Abstract
Concern regarding the potential for radiation exposure from accidents or nuclear and radiologic terrorism is increasing. The purpose of this study was to determine whether the addition of minimal supportive care consisting of hydration or nutritional gels could be used to reduce mortality in mice exposed to 60Co γ-radiation. Male CD2F1 mice received 0, 8.50, or 9.25 Gy 60Co at a dose rate of 0.6 Gy/min. These groups were further divided into 3 treatment groups that—in addition to pelleted food and water—received no supportive care, hydration gel, or nutritional gel. Overall survival, mean survival time, consumption of pelleted food and gel, and body weight were recorded for 30 d. Radiation caused dose-dependent decreases in overall survival, consumption of pelleted food and supplemental gel, and body weight. However, at each radiation dose (0, 8.50, 9.25 Gy), the type of supportive care did not modify overall survival, mean survival time, or changes in body weight. These results demonstrate that hydration and nutritional gels were not effective methods of supportive care after high-dose total body irradiation in mice.
Concern regarding the potential for radiation exposure from accidents or nuclear and radiologic terrorism is increasing.10,27 High-dose total-body irradiation exposure to mammals results in a severe, dose-dependent, and usually fatal illness known as acute radiation syndrome. In mice, this syndrome is characterized by hematopoietic (5 to 10 Gy), gastrointestinal (10 to 100 Gy), and cerebrovascular (greater than 100 Gy) syndromes.23
In vitro assays for radioprotection have not been able to predict protection from lethality in animals. Therefore, drugs must be evaluated for efficacy by using an in vivo model.28 To screen radiation countermeasure agents for their protective efficacy, animals must be exposed to high doses of radiation. Rodents exposed to doses of radiation that result in hematopoietic death exhibit clinical signs of radiation sickness, including decreased food consumption, weight loss, and impaired mobility.6,17,22 The primary cause of death from the hematopoietic syndrome is destruction of the bone marrow, resulting in significant reductions in neutrophils and platelets that can lead to infection and hemorrhage.26 In mice, mortality from hematopoietic syndrome typically occurs within 30 d after irradiation.23
In most laboratories investigating radiation-induced injury in mice, survival studies are commonly conducted without providing additional supportive care (nutritional supplements, antibiotics, intravenous fluids). Radiation sickness and the accompanying weakness reduce water intake and food consumption from the cage hopper. Mice have a high basal metabolic rate15 and, depending on the strain, require 3 to 6 g food and 4 to 8 mL water daily.2 Water intake and food consumption are directly related to the general health of the mouse. Mice deprived of water consume less food, and deaths have been attributed to both dehydration and starvation.3 In addition, food and water deprivation decrease the numbers of circulating lymphocytes and platelets, blood elements essential for recovery from radiation.1
A variety of methods have been used to provide additional hydration and nutrition to rodents during transport,24 after surgery,13 or under other stressful conditions.11 These methods include access to potatoes, wet mash, and gel packs.19 Providing mice hydration or nutritional gels may prolong survival times and promote recovery from an otherwise lethal dose of radiation. The purpose of this study was to determine whether the addition of minimal supportive care consisting of hydration or nutritional gels could be used to reduce mortality in mice exposed to 60Co γ radiation.
Materials and Methods
Animals and housing.
CD2F1 mice (Mus musculus; male; age, 10 to 12 wk; weight: mean, 27.9 g; range, 25.1 to 30.7 g) were obtained from Harlan Laboratories (Dublin, VA). Representative animals were screened and determined to be free of the following agents: Klebsiella pneumonia, Pasteurella spp., Sendai virus, pneumonia virus of mice, reovirus 3, mouse adenovirus types 1 and 2, mouse cytomegalovirus virus, ectromelia virus, K virus, lymphocytic choriomeningitis virus, epidemic diarrhea of infant mice, Hantaan virus, rotavirus, mouse parvovirus, polyoma virus, mouse minute virus, mouse thymic virus, Theiler mouse encephalomyelitis virus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus; Helicobacter spp. Mycoplasma pulmonis, Clostridium piliforme, endoparasites, and ectoparasites. Animals were maintained at temperature and humidity levels as set forth in the Guide for the Care and Use of Laboratory Animals.16 Mice were housed in groups of 4 in polycarbonate cages (11.5 × 7.5 × 5 in.) with microisolation filter tops on autoclaved rodent hardwood bedding (Laboratory-grade SaniChips, catalog no. 7090M, Harlan Teklad). They were provided ad libitum pelleted rodent food (Harlan Teklad Rodent Diet 8604) and a water bottle through the cage lid. Because Pseudomonas aeruginosa has been shown to adversely affect survivability in irradiated animals, mice were screened for the organism and then maintained on acidified water (pH, 2.5 to 3.0) to control for opportunistic infections.20 Subcutaneous microchips were implanted dorsally between the scapulae for unique identification of each animal (IPTT300 microchip, Bio Medic Data Systems, Rockville, MD). To ensure healing of the skin, microchips were implanted in the mice 2 wk prior to irradiation. All procedures used in these experiments were approved by the Institutional Animal Care and Use Committee of the Armed Forces Radiobiology Research Institute, which is AAALAC-accredited.
Supplemental gels.
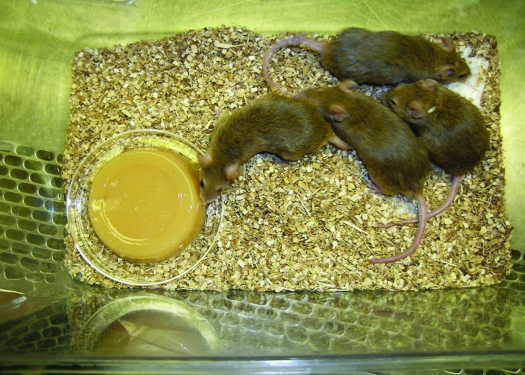
Hydration gel (HydroGel, Clear H2O, Portland, ME) and nutritional gel (DietGel R/E, Clear H2O) were provided as supplements in addition to pelleted food and water to designated treatment groups in this study. The hydration gel contained 6.3 kcal per 100 g consumed and consisted of 98% pure water. The nutritional gel was a combination hydration and caloric supplement with 155 kcal per 100 g consumed. The nutrient-fortified gel consisted of 60% pure water with added carbohydrates, proteins, fats, minerals, and electrolytes and was certified free of phytoestrogens and nitrosamines. The gels were placed in the cages 4 d prior to the start of the experiment to allow mice to acclimate and to prevent the development of taste aversions. Visual observation confirmed that all mice consumed portions of the gels provided. The gels were replaced every 48 to 72 h. Gels (2-oz portions) were removed from the manufacturer packaging and placed on the floor of the cage on the inverted lid of a 3-in. culture dish, to make them more readily accessible to the mice (Figure 1). Before new gel was placed in the cage, both the new and remaining gel were weighed separately.
Figure 1.
Nutritional gel supplement. Hydration and nutritional gels were removed from their packaging and placed on the floor of the cage on an inverted lid of a 3-in. culture dish and replaced and weighed every 48 to72 h during cage changing.
Radiation.
Mice were restrained in well-ventilated acrylic irradiation boxes and exposed to gamma-photons in our institution's 60Co radiation facility (Figure 2). This facility used a bilateral γ-radiation field. The total tissue dose of irradiation received was measured at the level of the abdominal core. Mice were exposed to 8.50 or 9.25 Gy total-body irradiation at a dose rate of 0.6 Gy/min. Control (sham-irradiated) animals were placed into the acrylic radiation boxes but were not irradiated.
Figure 2.
Acrylic mouse restraint box containing 3 phantoms. The box was portioned into 8 equal sections. Mice or acrylic phantoms (length, 3 in; diameter, 1 in.) were placed into each section.
An alanine electron-spin resonance dosimetry system (Standard E 1607, American Society for Testing and Materials, West Conshohocken, PA) was used to measure dose rates (to water) in the cores of acrylic mouse phantoms. To mimic the size of a mouse, each phantom was 3 in. in length and 1 in. in diameter. For field mapping, which was used to validate dosages throughout the radiation field prior to animal exposure, all exposure rack compartments contained phantoms, and alternate phantoms contained alanine dosimeters. Signals were measured by using a calibration curve based on standard calibration dosimeters (National Institute of Standards and Technology, Gaithersburg, MD). The overall uncertainty in the doses given to the calibration dosimeters was approximately 1.8% at 2 standard deviations. The accuracy of the calibration curve was verified by parallel measurements of doses to selected dosimeters at the Armed Forces Radiobiology Research Institute and National Physical Laboratory (Middlesex, UK). Appropriate corrections were applied to the measured phantoms dose rates to account for the decay of 60Co and differences in the mass energy-absorption coefficients for water and soft tissue.
Study design.
Mice were divided randomly into 3 experimental groups: a control group that was sham-irradiated (0 Gy), mice exposed to 8.50 Gy, and mice exposed to 9.25 Gy radiation. Each control or radiation group was further divided into 3 treatment groups: no gel (no support), supplemented with hydration gel, and supplemented with nutritional gel. Mice in all groups had free access to water bottles and pelleted food. Each of the 9 subgroups contained 20 mice. The same group served as controls for both radiation dosages, reducing the total number of mice required by 25%.
Body weights and weights of gel supplements and pelleted food were recorded at 48- to 72-h intervals (coinciding with routine facility cage changes) for 30 d after irradiation. Body weights of mice found deceased were not recorded due to the effects of postmortem dehydration. In these cases, the last recorded body weight was taken as the final data entry. Body weights of moribund mice were recorded prior to euthanasia. The weights of the pellets and gel consumed were divided by the number of days in the exposure period and the number of animals per cage to determine the average amount of feed or gel consumed (grams per day per mouse). The 48- to 72-h interval was selected to minimize animal handling and corresponded to the days on which cages were changed. The limited animal manipulation also minimized handling-associated stress in these already immunocompromised mice. Because a high drip rate from the water bottles was observed during animal handling and inadvertent movement of cage racks, water consumption was not recorded in this study.
The endpoint for the study was survival through day 30 after irradiation. The number of moribund or dead mice was recorded twice daily. Moribund mice were euthanized and recorded as deceased on the day euthanized. Mice were considered moribund when one or more of the following clinical signs were observed: excessive weight loss or emaciation, inability to remain upright, impaired ambulation, decreased or labored breathing, and no response to external stimuli. Overall survival (percentage of population alive on day 30) and mean survival (in days) were monitored for 30 d after irradiation. This time interval reflects the period during which radiation-induced hematopoietic death in the mouse is complete.12
Data analysis.
Survival curves for each group of mice were estimated by using the Kaplan–Meier method and compared by using the log-rank test. The quantities of gels and food pellets consumed were calculated as grams consumed per mouse per day over the study period and were compared among groups by using ANOVA. Post hoc comparisons were made by using the Fisher least significant difference test. The mean survival time during the 30-d postirradiation observation period was calculated for those groups of mice for which survival was less than 100%. The body weights were analyzed by ANOVA by using a mixed-models approach to estimate pooled differences across experimental groups. Data were analyzed by using SPSS version 14.0 (SPSS, Chicago, IL) and SAS version 9.1 (SAS Institute, Cary, NC) for Windows.
Results
Survival.
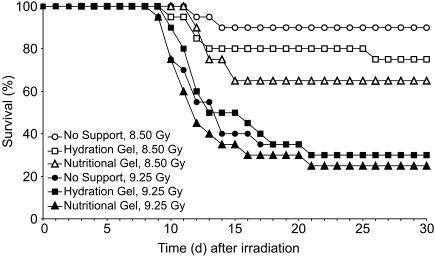
Survival of mice was evaluated at 30 d after irradiation to determine whether providing hydration or nutritional supplemental gels would reduce radiation-induced mortality. Mice at all 3 levels of supportive care (no support, hydration gel, nutritional gel) at 8.50 and 9.25 Gy displayed clinical signs of morbidity, including swollen faces, hunched posture, ruffled hair coat, weight loss, social isolation, and lethargy. Neither overall survival nor mean survival time differed between the no-support, hydration support, and nutritional support groups of mice irradiated with either 8.50 Gy or 9.25 Gy (Table 1). For mice exposed to 8.50 Gy, overall survival was 90%, 75%, and 60% respectively for the no-support, hydration gel, and nutritional gel groups. Mice irradiated with 9.25 Gy had survival rates of 30%, 25%, and 30%, for the no-support, hydration gel, and nutrition gel groups, respectively (Figure 3). Survival time did not vary significantly by group at either exposure level (8.50-Gy exposure, P = 0.196; 9.25-Gy exposure, P = 0.742). Overall, the mean duration of survival of decedents was 13.7 d for those exposed to 8.50 Gy compared with 12.6 d for those irradiated with 9.25 Gy (P = 0.267; Table 1).
Table 1.
Overall survival (% alive after 30 d) and survival duration (d, mean ± SEM; n = 20 per group) of decedents by type of supportive care provided
| No supportive care |
Hydration supportive care |
Nutritional supportive care |
|||||||
| Dose (Gy) | % | mean | SEMd | % | mean | SEM | % | mean | SEM |
| 0 | 100 | 100 | 100 | ||||||
| 8.50 | 90 | 13.0 | 1.0 | 75 | 14.6 | 2.9 | 65 | 13.3 | 0.5 |
| 9.25 | 30 | 12.2 | 0.8 | 30 | 13.4 | 0.9 | 25 | 12.1 | 0.8 |
Figure 3.
Survival of mice treated receiving 8.50 or 9.25 Gy 60Co total-body irradiation. In addition to pelleted food and water, mice received no supportive care, hydration gel, or nutritional gel. Overall, survival (percentage of mice alive on day 30 after irradiation) was significantly (P < 0.05) higher for mice irradiated with 8.50 Gy compared with those receiving 9.25 Gy. There were no significant differences in the 30-d survival between the no-support, hydration support, and nutritional support groups of mice (n = 20 per group) irradiated with either 8.50 Gy or 9.25 Gy.
Gel consumption.
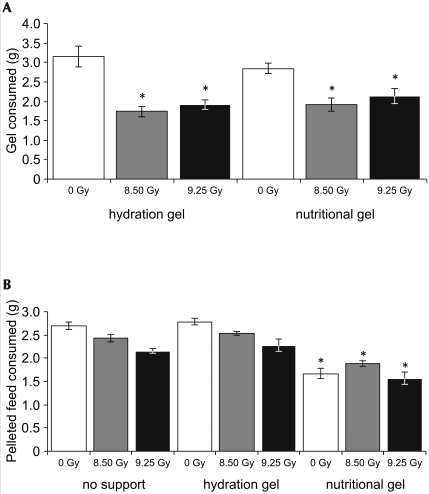
The amount of supplemental gel consumed was analyzed to determine differences in consumption between radiation exposure levels and the subgroups that received hydration or nutritional supplement gels. Across all study days, mice in the control group (0 Gy) consumed significantly (P < 0.001) more of the supplemental gels than did mice in either of the irradiated groups, with no significant difference in consumption of the 2 gel types. Gel consumption did not differ between the mice in the 8.50- or 9.25-Gy irradiation groups. On average, the control group consumed 2 to 3 g nutritional gel daily throughout the study, with no significant (P = 0.961) difference between days. Consumption of hydration gel was variable, with average daily consumption decreasing from 4.1 g per mouse to approximately 2.5 g per mouse at the end of the study (P = 0.026). Mice irradiated with 8.50 Gy 60Co consumed 1.7 g hydration gel and 1.9 g nutritional gel daily, on average. Mice exposed to 9.25 Gy had average consumption rates of 1.9 and 2.1 g daily for the hydration and nutritional gels, respectively. These 4 groups of irradiated mice did not differ significantly in the amount or type of gel consumed (P = 0.366; Figure 4 A).
Figure 4.
Average consumption of gels and food pellets over 30 d as a function of radiation dose (0, 8.50, or 9.25 Gy 60Co) and level of supportive care (no support, nutritional gel, hydration gel). (A) Gel consumption. Across all study days, mice in the irradiated groups consumed significantly (*, P < 0.001) less hydration and nutritional gels than did nonirradiated (0 Gy) mice. (B) Pelleted food consumption. Across all levels of radiation exposure, mice in the nutritional gel supplement subgroups consumed significantly (*, P < 0.001) less pelleted food than did animals in the no-support or hydration gel groups. Data are given as mean ± SEM of pellets or gel consumed.
Pelleted food consumption.
The amount of pelleted food consumed was analyzed to determine whether the mice demonstrated a preference for the gel supplements over the pelleted food. Overall, at all 3 levels of radiation exposure (0, 8.50, 9.25 Gy), mice in the nutritional gel subgroups consumed significantly (P < 0.05) less pelleted food than did animals in the no-support and hydration gel groups (Figure 4 B). In addition, pelleted food consumption showed a radiation-dose–dependent decrease in both no-support and hydration gel groups. The nutritional gel subgroups consumed an average of 1.7 g pelleted food per mouse daily, compared with 2.5 g among mice in the hydration gel and no-support subgroups (P < 0.001). Across all 3 levels of radiation exposure, nonirradiated animals consumed the greatest amount of pelleted food (2.4 g daily per mouse), compared with 2.3 and 2.0 g daily for mice that received 8.50 and 9.25 Gy, respectively (P < 0.001; Figure 4 B).
Body weight.
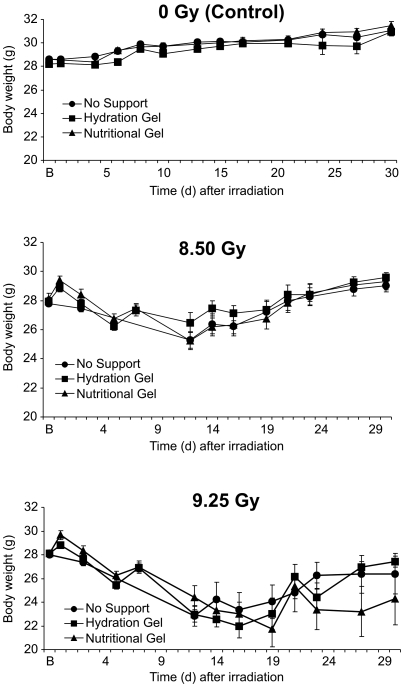
Mice were weighed at multiple time points during the 30-d study to determine changes in body weight as a function of radiation dose and level of supportive care. Body weight showed a radiation-dose–dependent decrease (P < 0.001), but the level of supportive care had no effect on body weight for the either the 0-Gy (P = 0.307), 8.50-Gy (P = 0.359), or 9.25-Gy (P = 0.198) groups (Figure 5). The control mice continued to gain weight over time, and by the end of the experiment, all 3 supportive care subgroups had gained weight compared with baseline measures. Mice irradiated with 8.50 Gy had significant (P < 0.05) reductions in body weight on days 5 and 14 to 19, and mice surviving until 30 d after irradiation exhibited significant (P < 0.028) increases in weight compared with baseline levels. Similarly mice in the 9.25-Gy group showed significant (P < 0.05) weight loss on days 5 and 14 to 30; surviving mice failed to return to baseline weights by the end of the 30-d observation period (P < 0.05).
Figure 5.
Body weights of the 3 subgroups of mice (no support, hydration support, nutritional support) for each radiation group (control nonirradiated mice [0 Gy], 8.50 Gy, or 9.25 Gy). The 3 panels illustrate a radiation dose-dependent decrease in body weight (P < 0.001). The level of supportive care had no effect on body weight for either the nonirradiated and irradiated mice. Data are given as mean ± SEM total body weight.
Discussion
The purpose of the current study was to determine whether providing supportive care in the form of easily accessible hydrational or nutritional gels would decrease or delay mortality in mice exposed to high doses of γ-irradiation. We found no significant difference in overall survival at 30 d after irradiation or in mean survival time across all treatment groups at either radiation dose (8.50 and 9.25 Gy). Therefore, the addition of hydration or nutrition gels, as methods of supportive care, did not modify radiation-induced mortality in CD2F1 mice.
A review of the literature on postirradiation supportive care indicated that reductions in mortality after an otherwise lethal dose of irradiation have been achieved in several species by using various treatment regimens.4,5,14,18 For example, studies using antimicrobial supportive care in mice have reported increases in survival after lethal doses of irradiation.4,5,14 Because the neutropenic phase of the hematopoietic syndrome is regarded as a primary cause of mortality at the levels of irradiation used in the present study, providing antimicrobial agents likely would lead to increased levels of survival. Significant decreases in postirradiation mortality in canines have been achieved by the addition of more intensive levels of supportive care beyond systemic antibiotics; these additional measures include intravenous fluids to improve intravascular volume and fresh platelets to replenish those lost to bone marrow destruction.18 In addition, use of the hematopoiesis-stimulating factor, recombinant granulocyte colony-stimulating factor, in the supportive care regimen further increased canine survival after lethal irradiation.18
Starvation and dehydration contribute to mortality of mice that are too fatigued to eat or drink.3 Anorexia and associated weight loss are common side effects of exposure to ionizing radiation in rodents.6,21,22 Without adequate food and water intake, circulating lymphocytes and platelets are decreased, preventing rapid recovery from irradiation.1 The clinical signs of morbidity in lethally irradiated mice (that is, swollen faces, hunched body position, social isolation, decreased activity) may hinder access to food and water in traditional cage designs. In the present investigation, hydrational and nutritional gels were easily accessible on the floor of the cage. Nonetheless, consumption of both pelleted food and the supportive gels were decreased significantly in irradiated mice, consistent with other studies.22,25 In the present study, the reduction in food consumption induced by exposure to lethal doses of γ-radiation was not altered by the easy accessibility and greater palatability of the supplemental gels.
The decrease in food consumption and subsequent reduction in body weight observed in the present study varied directly as a function of radiation dose. Although all mice in the study consumed the supplemental gels when provided, body weight did not change significantly within any of the 3 treatment groups (no support, hydration gel, nutritional gel) at any of the 3 radiation doses (0, 8.50, 9.25 Gy) in any way that could be attributed to use of the gels.
Although we did not investigate the cause of death for the CD2F1 mice in this study, others have reported this information for animals irradiated in the approximate dose range as that used in the current experiments. Radiation (8 to 9 Gy) has been shown to decrease the numbers of circulating neutrophils, lymphocytes, erythrocytes, and platelets.7,9 The reduction in platelets leads to hemorrhaging, and prolonged neutropenia is associated with increased susceptibility to endogenous infections, often of enteric origin. Irradiated (8 to 9 Gy) CD2F1 mice exhibit hematopoietic damage as well as some intestinal injury.9 At 9 Gy, bacterial translocation from the gastrointestinal tract was observed in this mouse strain.9 Although bacterial information is not available for the CD2F1 strain, the predominant microorganisms cultured from the liver of B6D2F1 mice receiving 10 Gy 60Co γ-photons (LD90/LD30) were gram-positive Streptococcus, Staphylococcus, and Enterococcus spp.8
In summary, we demonstrated that the supplemental gels were palatable, with mice observed to readily consume both the hydration and nutritional gels when offered. All groups of mice consumed less pelleted food when nutritional gel was available, demonstrating that the gels provided an alternative source of nutrition. Nonirradiated mice continued to grow at the same rate whether they had access to only pellets or pellets in combination with hydration gel or nutritional gel. Further research is needed to determine whether the gels may enable animals to recover more rapidly from the physiologic effects of sublethal doses of radiation. Areas for future investigation include determining whether the addition of antimicrobial agents4,5 or radiation-protective agents28 to the gel supplements could reduce mortality in mice after exposure to high-dose total-body irradiation.
Acknowledgments
We especially thank Drs Larry Shelton and Joseph Harre for their advice and support in completing this project; Marc Gonzalez, Benjamin Lowry, Jessica Adams, Sarita Copeland, Steve Fall, and Elizabeth McCart for their technical assistance; and Kevin Monfreda for his photographic contributions. The views in this article are those of the authors and do not necessarily reflect the views, official policy, or position of the Uniformed Services University of the Health Sciences, Armed Forces Radiobiology Research Institute, Department of Defense, or United States Federal Government.
References
- 1.Archambeau JO, Stryckmanns P, Brenneis H. 1968. The effect of food and water deprivation on the peripheral blood parameters of the mouse. Radiat Res 36:396–409 [PubMed] [Google Scholar]
- 2.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. 2002. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32:435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bing FC, Mendel LB. 1931. The relationship between food and water intake in mice. Am J sPhysiol 98:169–179 [Google Scholar]
- 4.Brook I, Elliott TB. 1991. Quinolone therapy in the prevention of mortality after irradiation. Radiat Res 128:100–103 [PubMed] [Google Scholar]
- 5.Brook I, Ledney GD. 1991. Ofloxacin and penicillin G combination therapy in prevention of bacterial translocation and animal mortality after irradiation. Antimicrob Agents Chemother 35:1685–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman WH. 1955. The weight and mortality response of male and female mice in the lethal X-ray dose range. Radiat Res 2:502–511 [PubMed] [Google Scholar]
- 7.Davis TA, Clarke TK, Mog SR, Landauer MR. 2007. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int J Radiat Biol 83:141–151 [DOI] [PubMed] [Google Scholar]
- 8.Elliott TB, Ledney GD, Harding RA, Henderson PL, Gerstenberg HM, Rotruck JR, Verdolin MH, Stille CM, Krieger AG. 1995. Mixed-field neutrons and gamma photons induce different changes in ileal bacteria and correlated sepsis in mice. Int J Radiat Biol 68:311–320 [DOI] [PubMed] [Google Scholar]
- 9.Fu Q, Berbée M, Boerma M, Wang J, Schmid HA, Hauer-Jensen M. 2009. The somatostatin analog SOM230 (pasireotide) ameliorates injury of the intestinal mucosa and increases survival after total-body irradiation by inhibiting exocrine pancreatic secretion. Radiat Res 171:698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goans RE, Waselenko JK. 2005. Medical management of radiological casualties. Health Phys 89:505–512 [DOI] [PubMed] [Google Scholar]
- 11.Gridley DS, Pecaut MJ, Dutta-Roy R, Nelson GA. 2002. Dose and dose-rate effects of whole-body proton irradiation on leukocyte populations and lymphoid organs: part I. Immunol Lett 80:55–66 [DOI] [PubMed] [Google Scholar]
- 12.Hall EJ, Giaccia AJ. 2006. Radiobiology for the radiologist, p 120–121 Philadelphia (PA): Lippincott, Williams, and Wilkins [Google Scholar]
- 13.Heiser A, Liu JHK. 2007. Rat jugular vein and carotid artery catheterization for acute survival studies: a practical guide, p 1–115 New York (NY): Springer [Google Scholar]
- 14.Hérodin F, Grenier N, Drouet M. 2007. Revisiting therapeutic strategies in radiation casualties. Exp Hematol 35:28–33 [DOI] [PubMed] [Google Scholar]
- 15.Hoyt RF, Jr, Hawkins JV, St. Clair MB, Kennett MJ. 2007. Mouse physiology, p 67. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research, vol 3 Burlington (MA): Academic Press [Google Scholar]
- 16.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 17.Landauer MR, Davis HD, Dominitz JA, Weiss JF. 1988. Long-term effects of radioprotector WR2721 on locomotor activity and body weight of mice following exposure to ionizing radiation. Toxicology 49:315–323 [DOI] [PubMed] [Google Scholar]
- 18.MacVittie TJ, Farese AM, Jackson W., 3rd 2005. Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of GCSF. Health Phys 89:546–555 [DOI] [PubMed] [Google Scholar]
- 19.Maher JA, Schub T. 2004. Laboratory rodent transportation supplies. Lab Anim (NY) 33:29–32 [DOI] [PubMed] [Google Scholar]
- 20.McPherson CW. 1963. Reduction of Pseudomonas aeruginosa and coliform bacteria in mouse drinking water following treatment with hydrochloric acid or chlorine. Lab Anim Care 13:737–744 [PubMed] [Google Scholar]
- 21.Nims LF, Sutton E. 1952. Weight changes and water consumption of rats exposed to whole-body X-irradiation. Am J Physiol 171:17–21 [DOI] [PubMed] [Google Scholar]
- 22.Smith DE, Tyree EB. 1954. Influence of X-irradiation upon body weight and food consumption of the rat. Am J Physiol 177:251–260 [DOI] [PubMed] [Google Scholar]
- 23.Storer JB, Fry RJ, Ullrich RL. 1982. Somatic effects of radiation, p 133–146 : Foster HL, Small JD, Fox JG. The mouse in biomedical research, vol 4 New York (NY): Academic Press [Google Scholar]
- 24.Tordoff MG, Alarcón LK, Byerly EA, Doman SA. 2005. Mice acquire flavor preferences during shipping. Physiol Behav 86:480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vesselinovitch D, Wissler RW, Doull J. 1968. Experimental production of atherosclerosis in mice. Part 1. Effect of various synthetic diets and radiation on survival time, food consumption and body weight in mice. J Atheroscler Res 8:483–495 [DOI] [PubMed] [Google Scholar]
- 26.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO, Dainiak N. 2004. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med 140:1037–1051 [DOI] [PubMed] [Google Scholar]
- 27.Weinstock DM, Case C, Jr, Bader JL, Chao NJ, Coleman CN, Hatchett RJ, Weisdorf DJ, Confer DL. 2008. Radiologic and nuclear events: contingency planning for hematologists–oncologists. Blood 111:5440–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss JF, Landauer MR. 2009. History and development of radiation protective agents. Int J Radiat Biol 85:539–573 [DOI] [PubMed] [Google Scholar]