Abstract
Acetaminophen induces the mitochondrial permeability transition (MPT) in hepatocytes. Reactive oxygen species (ROS) trigger the MPT and play an important role in AAP-induced hepatocellular injury. Because iron is a catalyst for ROS formation, our aim was to investigate the role of chelatable iron in MPT-dependent acetaminophen toxicity to mouse hepatocytes. Hepatocytes were isolated from fasted male C3Heb/FeJ mice. Necrotic cell killing was determined by propidium iodide fluorometry. Mitochondrial membrane potential was visualized by confocal microscopy of tetramethylrhodamine methylester. Chelatable ferrous ion was monitored by calcein quenching, and 70 kDa rhodamine-dextran was used to visualize lysosomes. Cell killing after acetaminophen (10mM) was delayed and decreased by more than half after 6 h by 1mM desferal or 1mM starch-desferal. In a cell-free system, ferrous but not ferric iron quenched calcein fluorescence, an effect reversed by dipyridyl, a membrane-permeable iron chelator. In hepatocytes loaded with calcein, intracellular calcein fluorescence decreased progressively beginning about 4 h after acetaminophen. Mitochondria then depolarized after about 6 h. Dipyridyl (20mM) dequenched calcein fluorescence. Desferal and starch-desferal conjugate prevented acetaminophen-induced calcein quenching and mitochondrial depolarization. As calcein fluorescence became quenched, lysosomes disappeared, consistent with release of iron from ruptured lysosomes. In conclusion, an increase of cytosolic chelatable ferrous iron occurs during acetaminophen hepatotoxicity, which triggers the MPT and cell killing. Disrupted lysosomes are the likely source of iron, and chelation of this iron decreases acetaminophen toxicity to hepatocytes.
Keywords: acetaminophen, cell death, glutathione, iron, mitochondria
Acetaminophen overdose is the leading cause of acute liver failure in North America (Fontana, 2008). In experimental animals and humans, cytochrome P-450 oxidation of acetaminophen forms N-acetyl-p-benzoquinonimine (NAPQI), which glutathione (GSH) detoxifies by conjugate formation (Mitchell et al., 1973; Nelson, 1990). After GSH exhaustion, continued NAPQI formation promotes oxidative stress (Bajt et al., 2004) and onset of the mitochondrial permeability transition (MPT), a phenomenon characterized by opening of high conductance permeability transition pores in the mitochondrial inner membrane that conduct solutes up to a molecular mass of about 1500 (Hanawa et al., 2008; Kon et al., 2004; Reid et al., 2005). In cultured mouse hepatocytes, MPT onset causes either necrotic cell death from ATP depletion or apoptosis secondary to cytochrome c release, both of which are delayed by the MPT inhibitors, cyclosporin A (CsA), and NIM811 (Kon et al., 2004).
Formation of reactive oxygen species (ROS) increases after acetaminophen exposure, and agents that augment antioxidant defenses and scavenge ROS protect against acetaminophen toxicity in vitro and in vivo (Jaeschke and Bajt, 2006; Jaeschke et al., 2002). The iron chelator, desferal, also protects hepatocytes after acetaminophen and in other models of cytotoxicity mediated by oxidative stress (Badr et al., 1986; Gerson et al., 1985; Nieminen et al., 1997; Sakaida et al., 1995; Schnellmann et al., 1999; Uchiyama et al., 2008). Here, we investigated the role of iron in acetaminophen-induced cytotoxicity to cultured mouse hepatocytes. The data indicate that acetaminophen exposure leads to release of chelatable ferrous iron from lysosomes, which then is taken up by mitochondria to promote mitochondrial ROS formation, the MPT, and cell death.
MATERIALS AND METHODS
Mouse hepatocytes.
Hepatocytes were isolated from 25 to 30 g overnight-fasted male C3Heb/FeJ mice (Jackson Laboratory, Bar Harbor, ME) by collagenase digestion and plated on type 1 collagen-coated 24-well microtiter plates, 60-mm culture dishes, or glass bottom Petri dishes in Waymouth’s medium MB-752/1 supplemented with 2mM L-glutamine, 10% fetal calf serum, 100nM insulin, 100nM dexamethasone, 100 U/ml penicillin, and 100 μg/ml streptomycin, as previously described (Qian et al., 1997). Cell viability was greater than 90% by trypan blue exclusion. After 4 h, hepatocytes were placed in hormonally defined medium (HDM) consisting of RPMI 1640 supplemented with 240nM insulin, 2mM L-glutamine, 1 μg/ml transferrin, 0.3nM selenium, 1.5μM free fatty acids, 100 U/ml penicillin, and 100 μg/ml streptomycin. Animal protocols were approved by the Institutional Animal Care and Use Committee.
Fluorometric assay of cell viability.
Loss of cell viability was assessed by propidium iodide (PI) fluorometry, as previously described (Nieminen et al., 1992). This assay correlates closely with trypan blue uptake and enzyme release as indicators of oncotic necrosis.
Laser scanning confocal microscopy.
Loading of hepatocytes with calcein and tetramethylrhodamine methylester (TMRM) for confocal microscopy was carried out as described previously (Kon et al., 2004). Briefly, hepatocytes plated on cover glasses were incubated in HDM with 50mM HEPES (pH 7.4) to stabilize pH. To stabilize the plasma membrane after acetaminophen-induced disruption of mitochondrial metabolism, in some experiments, hepatocytes were incubated with 20mM fructose plus 5mM glycine. After 2.5 h of treatment with 10mM acetaminophen or no addition, cells were loaded with 100nM TMRM, 1μM acetoxymethyl ester of calcein (calcein-AM), and 3μM PI in HDM at 37°C and then incubated in the presence of calcein-free acid (300μM). In experiments to visualize lysosomes, mice were injected with rhodamine-dextran (70 kDa, 5 mg/g body weight, ip) 12 h prior to hepatocyte isolation (Gores et al., 1989). Images were collected using Zeiss LSM 410 and LSM 510 laser scanning confocal microscopes (Zeiss, Germany). TMRM, PI, and rhodamine-dextran fluorescence was excited at 568 nm, and emission was imaged through a 590-nm long pass filter. Calcein fluorescence was excited at 488 nm, and emission was collected through a 515- to 560-nm band-pass filter. To evaluate total lysosome content, z stacks of rhodamine-dextran fluorescence were collected at 0.5-μm intervals through the entire thickness of cells and superimposed. To better visualize the dim fluorescence of rhodamine-dextran released into the cytosol from lysosomes, gamma in the red fluorescence channel was increased using Adobe Photoshop CS4 (Mountain View, CA). The gamma correction was applied equally to all compared images.
Materials.
Calcein-AM, calcein-free acid, and TMRM were purchased from Invitrogen-Molecular Probes (Eugene, OR). Starch-desferal was purchased from Biomedical Frontiers (Minneapolis, MN). Other chemicals were of analytical grade obtained from standard commercial sources. When used, acetaminophen was dissolved in absolute ethanol, and CsA was dissolved in dimethyl sulfoxide. Vehicle for other agents was water or HDM.
Statistics.
Differences between means were compared by ANOVA followed by Tukey’s multiple comparison procedure using p < 0.05 as the criterion for significance. Values of p < 0.01 were also noted. Data were expressed as means ± SE. When error bars in graphs are not shown, they are smaller than the size of the associated symbol. Imaging experiments are representative of three or more replicates.
RESULTS
Iron Chelators Decrease Acetaminophen-Induced Necrotic Cell Killing
When mouse hepatocytes in HDM were exposed to 10mM acetaminophen, cell killing began within 3 h and reached 70% after 9 h (Fig. 1), as observed previously (Kon et al., 2004). With treatment with 1mM desferal or 1mM starch-desferal beginning 30 min before acetaminophen addition, cell killing was delayed and decreased by more than half after 6 h (Fig. 1). Phenanthroline (25μM), another iron chelator, protected to a similar extent (data not shown). Because desferal and its starch conjugate are highly specific iron chelators, these results indicated that iron chelation was protective against acetaminophen cytotoxicity. However, both desferal and starch-desferal are poorly permeant to the plasma membrane, suggesting that these iron chelators protect after cell entry by a different pathway.
FIG. 1.
Acetaminophen-induced killing in mouse hepatocytes: protection by desferal and starch-desferal. Mouse hepatocytes were treated with desferal (1mM), starch-desferal (sDesferal, 1mM), or no addition 30 min before exposure to acetaminophen (AAP, 10mM). Cell viability was determined by propidium iodide fluorometry. Control represents hepatocytes not exposed to acetaminophen. Values are means ± SE from three or more hepatocyte isolations.
Ferrous Iron but Not Ferric Iron Quenches Calcein Fluorescence
To characterize the interaction of calcein with iron, the fluorescence of 2μM calcein-free acid in cell-free HDM was measured in a fluorescence plate reader before and after addition of 10μM Fe(NH4)2(SO4)2 (ferrous iron, Fe2+) or 10μM FeCl3 (ferric iron, Fe3+). Calcein fluorescence decreased rapidly and virtually completely after Fe2+ but not after Fe3+ (Fig. 2). Dipyridyl (DPD, 20mM), an iron chelator, partially restored calcein fluorescence. DPD also increased calcein fluorescence to a small extent in the absence of added Fe2+, suggesting that a small amount of contaminating iron was present in the buffer. Overall, these findings showed that quenching of calcein fluorescence reports an increase of chelatable Fe2+ but not Fe3+, consistent with a previous report showing that transition metals quench calcein fluorescence with the relative potency: Cu > Ni > Co > Fe2+ >> Mn > Zn > Pb > Fe3+ > Ca, Ca, Mg, Hg (Breuer et al., 1995).
FIG. 2.
Quenching of calcein fluorescence by ferrous iron but not ferric iron. Calcein-free acid (2μM) was added to HDM, and ferric chloride (FeCl3, 10μM) or ferrous ammonium sulfate ((Fe(NH4)2(SO4)2, 10μM) was subsequently added, followed by dipyridyl (DPD, 20mM) after 10 min. Fluorescence was measured with a plate reader, as described in “Materials and Methods” section.
Acetaminophen Causes Quenching of Calcein Fluorescence That is Reversed by Dipyridyl, a Membrane-Permeable Iron Chelator
Mouse hepatocytes were coloaded with TMRM and calcein-AM and then incubated with 300μM calcein-free acid and 3μM propidium iodide in the extracellular space. TMRM is a membrane-permeant monovalent cation that accumulates electrophoretically into mitochondria as an indicator of mitochondrial polarization. Ester-loaded calcein localizes to the cytosolic compartment and becomes quenched by an increase of chelatable Fe2+. Calcein in the extracellular space serves as an internal standard for calcein fluorescence intensity and assures that any decrease of cytosolic calcein is not because of passive leakage of calcein from the cells. Last, propidium iodide nuclear labeling identifies onset of necrotic cell death.
Hepatocytes were then exposed to acetaminophen (10mM) in the presence of fructose (20mM) and glycine (5mM) to prevent onset of necrotic cell death (Kon et al., 2004). After 3 h, mitochondria of the hepatocyte shown in Figure 3 remained polarized, as shown by mitochondrial retention of red TMRM fluorescence. Cytosolic calcein fluorescence was also bright and comparable with hepatocytes not treated with acetaminophen (data not shown, see references). At 4 h after acetaminophen (APAP) treatment, mitochondria began to lose TMRM fluorescence, indicating depolarization, and were completely depolarized after 6 h. However, cell viability was maintained because nuclear PI labeling did not occur. As mitochondria depolarized, cytosolic calcein fluorescence progressively decreased. Loss of calcein fluorescence could not be explained by passive leak because intracellular fluorescence decreased in intensity to less than the calcein fluorescence of the extracellular medium. In 14 cells, intracellular calcein fluorescence decreased by the equivalent of 198 ± 26μM, using extracellular calcein as an internal standard. Notably, addition of the membrane-permeant iron chelator, DPD, reversed calcein quenching and partially restored intracellular calcein fluorescence (Fig. 3). The reversal of calcein quenching by DPD was not simply the consequence of nonspecific plasma membrane permeabilization because the hepatocytes excluded propidium. Subsequent plasma membrane permeabilization with digitonin then led to nuclear labeling with propidium iodide. These data are consistent with the conclusion that acetaminophen treatment of hepatocytes leads to an increase of chelatable Fe2+ in the cytosol. Moreover, this increase of chelatable Fe2+ accompanied mitochondrial depolarization, which we previously showed to be because of onset of the MPT (Uchiyama et al., 2008).
FIG. 3.
Calcein quenching and mitochondrial depolarization after acetaminophen. Hepatocytes were exposed to acetaminophen (AAP, 10mM) in the presence of fructose (20mM) plus the glycine (5mM) to prevent plasma membrane failure and cell death after mitochondrial failure. After 2.5 h, hepatocytes were loaded with TMRM (100nM), PI (3μM), and calcein-AM (1μM), and calcein-free acid (300μM) was then added to the extracellular medium, as described in “Materials and Methods” section. After 6 h, dipyridyl (DPD, 20mM) was added. Lastly, digitonin (Dig, 375μM) was added to permeabilize the plasma membrane.
Desferal and Starch-Desferal Prevent Mitochondrial Depolarization and Calcein Quenching after Acetaminophen
To further investigate the role of increased chelatable Fe2+ in MPT onset after acetaminophen, TMRM- and calcein-loaded hepatocytes were treated with 1mM desferal beginning 30 min before acetaminophen addition. Desferal prevented acetaminophen-induced mitochondrial depolarization and decreased calcein quenching (increase of chelatable Fe2+) (Fig. 4A). Desferal also prevented mitochondrial inner membrane permeabilization after acetaminophen, and mitochondria continued to exclude calcein from their matrix space, as shown by dark voids in the green cytosolic calcein fluorescence that corresponded to TMRM-labeled mitochondria in the red images. Similarly, starch-desferal protected against calcein quenching, mitochondrial depolarization, and inner membrane permeabilization after acetaminophen exposure (Fig. 4B).
FIG. 4.
Inhibition of acetaminophen-induced calcein quenching, mitochondrial depolarization, and inner membrane permeabilization desferal and starch-desferal. Hepatocytes were incubated with desferal (1mM) (A) or starch-desferal (sDesferal, 1mM) (B) for 30 min before exposure to acetaminophen, as described in Figure 3.
Lysosomes Are a Reservoir of Chelatable Iron in Hepatocytes
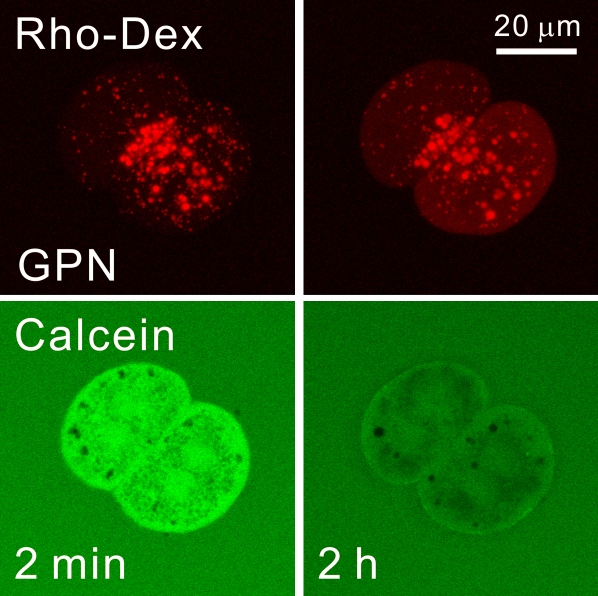
Calcein quenching and its prevention by desferal and starch-desferal indicated that an increase of chelatable iron occurred in the cytosol after acetaminophen exposure. Previous studies have indicated that lysosomes can release chelatable iron after oxidative stress, and in hepatocytes, lysosomal permeabilization occurs after a variety of cellular stresses (Kurz et al., 2007; Uchiyama et al., 2008; Werneburg et al., 2002; Wildenthal and Decker, 1980; Zahrebelski et al., 1995). To determine if lysosomes store chelatable iron in hepatocytes, we used glycylphenylalanine 2-napthylamide (GPN), a lysomotropic detergent that causes selective lysis of lysosomes (Jadot et al., 1990). To visualize lysosomes, mice were first injected with 70 kDa rhodamine-dextran 12 h prior to hepatocyte isolation. Rhodamine-dextran goes to the liver and is taken up by hepatocytes via endocytosis to be delivered to lysosomes. After hepatocyte isolation and ester loading of calcein, confocal microscopy revealed the distribution of red-fluorescing rhodamine-dextran in predominantly spherical organelles ranging in diameter from a few tenths of a micron to 1 or 2 μm that were scattered within the calcein-labeled cytosol (Fig. 5). The rhodamine-dextran–labeled compartment is a dynamic structure whose contents may intermix with late endosomes, autophagosomes, and other acidified compartments. However, for brevity and simplicity of expression, we will refer to rhodamine-dextran–labeled structures as lysosomes. After exposure of hepatocytes to 100μM GPN, swelling and distintegration of some but not all lysosomes occurred within 2 h, which caused an increase of diffuse red fluorescence in the cytosol. In parallel, calcein fluorescence in the cytosol decreased markedly (Fig. 5). These results indicated that lysosomes contained a pool of chelatable iron that was mobilized upon lysosomal disruption. In the time frame studied, GPN did not cause overt cellular stress, as manifested by cell swelling or bleb formation.
FIG. 5.
Release of chelatable iron after treatment with glycylphenylalanine 2-napthylamide. Mouse hepatocytes were isolated from mice injected with rhodamine-dextran (Rho-Dex) and then loaded with calcein-AM, as described in “Materials and Methods” section. Hepatocytes were then incubated with the lysomotropic detergent, GPN (100μM), and the red fluorescence of rhodamine-dextran and the green fluorescence of calcein were imaged by laser scanning confocal microscopy.
Acetaminophen Induces Lysosomal Degradation in Parallel with Calcein Quenching That is Prevented by Desferal
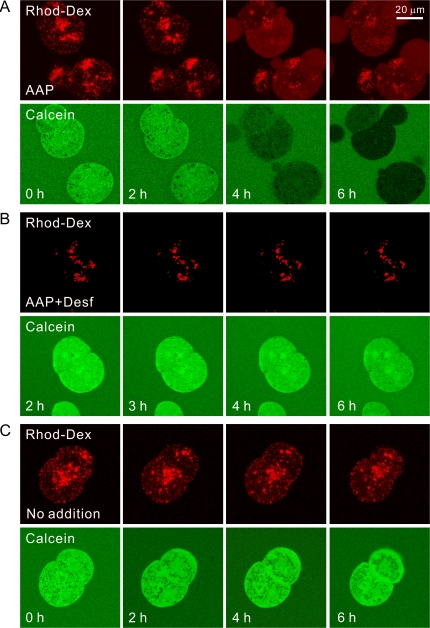
We also exposed rhodamine-dextran–labeled and calcein-loaded hepatocytes to acetaminophen. After 2 h of exposure, rhodamine-dextran–labeled lysosomes remained intact and calcein quenching had not yet occurred (Fig. 6A). Beginning between 2 and 4 h after acetaminophen, rhodamine-dextran–labeled lysosomes began to disappear in parallel with quenching of calcein fluorescence. As lysosomes disappeared, diffuse red fluorescence increased in the cytosol, signifying that acetaminophen caused breakdown of many lysosomes. Nonetheless, after 6 h when calcein quenching was marked and close to maximal, many rhodamine-labeled structures remained intact (Fig. 6A). This experiment was repeated in rhodamine-dextran–labeled hepatocytes treated with desferal beginning 30 min prior to acetaminophen exposure. With desferal treatment, lysosomal breakdown as well as calcein quenching did not occur (Fig. 6B). As a control, rhodamine-dextran–labeled hepatocytes were incubated without addition of acetaminophen or other compound. In the absence of acetaminophen, lysosomal breakdown and calcein quenching did not occur (Fig. 6C). Differences of initial intracellular rhodamine-dextran labeling in Figure 6 represent cell-to-cell variations.
FIG. 6.
Acetaminophen-dependent lysosomal degradation in parallel with calcein quenching: prevention by desferal. Mouse hepatocytes were isolated from mice injected with rhodamine-dextran and then loaded with calcein-AM, as described in “Materials and Methods” section. In the presence of fructose plus glycine, hepatocytes were then exposed to acetaminophen (AAP, 10mM) (A), acetaminophen after 30-min treatment with desferal (Desf, 1mM) (B), or no treatment (C). The red fluorescence of rhodamine-dextran and the green fluorescence of calcein were imaged by laser scanning confocal microscopy.
CsA Promotes Recovery from Calcein Quenching but Does Not Prevent Acetaminophen-Induced Lysosomal Disappearance
CsA is an MPT blocker that delays acetaminophen cytotoxicity to mouse hepatocytes (Kon et al., 2004; Reid et al., 2005). When rhodamine-dextran– and calcein-loaded hepatocytes were treated with 2μM CsA and exposed to acetaminophen, disappearance of punctate rhodamine-dextran fluorescence was not prevented (Fig. 7). CsA, however, did affect calcein quenching. Initially, in the presence of CsA, calcein fluorescence decreased, at least in some cells, but subsequently calcein fluorescence recovered. In other cells, calcein fluorescence did not appear to decrease, at least at the 30-min time intervals at which observations were made. Consistent with our previous observations (Kon et al., 2004), CsA prevented the MPT during the first 6 h of exposure to acetaminophen, as shown by the persistence of dark mitochondrial voids in the calcein images. Such voids demonstrated that mitochondrial inner membranes remained impermeable to calcein.
FIG. 7.
Lack of protection by cyclosporin A against acetaminophen-induced lysosomal breakdown and calcein quenching. Mouse hepatocytes were loaded with rhodamine-dextran and calcein, as described in Figure 6 and treated with acetaminophen (AAP) in the presence of fructose plus glycine and cyclosporin A (CsA, 1μM).
DISCUSSION
Acetaminophen is a commonly used analgesic drug that in overdose causes hepatic necrosis and fulminant liver failure requiring liver transplantation (Fontana, 2008). Acetaminophen metabolism by several cytochrome P-450 isoenzymes, especially CYP2E1, leads to formation of the reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI) (Nelson, 1990). GSH detoxifies NAPQI, but once GSH is depleted, NAPQI reacts covalently with other cellular components, in particular mitochondrial proteins, to produce mitochondrial dysfunction and cellular injury (Cohen and Khairallah, 1997; Jaeschke and Bajt, 2006; Jaeschke et al., 2003; Mitchell et al., 1973; Qiu et al., 2001; Tirmenstein and Nelson, 1989). Mitochondrial effects of APAP overdose include inhibition of mitochondrial respiration, enhanced formation of ROS and peroxynitrite, mitochondrial DNA damage, release of mitochondrial intermembrane proteins that translocate to the nucleus to cause nuclear DNA degradation, and ultimately mitochondrial membrane depolarization and onset of the MPT (Jaeschke and Bajt, 2006; Jaeschke et al., 2003; Kon et al., 2004). Results from the present study indicate that iron mobilization from lysosomes into mitochondria contributes to these pathophysiological changes.
In agreement with our earlier study (Kon et al., 2004), acetaminophen induced 70% necrotic cell killing after 9 h to cultured mouse hepatocytes. Despite the high concentration (10mM) of acetaminophen used, desferal and starch-desferal delayed cell killing substantially and to an equal extent (Fig. 1). Similarly, in vivo, desferal delays but does not necessarily prevent acetaminophen hepatotoxicity (Sakaida et al., 1995; Schnellmann et al., 1999). Starch-desferal is membrane impermeant and taken up into cells principally by endocytosis to localize in the lysosomal/endosomal compartment. Because previous studies identified the lysosomal/endosomal compartment as a source of mobilizable chelatable iron (Kurz et al., 2007; Uchiyama et al., 2008; Werneburg et al., 2002), the goal of this study became to characterize changes of chelatable iron after acetaminophen especially in relation to possible release from lysosomes.
To visualize changes of chelatable iron, we used the fluorophore calcein (Breuer et al., 1995; Petrat et al., 2002). Quenching of calcein was highly specific for ferrous iron, and ferric iron (Fe3+) produced virtually no change of calcein fluorescence (Fig. 2). After acetaminophen, cytosolic calcein fluorescence began to quench after between 3 and 4 h (Kon et al., 2004), a time corresponding to maximal GSH depletion (Bajt et al., 2004). Loss of calcein fluorescence did not represent passive leakage of calcein because fluorescence decreased to below that of calcein-free acid in the extracellular space. Using the fluorescence of extracellular calcein as an internal standard and assuming a one-to-one stoichiometry of Fe2+-calcein binding, chelatable Fe2+ increased by ∼200μM after acetaminophen. However, the increase of free Fe2+ was likely much less because most chelatable iron is bound to anionic metabolites like citrate and ATP. Nonetheless, both free iron and loosely bound iron are redox active. Thus, the overall increase in chelatable redox-active iron was quite substantial. The iron chelator, dipyridyl, reversed calcein quenching after acetaminophen. Moreover, desferal and starch-desferal both blocked calcein quenching after acetaminophen treatment. These findings confirm that calcium quenching signified an increase of chelatable Fe2+ because the only other biologically relevant cation besides iron that is chelated by desferal is aluminum, and calcein does not bind aluminum (Breuer et al., 1995; Liu and Hider, 2002).
Previous reports indicate that lysosomal disruption occurs after many cellular stresses and can be a source of chelatable iron in hepatocytes and other cells (Kurz et al., 2007; Uchiyama et al., 2008; Werneburg et al., 2002; Wildenthal and Decker, 1980; Zahrebelski et al., 1995). In confirmation, lysosomal disruption with the lysomotropic detergent GPN led to marked quenching of cytosolic calcein fluorescence, which indicated an increase in chelatable Fe2+ of magnitude similar to that observed after acetaminophen (Fig. 5). However, increased chelatable Fe2+ alone did not cause evident cellular stress. Similarly, overt cellular stress and cytotoxicity did not occur in hepatocytes after increasing cytosolic chelatable Fe2+ with the vacuolar proton pump inhibitor, bafilomycin (Uchiyama et al., 2008). Because disruption of lysosomes occurred after acetaminophen, the increase of chelatable Fe2+ after acetaminophen is most likely because of this lysosomal damage. Interestingly, desferal prevented lysosomal disruption after acetaminophen, suggesting that iron-dependent interactions with NAPQI are promoting lysosomal damage and fragility.
Previous reports have implicated the MPT in the mechanism of acetaminophen-dependent cytolethality to hepatocytes (Kon et al., 2004; Reid et al., 2005). In agreement, the MPT inhibitor CsA prevented mitochondrial permeabilization and delayed cell killing after acetaminophen treatment (Fig. 7). Moreover, chelatable iron promoted MPT onset because desferal and starch-desferal both prevented mitochondrial depolarization after acetaminophen (Fig. 4). In the presence of CsA, which prevented mitochondrial depolarization, cytosolic calcein fluorescence first decreased, indicating an increase of chelatable Fe2+, and then recovered in some cells, whereas in other cells, calcein quenching was not observed (Fig. 7). These observations suggest that the chelatable Fe2+ released from lysosomes into the cytosol was then taken up into polarized mitochondria when depolarization was prevented by CsA. However, CsA did not prevent lysosomal disruption, which is consistent with the conclusion that MPT onset is downstream of lysosomal damage and iron release.
In conclusion, our results indicate that mobilization of chelatable iron from lysosomes plays a key role in acetaminophen hepatotoxicity. Our working model is that after GSH depletion following acetaminophen exposure, excess NAPQI generated by cytochromes P-450 causes lysosomal damage with release of chelatable Fe2+ into the cytosol. This Fe2+ is then taken up into mitochondria to promote intramitochondrial ROS formation, which in turn causes MPT onset, mitochondrial depolarization, bioenergetic failure, and cell death. Desferal and starch-desferal by chelating chelatable iron block this progression and protect against injury. However, future studies will be needed to determine the specific route of Fe2+ uptake into mitochondria and whether lysosomal disruption and iron mobilization occur in vivo during acetaminophen hepatotoxicity.
FUNDING
National Institutes of Health (1 R01 DK070195, 2-R01 DK37034, and 1 R01 DK073336); National Institutes of Health Center (1P30 CA138313 to imaging facilities).
References
- Badr MZ, Belinsky SA, Kauffman FC, Thurman RG. Mechanism of hepatotoxicity to periportal regions of the liver lobule due to allyl alcohol: role of oxygen and lipid peroxidation. J. Pharmacol. Exp. Ther. 1986;238:1138–1142. [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Breuer W, Epsztejn S, Millgram P, Cabantchik IZ. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am. J. Physiol. 1995;268(Pt 1):C1354–C1361. doi: 10.1152/ajpcell.1995.268.6.C1354. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Khairallah EA. Selective protein arylation and acetaminophen-induced hepatotoxicity. Drug Metab. Rev. 1997;29:59–77. doi: 10.3109/03602539709037573. [DOI] [PubMed] [Google Scholar]
- Fontana RJ. Acute liver failure including acetaminophen overdose. Med. Clin. North Am. 2008;92:761–794. doi: 10.1016/j.mcna.2008.03.005. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson RJ, Casini A, Gilfor D, Serroni A, Farber JL. Oxygen-mediated cell injury in the killing of cultured hepatocytes by acetaminophen. Biochem. Biophys. Res. Commun. 1985;126:1129–1137. doi: 10.1016/0006-291x(85)90303-1. [DOI] [PubMed] [Google Scholar]
- Gores GJ, Nieminen AL, Wray BE, Herman B, Lemasters JJ. Intracellular pH during “chemical hypoxia” in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J. Clin. Invest. 1989;83:386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadot M, Bielande V, Beauloye V, Wattiaux-De CS, Wattiaux R. Cytotoxicity and effect of glycyl-D-phenylalanine-2-naphthylamide on lysosomes. Biochim. Biophys. Acta. 1990;1027:205–209. doi: 10.1016/0005-2736(90)90086-4. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol. Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Brunk UT. Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch. Biochem. Biophys. 2007;462:220–230. doi: 10.1016/j.abb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Liu ZD, Hider RC. Design of clinically useful iron(III)-selective chelators. Med. Res. Rev. 2002;22:26–64. doi: 10.1002/med.1027. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am. J. Physiol. 1997;272(Pt 1):C1286–C1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- Nieminen AL, Gores GJ, Bond JM, Imberti R, Herman B, Lemasters JJ. A novel cytotoxicity screening assay using a multiwell fluorescence scanner. Toxicol. Appl. Pharmacol. 1992;115:147–155. doi: 10.1016/0041-008x(92)90317-l. [DOI] [PubMed] [Google Scholar]
- Petrat F, de Groot H, Sustmann R, Rauen U. The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 2002;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am. J. Physiol. 1997;273(Pt 1):C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3′-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv. Exp. Med. Biol. 2001;500:663–673. doi: 10.1007/978-1-4615-0667-6_99. [DOI] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J. Pharmacol. Exp. Ther. 2005;312:509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- Sakaida I, Kayano K, Wasaki S, Nagatomi A, Matsumura Y, Okita K. Protection against acetaminophen-induced liver injury in vivo by an iron chelator, deferoxamine. Scand. J. Gastroenterol. 1995;30:61–67. doi: 10.3109/00365529509093237. [DOI] [PubMed] [Google Scholar]
- Schnellmann JG, Pumford NR, Kusewitt DF, Bucci TJ, Hinson JA. Deferoxamine delays the development of the hepatotoxicity of acetaminophen in mice. Toxicol. Lett. 1999;106:79–88. doi: 10.1016/s0378-4274(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48:1644–1654. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneburg NW, Guicciardi ME, Bronk SF, Gores GJ. Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G947–G956. doi: 10.1152/ajpgi.00151.2002. [DOI] [PubMed] [Google Scholar]
- Wildenthal K, Decker RS. The role of lysosomes in the heart. Adv. Myocardiol. 1980;2:349–358. [PubMed] [Google Scholar]
- Zahrebelski G, Nieminen AL, al Ghoul K, Qian T, Herman B, Lemasters JJ. Progression of subcellular changes during chemical hypoxia to cultured rat hepatocytes: a laser scanning confocal microscopic study. Hepatology. 1995;21:1361–1372. [PubMed] [Google Scholar]