Abstract
Aim
Resistance exercise performed at low loads (20-30% of maximal strength) with blood flow restriction (BFR) acutely increases protein synthesis and induces hypertrophy when performed chronically. We investigated myogenic and proteolytic mRNA expression 8 hrs following an acute bout of knee extension exercise
Methods
Fifteen subjects (22.8 ± 3.7 yrs, 8 men and 7 women) were randomized to two exercise conditions: BFR or control exercise. All participants performed 4 sets of exercise (30, 15, 15 & 15 repetitions) at 20% of maximal strength. Persons in the BFR group had a cuff placed on the upper thigh inflated to 1.5 times brachial systolic blood pressure (cuff pressure range: 135-186 mmHg). Muscle biopsies from the vastus lateralis were excised 24 hrs before and 8 hrs following the exercise.
Results
RT-PCR analysis demonstrated no change in myogenic gene expression (IGF-1, MyoD, Myogenin, Myostatin – a negative regulator) with either exercise condition (p > 0.123). However, BFR exercise downregulated mRNA expression in transcripts associated with proteolytic pathways (FOXO3A, Atrogin-1 and MuRF-1) with no change in the control exercise condition. Specifically, median mRNA expression of FOXO3A decreased by 1.92 fold (p = 0.01), Atrogin-1 by 2.10 fold (p = 0.01) and MuRF-1 by 2.44 fold (p = 0.01).
Conclusion
These data are consistent with the downregulation of proteolytic transcripts observed following high load resistance exercise. In summary, myogenic genes are unchanged and proteolytic genes associated with muscle remodeling are reduced 8 hours following low load BFR exercise.
Keywords: KAATSU, Blood flow restriction, gene expression, mRNA, muscle
INTRODUCTION
Muscle hypertrophy is effectively achieved through performance of resistance exercise with loads exceeding 60% of maximal strength (Kraemer et al., 2002). The mechanisms governing this response are complex translations of mechanical signals that lead to enhanced protein synthesis and ultimately to muscle hypertrophy (Tidball, 2005). Over the past decade, research has demonstrated that exercise performed at low loads (i.e. < 50% of maximal strength) with blood flow restriction (BFR) is effective at increasing muscle hypertrophy to a similar extent as high load paradigms (For review please see (Manini et al., 2009)). This observation suggests that low mechanical load BFR exercise may be an effective means of therapy for patients with conditions that require rehabilitation, but who cannot perform intense, high mechanical load exercise. Such conditions might include joint, tendon or ligament injury, arthroplastic reconstructive surgery and a variety of neurological conditions (stroke, Parkinson's Disease, Central Palsy). However, the potential physiological mechanisms that explain this response remain unclear.
The gene regulators responsible for the hypertrophic response of low load BFR resistance exercise might be learned from findings on high load resistance exercise, which has been more clearly delineated. Genes such as myogenic differentiation (MyoD), myogenin, myostain and insulin-like growth factor (IGF-1) are elevated following a bout of resistance exercise beginning 2 hours and peaking at 8 hours post exercise (Kim et al., 2005, Yang et al., 2005). The delayed gene response is thought to play a role in the cells’ stimulus for growth (Bamman et al., 2007). High load resistance exercise also causes a potent catabolic response that is thought to rid the cell of damaged cytoskeletal proteins through the ubiquitin-proteosome system (Louis et al., 2007, Mascher et al., 2008, Yang et al., 2006). This selective proteolytic pathway following resistance exercise is a key process for cell remodeling and adaptation (Louis et al., 2007). MuRF-1 and Atrogin-1 are E3 ligases that regulate the ubiquitin proteosome pathway and are elevated for up to 4 hrs following resistance exercise. However, the expression level completely reverses direction at 8 and 12 hrs post-exercise (Louis et al., 2007). Additionally, the FOXO family of genes target Atrogin-1 and MuRF-1, and partially control their function (Sandri et al., 2004, Waddell et al., 2008). Overall, high load resistance exercise is a potent stimulus for myogenic and proteolytic gene expression, but they have complex and variable temporal patterns following the bout of exercise (Louis et al., 2007, Yang et al., 2005).
Little is known regarding the muscle responses to low load BFR exercise. Recently, Fujita et al. reported that protein synthesis increased in young adults 3 hrs following an acute bout of BFR knee extension exercise performed at 20% of maximal strength (Fujita et al., 2007). Additionally, ribosomal S6-Kinase-1, a key regulatory protein in the mTOR pathway, was phosphorylated 3 hrs post-exercise suggesting activation of this pathway (Fujita et al., 2007). Drummond and colleagues demonstrated that mRNA expression for genes related to myogenesis (IGF-1 receptor, mechano-growth factor, myogenin, and myostatin, which is a negative regulator) and proteolysis (MuRF1 and MAFbx/Atrogin-1) 3 hrs after performing BFR knee extension exercise at 20% of maximal strength was no different than exercise without BFR at the same loading paradigm (Drummond et al., 2008). As demonstrated with high load resistance exercise, several genes are modulated later than 3 hours – many demonstrating a significant modulation 8-10 hours following the bout of exercise. However, no studies have examined these effects of low load BFR resistance exercise greater than 3 hours post-exercise. Therefore, previous research might have overlooked important changes that occur outside a 3-hour window. Such information would provide for a better understanding of the hypertrophic responses to low load BFR exercise.
The purpose of this study was to determine mRNA expression of genes within the protein synthesis and degradation pathways at 8 hrs following an acute bout of low-load resistance exercise with and without BFR. We hypothesized based on the purported effects on muscle hypertrophy with BFR exercise that mRNA expression would mimic the profile of an acute bout of high-load resistance exercise with an elevation of myogenic and a decrease in proteolytic gene expression 8 hrs post-exercise (Louis et al., 2007).
MATERIALS AND METHODS
Subjects
Fifteen men and women aged 20 to 40 years were recruited for the study (8 men and 7 women; mean age: 22.8 ± 3.7 yrs). Women taking oral contraceptives were excluded from the study. Each subject was screened for physical activity level, smoking status, medication usage, anthropometry and vital signs. Non-smoking individuals who were not actively performing resistance or aerobic exercise (recreational activity was permitted) for the past 6 months were eligible to participate. For enrollment, subjects also needed to have a body mass index < 30 kg/m2 with a resting heart rate < 90 beats per minute and a resting blood pressure < 140/90 mmHg. Subjects were informed of the study procedures and gave written consent to participate in the study. The Institutional Review Board at the University of Florida approved all study procedures. Subjects were compensated $200 for the completion of the protocol.
Experimental design
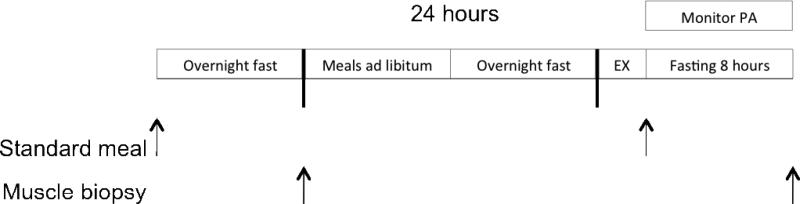
Subjects were randomized to either a BFR exercise group or control group who performed exercise without BFR. Subjects were randomized using a blocked design by sex to ensure men and women were distributed similarly within each group. Eligibility screening was performed at approximately 1 week prior to the start of the experiment at which point participants were asked to refrain from structured physical activity during their enrollment. Experimental procedures followed the schematic in Figure 1 where subjects performed study procedures over approximately 2 days and the muscle biopsies were spaced ≥ 8 hrs to the last feeding. To control for the effects of diet and timing of muscle biopsies and blood draws were scheduled following overnight fasts (8-10 hrs). In addition, subjects were provided a standardized isocaloric meal that was consumed the evening before the procedure (drinking water was permitted). Muscle biopsies were performed between 7 and 8 AM on the left vastus lateralis muscle. Subjects returned approximately 24 hrs later following another overnight fast to perform either BFR exercise or control exercise (exercise protocols described below). Thirty minutes following the exercise bout, subjects consumed a standardized isocaloric meal and then fasted for the next 7.5 hrs. Subjects were permitted to return to their usual daily activities following the exercise bout, but were asked to refrain from physical activities. During this period physical activity levels were monitored with an accelerometer placed on the hip. The number of steps taken were recorded and compared between groups. Subjects returned to the lab for a second biopsy that was performed on the right vastus lateralis muscle. Women were tested during the mid-follicular phase of their menstrual cycle to control for potential hormonal effects on mRNA expression. Scheduling was determined through conversations regarding the timing of their last menses and testing was conducted within a 3-12 day window following the cessation of menses.
Figure 1.
Schematic of experimental design. (EX = exercise condition and PA = physical activity).
Exercise protocols and diet
The contraction intensity and work performed in the exercise protocols were chosen based on several findings of enhanced muscle adaptations during low intensity resistance exercise with BFR. We therefore sought to understand the myogenic and proteolytic regulators to this response and compare results in gene expression with previous reports (Drummond et al., 2008).
During the screening visit, subjects were evaluated for maximal bilateral knee extension strength by the 1-repetition max (1-RM) method as described previously (Ploutz-Snyder et al., 2001). Group assignment was randomly administered and protocols were designed to equate the amount of muscle work performed in each group. Subjects in both conditions performed 4 sets of bilateral knee extension exercise at the 20% of 1-RM. Each subject performed 30 repetitions in the first set and 15 repetitions in the subsequent 3 sets. Thirty sec of rest was given between each set. Subjects randomized to the BFR group performed knee extension exercise with a narrow blood pressure cuff (11 cm wide) inflated around their upper thigh. The cuff was inflated to 1.5 times brachial systolic blood pressure (range: 135-186 mmHg) that was assessed prior to the start of the exercise. The cuff was inflated approximately 1-2 minutes prior to the start of exercise and remained inflated for the duration of the exercise and rest periods lasting approximately 8 minutes. Leg volume – measured using an anthropometric method (Katch et al., 1975) - partially dictates the degree of ischemic response due to the cuff pressures (Shaw et al., 1982) and was used to compare randomized groups.
Standardized meals were provided prior to each fasting condition to control for potential diet induced changes on gene expression. These meals contained 46% carbohydrate, 28% fat and 26% protein (Ensure Plus, Abbott Laboratories, Abbott Park Illinois). The caloric content of the meals was adjusted for estimated total energy expenditure using 2001 Food and Agriculture Organization, World Health Organization, and United Nations University (FAO/WHO/UNU) reference to human energy requirements (FAO/WHO/UNU, 2001). These guidelines determine total energy expenditure using the age-appropriate basal metabolic equations and adjustments for physical activity. Because subjects were considered sedentary during the eligibility screening, we multiplied the basal metabolic rate by 1.69 times to estimate total energy expenditure. This physical activity level is considered the upper tail of the category of “sedentary and light activity lifestyle” in the FAO/WHO/UNU guidelines.
Electromyographic activity
Muscle activity, assessed by electromyogram (EMG), was used to characterize the intensity of muscle contractions during each type of exercise. These measurements were included to also help explain gene expression responses. During the exercise session, EMG was recorded from the right vastus lateralis with surface electromyography as we have previously described (Clark et al., 2004). In brief, EMG signals were recorded using bipolar surface electrodes (Ag-AgCl) with an inter-electrode distance of 25 mm. The electrodes were placed parallel to the muscle fibers on the lateral and distal aspect of the vastus lateralis according to recommendations from Cram and Kasman (Cram et al., 1998). A reference electrode was placed on the patella. EMG signals were amplified 500 times, band-pass filtered between 10 and 500 Hz, and sampled at 1000 Hz (MP150 BioPac Systems Inc., Goleta, CA). EMG signals were postprocessed by taking the root-mean-squared value for every contiguous 50 ms epoch. Each repetition was evaluated for the maximal EMG amplitude over 50 ms and these amplitudes were averaged over the entire set. This value was normalized to the highest EMG amplitude found during a maximal voluntary isometric contraction performed immediately before the exercise bout. For the isometric contraction, each subject was asked to extend their knees at a maximal effort against the padded bar located at the ankles while a lock was applied to the movement arm. Subjects held the contraction for 5 sec while strong verbal encouragement was provided. Each subject performed three maximal efforts with 30 sec rest between each contraction. The effort that yielded the highest EMG amplitude was used to normalize the average EMG signal measured during each set of knee extension exercise. Equipment malfunction permitted data analysis of only 5 subjects (2 women and 3 men) in the BFR exercise and 6 subjects (3 women and 3 men) in the control exercise group.
Muscle biopsy
Two percutaneous needle biopsies were performed under local anesthesia on the vastus lateralis. Muscle was extracted from the left leg before the exercise bout and from the right leg 8 hrs following the exercise bout. The choice of biopsy sampling was determined using gene profiling studies where resistance exercise caused an upregulation in several myogenic genes and downregulation in proteolytic genes (Louis et al., 2007, Yang et al., 2005, Yang et al., 2006). Muscle samples were blotted dry of blood, weighed and dissected free of visible connective and adipose tissue. An aliquot of muscle tissue was placed in a cryovial with 0.50 ml of RNAlater (Ambion, Austin, TX), cooled in liquid nitrogen and stored at -80°C until RNA extraction.
RNA extraction and cDNA synthesis
Total RNA was isolated with TriReagent (Sigma-Aldrich). Briefly, 20-40 mg of tissue were homogenized in 1 mL of TriReagent using a glass on glass motorized mortar and pestle. The homogenate was cleared by centrifugation, and the RNA isolated from the supernatant according to the manufacturer's instructions. Total RNA was dissolved in nuclease-free water and quantified spectrophotometrically. Any contaminating DNA was removed via DNase digestion using the TURBO DNA-free kit from Ambion (Austin, TX). RNA quality was evaluated using the 2100 Nano Labchip Kit on an Agilent 2100 Bioanalyzer (Agilent Technologies Inc, Santa Clara, CA). Synthesis of cDNA was achieved from 2 μg of RNA using the High capacity cDNA reverse transcription kit (ABI, Foster City, CA). Briefly, 2 μL of 10X Buffer, 0.8 μL of 100 mM dNTP's, 2 μL of 10X random hexamers, 1 μL of 100 mM dNTP mix, 1 μL of 50 units/μL reverse transcriptase, 1 μL of 20 units/μL of RNase inhibitor, and nuclease-free water were mixed and added to total RNA. Samples were then incubated at 25°C for 10 min, followed by 37°C for 120 min. Enzyme activity was terminated by heating to 85°C for 5 min.
PCR and Primers
Primers were designed using the Applied Biosystems Primer Express 3.0 software (ABI, Foster City, CA). Q-PCR analysis was performed using the Power SYBR® Green PCR Master Mix (ABI, Warrington, UK), 0.2 nM primers and nuclease-free water in a 25 μL reaction. Relative expression was determined using the ABI 7500 real-time PCR system (ABI, Foster City, CA) with Universal cycling conditions (enzyme activation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, and anneal/extend at 60°C for 1 min). All samples were examined in triplicate and melt curves performed to verify primer specificity.
The mRNA expression of each gene investigated was normalized to 18S mRNA using the 2-ΔCt at each time point, where ΔCt = (Ctgene – Ctcontrol gene)time point is the relative expression for each individual at a specific time point. To confirm that the internal control was unchanged with the exercise the 2-ΔCt was calculated with (Ctcontrol gene post – Ctcontrol gene pre). The calculation should yield a 2-ΔCt that is close to zero (e.g. 20 = 1) if the exercise did not affect the internal control gene. The median and 95% confidence interval (CI) 2-ΔCt of 18S from pre to post was 1.11 (95% CI: 0.59-1.37) and thus was unaffected by the acute bout of exercise. The values of the median and 95% CI fold change were examined at baseline to evaluate the experimental variation using 2-ΔCt where Ct = (Ctgene – Ctcontrolgene)baseline. The average fold change at baseline across the genes should fall close to the value of 1.0 if errors among the samples were low. We found a median fold change of 1.04 (95% CI: 0.87-1.20) ensuring the quality of the experimental methods.
Statistical analysis
Subject characteristics were compared using two-sample t-tests. Normalized EMG values were compared across groups over the 4 sets of exercise using a repeated measures ANOVA with between subject (group) and within subjects factors (exercise set). For the primary outcomes of the study, the change in relative mRNA expression from pre to post exercise was examined. Relative mRNA expression values were non-normally distributed and attempts to transform the data were unsuccessful. Therefore, the Wilcoxon signed-rank test was used within groups to identify differences in pre- vs. post-exercise changes for both groups. Statistical significance was set at an alpha level of 0.05.
RESULTS
Exercise group characteristics and muscle activity
No differences were observed in age, body mass index, maximal knee extension strength or leg volume between randomized groups (see Table 1). All subjects completed the number of repetitions instructed and followed the dietary requirements outlined above.
Table 1.
Demographic and anthropometric characteristics of randomized groups.
| Randomized group | Blood flow restricted exercise (n = 8) | Control exercise (n = 7) | p-value |
|---|---|---|---|
| Age, years | 23.5 (4.8) | 22.0 (2.1) | 0.459 |
| Women, no (%) | 4 (50%) | 3 (43%) | 0.782 |
| Body mass index* | 24.5 (2.9) | 24.2 (3.5) | 0.891 |
| Maximal knee extension strength (kg) | 91.1 (34.8) | 99.6 (40.4) | 0.666 |
| 20% of maximal knee extension strength | 18.2 (6.9) | 19.9 (8.1) | 0.666 |
| Leg volume (cm3) | 6721 (901) | 8438 (2449) | 0.087 |
| Number of steps taken between exercise session and second biopsy† | 3010 (3069) | 2905 (2403) | 0.943 |
Values are means (standard deviation) unless noted otherwise
Body mass index = body mass in kilograms/(height in meters)2
An accelerometer was placed on the hip (Actigraph GT1M, Pensacola, FL) to monitor physical activity following the exercise session.
Electromyographic activity
The average normalized vastus lateralis EMG activity was similar between the two groups at the first set of exercise (BFR: 47.6 ± 13.6% of maximum vs. Control: 53.5 ± 13.5% of maximum, p = 0.494) (Figure 2). However, there was a significant increase in EMG activity with additional sets in the BFR exercise group (group by set effect: p = 0.007). Post-hoc analyses revealed a main exercise set effect where knee extension exercise increased EMG activity from 47.6 ± 13.6% (1st set) to 66.8 ± 18.9% (4th set) (p = 0.002) of maximal voluntary contraction in the BFR exercise group with no change in the control exercise group (53.5 ± 13.5% (1st set) to 58.9 ± 13.7% (4th set), p =0.084). Further analyses showed the BFR exercise group had higher EMG levels at the 4th set than the control group (66.8 ± 18.9% vs. 58.9 ± 13.7%, p < 0.05).
Figure 2.
Normalized electromyographic activity changes over subsequent sets of exercise for randomized experimental groups. Subjects performing blood flow restricted (BFR) knee extension exercise showed an increase in normalized electromyographic activity with additional sets of exercise (p = 0.002). No change was observed in the control group (p = 0.084). Note: asterisk (*) group difference at set 4. Note: electromyographic data were normalized to a maximal voluntary contraction prior to the exercise bout. Values are means (SEM).
Exercise-induced change in mRNA expression
mRNA expression was determined from biopsy samples taken before and 8 hrs following exercise. Expression of myogenic transcripts was not significantly altered with either type of exercise as listed in Table 2. Three of the four proteolytic transcripts assessed were preferentially downregulated in all participants undergoing BFR exercise (FOXO3A, Atrogin-1 and MuRF-1, but not Caspase-3; Figure 3). Median mRNA expression decreased in FOXO3A, Atrogin-1 and MuRF-1 (All changes p = 0.01). The control exercise group showed no significant changes in any of the proteolytic gene transcripts. Change values illustrated in Figure 3 show that all subjects randomized to the BFR group had decreased expression of FOXO3A, Atrogin-1 and MuRF-1, but only 3 out of the 7 subjects demonstrated a decline in the control exercise group.
Table 2.
Median mRNA levels of myogenic and proteolytic skeletal muscle transcripts before and after control and blood flow restricted exercise.
| Randomized group | Blood flow restricted exercise (n = 8) | Control exercise (n = 7) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | p-value | Pre | Post | p-value | |
| Transcripts associated with myogenesis | ||||||
| Insulin-like growth factor (IGF-1) | 0.095 (0.068-0.174) | 0.072 (0.047-0.093) | 0.093 | 0.075 (0.047-0.097) | 0.048 (0.029-0.120) | 0.866 |
| MyoD | 0.265 (0.189-0.498) | 0.240 (0.165-0.405) | 0.779 | 0.216 (0.139-0.301) | 0.290 (0.125-0.527) | 0.310 |
| Myogenin | 0.528 (0.399-0.787) | 0.559 (0.365-0.970) | 0.674 | 0.287 (0.204-0.463) | 0.369 (0.277-1.56) | 0.237 |
| Myostatin (negative regulator) | 0.049 (0.029-0.119) | 0.027 (0.016-0.058) | 0.123 | 0.039 (0.031-0.138) | 0.026 (0.011-0.037) | 0.237 |
| Transcripts association with proteolysis | ||||||
| FOXO3A | 0.115 (0.08-0.159) | 0.043 (0.023-0.061) | 0.011 | 0.107 (0.032-0.144) | 0.051 (0.015-0.116) | 0.735 |
| Atrogin-1 | 5.08 (3.80-6.45) | 1.79 (0.79-2.41) | 0.011 | 1.82 (1.04-2.68) | 1.89 (1.04-2.68) | 0.735 |
| MuRF-1 | 3.20 (2.15-4.11) | 1.58 (0.920-1.79) | 0.011 | 2.34 (1.18-2.64) | 1.38 (0.647-3.61) | 0.865 |
| Caspase-3 | 0.036 (0.029-0.053) | 0.032 (0.025-0.049) | 0.484 | 0.021 (0.012-0.065) | 0.020 (0.017-0.036) | 0.866 |
Relative expression values are expressed as arbitrary units in medians (25th and 75th percentiles). P-values are for comparisons within each group across time using Wilcoxon signed-ranks test.
Figure 3.
Relative changes (post – pre values) in mRNA expression for subjects performing blood flow restricted (BFR) (grey symbols) and control knee extension exercise (black symbols). The line represents the median change in relative expression.
DISCUSSION
Several studies have reported muscle hypertrophy and an increase in strength following chronic low-load exercise training with BFR (<50% of maximal) without a clear understanding of the mechanisms involved (Abe et al., 2006, Ohta et al., 2003, Takarada et al., 2000). The primary findings of this study are that myogenic transcripts were unaltered independent of the exercise type and that proteolytic transcripts were preferentially downregulated at 8 hrs following BFR exercise. Additionally, BFR exercise increased EMG patterns suggesting a higher level of motor unit activity with ischemia. Our hypotheses were partially confirmed in that transcripts associated with proteolysis were downregulated in a similar manner to high-loading resistance exercise 8 hrs post-exercise. However, because gene expression has a high temporal variability, it is cautioned that these results are a snapshot at 8 hours following exercise and it remains unclear how expression levels change at other periods.
A single bout of resistance exercise performed at relatively high loads (65-80% of maximal strength) initiates transcription in a number of genes associated with protein synthesis and degradation, as well as metabolism (Louis et al., 2007, Mascher et al., 2008, Yang et al., 2005, Yang et al., 2006). With regard to high load resistance exercise, transcripts associated with myogenesis (myoD, myogenin and myogenic regulatory factor-4) are upregulated as early as 2 hrs post exercise with some peaking at 8 hrs (Yang et al., 2005). Unexpectedly, our data suggest that these myogenic transcripts are unaltered 8 hrs following low-load resistance exercise with or without BFR. However, because mRNA expression can be affected by resistance exercise in a biphasic manner, the timing of the biopsy may have failed to observe a change in expression. For example, Drummond and colleagues demonstrated an increase in MyoD and MuRF-1, and a decrease in myostatin expression 3 hrs following resistance exercise performed at 20% of maximal strength with and without BFR (Drummond et al., 2008).
Proteolytic transcripts are altered with high-load exercise and change over time in a complex manner. For example, Yang et al. observed that MuRF-1 and caspase-3 were increased 4 hrs following high-load resistance exercise, but 24 hrs post-exercise MuRF-1 and Atrogin-1 were downregulated from baseline and caspase-3 remained elevated (Yang et al., 2006). However, in a follow-up study by the same group of investigators, Louis and co-workers found that MuRF-1 increased at 1-4 hrs post high-load exercise and reversed direction with a significant downregulation by 8 hrs post-exercise (Louis et al., 2007). At the same time, Atrogin-1 and FOXO3A, which were unaltered up to 4 hrs post-exercise demonstrated a decrease in mRNA expression at 8 hrs and 12 hrs post-exercise (Mascher et al., 2008). Our findings involving low-load resistance exercise with BFR are congruent with the latter work where mRNA expression of MuRF-1, Atrogin-1 and FOXO-3A were downregulated 8 hrs post-exercise (~2 fold decrease) (Drummond et al., 2008, Louis et al., 2007).
Several adaptive pathways that explain responses to BFR exercise have been proposed (Manini et al., 2009), the most fully developed originate from data demonstrating an acute increase in protein synthesis with activation of the mTOR pathway 3 hours following BFR exercise (Fry et al., 2010, Fujita et al., 2007). However, similar to high load resistance training, several adaptive responses are likely involved and our data suggest that downregulation of proteolytic markers is a response to BFR exercise. These data are supported by recent evidence of a reduction in atrogin-1 following 12 weeks of BFR exercise training in a patient with inclusion body myositis (Gualano et al., 2010). Additionally, we have recently shown that a single bout of low load BFR exercise increases delayed-onset of muscle soreness (Umbel et al., 2009), that is often times a result of ultra-structural damage of muscle fibers and is a stimulus for remodeling of muscle following resistance exercise (Hortobagyi et al., 1996). Therefore, ischemia and build-up of metabolic byproducts caused by venous occlusion might increase muscle remodeling and would partially explain adaptations seen with exercise coupled with ischemia. On another note, we found that BFR exercise caused an elevated muscle activity response that was likely related to the additional recruitment of motor units due to the BFR (or related hypoxia) inducing muscle fiber fatigue. However, activation patterns at the last set of BFR exercise were only modestly higher than control exercise and not correlated with gene expression (r < 0.18, p > 0.60). Future work should consider how BFR exercise modulates the balance and activation patterns of myogenic and proteolytic regulators that are involved in exercise-induced muscle remodeling responses.
There are several limitations of this study that need to be considered when interpreting the results. An inherent limitation of the study is that gene expression is only a small piece of the information needed to understand the pathways altered with BFR exercise. Additional data is needed to confirm these results with protein assays and evaluation of post-translational modifications. Regarding the standardized meal, the inclusion of a meal following the bout of exercise may have altered gene transcript profiles, but this was necessary to ensure the second biopsy was performed in fasted state similar to the first biopsy. It is also clear that gene expression is partially due to diurnal variations and the pre-intervention biopsy that confounds exercise-related gene expression (Vissing et al., 2005). We attempted to control for these effects by having both groups perform exercise and extract biopsies at the same time. Regarding the BFR protocol, the degree of venous occlusion and/or hypoxia that occurred at the muscle level was unknown. Additional variability may come from application of the cuff set at 1.5 brachial systolic blood pressure that is unlikely to cutoff blood flow in a large diameter leg (Shaw et al., 1982), but may completely occlude arterial flow in a small leg.
In conclusion, we investigated myogenic and proteolytic mRNA expression 8 hrs following an acute bout of low load knee extension exercise with and without BFR. Contradictory to our hypothesis, RT-PCR analysis revealed no change in genes associated with myogenesis (IGF-1, MyoD, Myogenin, and Myostatin which is a negative regulator myogenesis); however, BFR exercise decreased transcripts (~ 2-fold) associated with proteolytic pathways (FOXO3A, Atrogin-1 and MuRF-1). These data emulate the downregulation of proteolytic genes observed following high load resistance exercise, and help build a foundation for understanding the mechanisms involved in muscle growth with low-load BFR exercise.
Acknowledgements
This work was supported in part by the NIA Claude D. Pepper Center P30AG028740.
Footnotes
Conflict of interest.
No author reported a conflict of interest with the research presented in the manuscript.
REFERENCES
- Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100:1460–6. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol. 2007;102:2232–9. doi: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM, Ordway NR, Ploutz-Snyder LL. Leg muscle activity during walking with assistive devices at varying levels of weight bearing. Arch Phys Med Rehabil. 2004;85:1555–60. doi: 10.1016/j.apmr.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Cram JR, Kasman GS. Introduction to surface electromyography. Aspen; Gaithersburg: 1998. [Google Scholar]
- Drummond MJ, Fujita S, Takashi A, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. 2008;40:691–8. doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO/UNU Human energy requirements: Report of a joint FAO/WHO/UNU expert consultation. 2001. Rome FAO food and nutrition technical report series.
- Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108:1199–209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103:903–10. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- Gualano B, Neves M, Jr., Lima FR, Pinto AL, Laurentino G, Borges C, Baptista L, Artioli GG, Aoki MS, Moriscot A, Lancha AH, Jr., Bonfa E, Ugrinowitsch C. Resistance training with vascular occlusion in inclusion body myositis: acase study. Med Sci Sports Exerc. 2010;42:250–4. doi: 10.1249/MSS.0b013e3181b18fb8. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Hill JP, Houmard JA, Fraser DD, Lambert NJ, Israel RG. Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol. 1996;80:765–72. doi: 10.1152/jappl.1996.80.3.765. [DOI] [PubMed] [Google Scholar]
- Katch V, Weltman A. Predictability of body segment volumes in living subjects. Hum Biol. 1975;47:203–18. [PubMed] [Google Scholar]
- Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005;288:E1110–9. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744–51. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20:480–6. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- Manini TM, Clark BC. Blood flow restricted exercise and skeletal muscle health. Exerc Sport Sci Rev. 2009;37:78–85. doi: 10.1097/JES.0b013e31819c2e5c. [DOI] [PubMed] [Google Scholar]
- Mascher H, Tannerstedt J, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E43–51. doi: 10.1152/ajpendo.00504.2007. [DOI] [PubMed] [Google Scholar]
- Ohta H, Kurosawa H, Ikeda H, Iwase Y, Satou N, Nakamura S. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand. 2003;74:62–8. doi: 10.1080/00016470310013680. [DOI] [PubMed] [Google Scholar]
- Ploutz-Snyder LL, Giamis EL. Orientation and familiarization to 1RM strength testing in old and young women. J Strength Cond Res. 2001;15:519–23. [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JA, Murray DG. The relationship between tourniquet pressure and underlying soft-tissue pressure in the thigh. J Bone Joint Surg Am. 1982;64:1148–52. [PubMed] [Google Scholar]
- Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88:2097–106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol. 2005;98:1900–8. doi: 10.1152/japplphysiol.01178.2004. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Wolfe RR. Exercise, protein metabolism, and muscle growth. Int J Sport Nutr Exerc Metab. 2001;11:109–32. doi: 10.1123/ijsnem.11.1.109. [DOI] [PubMed] [Google Scholar]
- Umbel JD, Hoffman RL, Dearth DJ, Chleboun GS, Manini TM, Clark BC. Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur J Appl Physiol. 2009;107:687–95. doi: 10.1007/s00421-009-1175-6. [DOI] [PubMed] [Google Scholar]
- Vissing K, Andersen JL, Schjerling P. Are exercise-induced genes induced by exercise? Faseb J. 2005;19:94–6. doi: 10.1096/fj.04-2084fje. [DOI] [PubMed] [Google Scholar]
- Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab. 2008;295:E785–97. doi: 10.1152/ajpendo.00646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol. 2005;98:1745–52. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- Yang Y, Jemiolo B, Trappe S. Proteolytic mRNA expression in response to acute resistance exercise in human single skeletal muscle fibers. J Appl Physiol. 2006;101:1442–50. doi: 10.1152/japplphysiol.00438.2006. [DOI] [PubMed] [Google Scholar]