Abstract
Sex steroids are essential for reproduction and development in animals and humans, and sex steroids also play an important role in neuroprotection following brain injury. New data indicate that sex-specific responses to brain injury occur at the cellular and molecular levels. This review summarizes the current understanding of neuroprotection by sex steroids, particularly estrogen, androgen, and progesterone, based on both in vitro and in vivo studies. Better understanding of the role of sex steroids under physiological and pathological conditions will help us to develop novel effective therapeutic strategies for brain injury.
Keywords: P450 aromatas, estrogen, androgen, progesterone, brain ischemia, stroke, oxygen-glucose deprivation, EDHF, sex differences, neuroprotection
1. Introduction
Sex steroids are involved in functions that extend beyond reproduction. The effects of sex steroids are implicated in cognition, synaptic plasticity, memory, neurogenesis, and neuroprotection. In this article, we review the neuroprotective effects of estrogen, progesterone, and androgens following brain injury, particularly ischemic brain injury, and molecular mechanisms that are implicated in this neuroprotection.
Stroke is a major cause of death and disability worldwide. Ischemic stroke affects more than 700,000 individuals every year in the United States. Epidemiological studies have shown that stroke incidence and mortality rates are higher in men relative to age-matched women worldwide,1 suggesting that stroke is a sexually dimorphic disease. However, stroke event rates increase with age, and more women are affected by stroke after menopause.1 Long-term exposure to ovarian estrogens may protect against ischemic stroke, an effect that seems to cease with menopause. Thus, this loss of protection has been one reason for using postmenopausal hormone replacement therapy.
Several different animal models have been used for ischemic brain injury studies, including transient or permanent middle cerebral artery occlusion (MCAO), transient forebrain ischemia, and transient global ischemia. MCAO serves as a valuable in vivo model for human stroke. In addition, oxygen-glucose deprivation (OGD) has been used as an in vitro ischemic model. In experimental stroke, the interpretation of cellular and molecular data in animals is relatively straightforward and consistent with neuroprotection by estrogen. Despite these studies, recent clinical randomized controlled trials have failed to show the beneficial effects of hormonal replacement therapy in estrogen-treated groups. A possible explanation for the apparent discrepancy between clinical trials and animal studies has been ascribed to differences in timing of exposure, dose, duration, and route of administration. However, a careful review of the extensive literature suggests that data from clinical trials and other recent studies should be interpreted within the narrow context of the study design.2 For example, in some studies, beneficial effects of estrogen against stroke may not be observed because estrogen was introduced in most of the subjects after an extended period of hypoestrogenicity. Therefore, it is important to use appropriate models to perform studies on the effect of sex steroids on brain injury. This will not only advance our understanding of the relationship of sex steroids and neuroprotection but will also improve the clinical management of stroke thereby reducing brain damage and disability.
2. Estrogen and neuroprotection
Over the past several decades, estrogen has been extensively studied as an endogenous neuroprotective agent in cardiovascular and neurological diseases including ischemic brain injury. An accumulating body of evidence from in vivo (Table 1) and in vitro (Table 2) studies demonstrates that estrogen provides powerful protection against ischemic brain injury through multiple molecular mechanisms.
Table 1.
Neuroprotection of Estrogen in vivo Studies.
| Model | Protection | Mechanisms/Results | Sex | Species | References |
|---|---|---|---|---|---|
| Transit MCAO | Yes | Increases Aromatase | Female, OVX | Mouse | 49 |
| Transit MCAO | Yes | Increases EDHF | Male | Rat | 33 |
| Gonadectomy | Yes | Decreases ROS production | Female, male, OVX, ORX | Rat | 35, 36 |
| Transit MCAO | Yes | Increases BcI-2 | Female, Male, OVX | Rat, mouse | 3, 8 |
| Permanent MCAO | Yes | Reduces inflammation, iNOS | Female and OVX | Mouse | 24 |
| Permanent MCAO | Yes | Increases neurogenesis | Female, OVX | Mouse | 37 |
| Gonadectomy | Yes | Increases angiogenesis | Female, OVX | Rat | 38, 39 |
| Transit MCAO | Yes | Increases P-STAT3 | Female, OVX | Rat | 45 |
| Transit MCAO | Yes | Increases CART | Female, OVX | Rat | 43 |
| Transit MCAO | Yes | Increases blood flow, eNOS | Female, OVX | Rat | 3,23,40 |
| Transit MCAO | Yes | Decreases sEH | Female, OVX | Mouse | 40 |
| Transit MCAO | Yes | nNOS | Female, Male, OVX | Mouse | 28 |
| Permanent MCAO | Yes | Proestrus | Female | Rat | 5 |
| Permanent MCAO | Yes | Reduces edema | Female, OVX | Rat | 41 |
| Permanent MCAO | NO | N.D. | Female | Rat | 9 |
Abbreviations: OVX: ovariectomized; ORX: Orchiectomized; MCAO: middle cerebral artery occlusion; EDHF: endothelium-derived hyperpolarization factor; nNOS: neuronal nitric oxide synthase; N.D. not determined.
Table 2.
Neuroprotection of Estrogen in vitro Studies.
| Cell Type | Conditions | Protection | Mechanisms/Results | Sex | Species | References |
|---|---|---|---|---|---|---|
| Astrocytes | OGD | Yes | Increases aromatase | Female/Male cells | Rat and Mouse | 15, 16 |
| Astrocytes | 17β-estradiol | Yes | Reduces inflammation | Mixed cell | Rat | 26 |
| Astrocytes | 17β-estradiol | Yes | Increases TGF-β | Mixed cell | Rat | 42 |
| Neurons | 17β-estradiol | Yes | Reduces oxidative stress | Mixed cell | Rat | 35 |
| Neurons | Glutamate | Yes | Reduces apoptosis | Mixed cell | Rat, Mouse | 10, 11 |
| or Aβ exposure | ||||||
| Neurons | Glutamate | Yes | Increases BcI-2 | Mixed cell | Rat | 23 |
| NT2 Neurons | Glutamate | Yes | Increases BcI-2 | Mixed cell | Rat, Human | 11, 22 |
| H2O2 exposure | ||||||
| Endothelial cell | OGD | Yes | Estrogen reduces cell death | Mixed cell | Mouse | 17 |
| Endothelial cell | 17β-estradiol | Yes | Increases eNOS | Mixed cell | Human and Bovine | 27 |
| Oligodentricytes | Peroxynitrite | Yes | Decreases cytotoxicity | Mixed cell | Rat | 13 |
| Oligodentricytes | OGD | Yes | Estrogen reduces cell death | Mixed cell | Rat | 14 |
| Oligodentricytes | Cystine deprivation | Yes | Reduces oxidative stress | Mixed cell | Rat | 14 |
| Microglial | LPS | Yes | Suppresses iNOS | Mixed cell | Rat | 25 |
Abbreviations: OGD: oxygen glucose deprivation; Aβ: amyloid beta; eNOS: endothelial nitric oxide synthase; iNOS: inducible nitric oxide synthase; LPS: Lipopolysaccharides; H2O2: Hydrogen peroxide; TGF-β: Transforming growth factor β.
2.1. Estrogen and ischemic brain injury – in vivo studies
The neuroprotective effects of estrogen have been widely documented in various animal models of experimental stroke in different species and different genetic strains. Overall, adult female rats sustain smaller infarcts after experimental stroke induced by 2 hours middle cerebral artery occlusion (MCAO) compared to age-matched males.3 We have repeated this study in different animal strains under pathological conditions that are known to be high risk factors for stroke in humans, such as hypertension and diabetes. We found that this sex difference in ischemic brain injury persists in genetic models of hypertension3 and diabetes,4 suggesting that fundamental mechanisms and pathways of ischemic brain injury may be different between males and females. Moreover, in female animals, the estrous cycle also influences the outcome of ischemic brain injury, eg, the infarct size is smaller following ischemic brain injury induced by permanent MCAO during the proestrus (high endogenous estrogen levels) stage of the estrous cycle compared to the metestrus (low endogenous estrogen levels) stage.5 In addition, sex differences in infarct size disappear after MCAO in reproductively senescent rats.6 Taken together, these differences in outcome of cerebral ischemia are in part due to the protective effect of female sex hormones, especially estrogen,7 since ovariectomy increases ischemic brain damage in female rats3 and mice;8 and estrogen replacement is protective against cerebral ischemia in ovariectomized rats3 and mice8 and in reproductively senescent male and female animals.6 However, findings from some animal studies show that estrogen is not neuroprotective and can be deleterious under certain circumstances following stroke induced by permanent MCAO.9 Discrepancies in results among different studies have been attributed to differences in dose, formulation, route of administration, or length of treatment. As a whole, the above studies provide strong evidence that estrogen contributes to neuroprotection against cerebral ischemic damage in both male and female animals.
2.2. Estrogen and neuroprotection – in vitro studies
In vitro, estrogen protects neurons against insults induced by glutamate, glucose deprivation, and beta-amyloid peptide.10,11 A number of studies have suggested that estrogen may suppress microglial activation, an effect that could help mediate estrogen neuroprotection.12 Other studies demonstrate that 17β-estradiol protects against cell death after OGD in primary oligodendrocyte cultures and that protection by estrogen is dose-dependent.13,14 In both male and female astrocytes, physiological levels of 17β-estradiol added to the culture medium prevents cell death following OGD.15,16 Other evidence indicates that both long-term and short-term pretreatments with 10 nM 17β-estradiol protect cerebral endothelial cells after OGD.17
2.3. Mechanisms of neuroprotection by estrogen
Genomic and non-genomic actions mediated by estrogen receptors
Estrogen actions are mediated by two estrogen receptor (ER) isoforms – ERα and ERβ. Both of these subtypes are expressed in cortical astrocytes, neurons, and endothelial cells and thus could mediate neuroprotection by estrogen. Administration of the potent ER antagonist ICI 182,780 dramatically increases infarct size in intact female mice following MCAO,18 suggesting that the neuroprotective effect of estrogen is mediated via ER. Infarct size is reduced after permanent MCAO in estrogen-treated wild-type (WT) and ERβ knockout mice, but not in ERα knockout mice, suggesting that ERα mediates estrogen protection against ischemic brain injury.19 Furthermore, the protective effects of estrogen against endothelial cell death following OGD is mediated by ERα, but not ERβ.17 Upregulation of estrogen receptors influences the expression of genes involved in cellular proliferation and other physiological changes. There is now general acceptance that estrogen, like other steroid hormones, can act in a classical way as a transcription factor by binding to nuclear receptors, translocating to the nucleus, and interacting with estrogen response elements located in the promoter of target genes.
Recent work has led to the discovery of a third estrogen receptor, the membrane-associated G-protein-coupled receptor 30 (GPR30). GPR30 binds estradiol with high affinity and is expressed in breast cancer cells and various other tissues in the body including brain.20 GPR30 has been recognized as a putative membrane receptor for estrogen that mediates a series of non-genomic signals from estrogen.20 Non-genomic actions of estrogen have a rapid onset and include modification of protein phosphorylation and levels of intracellular second messengers such as cyclic adenosine monophosphate (cAMP) and calcium.21 However, the role of GPR30 in neuroprotection by estrogen against ischemic brain injury is unknown.
Increases Bcl-2
Bcl-2 and cell survival in cerebral ischemia and apoptosis has been extensively studied. Estrogen exhibits neuroprotection by increasing Bcl-2 expression in NT2 neurons22 and in primary cortical neurons11 when these cells are exposed to hydrogen peroxide or glutamate. In an ischemic stroke animal model, Bcl-2 mRNA and protein expression increases accompanied by smaller infarct size in female or 17β-estradiol-treated rats compared with WT males and estrogen-deficient animals.3 Furthermore, ovariectomy increases infarction after MCAO in WT females, but not in ovariectomized female mice overexpressing Bcl-2.8 These results suggest that estrogen prevents ischemic injury, at least in part, by mediating Bcl-2 upregulation.
Regulates MAPK /PI3K/AKT signaling pathways
In the brain, the mitogen-activated protein kinases (MAPK) and the phosphatidylinositol-3 kinase (PI3K/AKT) pathways are associated with the beneficial effect of estrogen.23 Several studies report that estrogen enhances activation of PI3K/AKT, which is an important mediator of cell survival signaling pathways,21 i.e., in cultured neurons, 17β-estradiol reduces cell death via the PI3K/AKT signaling pathway following various injuries, such as glutamate toxicity.
Reduces inflammatory response
Ovariectomy with estrogen treatment reduces infarct size following permanent MCAO in WT female mice, but not in inducible nitric oxide synthase (iNOS) knockout female mice24, suggesting that estrogen-mediated neuroprotection may involve suppressing inflammatory reactions. Physiological levels of estradiol suppress both microglial activation and the iNOS-mediated immune response as well as the production of several pro-inflammatory mediators including metalloproteinase, prostaglandin E2 (PGE2), and cyclooxygenase 2 (COX-2).25 In addition to microglial cells, astrocytes have been shown to mediate inflammatory action following injury. One study shows that 17β-estradiol pretreatment reduces the protein expression of interleukin 1-beta (IL-1β) and tumor necrosis factor-α (TNFα) and suppresses matrix metallopeptidase 9 (MMP-9) activity in astrocyte media following a 24-hour treatment with lipopolysaccharide (LPS).26 Together, these studies demonstrate that post-ischemic inflammation strongly contributes to the extent of brain injury, and estradiol exhibits anti-inflammatory effects that protect against ischemic injury.25
Modulates nitric oxide synthase
Endothelial dysfunction is an important component of initiating and contributing to the pathogenesis of ischemic damage. In ischemic brain injury, estrogen effects have been linked to nitric oxide (NO). NO is produced from L-arginine by nitric oxide synthase (NOS). Endothelial injury may increase the inflammatory response through loss of normal NO production due to inhibition of NOS. One mechanism by which estrogen protects the ischemic brain may involve increasing cerebral blood flow by enhancing the activity of endothelial NOS (eNOS). This effect has been demonstrated both in vivo and in vitro.23,27 Indeed, treatment of ovariectomized rats with estrogen activates eNOS in cerebral blood vessels, enhancing NO production23; and eNOS protein expression increases in endothelial cell cultures after incubation with physiological concentrations of estrogen.27
Another study shows that histological brain injury is significantly increased after MCAO in female neuronal NOS knockout (nNOS−/−) mice compared to WT females. In contrast, ovariectomy with estradiol treatment has no effect on ischemic damage compared to vehicle-treated ovariectomized female nNOS−/− mice. These results suggest that the neuroprotective effect of estradiol following ischemic brain injury is mediated via an nNOS pathway in the female.28
Regulates endothelium-derived hyperpolarization factor
Estrogen plays an important role in the regulation of endothelial-dependent vasodilation. Endothelium-dependent relaxation is an important endothelial function and is known to be mediated by three different endothelium-dependent relaxing factors: prostacyclin (PGI2), nitric oxide (NO), and endothelium-derived hyperpolarizing factor (EDHF). Although NO is recognized as the primary relaxing factor at the level of conduit arteries, a growing body of evidence suggests that EDHF also plays an important role in the regulation of regional vascular resistance and blood flow, especially prominent in resistance arteries.29 The EDHF-mediated response involves hyperpolarization with subsequent relaxation maintained even when NO and PGI2 synthesis is inhibited.
EDHF-mediated vasodilations have been studied in both peripheral and cerebral circulations. Estrogen deficiency has no effect on NO-mediated endothelial relaxation in rat mesenteric arteries. However, EDHF-mediated relaxations and hyperpolarization induced by acetylcholine are significantly increased in arteries from intact females and overiectomized rats treated with estrogen compared to male and untreated ovariectomized rats.30,31 Similarly, the EDHF response is significantly reduced in mesenteric arteries during the diestrus stage, a good experimental model of short-term estrogen deficiency, when compared with estrus controls.30 These results suggest that both long-term and short-term estrogen deficiency can alter EDHF-mediated relaxation and hyperpolarization.30
Although the actual identity of EDHF remains unknown, several putative candidate factors have been proposed including potassium ions, c-type natriuretic peptide (CNP), hydrogen peroxide (H2O2), and metabolites of arachidonic acid such as epoxyeicosatrienoic acids (EETs). Alternatively, EDHF may be an electrical coupling through myoendothelial junctions.29 These gap junction structures are composed of several different members of the connexin protein family including connexin-37, connexin-40, and connexin-43. In mesenteric arteries, ovariectomy significantly reduces connexin-43 protein expression; and treatment with estradiol prevents the reduced expression of connexin-43 protein, indicating that estrogen deficiency may impair the EDHF-mediated response by changing the expression of connexin-43.32 This data suggests that estrogen alters EDHF-mediated relaxation in part by enhancing gap junctional communication.32
Others have shown upregulation of EDHF-dependent vasodilation in rat middle cerebral arteries after 2 hours MCAO and 24 hours reperfusion compared with control and that potentiated EDHF-mediated dilations after ischemia/reperfusion are due to altered endothelial Ca2+ regulation.33 These results suggest that EDHF plays an important role in cerebral blood flow after cerebral ischemia and may be neuroprotective. Elucidating the impact of estrogen on the cerebral vasculature after ischemic stroke is very important. More investigation is needed to better understand how estrogen affects cerebrovascular function under different physiological and pathological conditions.
Reduces oxidative stress
Cerebral ischemia and reperfusion are responsible for oxidative stress due to the generation of free radicals, which cause deleterious effects during pathogenesis. Mitochondria are a major source of reactive oxygen species (ROS) and oxidative stress and a key player in apoptosis.34 Neurons are particularly sensitive to oxidative stress due to their high energy demand. Damage to mitochondria causes disruptions in ATP production and an increase in ROS that compromise antioxidant defense systems of the cell and lead to both necrosis and apoptosis. Studies have shown that estrogen suppresses brain mitochondrial ROS production in both male and female rats, and similar effects of estrogen on neural-like PC-12 cells confirmed that estrogen inhibits mitochondrial superoxide production.35 Estrogen increases mitochondrial efficiency and reduces oxidative stress by stabilizing ATP production, promoting cell survival.34 Furthermore, a physiological concentration of estrogen is neuroprotective as it modulates antioxidant enzyme activity, including superoxide dismutase, catalase, and glutathione.36
Increases neurogenesis
The investigation of neuroprotective effects of estrogen in neurogenesis is new and interesting. Neurogenesis is restricted to two major brain regions – the dentate gyrus of the hippocampus and the subventricular zone (SVZ) lining the lateral ventricle.37 Adult brain generates new neurons under normal and neurodegenerative conditions. Estrogen influences the process of adult neurogenesis.37 Estrogen promotes the migration of newly generated neurons toward the damaged brain region, eg, from the SVZ to ischemic regions, thus facilitating brain remodeling and repair after ischemic injury. Suzuki et al showed that the number of newborn neurons in the SVZ significantly increased in estrogen-treated ovariectomized mice compared to vehicle-treated ovariectomized mice at 96 hours after permanent MCAO,37 indicating that estrogen enhances neurogenesis following ischemic stroke. Since these newborn neurons of the SVZ can migrate to ischemic regions to potentially replace damaged neurons, this finding may have clinical implications. More research is needed to determine whether these newborn neurons have any effect on functional recovery following ischemic brain injury.
Increases angiogenesis
Angiogenesis, the growth of new blood vessels, is essential for organ development and plays a critical role in the long-term outcome following ischemic injury. Estrogen modulates angiogenesis under physiological and pathological conditions. In the brain, cerebral capillary density in the frontal cortex is significantly increased in estrogen-treated ovariectomized females compared to vehicle-treated ovariectomized female rats.38 In addition, the levels of mRNA and protein expression of vascular endothelial growth factor (VEGF) in cerebral vessels are significantly decreased in ovariectomized female rats, and estrogen replacement increases VEGF expression to levels similar to intact females.38 Others have shown that ovariectomy with estrogen treatment increases angiopoietin-1 mRNA and protein expression compared to vehicle-treated ovariectomized animals.39 Both VEGF and angiopoietin-1 are endothelial cell-specific growth factors and can promote angiogenesis. Thus, these studies demonstrate that estrogen enhances pro-angiogenic molecular expression and promotes cerebral angiogenesis. However, whether angiogenesis will promote functional recovery following ischemic brain injury is unclear.
Other mechanisms
Other potential mechanisms by which estrogen mediates neuroprotection have been studied. (1) Estrogen influences blood flow during ischemic stress and reperfusion. In both normotensive wistar and stroke-prone spontaneously hypertensive rats, females subjected to ischemia have a smaller infarct size and higher cerebral blood flow during ischemia compared with males and ovariectomized females, suggesting that neuroprotection by estrogen is mediated by cerebral blood flow enhancement.3 (2) Estrogen suppresses soluble epoxide hydrolase (sEH) expression, enhancing the levels of epoxyeicosatrienoic acid (EET), which leads to increased blood flow during MCAO in female mice compared to male and ovariectomized female mice. Sex differences in blood flow disappear in sEH knockout mice (sEHKO) using in vivo quantitative optical microangiography,40 suggesting that EET is involved in the neuroprotective action of estrogen. (3) Estrogen also modulates blood-brain-barrier permeability and attenuates stimulation of BBB cotransporter activity, reducing edema formation during stroke.41 (4) In vitro, 24 hours pretreatment with 10 nM of 17β-estradiol significantly reduces cortical neuronal cell death induced by toxicity from glutamate,11 suggesting that estrogen protects primary cortical neurons from glutamate toxicity. (5) Another mechanism involved in the neuroprotective actions of estrogen is the release of growth factors such as glial-derived transforming growth factor-β (TGF-β), which promote neuronal survival.42
Studies have shown that estrogen increases cocaine- and amphetamine-regulated transcript (CART) mRNA and protein expression in cortical neuronal cultures after OGD; and estrogen also increases CART expression in ischemic cerebral cortex and reduces cortical infarct size after MCAO,43 suggesting that CART plays a role in neuroprotection. More recently, Mao et al reported that CART can directly interact with subunit B of the mitochondrial enzyme succinate dehydrogenase (SDHB) based on yeast two-hybrid system screening and in vitro pull-down assay.44 Interestingly, treatment with nanomolar (nM) concentrations of CART significantly increases SDH function, complex II activity, and ATP production in purified mitochondria and protects primary cultured cortical neurons at baseline and after OGD.44 Collectively, these results suggest that CART may have a mitochondrial protective function through directly interacting with SDH after cerebral ischemic injury.
Ovariectomized rats treated with 17β-estradiol have increased phosphorylation of the signal transducer and activator of transcription-3 (P-STAT3) and reduced infarct size after transient focal cerebral ischemia compared to vehicle-treated ovariectomized female rats. This protective effect of estradiol on infarct size is abolished in the presence of P-STAT3 inhibitor, suggesting that estrogen mediates protection against cerebral ischemic injury via P-STAT3. Furthermore, immunohistochemistry shows that P-STAT3 is expressed in cells staining positive for microtubule-associated protein 2 (MAP2) and Bcl-2, demonstrating that P-STAT3 is expressed in surviving neurons.45
3. Aromatase and neuroprotection
In addition to circulating estrogen, growing evidence indicates that local estrogen synthesized by aromatase also protects against cerebral ischemic injury. Aromatase, cytochrome P450 19 (P450 aromatase), is encoded by the cyp19 gene and has been implicated as beneficial in injured brain because it can synthesize protective estrogen from androgen precursors.46,47 P450 aromatase expression and activity have been detected in multiple brain regions of animals including the cerebral cortex. P450 aromatase expression has also been detected in the human cerebral cortex.48
Previously, the role of brain aromatase was believed to be restricted to regulating neuroendocrine events and behavior linked to reproduction. Recent findings have revealed a novel neuroprotective role for brain aromatase. Brain injury induces P450 aromatase expression in astrocytes, which may be neuroprotective.47 More recently, Liu et al first reported that female astrocytes sustain less cell death from OGD than do male astrocytes. This endogenous protection in female astrocytes is associated with greater basal P450 aromatase activity and higher P450 aromatase mRNA and protein expression relative to male astrocytes. Furthermore, the sex-dependent response to OGD disappears in male and female cells treated with a selective P450 aromatase inhibitor Arimidex.15 Interestingly, astrocyte-conditioned media from female cultures protects against OGD-induced cell death in male cells, whereas astrocyte-conditioned media from male cultures has no effect on OGD-induced cell death in female cells. However, the protection of male astrocytes is lost in media from female astrocytes treated with P450 aromatase inhibitor.15 These results strongly suggest that endogenous P450 aromatase and local estrogen synthesis protect astocytes following OGD.
These new and exciting observations of cytoprotection by aromatase are also confirmed in astrocyte cultures from WT mice and mice with targeted deletion of the P450 aromatase gene (ArKO). ArKO mice of both sexes have abnormal reproductive phenotypes with plasma estradiol levels below detection. Consistent with data generated in sex-specific rat astrocytes, cell death is significantly reduced in WT female mice astrocytes following OGD compared to WT male cells.16 It is important to note that this sex-dependent response to OGD is observed across species. We also demonstrated that cell death significantly increases in ArKO female astrocytes compared to WT female astrocytes, but not in ArKO male astrocytes vs. WT male cells; and sex differences in astrocyte cell death are not observed in ArKO cells.16 These findings demonstrate that P450 aromatase is expressed in astrocytes and confirm that aromatase is instrumental in mediating astrocyte survival following OGD. Therefore, P450 aromatase plays a critical role in endogenous neuroprotection in females. These data are consistent with our in vivo findings: brain injury is significantly increased in female ArKO mice vs. WT females after experimental stroke and in female WT mice chronically treated with fadrozole, a well known aromatase inhibitor, compared to female WT mice treated with vehicle. Furthermore, female ArKO mice sustain more brain damage than ovariectomized WT animals, demonstrating that extragonadal synthesis of estradiol is involved in neuroprotection.49 Taken together, these studies demonstrate that brain P450 aromatase is an important factor for regulating brain function under physiological as well as pathological conditions.
In summary, both in vivo and in vitro studies demonstrate that estrogen exerts powerful neuroprotective actions following ischemic brain injury. The role of other sex steroids in neuroprotection has also been studied. Below, we will review the evidence for neuroprotection by progesterone and androgens following ischemic brain injury.
4. Progesterone and neuroprotection
The relative “female protection” observed following experimental stroke has been largely attributed to protective effects of estrogen (see above). For this reason, progesterone has been understudied in the context of cerebral ischemia. Nonetheless, the past decade has provided evidence that progesterone is a strong neuroprotectant in various models of brain injury and disease.
4.1. Progesterone and neuroprotection – in vivo studies
To date, over 20 published studies have used animal models to examine the neuroprotective effects of progesterone against cerebral ischemic injury. Only a few of these studies report that progesterone does not significantly decrease damage (see table 3 for references). Interestingly, most studies have focused on male animals; however, progesterone has been shown to protect both male and female brain from damage following MCAO. The duration of the insult is typically 1 or 2 hours. Histological improvement (infarct size) was observed as late as 7 days post ischemia, and cognitive improvement was observed up to 21 days after MCAO. The level of protection varied depending on the duration of ischemia and the dose of progesterone, but typically ranged between 20% and 50% reduction in infarct size. Significant neuroprotection by progesterone has also been observed following global cerebral ischemia (see Table 5). Using a 4-vessel occlusion model (4-VO) in male rats, 8 mg/kg progesterone significantly reduced hippocampal damage 21 days after global ischemia.50 Similarly, progesterone completely prevented neuronal death in the caudate nucleus51 and significantly reduced neuronal death in the hippocampus52 following 15 minutes cardiac arrest in male cats. Our group observed that 8 mg/kg provided only modest protection to cerebellar Purkinje cells in male mice following cardiac arrest (unpublished observation), yet the progesterone metabolite allopregnanolone (8 mg/kg) significantly decreased Purkinje cell damage.53
Table 3.
Neuroprotection of Progesterone in vivo Studies.
| Model | Protection | Mechanisms/Results | Sex | Species | References |
|---|---|---|---|---|---|
| Transit MCAO | Yes | Reduces inflammation | Male | Rat | 54-57,62,64,65 |
| Reduces edema | Mouse | ||||
| Reduces NO synthase | |||||
| Transit MCAO | Yes | N.D. | Female, OVX | Rat | 6, 61 |
| Transit MCAO | NO | N.D. | Female, OVX | Rat | 63 |
| Permanent MCAO | Yes | Reduces edema | Male | Rat | 58, 65-70 |
| Reduces inflammation, Reduces iNOS | Mouse | ||||
| Global ischemia | Yes | N.D. | Male, Female, OVX | Cat, Rat | 50-52, 59 |
| Partial | Yes | Reduces inflammation, | Male | Mouse | 60 |
| Global Ischemia | Increases antioxidants | ||||
| (BCAO) |
Abbreviations: MCAO: middle cerebral artery occlusion; OVX: ovariectomized; NO: nitric oxide; iNOS: inducible nitric oxide synthase; N.D. not determined; BCAO: bilateral common carotid artery occlusion.
Table 5.
Neuroprotection of Androgen in vivo Studies.
| Model | Protection | Mechanisms/Results | Sex | Species | References |
|---|---|---|---|---|---|
| Transit MCAO | Yes (low doses) | Low doses of T and DHT protect | Male | Mouse | 87 |
| T and DHT | NO (high doses) | High doses exacerbate ischemic damage. | |||
| pre-injury treatment | |||||
| Transit MCAO | Yes | T removal decreases tissue damage | Male, | Rat | 83 |
| Remove of T before injury | as compared to animals with endogenous T | ||||
| Transit MCAO | NO | Positive correlation | Male | Rat | 84 |
| Plasma T and tissue damage | (castration with or without T repletion) | ||||
| Transit MCAO | NO | NO change in tissue damage, | Male | Rat | 86 |
| but improved behavioral | (castration with or without T repletion) | ||||
| Transit MCAO | NO | DHT increase damage | Male | Rat | 89 |
| (castration with or without T repletion) | |||||
| Transit MCAO | Yes | T and DHT reduces tissue damage | Male | Rat, Mouse | 88 |
| (Aged animals received T or DHT) | |||||
Abbreviations: AMPA: α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; Aβ: amyloid beta; DHT: dihydrotestosterone; NMDA: N-methyl-D-aspartic acid; T: testosterone.
Jiang et al first reported that a dose of 8 mg/kg progesterone, by intraperitoneal injection, significantly reduces infarct size; most subsequent studies have used that dose and route of administration.54 Significant neuroprotection was not observed with 4 mg/kg.54-56 However, Cai et al reported significant neuroprotection with 4 mg/kg progesterone following 1 hour MCAO,57 and Betz and Coester found that 2 mg/kg progesterone reduces brain edema following 4 hours MCAO.58 Neuroprotection has also been observed using higher doses of progesterone: 10 mg/kg with a 4-VO59 model and 15 mg/kg with a bilateral common carotid artery occlusion (BCAO) model.60 Interestingly, 20, 30, and 60 mg/kg doses of progesterone do not protect against MCAO and may even exacerbate infarct levels.61
Pre- and post-treatment regimens have been used to test the ability of progesterone to protect against ischemic damage. With the exception of a few studies that administered progesterone intravenously,50,55 subcutaneously,51,52 or with subcutaneous pellets,6 most studies used intraperitoneal injections. Pre-treatments have consisted of administering progesterone 7 days, 6,51,52,59,62 48 hours,57 1 hour, 57,58 or 30 minutes54,60,61,63 prior to the ischemic insult. Post-treatments have been initiated at reperfusion54,56,62,64,65 or at occlusion66 and 1 hour post-occlusion65,67-70 in permanent MCAO models. One study administered progesterone 20 minutes after reperfusion in a 4-VO model and achieved significant neuroprotection.50 The neuroprotective potential of progesterone in experimental ischemia is clear; however, further research is needed to optimize dose, timing, and route of administration. Regardless, these findings suggest great therapeutic potential for treating ischemic stroke with progesterone.
4.2 Progesterone and neuroprotection – in vitro studies
The protective efficacy of progesterone has been tested in a variety of in vitro models that mimic various damaging insults experienced following cerebral ischemia (Table 4). Specifically, progesterone protects primary cultured cortical neurons,41,71,72 hippocampal neurons,73 and cerebellar Purkinje cells74 from glutamate toxicity and in vitro ischemia (OGD).74 Progesterone can also protect primary astrocytes,66 the macrophage cell line RAW264.7,66 and the microglial cell line BV-275 from inflammatory models of ischemia in vitro.
Table 4.
Neuroprotection of Progesterone in vitro Studies.
| Cell Type | Conditions | Protection | Mechanisms/Results | Sex | Species | References |
|---|---|---|---|---|---|---|
| Neurons | OGD | Yes | Increases GABAA | Female/Male cells | Rat | 74 |
| Receptor activity | Mixed cell | |||||
| Neurons | Glutamate/ | Yes | Increases BDNF, | Mixed cell | Rat | 71-73 |
| NMDA, Toxicity | Increases BcI-2 | Mouse | ||||
| Astrocytes | IFNγ, IL-1β | Yes | Reduces iNOS | Mixed cell | Mouse | 66 |
| Macrophages | IFNγ, LPS | Yes | Reduces iNOS | Mixed cell | Mouse | 66 |
| Microglia | LPS | Yes | Reduces iNOS | Mixed cell | Mouse | 75 |
Abbreviations: OGD: oxygen glucose deprivation; GABAA receptor: γ-Aminobutyric acid A receptor; iNOS: inducible nitric oxide synthase; LPS: Lipopolysaccharides; BDNF: Brain-derived neurotrophic factor; NMDA: N-methyl-D-aspartic acid; IFNγ: Interferon-gamma; IL-1β: Interleukin-1.
4.3. Mechanisms of neuroprotection by progesterone
Decreases inflammation
An anti-inflammatory role for progesterone has been reported. In particular, progesterone has been observed to suppress the expression of various pro-inflammatory cytokines following cerebral ischemia, minimizing the ischemia-induced increase in IL-1β,65 TNF-α,60 and TGF-β.65 Additionally, progesterone can decrease the expression of inducible nitric oxide synthase, iNOS or NOS-2, in vivo 65,66 and in vitro. 66,75
Reduces oxidative stress
Progesterone can reduce the level of malondialdehyde, a marker of oxidative stress, and prevent ischemia-induced decreases in glutathione following 10 minutes 4-VO.59 Similarly, increased expression of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, and catalase have been reported following progesterone administration and bilateral common carotid artery occlusion (BCAO).60 As a result, progesterone attenuation of oxidative stress may contribute to decreased inflammation.
Decreases edema
Progesterone can decrease cerebral edema following MCAO58,65,68,70 and traumatic brain injury.76 Suppression of injury-induced inflammation by progesterone likely contributes to reduced cerebral edema formation. However, there may be other progesterone- mediated mechanisms of osmoregulation. For example, following traumatic brain injury, progesterone can regulate expression of a water-permeable channel, aquaporin-4, in a time- and region-specific manner.77
Other mechanisms
Various molecular targets and pathways have been proposed to underlie progesterone-mediated protection. For example, Kaur et al found that progesterone protection from glutamate toxicity is associated with increased levels of BDNF transcript and protein in cortical neurons using an organotypic slice model.72 Activation of mitogen-activated protein kinase (MAPK)71,72 and phosphoinositide-3 kinase (PI3K)72 are also associated with, and required for, progesterone-mediated neuroprotection against glutamate toxicity. In addition, Cai et al reported that the timing of progesterone administration alters the mechanism of protection.57 Specifically, progesterone receptor-mediated neuroprotection is the major contributor when progesterone is administered 48 hours prior to MCAO. In contrast, administration of progesterone 1 hour prior to insult results in progesterone receptor-independent protection, mediated via antagonizing the σ receptor, which inhibits the NMDA receptor thereby reducing the injury-induced rise in intracellular calcium.
Another mechanism for neuroprotection by progesterone is attributed to its metabolism to allopregnanolone, a strong neuroprotectant78 and potent modulator of GABAA receptors that can increase the inhibitory drive to offset excitotoxicty. In cerebellar Purkinje neurons, progesterone can protect against ischemic injury in a GABAA receptor- dependent manner; and importantly, protection is abolished by finasteride, an inhibitor of 5α-reductase that metabolizes progesterone to allopregnanolone.74 Ischemia can also cause a downregulation of GABAA receptors early in reperfusion that is prevented by allopregnanolone.53 Aside from acting to stimulate and stabilize GABAA receptors, there is evidence that allopregnanolone acts as a neuroprotectant by activating various effector pathways to decrease apoptotic and necrotic cell death.78
5. Androgens and neuroprotection
Male sex steroids have been much less studied than those of the female. This statement is surprising because male sex is a well-recognized stroke risk factor in humans. Stroke sensitivity, e.g., brain damage that occurs in response to a known ischemic insult, is also greater in male animals and male cultured cells. Whether these male characteristics are under the control of androgen availability remains highly unclear. For example, low circulating testosterone levels are associated with risk for stroke and worse outcome in stroke survivors.79-82 However, androgen levels are known to be stress-sensitive, so this evidence may reflect a generalized response to acute insult.
5.1 Androgens and neuroprotection – in vivo studies
In bench studies that control steroids, androgens have been reported to either protect or exacerbate ischemic damage (Table 5). In overview, animal data suggest that reduction of androgens via stress or castration improves histological damage after cerebral ischemia.83-85 On the other hand, exogenous androgens administered after experimental stroke accelerate functional recovery,86 an apparent inconsistency that we currently do not understand. We have recently shown that maintaining testosterone or dihydrotestosterone (DHT) plasma levels within the low physiological range during stroke confers protection to both adult castrates and gonadally intact aged rodents with naturally declining androgens. The protection is presumably mediated via androgen receptor (AR) mechanisms because improved tissue outcomes are reversed by administration of the AR antagonist flutamide.87,88 Using a similar hormone implantation technique, we also observed that high but physiologically relevant androgen levels exacerbate ischemic damage in castrated males,89 emphasizing that androgens have complex dose-dependent effects in vivo.
5.2. Androgens and neuroprotection – in vitro studies
As in the animal work, both beneficial and deleterious effects of androgens have been reported in neuronal and glial cultures exposed to injury conditions such as oxidative stress, excitotoxicity, serum deprivation, and amyloid β (Aβ) exposure (Table 6).90-92 In primary neurons, testosterone either protects against or exacerbates damage depending on concentration. For example, supra-physiological concentrations (10 μM) amplify glutamate-induced excitotoxic neuronal death, while protection is observed at 10 nM because testosterone is aromatized to 17β-estradiol.93 In other studies, testosterone and non-aromatizable DHT salvage injured neurons, suggesting direct protection rather than actions through aromatization.94-96 One interpretation of the apparent paradox of beneficial vs. detrimental effects of androgens is that two potentially competing signaling mechanisms are engaged in vitro. Such mechanisms remain to be elucidated.
Table 6.
Neuroprotection of Androgen in vitro Studies.
| Cell Type | Conditions | Protection | Mechanisms/Results | Sex | Species | References |
|---|---|---|---|---|---|---|
| HT22 Neurons | Glutamate exposure | NO | Exacerbates cell death | Mixed cell | Mouse | 83 |
| T pre-injury treatment | ||||||
| Oligodendrocytes | AMPA or kainite receptor | NO | Enhances excitotoxicity; | Mixed cell | Rat | 90 |
| T pre-injury treatment | Reverse by flutamide; | |||||
| But not by aromatase inhibiton | ||||||
| Neurons | NMDA exposure/ | Yes | Enhances excitotoxicity and | Mixed cell | Mouse | 93 |
| Astrocytes | T pre-injury treatment | protection, depending on T concentration. | ||||
| Cerebellar | H2O2 exposure/ | Yes | Protects against oxidant injury; | Mixed cell | Rat | 91 |
| granule cells | T pre-injury treatment | reverse by flutamide | ||||
| Neurons | Aβ exposure/T or DHT | Yes | Protect across 1-1000 nM range | Mixed cell | Rat | 95, 96 |
| Pre-injury treatment | ||||||
| Neurons | Serum deprivation/ | Yes | Reduces apoptosis after | Mixed cell | Human | 92 |
| T co-treatment | serum deprivation; reveres by flutamide. |
Abbreviations: AMPA: α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; Aβ: amyloid beta; DHT: dihydrotestosterone; NMDA: N-methyl-D-aspartic acid; T: testosterone; H2O2: Hydrogen peroxide.
5.3. Mechanisms of neuroprotection by androgens
ARs are expressed in neurons throughout the brain, including cortical and striatal regions impacted by our focal ischemia models.97 Therefore, it is possible that AR-regulated transcription is an important mechanism underlying androgen actions. We used microarray and real time PCR to identify gene candidates induced by non-aromatizable DHT and observed that high physiological doses enhance pro-inflammatory gene expression after ischemic injury. (For full list of genes, see reference 89.) Non-genomic, rapid signaling pathways also have been implicated in androgen neuroprotection in cerebral ischemia via MAPK/MEK and PI3K/AKT signaling, and CREB activation.96,98-100 Continued investigation of transcriptional and non-genomic signaling may provide important insights into how androgens impact evolving brain damage.
6. Conclusions
Significant sex differences in brain injury have been observed in animal models as well as in clinical and epidemiological studies. Since sex steroids play a critical role in brain function under physiological and pathological conditions, they represent the most salient factor related to sex differences in brain injury. Physiological levels of the female sex steroids 17β-estradiol and progesterone are neuroprotive both in vivo and in vitro. The role of male steroids in neuroprotection is less clear. More recently, sex differences in the response to ischemic injury have also been found in male and female cells. Therefore, further studies on the effects of sex steroids on brain injury will help us to better understand the cellular and molecular basis of sex differences in susceptibility to stroke injury. Elucidating sex-specific mechanisms of brain injury will advance the development of more effective neuroprotective therapies.
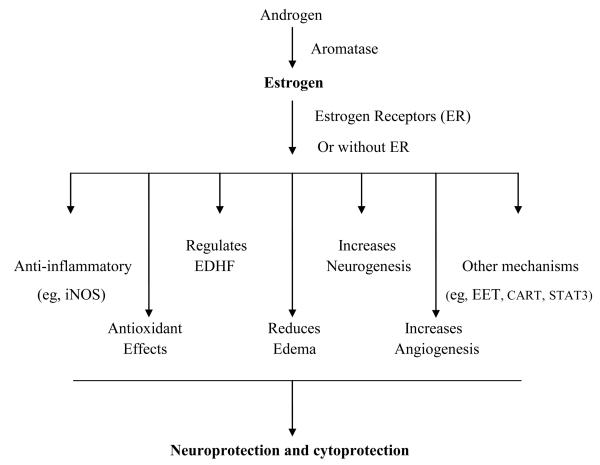
Figure 1.
Mechanisms of neuroprotection by estrogen in the brain.
Acknowledgments
Support for this research was provided by American Heart Association grant 0535284N and the Medical Research Foundation of Oregon and National Institutes of Health grants NR03521, NS49210 and NS058792. The authors thank Ms. Kathy Gage, grant and publications writer for the Department of Anesthesiology and Perioperative Medicine, for her editorial assistance in the preparation of this review.
References
- 1.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 2.Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr.Rev. 2006;27(6):575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- 3.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–166. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 4.Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J.Cereb.Blood Flow Metab. 2001;21(0271-678; 1):52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am.J.Physiol.Heart Circ.Physiol. 2000;278(0363-6135; 1):H290–H294. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- 6.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(0039-2499; 1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Dziennis S, Hurn PD, Alkayed NJ. Mechanisms of gender-linked ischemic brain injury. Restor.Neurol.Neurosci. 2009;27(3):163–179. doi: 10.3233/RNN-2009-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, Bernard O, Traystman RJ, Hurn PD. Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke. J.Neurosci. 2001;21(19):7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macrae IM, Carswell HV. Oestrogen and stroke: the potential for harm as well as benefit. Biochem.Soc.Trans. 2006;34(Pt 6):1362–1365. doi: 10.1042/BST0341362. [DOI] [PubMed] [Google Scholar]
- 10.Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. J.Neurosci. 2007;27(6):1422–1433. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sribnick EA, Ray SK, Nowak MW, Li L, Banik NL. 17beta-Estradiol Attenuates Glutamate-Induced Apoptosis and Preserves Electrophysiologic Function in Primary Cortical Neurons. J.Neurosci.Res. 2004;76(5):688–696. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- 12.Mor G, Nilsen J, Horvath T, Bechmann I, Brown S, Garcia-Segura LM, Naftolin F. Estrogen and microglia: A regulatory system that affects the brain. J.Neurobiol. 1999;40(0022-3034; 4):484–496. doi: 10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross KJ. 17beta-Estradiol Protects Oligodendrocytes from Cytotoxicity Induced Cell Death. J.Neurochem. 2004;89(3):660–673. doi: 10.1111/j.1471-4159.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerstner B, Lee J, DeSilva TM, Jensen FE, Volpe JJ, Rosenberg PA. 17beta-Estradiol Protects Against Hypoxic/ischemic White Matter Damage in the Neonatal Rat Brain. J.Neurosci.Res. 2009;87(9):2078–2086. doi: 10.1002/jnr.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J.Cereb.Blood Flow Metab. 2007;27(1):135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Oyarzabal EA, Yang R, Murphy SJ, Hurn PD. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. J.Neurosci.Methods. 2008;171(2):214–217. doi: 10.1016/j.jneumeth.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo J, Krause DN, Horne J, Weiss JH, Li X, Duckles SP. Estrogen-receptor-mediated protection of cerebral endothelial cell viability and mitochondrial function after ischemic insult in vitro. J.Cereb.Blood Flow Metab. 2010;30(3):545–554. doi: 10.1038/jcbfm.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J.Cereb.Blood Flow Metab. 2000;20(1):112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc.Natl.Acad.Sci.U.S.A. 2001;98(4):1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol.Cell.Endocrinol. 2007;265-266:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29(2):199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- 22.Singer CA, Rogers KL, Dorsa DM. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9(11):2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- 23.Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol.Pharmacol. 2005;67(1):105–113. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- 24.Brown CM, Dela Cruz CD, Yang E, Wise PM. Inducible nitric oxide synthase and estradiol exhibit complementary neuroprotective roles after ischemic brain injury. Exp.Neurol. 2008;210(2):782–787. doi: 10.1016/j.expneurol.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front.Neuroendocrinol. 2008;29(4):507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J.Neuroimmunol. 2008;195(1-2):47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem.Biophys.Res.Commun. 1995;214(3):847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- 28.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J.Cereb.Blood Flow Metab. 2005;25(4):502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 29.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Ann.Med. 2007;39(7):495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- 30.Liu MY, Hattori Y, Fukao M, Sato A, Sakuma I, Kanno M. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br.J.Pharmacol. 2001;132(5):1035–1046. doi: 10.1038/sj.bjp.0703899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakuma I, Liu MY, Sato A, Hayashi T, Iguchi A, Kitabatake A, Hattori Y. Endothelium-dependent hyperpolarization and relaxation in mesenteric arteries of middle-aged rats: influence of oestrogen. Br.J.Pharmacol. 2002;135(1):48–54. doi: 10.1038/sj.bjp.0704444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu MY, Hattori Y, Sato A, Ichikawa R, Zhang XH, Sakuma I. Ovariectomy attenuates hyperpolarization and relaxation mediated by endothelium-derived hyperpolarizing factor in female rat mesenteric artery: a concomitant decrease in connexin-43 expression. J.Cardiovasc.Pharmacol. 2002;40(0160-2446; 6):938–948. doi: 10.1097/00005344-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Marrelli SP. Altered endothelial Ca2+ regulation after ischemia/reperfusion produces potentiated endothelium-derived hyperpolarizing factor-mediated dilations. Stroke. 2002;33(9):2285–2291. doi: 10.1161/01.str.0000027439.61501.39. [DOI] [PubMed] [Google Scholar]
- 34.Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res.Rev. 2008;57(2):421–430. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azevedo RB, Lacava ZG, Miyasaka CK, Chaves SB, Curi R. Regulation of antioxidant enzyme activities in male and female rat macrophages by sex steroids. Braz.J.Med.Biol.Res. 2001;34(5):683–687. doi: 10.1590/s0100-879x2001000500018. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J.Comp.Neurol. 2007;500(6):1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- 38.Jesmin S, Hattori Y, Sakuma I, Liu MY, Mowa CN, Kitabatake A. Estrogen deprivation and replacement modulate cerebral capillary density with vascular expression of angiogenic molecules in middle-aged female rats. J.Cereb.Blood Flow Metab. 2003;23(2):181–189. doi: 10.1097/01.WCB.0000043341.09081.37. [DOI] [PubMed] [Google Scholar]
- 39.Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke. 2005;36(2):337–341. doi: 10.1161/01.STR.0000153795.38388.72. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, Alkayed NJ. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. J.Cereb.Blood Flow Metab. 2009;29(8):1475–1481. doi: 10.1038/jcbfm.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J.Cereb.Blood Flow Metab. 2006;26(10):1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 42.Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146(0013-7227; 6):2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, Hurn PD, Martinez-Murillo F, Alkayed NJ. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc.Natl.Acad.Sci.U.S.A. 2006;103(39):14489–14494. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao P, Ardeshiri A, Jacks R, Yang S, Hurn PD, Alkayed NJ. Mitochondrial mechanism of neuroprotection by CART. Eur.J.Neurosci. 2007;26(3):624–632. doi: 10.1111/j.1460-9568.2007.05691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dziennis S, Jia T, Ronnekleiv OK, Hurn PD, Alkayed NJ. Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J.Neurosci. 2007;27(27):7268–7274. doi: 10.1523/JNEUROSCI.1558-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M, Hurn PD, Alkayed NJ. Cytochrome P450 in neurological disease. Curr.Drug Metab. 2004;5(3):225–234. doi: 10.2174/1389200043335540. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog.Neurobiol. 2003;71(0301-0082; 1):31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138(2):389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 49.McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J.Neurosci. 2003;23(25):8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morali G, Letechipia-Vallejo G, Lopez-Loeza E, Montes P, Hernandez-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci.Lett. 2005;382(3):286–290. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 51.Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch.Med.Res. 2002;33(1):6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Vidal MD, Cervera-Gaviria M, Ruelas R, Escobar A, Morali G, Cervantes M. Progesterone: protective effects on the cat hippocampal neuronal damage due to acute global cerebral ischemia. Arch.Med.Res. 1998;29(2):117–124. [PubMed] [Google Scholar]
- 53.Kelley MH, Taguchi N, Ardeshiri A, Kuroiwa M, Hurn PD, Traystman RJ, Herson PS. Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and allopregnanolone neuroprotection is associated with GABAA receptor stabilization. J.Neurochem. 2008;107(3):668–678. doi: 10.1111/j.1471-4159.2008.05617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735(1):101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Chopp M, Li Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J.Neurol.Sci. 1999;171(0022-510; 1):24–30. doi: 10.1016/s0022-510x(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 56.Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J.Neurosurg. 2000;92(0022-3085; 5):848–852. doi: 10.3171/jns.2000.92.5.0848. [DOI] [PubMed] [Google Scholar]
- 57.Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. 2008;55(2):127–138. doi: 10.1016/j.neuropharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 58.Betz AL, Coester HC. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990;21(8):1199–1204. doi: 10.1161/01.str.21.8.1199. [DOI] [PubMed] [Google Scholar]
- 59.Ozacmak VH, Sayan H. The effects of 17beta estradiol, 17alpha estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol.Res. 2009;58(6):909–912. doi: 10.33549/physiolres.931647. [DOI] [PubMed] [Google Scholar]
- 60.Aggarwal R, Medhi B, Pathak A, Dhawan V, Chakrabarti A. Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. J.Pharm.Pharmacol. 2008;60(6):731–737. doi: 10.1211/jpp.60.6.0008. [DOI] [PubMed] [Google Scholar]
- 61.Murphy SJ, Littleton-Kearney MT, Hurn PD. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J.Cereb.Blood Flow Metab. 2002;22(10):1181–1188. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- 62.Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J.Cereb.Blood Flow Metab. 2004;24(0271-678; 7):805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- 63.Murphy SJ, Traystman RJ, Hurn PD, Duckles SP. Progesterone exacerbates striatal stroke injury in progesterone-deficient female animals. Stroke. 2000;31(0039-2499; 5):1173–1178. doi: 10.1161/01.str.31.5.1173. [DOI] [PubMed] [Google Scholar]
- 64.Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann.Emerg.Med. 2006;47(4):381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp.Neurol. 2005;193(2):522–530. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Coughlan T, Gibson C, Murphy S. Modulatory effects of progesterone on inducible nitric oxide synthase expression in vivo and in vitro. J.Neurochem. 2005;93(4):932–942. doi: 10.1111/j.1471-4159.2005.03068.x. [DOI] [PubMed] [Google Scholar]
- 67.Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, Jiang C, Liu C, Li X, Chen N, Hao Y. Neuroprotective effects of progesterone following stroke in aged rats. Behav.Brain Res. 2010;209(1):119–122. doi: 10.1016/j.bbr.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 69.Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor.Neurol.Neurosci. 2007;25(2):151–159. [PubMed] [Google Scholar]
- 70.Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm.Res. 2009;58(9):619–624. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 71.Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Mol.Med. 2009;15(9-10):328–336. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J.Neurosci.Res. 2007;85(11):2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lockhart EM, Warner DS, Pearlstein RD, Penning DH, Mehrabani S, Boustany RM. Allopregnanolone attenuates N-methyl-D-aspartate-induced excitotoxicity and apoptosis in the human NT2 cell line in culture. Neurosci.Lett. 2002;328(1):33–36. doi: 10.1016/s0304-3940(02)00448-2. [DOI] [PubMed] [Google Scholar]
- 74.Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS. Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. Eur.J.Neurosci. 2006;24(9):2567–2574. doi: 10.1111/j.1460-9568.2006.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muller E, Kerschbaum HH. Progesterone and its metabolites 5-dihydroprogesterone and 5-3-tetrahydroprogesterone decrease LPS-induced NO release in the murine microglial cell line, BV-2. Neuro Endocrinol.Lett. 2006;27(5):675–678. [PubMed] [Google Scholar]
- 76.Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607(0006-8993; 1-2):333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- 77.Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp.Neurol. 2006;198(2):469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 78.Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin.Reprod.Med. 2009;27(3):229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, Flicker L, Hankey GJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J.Clin.Endocrinol.Metab. 2009;94(7):2353–2359. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- 80.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J.Neurol.Neurosurg.Psychiatry. 2003;74(3):317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeppesen LL, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler.Thromb.Vasc.Biol. 1996;16(6):749–754. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- 82.Dash RJ, Sethi BK, Nalini K, Singh S. Circulating testosterone in pure motor stroke. Funct.Neurol. 1991;6(1):29–34. [PubMed] [Google Scholar]
- 83.Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J.Appl.Physiol. 2002;92(1):195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- 84.Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796(1-2):296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- 85.Yang SH, Liu R, Wen Y, Perez E, Cutright J, Brun-Zinkernagel AM, Singh M, Day AL, Simpkins JW. Neuroendocrine mechanism for tolerance to cerebral ischemia-reperfusion injury in male rats. J.Neurobiol. 2005;62(3):341–351. doi: 10.1002/neu.20103. [DOI] [PubMed] [Google Scholar]
- 86.Pan Y, Zhang H, Acharya AB, Patrick PH, Oliver D, Morley JE. Effect of testosterone on functional recovery in a castrate male rat stroke model. Brain Res. 2005;1043(1-2):195–204. doi: 10.1016/j.brainres.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 87.Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J.Cereb.Blood Flow Metab. 2009;29(8):1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng J, Hu W, Toung TJ, Zhang Z, Parker SM, Roselli CE, Hurn PD. Age-dependent effects of testosterone in experimental stroke. J.Cereb.Blood Flow Metab. 2009;29(3):486–494. doi: 10.1038/jcbfm.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J.Cereb.Blood Flow Metab. 2007;27(9):1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caruso A, Di Giorgi Gerevini V, Castiglione M, Marinelli F, Tomassini V, Pozzilli C, Caricasole A, Bruno V, Caciagli F, Moretti A, Nicoletti F, Melchiorri D. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J.Neurochem. 2004;88(5):1179–1185. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 91.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892(2):255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J.Neurosci. 2004;24(23):5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orlando R, Caruso A, Molinaro G, Motolese M, Matrisciano F, Togna G, Melchiorri D, Nicoletti F, Bruno V. Nanomolar concentrations of anabolic-androgenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res. 2007;1165:21–29. doi: 10.1016/j.brainres.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 94.Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919(0006-8993; 1):160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen TV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148(6):2936–2943. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J.Neurochem. 2005;94(6):1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 97.DonCarlos LL, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer C, Garcia-Segura LM. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience. 2006;138(3):801–807. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 98.Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J.Clin.Invest. 2003;111(11):1651–1664. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64(19):7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 100.Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm.Behav. 2008;53(5):693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]