Abstract

The oxidative cycloaddition of benzamides and alkynes has been developed. The reaction utilizes Rh(III) catalysts in the presence of Cu(II) oxidants, and is proposed to proceed by N-H metalation of the amide followed by ortho C-H activation. The resultant rhodacycle undergoes alkyne insertion to form isoquinolones in good yield. The reaction is tolerant of extensive substitution on the amide, alkyne and arene, including halides, silyl ethers, and unprotected aldehydes as substituents. Unsymmetrical alkynes proceed with excellent regioselectivity, and heteroaryl carboxamides are tolerated leading to intriguing scaffolds for medicinal chemistry. A series of competition experiments shed further light on the mechanism of the transformation and reasons for selectivity.
Keywords: rhodium, C–H activation, rhodacycle, heterocycles, cycloaddition
Introduction
Metal-catalyzed cycloadditions have proven reliable to form heterocycles1 with rhodium playing a prominent role.2 We have previously demonstrated that interception of rhodacyclic intermediates in a [2+2+2] cycloaddition provides access to indolizidines and quinolizidines from alkynes and alkene tethered isocyanates (eq 1).3 Although the described [2+2+2] cycloaddition methodology tolerates a range of aryl and alkyl alkynes, benzyne does not participate in metalacycle formation. To address this limitation, we imagined accessing similar products via a C–H activation strategy. Treatment of benzamides with catalytic amounts of rhodium (III) would generate rhodacycle A, which would undergo alkyne insertion to provide isoquinolones (eq 2).4
 |
(1) |
 |
(2) |
Similar intermediates have been invoked in isoquinolone synthesis from benzotriazones and phthalimides, with concomitant extrusion of either N2 or CO.5 Rhodium(III) catalysis forming similar metallacycles via C–H activation is precedented, with the added advantage of using unfunctionalized arenes.6 Excellent work by Miura and Satoh7 revealed that benzoic acid is able to direct C–H insertion in the presence of alkynes under rhodium (III) catalysis to yield isocoumarins (eq 3). Miura and Satoh expanded on this reactivity by using mildly acidic N–H bonds as directing groups for rhodium(III) catalyzed C–H activation, which in the presence of alkynes results in the cyclized product from a C–H/N–H activation (eq 4, 6, 7).8 In a spectacular application of this approach, Fagnou has demonstrated a regioselective indole synthesis by coupling N-aryl acetamides and alkynes under cationic rhodium (III) conditions (eq 5).9 We envisioned using an amide as a directing group with selective formation of the isoquinolone motif, a scaffold found in a variety of natural products.10 Herein we provide a complete description of our development of the methodology, a brief derivation of the isoquinolone scaffold, and some mechanistic insights.
 |
(3) |
 |
(4) |
 |
(5) |
 |
(6) |
 |
(7) |
Initial Conditions Screen
Our studies began with N-methyl benzamide and diphenylacetylene. When [RhCp*Cl2]2 is used as catalyst with Cu(OAc)2 as stoichiometric oxidant and AgSbF6 to sequester the halides, the desired isoquinolone product is obtained in 23% yield (Table 1). t-Amyl alcohol was found to be the ideal solvent for the reaction providing isoquinolone 3a in 65% yield. Substitution of Cu(OAc)2 with Cu(OTf)2 resulted in the exclusive recovery of starting material. However, addition of pivalic acid and potassium carbonate to the Cu(OTf)2 reaction affords 3a in 65% yield, suggesting the oxidant is a source of carboxylate necessary for the reaction to proceed.11 Ultimately the exclusion of AgSbF6 gave the optimal conditions with a yield of 82%. In the course of reaction optimization we observed that when AgSbF6 was added to the reaction a naphthalene product resulting from two C-H activations and two alkyne insertions is observed (Figure 1).12 We speculate that the cationic rhodium is better able to coordinate an additional equivalent of alkyne resulting in a side pathway leading to the napthalene product.
Table 1.
Conditions Screen
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | additive | oxidant | solvent | yield (%) |
| 1 | [RhCp*Cl2]2 | AgSbF6 | Cu(OAc)2 | PhMe | 23 |
| 2 | [RhCp*Cl2]2 | AgSbF6 | Cu(OAc)2 | t-AmOH | 65 |
| 3 | [RhCp*Cl2]2 | none | Cu(OAc)2 | t-AmOH | 82 |
| 4 | [RhCp*Cl2]2 | AgSbF6 | Cu(OTf)2 | t-AmOH | 0 |
| 5a | [RhCp*Cl2]2 | AgSbF6 | Cu(OTf)2 | t-AmOH | 65 |
| 6b | [RhCp*Cl2]2 | none | Cu(OTf)2 | t-AmOH | 0 |
50 mol % PivOH, and 250 mol % K2CO3 were added.
300 mol % Et3N was added.
Figure 1.
Additive Effect
Substrate Scope
An examination of the scope revealed that electron-rich and electron-poor N-methyl benzamides participate in good yield (Chart 1). The reaction is tolerant of aryl bromides and unprotected aldehydes (3c and 3f). The use of other substituents on nitrogen is also tolerated including N-ethyl, N-phenyl and N-benzyl amides, the latter proceeding in a depressed yield.13 It is interesting to note that we do not observe an indole product when subjecting 1g to these reaction conditions, as per the precedent of Fagnou.6a Apparently, benzamide C-H activation is faster than N-phenyl C-H activation under these neutral catalyst conditions. Substitution at the meta position is well tolerated and leads to isoquinolone products as single regioisomers (3j and 3k). ortho-Methyl benzamide affords the isoquinolone product in slightly depressed yield.14
Chart 1.
Benzamide Screen
The examination of the benzamide scope revealed three substrates that afforded anomalous results. Whereas ortho-methyl benzamide produces the isoquinolone product, the corresponding ortho-methoxy benzamide leads to clean conversion to the isocoumarin (eq 8, Figure 2). We suggest that this dichotomy is electronic in nature and that there is an intramolecular hydrogen bond between the amide and the ortho-methoxy group altering the chemoselectivity of the cyclization forming an imido ester product (Figure 2). The imido ester is hydrolyzed to form the isocoumarin product in situ or in the workup. Furthermore, the use of the more electron-deficient benzyl or trifluoroethyl amide leads to a mixture of isoquinolone and isocoumarin products (eq 9, Figure 2).
Figure 2.
Competitive Isocoumarin Formation
Extension of this reaction to heteroaryl carboxamides proved successful under these reaction conditions (Chart 2).15 With 3-heteroaryl carboxamides 4b and 4c, the reaction affords a single regioisomer as product resulting from C-H activation at the more activated 2-position (5b and 5c, Chart 2). Thiophene and indolyl carboxamides 4a and 4f provide the corresponding adducts in excellent yield using the neutral catalyst. However, 2-furyl and 2-pyrrolyl carboxamides lead to low yields under the neutral pre-catalyst conditions.16 This situation was rectified with the use of the more activated cationic precatalyst [RhCp*(MeCN)3](SbF6)2., leading to products 5d, 5e and 5g (Chart 2). The use of unsymmetrical alkynes with heteroaryl carboxamides led to products 5e-5g as single regioisomers, paralleling results with those found with benzamides (Chart 2 and see below).
Chart 2.
Heteroaryl Screen
a5 mol % RhCp*(MeCN)3(SbF6)2 used as catalyst
With respect to the alkyne substituent, the reaction shows broad substrate tolerance among internal alkynes (Table 2).17 Electron-rich tolanes participate in excellent yield while electron-deficient systems are somewhat more recalcitrant (contrast entries 1 and 2, Table 2). Heteroaryl and aliphatic alkynes are also tolerated. Acetals are deprotected under the reaction conditions to yield the corresponding aldehyde (entry 7, Table 2).18 When unsymmetrical alkynes are employed, largely a single regioisomer is observed. An unsymmetrical dialkyl alkyne also participates, with 4-methyl-2-pentyne surprisingly affording 3u bearing the bulky isopropyl group distal to the isoquinolone nitrogen (entry 9, Table 2).
Table 2.
Alkyne Screen
 |
Mechanistic Studies
We conducted a series of experiments to probe the reaction mechanism. Fagnou has shown that the C–H insertion step is reversible with electron rich N-phenyl acetamide.6a When our reaction is conducted in t-AmOD in the absence of alkyne, 73% deuterium incorporation is observed at the two ortho positions (Figure 3). If the same reaction is conducted in the presence of diphenylacetylene, no deuterium incorporation is observed in unreacted 1a. These experiments suggest that under the reaction conditions the C–H insertion step is largely irreversible.19
Figure 3.
Reversible C–H Activation
Further support for this step in the mechanism was gained from a series of competition experiments. Equimolar amounts of benzamides 1b and 1e were subjected to the standard reaction conditions with a single equivalent of alkyne. The product formation favors isoquinolone 3e derived from the more electron-deficient benzamide (Figure 4). These results suggest that either N-H activation or C-H activation could be turnover limiting. In order to distinguish between these two possibilities, we also conducted a competition experiment between 1c and 1e, two para substituents that have disparate σp values but similar σm values. In the event, 3c and 3e are formed in nearly equal amounts consistent with the hypothesis that C-H activation is the first irreversible step. If N-H activation was the first irreversible step, we should see a greater difference in product distribution given the increased acidity of amide 1e relative to 1c. Importantly, this suggests a mechanistic dichotomy between this manifold and Fagnou's Rh(III)-catalyzed C-H/N-H activation of N-acetyl anilides leading to indoles.6a
Figure 4.
Benzamide Competition Experiments
In order to learn about the subsequent steps in the catalytic cycle, we conducted a series of competition experiments between alkynes. Equimolar amounts of 1a, 5-decyne and diphenylacetylene afford isoquinolone 3a as a single product (eq 12, Figure 5). A competition experiment between electron-rich (p-methoxy) and electron-deficient (p-trifluoromethyl) tolanes provides only the isoquinolone derived from the electron-rich alkyne (eq 13, Figure 5). Lastly, an unsymmetrical tolane gives two regioisomeric isoquinolones in good yield (eq 14, Figure 5). Taken together, these results suggest that more electron-rich alkynes are favored in the insertion event but the regioselectivity of insertion appears to be dictated largely by steric factors.20
Figure 5.
Alkyne Competition Experiments
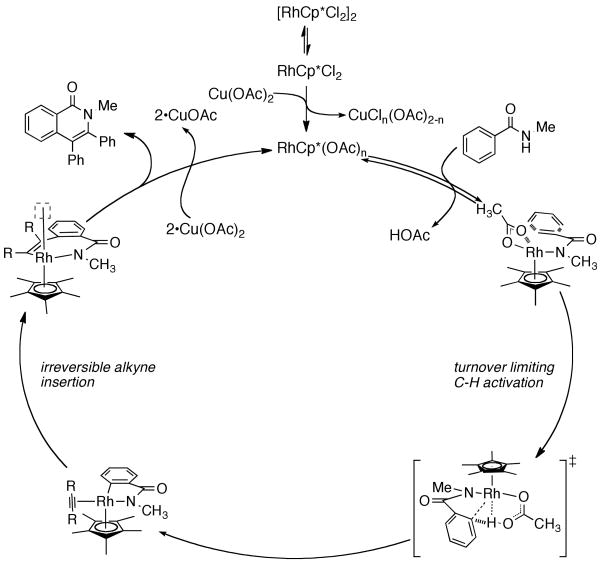
In light of these experiments we propose the following mechanism. The rhodium dimer precatalyst presumably dissociates into the coordinatively unsaturated monomer, which can exchange ligands to form an acetate-ligated species with the copper oxidant. Coordination of an equivalent of benzamide then initiates either N- or O-metalation before a turnover limiting C–H activation can occur to form a 5-membered rhodacycle, with concomitant formation of acetic acid. This rhodacycle has an open coordination site which can regioselectively and irreversibly insert an equivalent of alkyne to form a 7-membered rhodacycle with an open coordination site. If this coordination site is occupied by an equivalent of alkyne this can lead to the naphthalene side product; alternately, the rhodium can reductively eliminate to form the desired isoquinolone and generate a rhodium (I) species which can undergo oxidation to regenerate the catalytically active rhodium (III) complex.
Conclusion
In conclusion, we have developed a Rh(III) catalyzed oxidative isoquinolone synthesis using a transient 5-membered rhodacycle accessed from ortho C–H/N-H activation.21 We found that heterocycles are well tolerated in the reaction allowing access to a number of unique molecular scaffolds. Additionally unsymmetrical alkynes are tolerated in high yield and high regioselective with high functional group tolerance. The mechanism of the reaction was probed to find that C–H activation is the turnover-limiting step. A series of competition experiments shed light on this mechanism, suggesting alkyne insertion is largely governed by steric factors and alkyne coordination plays a central role in product selectivity.
Supplementary Material
Figure 6.
Proposed Mechanism
Acknowledgments
We thank NIGMS (GM80442), Amgen and Roche for support. We thank Johnson Matthey for a loan of rhodium salts.
Footnotes
Supporting information available. Experimental procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Nakamura I, Yamamoto Y. Chem Rev. 2004;104:2127. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]; (b) D'Souza DM, Müller TJJ. Chem Soc Rev. 2007;36:1095. doi: 10.1039/b608235c. [DOI] [PubMed] [Google Scholar]
- 2.For recent examples, see: Komine Y, Tanaka K. Org Lett. 2010;9:1312. doi: 10.1021/ol100182u.Saito T, Sugizaki K, Otani T, Suyama T. Org Lett. 2007;9:1239. doi: 10.1021/ol063123i.Tanaka K, Mimura M, Hojo D. Tetrahedron. 2009;65:9008.
- 3.(a) Yu RT, Rovis T. J Am Chem Soc. 2006;128:2782–2783. doi: 10.1021/ja057803c. [DOI] [PubMed] [Google Scholar]; (b) Yu RT, Rovis T. J Am Chem Soc. 2006;128:12370–12371. doi: 10.1021/ja064868m. [DOI] [PubMed] [Google Scholar]; (c) Lee EE, Rovis T. Org Lett. 2008;10(6):1231–1234. doi: 10.1021/ol800086s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yu RT, Rovis T. J Am Chem Soc. 2008;130:3262–3263. doi: 10.1021/ja710065h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yu RT, Lee EE, Malik G, Rovis T. Angew Chem Int Ed. 2009;48:2379–2382. doi: 10.1002/anie.200805455. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Keller Friedman R, Rovis T. J Am Chem Soc. 2009;131:10775–10782. doi: 10.1021/ja903899c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Oinen ME, Yu RT, Rovis T. Org Lett. 2009;12:4943. doi: 10.1021/ol9020805. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Yu RT, Keller Friedman R, Rovis T. J Am Chem Soc. 2009;131:13250. doi: 10.1021/ja906641d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Dalton DM, Oberg KM, Yu RT, Lee EE, Perreault S, Oinen ME, Pease ML, Malik G, Rovis T. J Am Chem Soc. 2009;131:15717. doi: 10.1021/ja905065j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Perreault S, Rovis T. Chem Soc Rev. 2009;38:3149. doi: 10.1039/b816702h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For a recent review of rhodium catalyzed C–H activation in C–C bond formation see: Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. doi: 10.1021/cr900005n.
- 5.For recent examples, see: Kajita Y, Matsubara S, Kurahashi T. J Am Chem Soc. 2008;130:6058. doi: 10.1021/ja7114426.Miura T, Yamachi M, Murakami M. Org Lett. 2008;10:3085. doi: 10.1021/ol8010826.
- 6.For a recent stoichiometric example see: Li L, Brennessel WW, Jones WD. J Am Chem Soc. 2008;130:12414. doi: 10.1021/ja802415h.
- 7.Ueura K, Satoh T, Miura M. Org Lett. 2007;9:1407. doi: 10.1021/ol070406h. [DOI] [PubMed] [Google Scholar]
- 8.(a) Morimoto K, Hirano K, Satoh T, Miura M. Org Lett. 2010;12:2068. doi: 10.1021/ol100560k. [DOI] [PubMed] [Google Scholar]; (b) Fukutani T, Umeda N, Hirano K, Satoh T, Miura M. Chem Commun. 2009:5141. doi: 10.1039/b910198e. [DOI] [PubMed] [Google Scholar]; (c) Umeda N, Tsurugo H, Satoh T, Miura M. Angew Chem Int Ed. 2008;47:4019. doi: 10.1002/anie.200800924. [DOI] [PubMed] [Google Scholar]
- 9.Stuart DR, Bertrand-Laperle M, Burgess KMN, Fagnou K. J Am Chem Soc. 2008;130:16474. doi: 10.1021/ja806955s. For an additional rhodium catalyzed cyclization by Fagnou see: Guimond N, Fagnou K. J Am Chem Soc. 2009;131:12050. doi: 10.1021/ja904380q.
- 10.For a review of isoquinolone synthesis, see: Glushkov VA, Shklyaev YV. Chem Heterocycl Compd. 2001;37:663.
- 11.This has been observed in palladium C–H functionalization; see: Lafrance M, Lapointe D, Fagnou K. Tetrahedron. 2008;129(64):6015.
- 12.Similar reactivity was observed by Miura and Satoh when examining 2-aryl oxazole substrates; see ref. 8c.
- 13.I-Pr and t-Bu benzamides provide no cycloadduct under these conditions.
- 14.N-acyl benzyl amines do not undergo the cycloaddition under these conditions.
- 15.Under these conditions, N-methylpyridine-3-carboxamide and N,2-dimethyloxazole-4-carboxamide afford cycloadduct in 0% and 8% yield respectively.
- 16.For example, 2-furyl carboxamide 4d leads to 5d in 13% yield using the [RhCp*Cl2]2 conditions.
- 17.Terminal alkynes are not tolerated under the current reaction conditions.
- 18.Acetic acid is generated during the course of the reaction and the Cu salt is used as its hydrate, presumably providing the necessary water and acid to lead to hydrolysis of the acetal.
- 19.This deuteration experiment was also conducted on N-t-Bu benzamide. In the absence of alkyne, no deuterium incorporation was observed after 16 h under these conditions suggesting the sterics of the tert-butyl group negatively affects its ability to metalate by rhodium. This also suggests that metalation occurs on nitrogen rather than oxygen.
- 20.The apparent reversed regioselectivity in product 3u seems to contradict this model; further studies to address this dichotomy are ongoing.
- 21.During the preparation and review of this manuscript, similar and complementary studies leading to isoquinolone synthesis were reported by the groups of Keith Fagnou and Masahiro Miura; see: Guimond N, Gouliaras C, Fagnou K. J Am Chem Soc. 2010;132:6908. doi: 10.1021/ja102571b.Mochida S, Umeda N, Hirano K, Satoh T, Miura M. Chem Lett. 2010;39:744.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.