Summary
We evaluate endovascular treatment (EVT) as an option to deal with multiple intracranial aneurysms(MA). From 1994 to 2001,24 patients underwent EVT for 59 MA. Patients were followed-up clinically and angiographically in a period ranging from 6 to 93 months (mean time of 22.2) and from 4 to 69 months (mean time of 19.3), respectively.
Ten patients (41.6%) were treated either by EVT (n=7, 29,16%) or by mixed treatment (EVT and surgery; n=3, 12.5%). Reasons for treating just ruptured aneurysms: six (25%) had aneurysms smaller than 5 mm; three (12.5%) deaths; two (8.33%) were in the subacute period; two (8.33%) lost to follow-up; one (4.17%) authorised no procedure. No rebleeding was detected at the clinical follow-up, but there were five deaths. At immediate arteriographic control: 28 (85%) aneurysms were fully occluded, four (12%) with neck flow and one (03%) with sac flow. For 20 aneurysms followed-up: stability of occlusion was reached in seven cases (35%) and repermeabilization in 13 (65%). Management of recanalization was close arteriography in seven (54%), re-embolization in five (38%) and surgery in one (08%).
When treating MA, EVT is advisable either alone or in mixed therapy. As a high degree of repermeabilization was disclosed, strict arteriographic control is required. The mechanisms underlying aneurysmal formation may be also involved in the recanalization phenomenon , a possible new manifestation of the fragility of the arterial wall.
Key words: multiple intracranial aneurysms, endovascular treatment, subarachnoid hemorrhage
Introduction
Subarachnoid haemorrhage (SAH) is the most common presentation of intracranial aneurysms and counts for a high overall mortality rate (about 40 to 50%)1. Due to the potential gravity, prompt medical intervention is the cornerstone of treatment to avoid the risk of re-bleeding from ruptured aneurysms and prevention of bleeding from unruptured ones.
Surgical intervention is the accepted treatment for many teams. However, some patients may be surgically inoperable, so since the eighties many techniques have been presented to allow endovascular access to this vasculopathy2-6. The technical development and expertise of many groups have made endovascular treatment (EVT) a feasible worthwhile option, mainly considering embolization by means of an electrical4,7,8 or mechanical coil detachment system 9,10.
The choice of either surgical or EVT treatment is still debated and controversial. The present study analyses our experience in patients with multiple aneurysms ( MA), accessing the role of the EVT.
Material and Methods
Patient population
Between February 1994 and July of 2001, 24 patients were admitted to our service with MA (59 aneurysms). Patients' ages ranged from 31 to 79 years (mean age 45.7). There were 13 women and 11 men.
Clinical presentation was SAH in all of them, whose clinical status was assessed by Hunt and Hess scale (HH) 11: grade 1 in three patients (12.5%), grade 2 in six (25%), grade 3 in 11 (46%), grade 4 in one (4%) and grade 5 in three (12.5%). Quantification of the blood in the initial computed tomography (CT) scan was made by Fisher scale 12 and presented the following results: one patient (04%) in grade I, nine (37%) in grade II, six (25%) in grade III and eight (33%) in grade 4.
Aneurysms characteristics
Association: 18 out of 24 patients presented two aneurysms, which represents 75% of the cases; three patients (12%) had three aneurysms, one patient (4%) had four aneurysms and two patients (9%) had five aneurysms (table 1).
Table 1.
Number and association of aneurysms per patient
| Aneurysmal association | Number of aneurysms | N° of patients | % |
|---|---|---|---|
| 2 | 18 | 75 | |
| Anterior communicating artery and internal carotid artery (2 at the supraclinoid and 1 at the juxtasellar segments) |
3 | 12.5 | |
| Mirror aneurysms of middle cerebral artery | 3 | 12.5 | |
| Mirror aneurysms of internal carotid artery supraclinoid segment | 2 | 8.33 | |
| Middle cerebral artery and internal carotid artery supraclinoid segment |
2 | 8.33 | |
| Postero-inferior cerebellar artery and internal carotid artery (supraclinoid segment) |
2 | 8.33 | |
| Anterior communicating artery and middle cerebral artery | 2 | 8.33 | |
| Anterior communicating artery and middle cerebral artery | 2 | 8.33 | |
| Anterior cerebral artery and middle cerebral artery | 1 | 4.16 | |
| At the same supraclinoid segment of internal carotid artery | 1 | 4.16 | |
| At the same middle cerebral artery | 1 | 4.16 | |
| Supraclinoid segment of internal carotid artery and Basilar artery | 1 | 4.16 | |
| 3 | 3 | 12.5 | |
| Anterior communicating artery and middle cerebral artery | 1 | 4.16 | |
| Postero-inferior cerebellar artery, supraclinoid segment of internal carotid artery and middle cerebral artery |
1 | 4.16 | |
| At the same supraclinoid segment of the internal carotid artery | 1 | 4.16 | |
| 4 | 2 | 8.33 | |
| Mirror aneurysms of middle cerebral artery | 1 | 4.16 | |
| Juxtasellar segment of the internal carotid artery, anterior communicating artery, anterior cerebral artery and middle cerebral artery |
1 | 4.16 | |
| 5 | 1 | 4.16 | |
| Mirror aneurysms of middle cerebral artery and anterior cerebral artery |
1 | 4.16 | |
Localisation: The distribution of the aneurysms was as follows: middle cerebral artery 26 (44%), supraclinoid segment of the internal carotid artery 17 (29%), anterior cerebral artery ten (17%), postero-inferior cerebellar artery three (5%), juxtasellar (intracavernous) segment of internal carotid artery two (3%) and basilar artery one (2%).
Of the 59 aneurysms, 55 were located in the carotid system (93%) and four in the vertebrobasilar system (7%). None of them were in distal arteries.
Size: The size of the aneurysmal dilatation ranged from 2 mm to 23 mm and was estimated smaller than 5 mm in 27 (46%), 5 to 10 mm in 25 (42%), 10 to 20 mm in six (10%) and more than 20 mm in one patient (2%).
Endovascular treatment
Procedure: All the procedures were performed under general anesthesia and full systemic heparinization to activated clotting time were kept around twice the base value (Hence, intravenous bolus of 50 mg of Heparin, then 1 to 2 mg/kg/hour intra-venous). All cases were infused by Nimodipine (Nimotop, Bayer, Sens, France) at a rate of 0.03 mg/kg/hour, as part of the treatment for SAH.
Through a 6F introducer by femoral route (Terumo, Belgium), a 5 or 6F guiding catheter (Cas. Balt, France), or Envoy (Cordis Endovascular System, USA) was gently pushed selectively into the carotid or vertebral artery.
All the procedures were analyzed using the Integris V3000 System (Philips Medical System, The Netherlands) with both fast subtracted rotation system and selective road mapping to allow satisfactory analysis of aneurysm and the parent vessels. In some cases a biplane view was used by a supplementary acquisition with BV 29 (Philips Medical System, The Nederlands). Recently, we acquired an Integris 3D-RA workstation (Philips Medical System, The Nederlands), which has given additional more precise information on the angioarchitecture provided by a three-dimensional reconstruction.
The microcatheter (Marco 10 or 18, Balt, France) (Prowler 10 or 14 Cordis Endovascular System, USA) was gently advanced into the bulk of the aneurysm, using a 0.12 micro-guidewire (Terumo, Belgium) with 45° or 90° tip angulation.
In some cases, a gentle curve was steam-shaped at the tip of the microcatheter according to the angulation of both the parent vessels and the collar of the aneurysm.
Management: Patients with MA in our service were treated by means of one intervention per aneurysm, reserving the first procedure for the symptomatic aneurysm. However, in one patient we performed embolization of two aneurysms in the same time, due to the fast treatment of the first and ready accessibility of the other.
Coil systems: For aneurysmal occlusion, we used detachable coil systems for all patients. In 19 patients (80%), we used mechanical systems: 12 mechanically detachable systems from Balt (MDS®, 42%) and seven detachable coil systems from Cook (DCS® 38%). In two, the electrical system with Guglielmi detachable system from Target (GDC® 8%). In three patients (12%), we used two systems: two patients (8%) MDS and DCS; another one (4%) MDS and GDC (table 2).
Table 2.
Coils employed
| Material | N° of patients | % |
|---|---|---|
| MDS | 12 | 42 |
| DCS | 7 | 38 |
| GDC | 2 | 8 |
| MDS/DCS | 2 | 8 |
| MDS/GDC | 1 | 4 |
Follow-up
The clinical follow-up was performed in a period ranging from six to 93 months (mean time of 22.2).
In addition, we performed angiography in a period ranging from four to 69 months (mean time of 19.3 months).
Occlusion rate
The evaluation of the embolization was assessed immediately and by angiographic follow-up and was classified as follows: a) full occlusion: total packing, no contrast visible at the neck or inside the sac; b) partial occlusion with neck flow: partial packing, contrast visible in the neck but not inside the sac; c) partial occlusion with sac flow: partial packing, contrast visible in the neck as well as inside the sac. Repermeabilization was considered when an involution at the degree of occlusion was detected at follow-up.
Results
Endovascular treatment
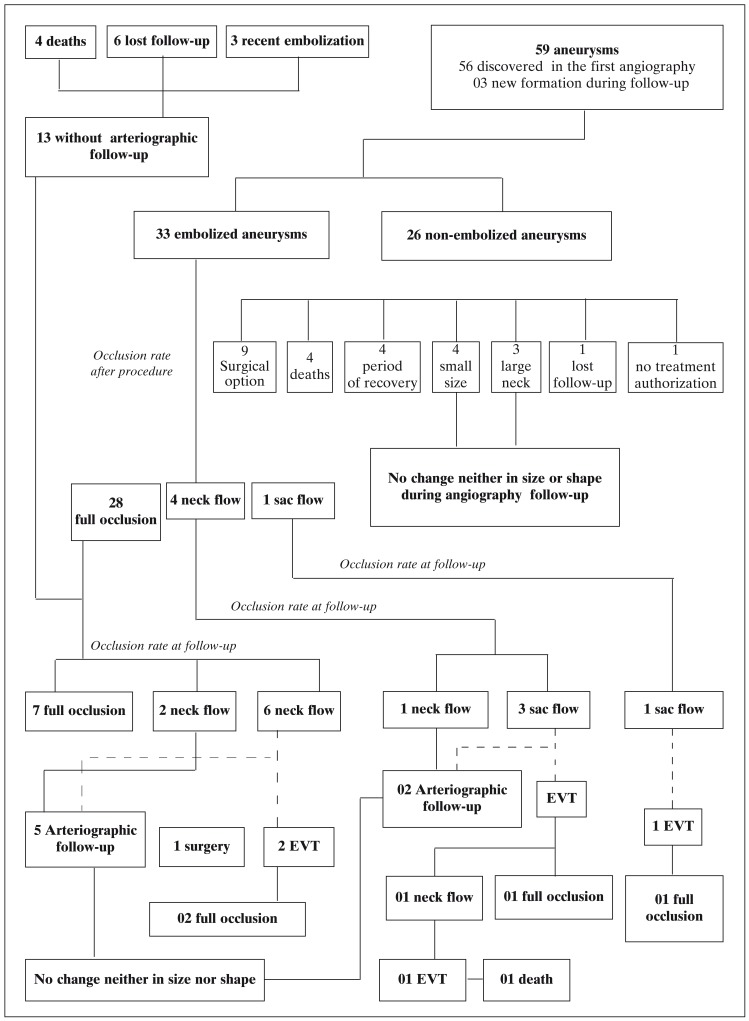
Feasibility: 33 out of the 59 aneurysms diagnosed were treated by EVT, however 26 aneurysms (44%) were not submitted to EVT due to: surgical option in nine cases (35% not embolized), small size in four cases (15%), death of four patients (15%), four cases (15%) in the period of recovery from SAH, large neck in three cases (12%), one lost to follow-up (4%) and another (4%) refused to authorise treatment. All ruptured aneurysms were treated by EVT. One patient during a second procedure had a technical complication, resulting in sac perforation with re-bleeding and death (graph 1).
Graph 1.
General vision of the population studied
Efficacy: For the 33 aneurysms treated, at the end of the procedure we have obtained: 28 (85%) full occlusion, four (12%) partial occlusion with neck flow and one (03%) partial occlusion with sac flow (table 3, graph 1).
Table 3.
Occlusion rate after embolization and at follow-up
| Occlusion rate | |||
|---|---|---|---|
| Full occlusion | Neck flow | Sac flow | |
| After embolization (n=33) | 28 (85%) | 04 (12%) | 01 (03%) |
| At follow-up1(n=20) | 07 (35%) | 03 (15%) | 10 (50%) |
| After re-embolization (n=05) | 04 (80%) | 01 (20%) | 0 |
|
Final angiographic result 2 within 19 months (n=18) |
11 (60%) | 03 (17%) | 04 (22%) |
|
Obs: 1 Arteriographic control was not performed due to patient's death (04), recent procedure (03) and lost to follow-up (06). 2 One patient died during a second embolization and another made a neurosurgical option. | |||
Follow-up
Clinical results (table 4): The clinical follow-up detected no rebleeding and a mortality rate of 20.83% (five patients). All patients but one died as a result of the disease itself and its complications. The overall outcome was a direct result of clinical status at admission.
Table 4.
Clinical follow-up after embolization
| Number of patients (%) | |
|---|---|
|
Asymptomatic (no re-bleeding) |
131 (54) |
| Death1 | 05 (21) |
| Lost to follow-up | 06 (25) |
|
Obs: 1 All deaths were related to the gravity of SAH and clinical complications, but one re-bleeding occurred due to perforation of the aneurysmal sac with death complication. | |
Angiographic results (graph 1, tables 4,5): In a mean angiographic follow-up of 19 months, 20 aneurysms were re-evaluated, the stability of the full occlusion was achieved in seven aneurysms (35%). However, 13 (65%) aneurysms presented repermeabilization: three (15%) had neck flow disclosed and ten sac flow (50%). The option for repermeabilization was close arteriographic control to detect any change in the degree of repermeabilization and shape / size of aneurysms in seven out of 13 (54%), EVT in five (38%) and surgery in one (8%) (table 5). Consequently, the final angiographic result of embolized and controlled aneurysms in our series is: 11 aneurysms (60%) were fully occluded, three aneurysms (17%) were partially occluded with neck flow and four aneurysms (22%) were partially occluded with sac flow. In one patient (5%), an asymptomatic occlusion of the parent vessel was detected. In two (8%) patients, three “de novo” aneurysmal formations were detected (5% of all aneurysms diagnosed).
Table 5.
Therapeutic option for repermeabilization considering the angiographic state at follow-up
| Angiographic State at Follow-up |
Number of aneurysms (%) |
|---|---|
| Overall view | (n=13) |
| EVT | 05 (38) |
| Surgery | 01 (8) |
| Angiographic follow-up | 07 (54) |
| Neck flow | (n=03) |
| EVT | 0 |
| Surgery | 0 |
| Angiographic follow-up | 03 (100) |
| Sac flow | (n=10) |
| EVT | 05 (50) |
| Surgery | 01 (10) |
| Angiographic follow-up | 04 (40) |
Discussion
It is necessary to treat patients with ruptured aneurysms presenting with SAH efficiently to prevent the risk of rebleeding, because the morbidity and mortality of neurosurgical or endovascular treatment are lower than the risk of rebleeding: 4.1% and 1.0% for surgery13 and 3.6% and 0.0% for EVT7, respectively . However, some special difficulties may be faced when dealing with MA, whose frequency can range from 5% to 30% 14-18: in our data 10% of cases.
Firstly, it is crucial to define and diagnose which aneurysm was responsible for SAH. For this purpose the current practice accepts the Nehls’ algorithm19 as a reasonable and satisfactory method for prediction of the ruptured site, in which the analysis of clot distribution at CT and angiographic findings (size/ shape of aneurysms and vasospasm distribution) are the key points for diagnosis. By this means, they were able to identify the site of rupture in 98% of patients. Another study20 claimed 95%, but the main cause of misjudgement was improper diagnosis of a hidden aneurysm missed in the initial radiological study.
Based on this rule, we assumed to find out the aneurysm to be treated in all cases but one, in which a diffuse SAH was detected and one aneurysm in the internal cerebral artery and the other in the middle cerebral artery with the same size / shape and no vasospasm. The former underwent EVT and the latter surgery, because there were many branches related to the neck.
As no re-bleeding was detected in our study, the EVT for MA proved to protect the risk of rebleeding, which alone counts for the main reason of mortality in the acute phase and the main danger in long term follow-up. Although much controversy surrounds the intensity of the packing7,21, we did not notice any relation of this with the protective measure reached for EVT. Regardless of these opinions, a dense packing was sought in all cases.
Additionally, once the problem of the acute phase is solved, there remains the decision to treat the unruptured aneurysm or not. This question is one of the controversies in aneurysmal disease and is why many studies have already been carried out to give some specific parameters to define which aneurysms should be treated.
In a study on the natural history of unruptured aneurysms, Juvela et Al1 described an average annual rate of rupture of 1.4%, the cumulative rate of rupture of 10% at ten years after diagnosis, 26% at 20 years and 32% at 30 years. Wiebers et Al22, after a multivariate discriminate analysis of several independent variables of incidence of rupture, described an aneurysmal sac size larger than 10 mm as the only significant variable.
Conversely, other articles 1,23 have shown that sometimes the diameter of the ruptured aneurysm can be smaller than 10 mm, despite the very low probability of bleeding. Others have described the growth in aneurysmal sac as a specific risk for rupture irrespective of the initial size 1,23-25.
In the case of MA, Heiskanen 14 followed a group of 61 patients with proved SAH and at least two aneurysms, in whom just the ruptured lesion was surgically treated. After ten years he found a risk of hemorrhage of 11.5% (7 cases) and 21.3% up to twenty years, with fatal bleedings of 6.6% and 8.1%, respectively. However, no mention of aneurysmal size was made, not even aneurysmal growth.
Although it was controversial for many years, and treatment of unruptured aneurysms was not recommended in the case of MA15, it has been the trend to recommend either surgery14-18 or EVT for all aneurysms8,26,27.
In our institution, the management of ruptured and unruptered aneurysms is always in a multidisciplinary decision involving neurosurgical and neuroradiological teams. Even though it is advocated that surgery is the first and definitive treatment for aneurysms, we have been offering EVT as the first therapy, when there is a detailed analysis of the angioarchitecture, morphology of aneurysmal dilatation and no need for surgical evacuation of an hematome. We are experiencing good results for protection against re-bleeding 9,10, as are many other teams 7,8,21, to support this choice.
In the special case of MA, our strategy was to treat the ruptured aneurysm in the acute phase, unless some doubt on the topography is present or if the treatment of the symptomatic lesion was done in such a rapid way as to allow intervention in the unruptured aneurysm, only when its endovascular access and treatment seem easy. If EVT failed during the emergency setting, it was followed by a surgical approach.
Based on the already described works, we have defined a policy to treat all unruptured aneurysms, mainly because MA seems to result in a higher risk of SAH. In the vast majority of cases, the therapeutic choice was EVT, but in some we performed regular angiographic control and if modification of shape and size was detected during follow-up, either surgery or EVT was proposed. To sum up the final result of the 24 patients, ten patients (41.6%) had all aneurysms treated: seven (29.16%) by EVT and three (12.5%) by mixed treatment (surgery + EVT). For the 14 treated just for the ruptured aneurysm (58.33%): six (25%, three of which had a large neck) had aneurysms smaller than 5 mm with stable shape and size during regular angiographic control; three (12.5%) died in the acute period; two (8.33%) had the ruptured aneurysm recently treated; two (8.33%) were lost to follow-up; one (4.17%) patient after being embolized once and operated for “de novo” aneurysmal formation three times refused to continue.
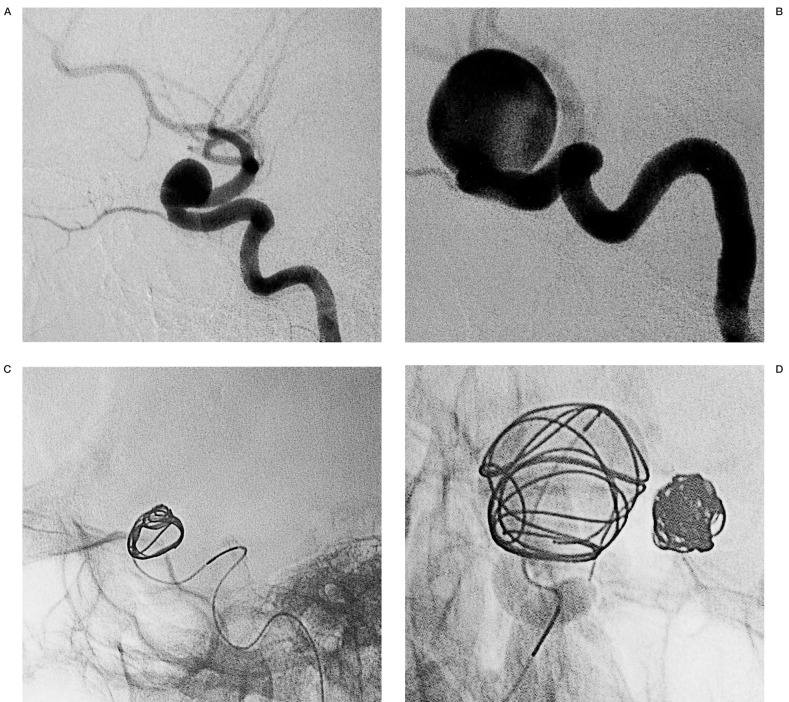
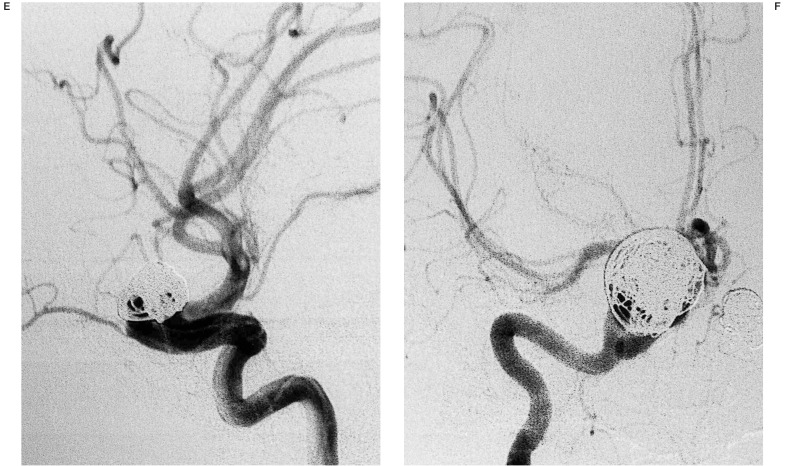
In six (85%) out of the seven patients treated by EVT for all aneurysms, the unruptured aneurysm was the object of a different session (figure 1). Just one case (15%) was treated at the same time (figure 2). One of three patients treated by mixed option had both aneurysms managed in the acute period. We advocate, however, when indicated, that other aneurysms be treated as soon as the clinical condition of the patient has stabilised.
Figure 1.
Embolization of two aneurysms of the supraclinoid segment of left (A) and right internal carotid artery during different procedures (B). For both we used DCS, firstly choosing J-shaped coils for internal remodelling (C,D) and then we had put inside this cage spiral-shaped ones intending to have a dense packing (E,F).
Figure 2.
Embolization of an aneurysm at A1-A2 segment of left anterior cerebral artery (A) and another at the trifurcation of the left middle cerebral artery during the same session (B), using DCS spiral shaped coils (C,D).
The angiographic follow-up disclosed a high rate of repermeabilization in MA, mainly when partial occlusion was encountered, supporting the already known aim of a complete aneurysmal occlusion in the first embolization, whenever possible. Additionally, we should mention a clear tendency towards recanalization, besides the full occlusion obtained.
When repermeabilization was seen, the same principles already discussed for treating ruptured and unruptured aneurysms were used. As a result, we chose a close arteriographic follow-up for repermeabilized aneurysms, in which the remnant sac was small in such a morphology that EVT was not allowed. When no alteration of the aneurysm was detected in either size or shape, the patients were kept in the follow-up choice. The repermeabilized aneurysms that did not fulfil these criteria or whose follow-up showed modification of size or shape were addressed either to EVT or to surgery after detailed multidisciplinary discussion.
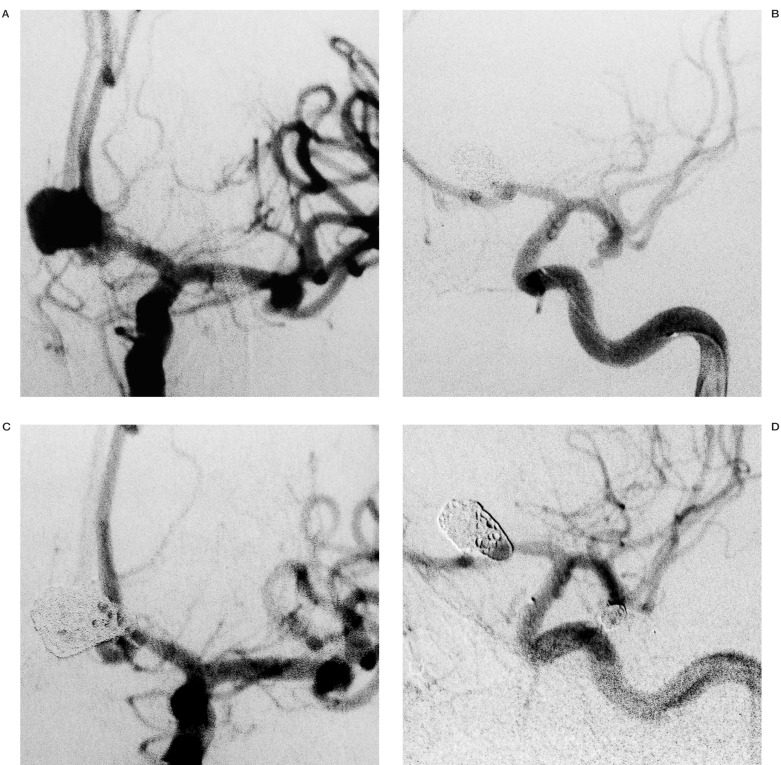
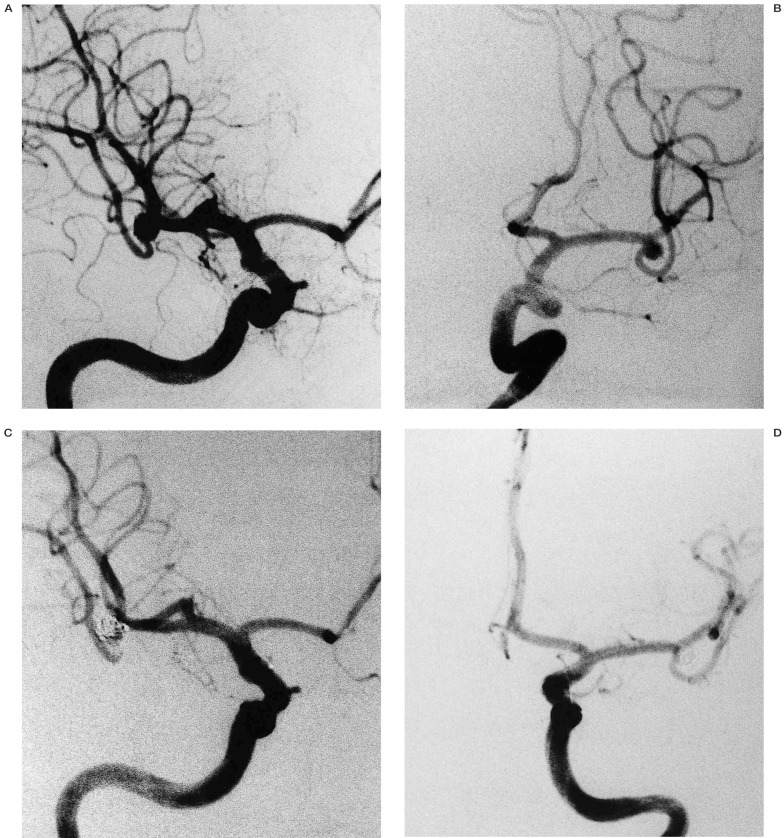
Regarding this strategy, from the 13 repermeabilized aneurysms (65% of all embolizations) (table 5), seven were maintained in the follow-up and six underwent a new intervention, by means of an endovascular route in five patients (figure 3) and neurosurgery in one. As no rebleeding was detected, this seems a safe policy for repermeabilization.
Figure 3.
A case of full repermeabilization of mirror aneurysms of middle cerebral artery (A,B), whose therapeuthic option was EVT in different sessions (C,D).
The high incidence of repermeabilization in this article raised the question of whether this tendency was a consequence of the natural fragility of the parietal wall, which was responsible for the origin of the MA.
Lasjaunias28,29 described the concept of vascular phenotypic vulnerability and of aneurysmal vasculopathies. The former presents the susceptibility of a vascular segment as an eventual hypothesis for some arterial diseases and the latter presents the differences among aneurysmal diseases. Based on this, multiple aneurysms could be either a phenotype of a genetic disorder accompanied sometimes by other systemic manifestations, as in other connective tissue disorders 30, sickling disorders 31, polycystic kidney disease 32, or an acquired disease after exposure to some risk factors as described for smoking, hypertension and female sex 33-35.
Whatever the facts may be, many teams have already been working on the mechanisms of the physiopathology for aneurysmal formation and rupture. After studies with experimental models 36 or in human beings 37-40, some have proposed that a defect in the structure of the extracellular matrix at the level of production or overdestruction, or even in the matrix remodelling after tissue injury, could explain aneurysmal formation and rupture. The problem could be the result of an impaired production of proteins involved in this process, due to an inherited gene or as a result of action of environmental factors. In some cases, defects involving collagen type III 39 and alpha1 antitrypsin deficiency were detected 38,39.
In the same way, there is already convincing evidence of a genetic component for intracranial aneurysms, and a considerable number of studies have analysed the role of the following genes, among others, in the predisposition for aneurysmal development: promoter of metalloproteinase-9 (MMP9) 37, collagen production (COL3A1, COL1A,COL1A2, etc.)41, elastin production 41, extracellular matrix turnover (TIMP-3, OSF-2)41.
On the other hand, some teams have proposed apopotosis 42,43, a special phenotype of smooth muscle cells in the arterial walls 44 and nitric oxide 45 as key requirements for aneurysmal formation and growth.
With reference to these considerations, a similar process may account for the loss of elastibility and weakness of the arterial wall, leading to a predisposition for aneurysmal formation, aneurysmal regrowth and “de novo” aneurysmal formation after embolization in the presented data.
Despite our observation, more detailed studies are required focusing on the follow-up and histological aspects of MA and also single aneurysms, to evaluate this hypothesis and per-haps to disclose another phenotype of this peculiar aneurysmal disease. In spite of this, a larger study is warranted to understand the physiopathology of single and multiple aneurysms, and to find clues to the challenge of dealing with MA.
Conclusions
EVT is a reliable option to deal with MA, mainly due to the low-risk and fast procedure, allowing protection from re-bleeding which is the main cause of morbidity and mortality among patients with SAH. The question to treat or not to treat unruptured aneurysms will still arouse controversy, chiefly in MA, but a multidisciplinary staff must be always analyse the best choice, either therapeutic (surgery or EVT) or radiological follow-up.
As we found a high propensity to recanalization and in some cases new aneurysmal formation, patients with MA must be followed-up by a regular angiography after treatment.
The possibility of a greater fragility of the arterial wall of patients with multiple aneurysms as the pathologic reason for repermeabilization and “de novo” aneurysmal formation, should encourage research into the exact physiopathology of multiple aneurysms for future genetic treatment or at least to disclose genetic and phenotypic markers at extracellular matrix or intracellular level.
Acknowledgments
We thank MD Reda El Bayoumy for revision of the language.
References
- 1.Juvella S, Porras M, Heiskanen O. Natural history of unruptured intracranial aneurysms: a long term follow-up study. J Neurosurg. 1993;79:174–182. doi: 10.3171/jns.1993.79.2.0174. [DOI] [PubMed] [Google Scholar]
- 2.Fox AJ, Drake CG. Detachable balloon embolization for intracranial aneurysms. J Neurosurg. 1990;73:157–159. doi: 10.3171/jns.1990.73.1.0157. [DOI] [PubMed] [Google Scholar]
- 3.Fox AJ, Vinuela F, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg. 1987;66:40–46. doi: 10.3171/jns.1987.66.1.0040. [DOI] [PubMed] [Google Scholar]
- 4.Guglielmi G, Vinuela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 5.Higashida R, Halbach VV, et al. Intracranial aneurysms: interventional neurovascular treatment with detachable balloons results in 215 cases. Radiology. 1991;178:663–670. doi: 10.1148/radiology.178.3.1994399. [DOI] [PubMed] [Google Scholar]
- 6.Kinugasa K, Mandai S, et al. Direct thrombosis of aneurysms with cellulose acetate polymer. Part II: Preliminary clinical experience. J Neurosurg. 1992;77:501–507. doi: 10.3171/jns.1992.77.4.0501. [DOI] [PubMed] [Google Scholar]
- 7.Fuse A, Rodesch G, et al. Endovascular management of intradural berry aneurysm. Review of 203 consecutive patients managed between 1993 and 1998. Morphological and clinical results at mid-term follow-up. Interventional Neuroradiology. 2000;6:27–39. doi: 10.1177/159101990000600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solander S, Ulhoa A, et al. Endovascular treatment of multiple intracranial aneurysms by using Guglielmi detachable coils. J Neurosurg. 1999;90(5):857–864. doi: 10.3171/jns.1999.90.5.0857. [DOI] [PubMed] [Google Scholar]
- 9.Tournade A, Courtheoux P, et al. Saccular intracranial aneurysms: endovascular treatment with mechanical detachable spiral coils. Radiology. 1997;202:481–486. doi: 10.1148/radiology.202.2.9015078. [DOI] [PubMed] [Google Scholar]
- 10.Tournade A, Riquelme C, et al. Endovascular treatment of berry intracranial aneurysms using a new detachable coils system (DCS) Interventional Neuroradiology. 2001;7:93–102. doi: 10.1177/159101990100700201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid haemorrhage visualised by computerised topography scanning. Neurosurgery. 1980;6:19. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 13.King JT, Jr, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for assymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg. 1994;81:837–842. doi: 10.3171/jns.1994.81.6.0837. [DOI] [PubMed] [Google Scholar]
- 14.Heiskanen O. Risk of bleeding from unruptured aneurysms in cases with multiple intracranial aneurysms. J Neurosurg. 1981;55:524–526. doi: 10.3171/jns.1981.55.4.0524. [DOI] [PubMed] [Google Scholar]
- 15.McKissock W, Richardson A, Walsh L. Multiple intracranial aneurysms. Lancet. 1964;1:623–626. doi: 10.1016/s0140-6736(64)91449-7. [DOI] [PubMed] [Google Scholar]
- 16.Mizoi K, Suzuki J, Yoshimoto T. Surgical treatment of multiple aneurysms: Review of experience with 372 cases. Acta Neurochiru (Wien) 1990;96:8–14. doi: 10.1007/BF01403489. [DOI] [PubMed] [Google Scholar]
- 17.Mount L, Brisman R. Treatment of multiple intracranial aneurysms. J Neurosurg. 1990;35:728–730. doi: 10.3171/jns.1971.35.6.0728. [DOI] [PubMed] [Google Scholar]
- 18.Orz Y, Osawa M, et al. Surgical outcomes for multiple aneurysms. Acta Neurochir (Wien) 1996;138:411–417. doi: 10.1007/BF01420303. [DOI] [PubMed] [Google Scholar]
- 19.Nehls DG, Flom RA, et al. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg. 1985;63:342–348. doi: 10.3171/jns.1985.63.3.0342. [DOI] [PubMed] [Google Scholar]
- 20.Hino A, Fujimoto M, et al. False localisation of rupture site in patients with multiple cerebral aneurysms and subarachnoid haemorrhage. Neurosurgery. 2000;46:825–830. doi: 10.1097/00006123-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Cognard C, Weill A, et al. Intracranial berry aneurysms: Angiographic and clinical results after endovascular treatment. Radiology. 1998;206:499–510. doi: 10.1148/radiology.206.2.9457205. [DOI] [PubMed] [Google Scholar]
- 22.Wiebers DO, Whisnant JP, et al. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987;66:23–29. doi: 10.3171/jns.1987.66.1.0023. [DOI] [PubMed] [Google Scholar]
- 23.Yasui N, Magarisawa S, et al. Subarachnoid haemorrhage caused by previously diagnosed, previously unruptured intracranial aneurysms: A retrospective analysis of 25 cases. Neurosurgery. 1996;39:1096–1101. doi: 10.1097/00006123-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Allcock JM, Canham PB. Angiographic study of the growth of intracranial aneurysms. J Neurosurg. 1976;45:617–621. doi: 10.3171/jns.1976.45.6.0617. [DOI] [PubMed] [Google Scholar]
- 25.Sampei T, Mizuno M, Nakajima S, et al. Clinical study of growing up aneurysms: Report of 25 cases. Neurol Surg. 1991;19:825–830. [PubMed] [Google Scholar]
- 26.Pierot L, Boulin A, et al. The endovascular approach in the management of patients with multiple intracranial aneurysms. Neuroradiology. 1997;39:361–366. doi: 10.1007/s002340050425. [DOI] [PubMed] [Google Scholar]
- 27.Solander S, Ulhoa A, et al. Endovascular treatment of multiple intracranial aneurysms by using Guglielmi detachable coils. J Neurosurg. 1999;90(5):857–864. doi: 10.3171/jns.1999.90.5.0857. [DOI] [PubMed] [Google Scholar]
- 28.Lasjaunias PL. From aneurysms to aneurysmal vasculopathies. Interventional Neuroradiology. 1999;5:97–108. doi: 10.1177/159101999900500201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasjaunias PL. Segmental identity and vulnerability in cerebral arteries. Interventional Neuroradiology. 2000;6:113–124. doi: 10.1177/159101990000600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Paepe A, Van Landegem W, et al. Association of multiple intracranial aneurysms and collagen type III deficiency. Clin Neurol Neurosurg. 1988;90:53–56. doi: 10.1016/s0303-8467(88)80010-6. [DOI] [PubMed] [Google Scholar]
- 31.Diggs LW, Brookoff D. Multiple Cerebral aneurysms in patients with sickle cell disease. South Med J. 1993;86:480–482. doi: 10.1097/00007611-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Chapman AB, Rubinstein D, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J. 1992;327:916–920. doi: 10.1056/NEJM199209243271303. [DOI] [PubMed] [Google Scholar]
- 33.Connoly ES, Jr, Choudrhri TF, et al. Influence of smoking, hypertension and sex on the phenotypic expression of intracranial aneurysms on siblings. Neurosurgery. 2001;48:64–68. doi: 10.1097/00006123-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Juvela S. Risk factors for multiple intracranial aneurysms. Stroke. 2000;31:392–397. doi: 10.1161/01.str.31.2.392. [DOI] [PubMed] [Google Scholar]
- 35.Leblanc R. Familial cerebral aneurysms. A bias for women. Stroke. 1996;27:1050–1054. doi: 10.1161/01.str.27.6.1050. [DOI] [PubMed] [Google Scholar]
- 36.Coutard M. Experimental cerebral aneurysms in the female heterozygous Bloutchy mouse. Int J Exp Pathol. 1999;80:357–367. doi: 10.1046/j.1365-2613.1999.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters DG, Kassam A, et al. Functional polymorphism in the matrix metalloproteinase-9 promoter as a potential risk factor for intracranial aneurysms. Stroke. 1999;30:2612–2616. doi: 10.1161/01.str.30.12.2612. [DOI] [PubMed] [Google Scholar]
- 38.Schievink WI, Katzmann JA, et al. Alpha-1 anitrypsin phenotypes among patients with intracranial aneurysms. J Neurosurg. 1996;84:781–784. doi: 10.3171/jns.1996.84.5.0781. [DOI] [PubMed] [Google Scholar]
- 39.Schievink WI. Genetics and aneurysmal formation. Neurosurg Clin N Am. 1998;9:485–495. [PubMed] [Google Scholar]
- 40.Van den Berg JS, Pals G, et al. Type III collagen deficiency in saccular intracranial aneurysms. Defect in gene regulation? Stroke. 1999;30:1628–1631. doi: 10.1161/01.str.30.8.1628. [DOI] [PubMed] [Google Scholar]
- 41.Peters DG, Kassam AB, et al. Molecular anatomy of an intracranial aneurysm: Coordinated expression involved in wound healing and tissue remodelling. Stroke. 2001;32:1036–1042. doi: 10.1161/01.str.32.4.1036. [DOI] [PubMed] [Google Scholar]
- 42.Kondo S, Hashimoto N, et al. Apoptosis of medial smooth muscle cells in the development of saccular cerebral aneurysms in rats. Stroke. 1998;29:181–188. doi: 10.1161/01.str.29.1.181. [DOI] [PubMed] [Google Scholar]
- 43.Hara A, Yoshimi N, Mori H. Evidence for apoptosis in human intracranial aneurysms. Neurol Res. 1998;20(2):127–130. doi: 10.1080/01616412.1998.11740494. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima N, Nagahiro S, et al. Phenotype modulation of smooth muscle cells in human cerebral aneurysmal walls. Acta Neuropathol (Berl) 2000;100:457–480. doi: 10.1007/s004010000220. [DOI] [PubMed] [Google Scholar]
- 45.Fukuda S, Hashimoto N, et al. Prevention of rat cerebral formation by inhibition of nitric oxide synthase. Circulation. 2000;101:2532–2538. doi: 10.1161/01.cir.101.21.2532. [DOI] [PubMed] [Google Scholar]