-003-0607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehdizade A, Lovblad KO, et al. Radiation dose in vertebroplasty. Neuroradiology. 2004;46:243–245. doi: 10.1007/s00234-003-1156-0. [DOI] [PubMed] [Google Scholar]
- 35.Minart D, Vallee JN, et al. Percutaneous coaxial transpedicular biopsy of vertebral body lesions during vertebroplasty. Neuroradiology. 2001;43:409–412. doi: 10.1007/s002340000423. [DOI] [PubMed] [Google Scholar]
- 36.Muto M, Bonsignore R, Palmieri A. La vertebroplastica nel trattamento delle lombalgie da osteoporosi e delle lesioni secondarie vertebral. Rivista di Neuroradiologia. 2001;14:187–290. [Google Scholar]
- 37.Peh WC, Gelbart MS, et al. Percutaneous vertebroplasty: treatment of painful vertebral compression fractures with intraosseous vacuum phenomena. Am J Roentgenol. 2003;180:1411–1417. doi: 10.2214/ajr.180.5.1801411. [DOI] [PubMed] [Google Scholar]
- 38.Peh WC, Gilula LA. Percutaneous vertebroplasty: indications, contraindications, and technique. Br J Radiol. 2003;76:69–75. doi: 10.1259/bjr/10254271. [DOI] [PubMed] [Google Scholar]
- 39.Peh WC, Gilula LA, Peck DD. Percutaneous vertebroplasty for severe osteoporotic vertebral body compression fractures. Radiology. 2002;223:121–126. doi: 10.1148/radiol.2231010234. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Higueras A, Alvarez L, et al. Percutaneous vertebroplasty: long-term clinical and radiological outcome. Neuroradiology. 2002;44:950–954. doi: 10.1007/s00234-002-0856-1. [DOI] [PubMed] [Google Scholar]
- 41.Peters KR, Guiot BH, et al. Vertebroplasty for osteoporotic compression fractures: current practice and evolving techniques. Neurosurgery. 2002;51:96–103. [PubMed] [Google Scholar]
- 42.Robert YC, Haleh G, et al. Osteoporotic vertebral collapse: percutaneous vertebroplasty and local kyphosis correction. Radiology. 2004;233:891–898. doi: 10.1148/radiol.2333030400. [DOI] [PubMed] [Google Scholar]
- 43.Sarzier JS, Evans AJ. Intrathecal Injection of Contrast Medium to Prevent Polymethylmethacrylate Leakage during Percutaneous Vertebroplasty. Am J Neuroradiol. 2003;24:1001–1002. [PMC free article] [PubMed] [Google Scholar]
- 44.Schaefer O, Lohrmann C, et al. Technical innovation. Combined treatment of a spinal metastasis with radiofrequency heat ablation and vertebroplasty. Am J Roentgenol. 2003;180:1075–1077. doi: 10.2214/ajr.180.4.1801075. [DOI] [PubMed] [Google Scholar]
- 45.Scroop R, Eskridge J, Britz GW. Paradoxical cerebral arterial embolization of cement during intraoperative vertebroplasty: case report. Am J Neuroradiol. 2002;23:868–870. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Shapiro S, Abel T, Purvines S. Surgical removal of epidural and intradural polymethylmethacrylate extravasation complicating percutaneous vertebroplasty for an osteoporotic lumbar compression fracture. Case report. J Neurosurg. 2003;98:90–92. doi: 10.3171/spi.2003.98.1.0090. [DOI] [PubMed] [Google Scholar]
- 47.Shimony JS, Gilula PL, et al. Percutaneus vertebroplasty for malignant compression fractures with epidural involvement. Radiology. 2004;232:846–853. doi: 10.1148/radiol.2323030353. [DOI] [PubMed] [Google Scholar]
- 48.Togawa D, Bauer TW, et al. Histologic evaluation of human vertebral bodies after vertebroplasty augmentation with polymethyl methacrylate. Spine. 2003;28:1521–1527. [PubMed] [Google Scholar]
- 49.Tohmeh AG, Mathis JM, et al. Biomechanical efficacy of unipedicular versus bipedicular vertebroplasty for the management of osteoporotic compression fracture. Spine 1; 1999;24:1772–1776. doi: 10.1097/00007632-199909010-00004. [DOI] [PubMed] [Google Scholar]
- 50.Tomita S, Molloy S, et al. Ex vivo mesurement of intravertebral pressure during vertebroplasty. Spine. 2004;29:723–725. doi: 10.1097/01.brs.0000116985.86437.04. [DOI] [PubMed] [Google Scholar]
- 51.Uppin AA, Hirsch JA, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 52.Vasconcelos C, Gailloud P, et al. Is percutaneous vertebroplasty without pretreatment venography safe? Evaluation of 205 consecutive procedures. Am J Neuroradiol. 2002;23:913–917. [PMC free article] [PubMed] [Google Scholar]
- 53.Vasconcelos C, Gailloud P, et al. Transient arterial hypotension induced by polymethylmethacrylate injection during percutaneous vertebroplasty. J Vasc Interv Radiol. 2001;12:1001–1002. doi: 10.1016/s1051-0443(07)61584-x. [DOI] [PubMed] [Google Scholar]
- 54.Weill A, Chiras J, et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241–247. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]
- 55.Wong W, Mathis J. Is intraosseous venography a significant safety measure in performance of vertebroplasty? J Vasc Interv Radiol. 2002;13:137–138. doi: 10.1016/s1051-0443(07)61929-0. [DOI] [PubMed] [Google Scholar]
- 56.Yeom JS, Kim WJ, et al. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br. 2003;85:83–89. doi: 10.1302/0301-620x.85b1.13026. [DOI] [PubMed] [Google Scholar]