Abstract
Hepatitis C is caused by an enveloped virus whose entry is mediated by two glycoproteins, namely, E1 and E2, which have been shown to assemble as a noncovalent heterodimer. Despite extensive research in the field of such an important human pathogen, hepatitis C virus (HCV) glycoproteins have only been studied so far in heterologous expression systems, and their organization at the surfaces of infectious virions has not yet been described. Here, we characterized the envelope glycoproteins associated with cell-cultured infectious virions and compared them with their prebudding counterparts. Viral particles were analyzed by ultracentrifugation, and the envelope glycoproteins were characterized by coimmunoprecipitation and receptor pulldown assays. Furthermore, their oligomeric state was determined by sedimentation through sucrose gradients and by separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions. In sucrose gradient analyses, HCV envelope glycoproteins were associated with fractions containing the most infectious viral particles. Importantly, besides maturation of some of their glycans, HCV envelope glycoproteins showed a dramatic change in their oligomeric state after incorporation into the viral particle. Indeed, virion-associated E1 and E2 envelope glycoproteins formed large covalent complexes stabilized by disulfide bridges, whereas the intracellular forms of these proteins assembled as noncovalent heterodimers. Furthermore, the virion-associated glycoprotein complexes were recognized by the large extracellular loop of CD81 as well as conformation-sensitive antibodies, indicating that these proteins are in a functional conformation. Overall, our study fills a gap in the description of HCV outer morphology and should guide further investigations into virus entry and assembly.
Hepatitis C virus (HCV) infects 3% of the world population. It represents a major health burden due to its high propensity to cause chronic infections and the subsequent liver cirrhosis and carcinoma (for a review, see reference 31). Discovered in 1989, it is a small enveloped virus, classified into the Flaviviridae family. Its positive-strand RNA genome encodes a single polyprotein that is co- and posttranslationally processed into 10 mature proteins. From the N terminus to the C terminus of the polyprotein are located the precursors for three structural proteins, namely, the core protein (capsid protein) and E1 and E2 (envelope glycoproteins), and for seven nonstructural proteins (p7, NS2, 3, 4A, 4B, 5A, and 5B) (for a review, see reference 43).
HCV circulates in infected patients at relatively low titers and forms complexes with serum components such as lipoproteins, antibodies or rheumatoid factors, and cryoglobulins (45). Furthermore, for reasons that remain partly unknown, serum-derived HCV cannot be propagated in cell culture. HCV research has been hindered by these restrictions and has largely depended on surrogate systems. For instance, replication-deficient retroviruses pseudotyped with HCV envelope glycoproteins (HCV pseudoparticles [HCVpp]) (6, 32) were used to study HCV entry (8, 41). Similarly, replicon systems allowed investigation of HCV replication (for a review, see reference 2). However, until recently, there was no system available to reproduce HCV assembly and secretion, which remained mostly elusive. Likewise, 21 years after the HCV discovery, its structure remains unknown. Nevertheless, in 2005, several teams finally succeeded in propagating HCV in cell culture (cell-cultured HCV [HCVcc]) (39, 60, 62), which allowed verification of most of the previous data on viral entry and which facilitated antiviral testing. Moreover, this system allowed for the first time the investigation of virus assembly and the production of virions in quantities amenable to study their composition and structure.
Our study focuses on HCV-encoded envelope glycoproteins E1 and E2. These proteins are sufficient to confer infectivity to HCVpp (6, 32), and E1- or E2-specific antibodies have been found to neutralize both HCVpp and HCVcc infectivity (5, 6, 32, 35, 47, 48, 50). Each protein consists of a large N-terminal ectodomain, responsible for virus attachment to its receptors, and a C-terminal transmembrane domain, anchoring each glycoprotein in a lipid bilayer. The ectodomains are heavily glycosylated (25), and their structure is stabilized by disulfide bridges (36). For instance, E1 and E2 from the JFH-1 isolate respectively harbor 4 and 11 glycans and contain 8 and 18 cysteine residues in their ectodomains (14, 34). The transmembrane domains contain retention signals for the endoplasmic reticulum (ER) (12, 13). From the start, the fates of E1 and E2 glycoproteins are closely linked, as both proteins are expressed consecutively in the context of the viral polyprotein. Previous characterization of these glycoproteins using heterologous expression systems led us and others to propose that an E1E2 noncovalent heterodimer would be the functional complex for HCV entry (16, 46). However, data showing the relevance of this model in the genuine infectious HCV system are missing.
In this work, we address the characterization of the glycoproteins incorporated into the envelopes of infectious HCVcc virions, specifically, their maturation, glycosylation patterns, and oligomerization. Our results indicate that intracellular and secreted envelope glycoproteins were able to bind CD81, a HCV receptor, as well as heparin, a heparan sulfate homolog. Furthermore, virion-associated glycoproteins harbored both high-mannose and complex glycans. Finally, the intracellular forms of E1 and E2 envelope glycoproteins assembled as noncovalent heterodimers, whereas virion-associated envelope glycoproteins formed large covalent complexes stabilized by disulfide bridges.
MATERIALS AND METHODS
Antibodies.
For Western blot analysis, E1 and E2 glycoproteins were detected with mouse A4 (18) and rat 3/11 (22) monoclonal antibodies (MAbs), respectively. Core protein was stained with R526 rabbit antiserum (11). Rabbit antibody to calnexin was obtained from Stressgen. Anti-NS5A 9E10 antibody (40) was generously provided by C. M. Rice (Rockefeller University). Anti-E2 CBH-5 and CBH-7 MAbs (27, 35) were kind gifts from S. Foung (Stanford University).
Cell culture, generation of viral stocks, and HCVcc titration.
The Huh-7 hepatoma cell line (44) and HEK 293T cells were grown in Dulbecco's modified essential medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum.
In this work, we used a modified version of the plasmid carrying the JFH-1 genome (genotype 2a) (GenBank accession number AB237837), kindly provided by T. Wakita (National Institute of Infectious Diseases, Tokyo, Japan) (60). The JFH-1-derived plasmid pJFH1/CS-A4 contains two amino acid changes, F172C and P173S, in the viral core protein which have been shown to increase infectious titers (17). It also has a reconstituted A4 epitope in the N-terminal region of E1, which allows the detection of E1 with the A4 MAb, as described previously (26). Virus stocks were generated in two steps. First, JFH1/CS-A4 RNA was produced and electroporated into Huh-7 cells as described previously (17). The infectious supernatant was used to infect naïve Huh-7 cells, which were serially passaged twice or thrice in order to harvest high-titer supernatant. Supernatants were filtered through 0.45-μm-pore-size membranes. Unless otherwise stated, cleared supernatants were pelleted by ultracentrifugation over a 20% sucrose cushion in sterile phosphate-buffered saline (PBS), in a SW 28 rotor (Beckman), for 4 h at 110,000 × g. Virus pellets were resuspended in PBS, aliquoted, and frozen at −80°C or used fresh. On average, virus stocks were concentrated 300 times in volume. In parallel, infected cells were harvested to study intracellular glycoproteins.
HCVcc infectivity was determined by focus-forming assay as follows. Infectious samples were serially diluted and inoculated onto Huh-7 cells in 96-well dishes for 3 h at 37°C. After the inoculation period, the inoculum was replaced by fresh supernatant. At 2 days postinfection, cells were fixed in methanol and immunostained with the anti-E1 MAb A4. The number of HCV-positive cells was counted by fluorescence microscopy as described previously (62).
HCV pseudoparticles were produced as previously reported (6, 46). Briefly, HEK 293T cells were cotransfected with the murine leukemia virus (MLV) Gag-Pol packaging vector, the MLV-luciferase transfer construct, and a plasmid encoding E1 and E2 (genotype 1a, H strain), using Exgen reagent (Euromedex). A total of 2 to 3 days following transfection, the medium containing HCVpp was collected and treated similarly as HCVcc-containing supernatant (see above).
Pulldown assays, immunoprecipitations, and endoglycosidase digestions.
Unless otherwise stated, cells or concentrated supernatants were lysed in the following buffer (buffer A): TNE buffer (50 mM Tris at pH 7.5, 100 mM NaCl, 1 mM EDTA) in the presence of 0.5% Triton X-100, protease inhibitor cocktail (Complete; Roche), 1 mM phenylmethylsulfonyl fluoride (PMSF; Sigma), and 20 mM iodoacetamide (Sigma). Cell lysates were precleared by centrifugation at 13,000 × g for 15 min at 4°C. For protein analysis under native conditions, Triton X-100 was replaced by n-dodecyl-β-d-maltopyranoside (0.1%) (Affymetrix).
Heparin pulldown assays were performed with heparin-Sepharose (CL-6B) beads (Pharmacia), and Galanthas nivalis agglutinin (GNA) pulldown assays were performed with agarose-conjugated lectin (Galanthus nivalis) (Sigma). Soluble heparin extracted from bovine lungs was purchased from Sigma and incubated with E1E2-containing lysates overnight at 4°C when relevant. For the CD81 pulldown assay, glutathione-Sepharose (GS4B) beads were obtained from Amersham Biosciences. Mouse or human recombinant CD81 large extracellular loops in fusion with glutathione S-transferase (GST) were produced as already described (30). Protein A-Sepharose beads (GE Healthcare) were used for CBH-5 and CBH-7 immunoprecipitations. Typically, when relevant, beads were first coated with recombinant CD81 or antibodies for 2 h at 4°C and washed twice in PBS-1% Triton X-100. They were then further incubated for 2 to 4 h with E1E2-containing lysates and washed six times in PBS-1% Triton X-100 and once in water before addition of Laemmli buffer.
Protein deglycosylation by PNGase F or Endo H (New England Biolabs) was carried out on denatured samples for 2 h at 37°C in the presence of a protease inhibitor cocktail (Complete; Roche) and 1 mM PMSF (Sigma), according to the manufacturers' instructions.
Protein analysis by SDS-PAGE and Western blotting.
Protein samples were heated for 7 min at 70°C in Laemmli sample buffer before sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For reducing conditions, dithiothreitol (DTT; Sigma) was added to the Laemmli buffer at a working concentration of 100 mM. After separation by SDS-PAGE on 12% acrylamide gels, proteins were transferred onto nitrocellulose membranes (Hybond-ECL; Amersham) and probed with specific antibodies. Corresponding peroxidase-conjugated anti-species antibodies were used at 1/5,000 (anti-rat [Jackson]; anti-rabbit [Amersham]) or 1/10,000 (anti-mouse; Dako) dilutions. Peroxidase activity was revealed by enhanced chemiluminescent (ECL) detection (ECL Plus [Amersham]; LumiGlo or LumiGlo Reserve substrates [KPL]).
Separation of HCVcc on sucrose gradients.
Continuous 20 to 60% sucrose gradients were formed by equal volume steps of 20-to-60% sucrose solutions in PBS (incremented by 5%) and incubation at 4°C for at least 4 h. A total of 500 μl of infectious viral supernatant, concentrated beforehand by ultracentrifugation without the sucrose cushion, was deposited onto the top of the gradient. Gradients were spun for 16 h at 210,000 × g in a SW 41 rotor (Beckman) and fractionated from the top. Each 1-ml fraction was analyzed for its infectivity (50-μl sample), viral RNA content (100-μl sample), and protein content (200 plus 600 μl). Fraction densities were determined by measuring the sucrose content in similar fractions of a control gradient with a refractometer.
Infectivity was assessed by a focus-forming assay as described above. RNA was extracted from the sucrose gradient fractions and quantified by reverse transcription and quantitative real-time reverse transcription-PCR (RT-PCR) assay, as described previously (17).
In order to precipitate total proteins, protease inhibitors, PMSF, and 10 μg of carrier protein (alcohol dehydrogenase) were added to a 200-μl aliquot (corresponding to 1 volume) of each fraction. Total proteins were then precipitated according to the methanol-chloroform method (61). Briefly, 4 volumes of methanol, 1 volume of chloroform, and 3 volumes of water were added sequentially to each 200-μl aliquot and mixed thoroughly between each step. Samples were then centrifuged at 13,000 × g for 5 min at room temperature; 4 volumes of methanol were added to the lower organic layer. Samples were mixed and centrifuged (13,000 × g, 5 min, room temperature), and dried pellets were resuspended in reducing Laemmli buffer and analyzed by SDS-PAGE, followed by Western blotting for core protein, NS5A, or calnexin content.
Last, 600 μl of each fraction was lysed in buffer A (see above) and used in a CD81 (300-μl aliquot) or a GNA (300-μl aliquot) pulldown assay. GNA-pulled-down samples were divided into two equal parts and analyzed under reducing or nonreducing SDS-PAGE, and CD81 pulled-down samples were analyzed under reducing SDS-PAGE only.
Separation of E1E2 complexes on sucrose gradients.
Sucrose solutions were made in TNE-0.1% n-dodecyl-β-d-maltopyranoside-20 mM iodoacetamide. Continuous 5-to-20% sucrose gradients were formed by equal volume steps of 5-to-20% sucrose solutions (incremented by 2.5%) and incubation at 4°C for at least 4 h. Lysed concentrated viral supernatant or cell lysates were overlaid onto the top of the gradient. Gradients were spun for 16 h at 210,000 × g in a SW 41 rotor (Beckman). Twelve 1-ml fractions were harvested from the top, and the pellet was resuspended in the gradient buffer. In parallel, a control calibration gradient was run with 250 μg of each of the following proteins: carbonic anhydrase (29 kDa), ovalbumin (44 kDa), bovine serum albumine (BSA; 66 kDa), conalbumin (75 kDa), aldolase (158 kDa), β-amylase (200 kDa), ferritin (440 kDa), and thyroglobulin (669 kDa) (Sigma and GE Healthcare). After similar fractionation, 30 μl of each fraction was analyzed by reducing SDS-PAGE and Coomassie blue staining to determine the sedimentation pattern of each marker protein.
RESULTS
HCV structural proteins are detectable in infected cell culture supernatant.
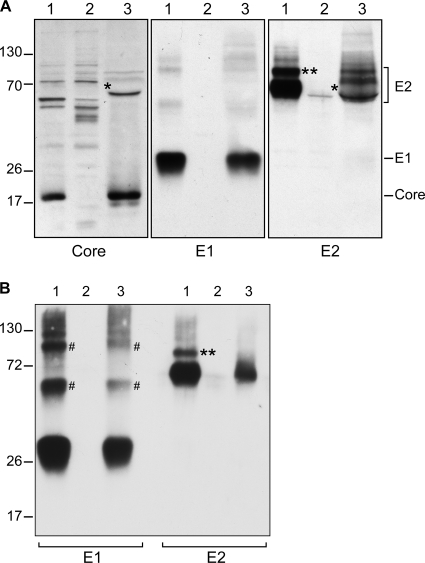
To characterize virion-associated envelope glycoproteins, we first needed to set up highly sensitive conditions, allowing their detection in a small amount from concentrated supernatant of HCV-infected cells. To this aim, we used a modified JFH-1 infectious clone which contains the MAb A4 epitope in E1 glycoprotein (26) and the adaptative F172C and C173S mutations in the core protein coding sequence in order to increase virus release (17). Viral stocks were produced by electroporation of Huh-7 cells with the corresponding RNA and subsequent amplification by infection of naïve Huh-7 cells. Secreted virus was pelleted by ultracentrifugation in order to concentrate the virions and to discard nonparticulate matter. Cell lysate and concentrated supernatant were analyzed for the presence of structural proteins by SDS-PAGE, followed by Western blotting. As expected, core, E1, and E2 proteins could be readily detected in cell lysates and supernatants (Fig. 1A). Despite virus ultracentrifugation over a sucrose cushion, our virus preparations were contaminated with BSA present at high concentrations in the fetal calf serum. BSA has roughly the same molecular mass as the E2 protein and, therefore, disturbed the E2 migration profile (Fig. 1A, single asterisk). For this reason, envelope glycoproteins were also analyzed by GNA pulldown. This lectin is indeed known to bind HCV envelope glycoproteins (52) by interacting with high-mannose-type glycans. As shown in Fig. 1B, this treatment removed the contaminant BSA, which tends to disturb the E2 migration profile, and it also resulted in glycoprotein enrichment. A potential concern with this approach is that it might lead to the selection of a particular subset of glycoproteins. However, the presence of high-mannose-type glycans on both E1- and E2-secreted proteins (see below and Fig. 4) suggests that GNA has the potential to interact with all the glycoforms of HCV envelope glycoproteins. Therefore, the envelope glycoproteins were precipitated with the GNA lectin in several subsequent experiments.
FIG. 1.
Detection of HCV structural proteins in infected cell lysate and supernatant. (A) Lysates obtained from infected cells (lane 1), naïve cells (lane 2), or concentrated infectious supernatant (lane 3) were analyzed by reducing SDS-PAGE and Western blotting with specific anti-core, anti-E1, or anti-E2 antibodies. Molecular mass markers (in kDa) are indicated on the left. In supernatant, the asterisk corresponds to a disturbance in the E2 migration profile, likely due to the presence of the BSA protein, as seen by membrane staining with Ponceau solution (data not shown). The double asterisk indicates the slow-migrating band detected by the anti-E2 antibody in cell lysate which likely corresponds to the E2-p7-NS2 precursor, as previously reported (57). (B) GNA pulldown assay. Lysates obtained from infected cells (lane 1), naïve cells (lane 2), or concentrated infectious supernatant (lane 3) were incubated with GNA-conjugated beads. Proteins were eluted from the beads in reducing Laemmli buffer and analyzed by SDS-PAGE and Western blotting. #, the additional bands detected by the anti-E1 antibody might correspond to E1 dimers and trimers, as already observed in recombinant and HCVcc systems (7).
Interestingly, no diminution of size was apparent between the intracellular prebudding and the secreted virion-associated forms of HCV glycoproteins. This does not rule out a cleavage of HCV glycoproteins during secretion; however, if such a cleavage occurred, it would be close to the extremities of the glycoprotein. Moreover, E1 recognition by A4 antibody indicated that the corresponding epitope, which is located near the E1 N terminus, remained intact during virus maturation.
Secreted HCV envelope glycoproteins cosediment with infectious viruses.
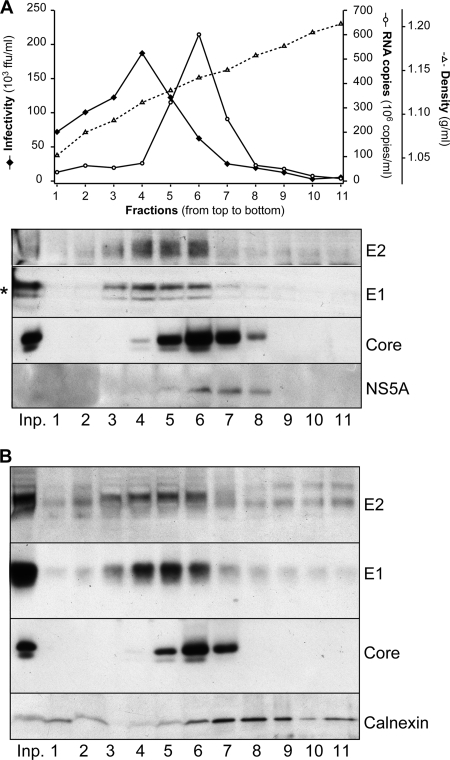
To determine whether E1 and E2 glycoproteins detected in infected cell culture supernatant were associated with infectious viral particles, we separated concentrated viral supernatant in a sucrose gradient. Fractions of different densities were analyzed for their infectivity as well as their content in viral RNA and structural proteins. As shown in Fig. 2 A, there was a broad distribution of infectious particles, with a peak of infectivity corresponding to a density of 1.112 g/ml. As previously reported (24), the viral genomic RNA sedimented in heavier fractions, with an average density of 1.141 g/ml. Also, as expected for an RNA binding protein (55), the core protein sedimented together with the viral RNA (Fig. 2A). In contrast, E1 and E2 glycoproteins were found mainly in the most infectious fractions of the gradient (Fig. 2A). Therefore, two populations of viral particles could be detected in the supernatant. The first population consisted of high-density particles, accounting for a large proportion of secreted viral RNA and core proteins but poorly infectious. The second population corresponded to infectious particles with lower densities and accounted for most of secreted E1 and E2 glycoproteins.
FIG. 2.
Secreted E1 and E2 glycoproteins are associated with infectious particles. (A) Separation of concentrated HCVcc in a sucrose gradient. (Top) Concentrated cell-cultured HCVs were separated by sedimentation through a 20-to-60% sucrose gradient. Fractions were collected from the top and analyzed for their infectivity by focus-forming assay (black diamonds) and for their viral RNA content by reverse transcription and quantitative PCR (white circles). Fraction density was measured in a control gradient spun in parallel (dotted line with white triangles). ffu, focus-forming units. (Bottom) Viral proteins were detected following total protein precipitation (core and NS5A proteins) or CD81 pulldown (E1 and E2 proteins), SDS-PAGE, and Western blotting with specific antibodies. The input virus preparation (inp.) was analyzed in parallel. The lower-molecular-weight band detected by the anti-E1 MAb is likely an artifact due to the comigration of one CD81 form (*) during SDS-PAGE (as observed by Ponceau staining) (data not shown); note that only one band is detectable after GNA pulldown (see panel B). (B) Analysis of cell contaminants in sucrose gradient-separated cell-cultured HCVs. In an experiment similar to that described in the legend to panel A, the cellular chaperone calnexin and the viral core protein were detected on the same membrane with specific antibodies. As a comparison, the E1 and E2 sedimentation profiles are also depicted; in that case, viral glycoproteins were precipitated from gradient fractions using the GNA lectin. Note that the distortion of the calnexin band in the first three lanes is likely due to the comigration of contaminant proteins such as BSA, as observed by membrane staining with Ponceau solution (data not shown).
To verify that HCV envelope glycoproteins detected in the supernatant of infected cells did not correspond to the release of cell debris, we analyzed the presence of calnexin and NS5A in the gradient fractions. Calnexin is an ER-associated chaperone protein, which colocalizes with HCV envelope glycoproteins in infected cells (53), and NS5A is a viral nonstructural protein, which also partially colocalizes with HCV envelope glycoproteins in infected cells. As shown in Fig. 2B, calnexin could be detected in concentrated cell supernatant, probably associated with microsomes or other cell debris. However, it sedimented to higher densities than infectious viruses or the E1 and E2 glycoproteins (Fig. 2A). NS5A, which is expected to be restricted to the cells, was also detected, albeit very faintly, in the cell supernatant (Fig. 2A). Moreover, as for calnexin, it sedimented in higher-density fractions than those of infectious viruses and the bulk of detected E1 and E2 proteins. It is also worth noting that the difference in the glycosylation profiles of E2 between the intra- and extracellular forms also exclude contamination from cell-associated envelope proteins (see below).
Importantly, viral preparations were concentrated beforehand by ultracentrifugation without a sucrose cushion, so as not to lose the lowest-density particles. Indeed, in Fig. 2A, fractions 1 and 2 contain 20% sucrose or less, and their protein content would presumably have been lost during ultracentrifugation over a 20% sucrose cushion. However, these fractions contribute little to the total amount of secreted E1E2 (Fig. 2A). Therefore, for the following experiments, virus was concentrated over a sucrose cushion to optimize the removal of cell contaminants and facilitate viral protein analyses.
All together, these data indicated that the bulk of E1 and E2 proteins detected in concentrated cell supernatant was associated with infectious virus and did not correspond to proteins associated with cell debris. In consequence, further E1 and E2 characterization was performed on supernatants partially purified on a sucrose cushion.
HCV envelope glycoproteins interact with the CD81 receptor and with the heparan sulfate homolog heparin.
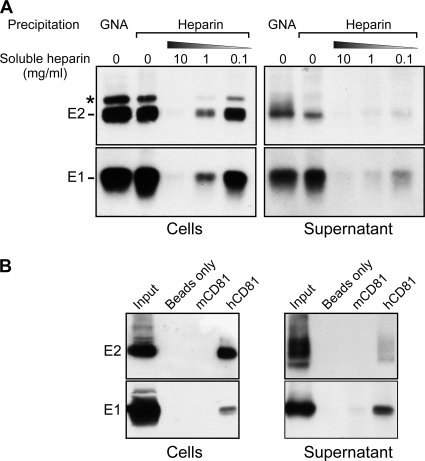
A number of cellular factors play a role in HCV entry as attachment molecules or active entry factors. Among them, heparan sulfates are able to capture a variety of viruses, including Flaviviridae members (dengue, classical swine fever, and tick-borne encephalitis viruses), Herpesviridae, papillomavirus, and respiratory syncytial virus (for a review, see reference 56). Heparan sulfates also bind HCV glycoproteins and were suggested to play a role in HCV capture from the bloodstream onto hepatocytes (3, 4). Heparan sulfates are glycosaminoglycans, which are long linear polysaccharides covalently linked to a core protein and together forming a proteoglycan. Heparin is a heparan sulfate homolog that is naturally synthesized by mast cells, which can be extracted from animal mucosa and used in in vitro assays. It was already demonstrated to bind intracellular recombinant forms of E1 and E2 glycoproteins but not the HCVpp-associated form (10). As shown by pulldown assay, both intra- and extracellular E1 and E2 glycoproteins were able to bind heparin-conjugated beads (Fig. 3A ). Preincubation of E1E2-containing extracts with 0.1 to 10 mg/ml soluble heparin abolished this interaction (but not the interaction with the GNA lectin [data not shown]), comforting its specificity. It is worth noting that, after GNA pulldown, E2 was detected as a more diffuse band corresponding to different glycoforms, whereas only a sharp fast-migrating band was detected after precipitation with heparin beads, suggesting that heparin might recognize only a particular subset of glycoproteins.
FIG. 3.
Interaction of viral envelope glycoproteins with HCV entry factors. (A) Heparin pulldown assay. Infected cells or viral supernatants were lysed and incubated with heparin-conjugated beads or GNA-conjugated beads as a positive control. As a specificity control, E1E2-containing extracts were incubated beforehand with soluble heparin at various concentrations. *, the slow-migrating band detected by the anti-E2 antibody in cell lysate might correspond to the E2-p7-NS2 precursor. (B) CD81 pulldown assay. Infected cells or viral supernatants were lysed and incubated with GS4B beads or GS4B beads incubated beforehand with a recombinant form of mouse CD81 protein (mCD81) or human CD81 protein (hCD81). (A, B) Pulled-down proteins were eluted from the beads in Laemmli buffer and analyzed by SDS-PAGE and Western blotting.
Moreover, intra- and extracellular E1 and E2 were able to bind a recombinant form of human CD81 but not, or to a much lesser extent, the murine homolog (Fig. 3B). Importantly, CD81 has been shown to interact directly with the E2 glycoprotein but not with E1 (51). Therefore, this experiment indicated that E1 coprecipitated with E2 and, hence, that at least a fraction of E1 proteins formed complexes with E2 in infected cells and on virions.
These pulldown experiments confirmed the role of CD81 and heparan sulfates in HCVcc infection. They also indicated that intracellular E1 and E2 were already competent for interaction with some cellular entry factors.
Virion-associated envelope glycoproteins display a mixture of high-mannose- and complex-type glycans.
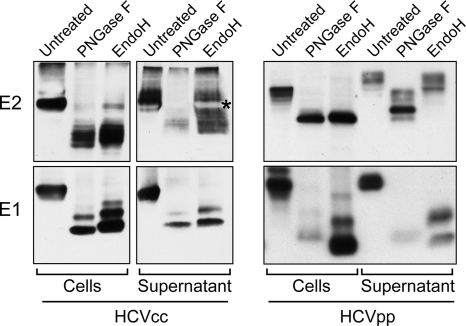
Contrary to most flaviviruses, HCV envelope proteins are heavily glycosylated (25). For instance, in the JFH-1 HCVcc system, E1 and E2 harbor 4 and 11 glycans, respectively. To characterize E1- and E2-associated glycans, infected cell lysates or concentrated supernatants were digested by endoglycosidases (Fig. 4, left). To rule out a selective pulldown of particular E1 and E2 glycoforms in these experiments, crude lysates of infected cells or supernatants were used rather than GNA-pulled-down glycoproteins. PNGase F was used as a control, as it cleaves both high-mannose- and complex-type glycans, yielding the deglycosylated protein. However, Endo H specifically cleaves high-mannose-type glycans and some hybrid glycans.
FIG. 4.
Glycosylation pattern of HCV envelope glycoproteins. (Left) E1E2-containing extracts from HCVcc-infected cells or viral concentrated supernatant were denatured and divided into three equal aliquots. The first aliquot was kept untreated, the second one was digested with PNGase F, and the third one was digested with Endo H. The samples were analyzed by SDS-PAGE and Western blotting. The white band labeled with an asterisk corresponds to a disturbance in the E2 migration profile, likely due to the BSA protein, as seen by membrane staining with Ponceau solution (data not shown). (Right) A similar analysis was conducted with HCVpp-producing cells or secreted HCVpp after partial purification by ultracentrifugation over a sucrose cushion.
PNGase F digestion confirmed the heavy glycosylation status of intracellular and secreted E1 and E2. While the undigested E1 and E2 glycoproteins migrated as ∼65- and ∼30-kDa proteins, respectively, their deglycosylated forms were found to migrate around ∼37 and ∼17 kDa, respectively. The doublet of bands observed after E1 deglycosylation with either PNGase F or Endo H had already been observed in the HCVpp system (46). It likely corresponds to the presence of a glycosidase-resistant glycan due to a poor accessibility on the E1 glycoprotein despite sample denaturation.
Intracellular E1 and most intracellular E2 were sensitive to Endo H digestion, suggesting that they harbored high-mannose-type glycans. Secreted E1 remained Endo H sensitive, whereas digestion of E2 with Endo H yielded a ladder of glycoforms. This indicated that glycans associated with E2 were only partially matured, with some glycans being modified with Golgi enzymes while others remained of the high-mannose type. It is worth noting that the glycans associated with E2 ranged from completely Endo H resistant to completely Endo H sensitive, suggesting that most E2 glycosylation sites can harbor either a high-mannose-type glycan or a modified glycan. As a comparison, the same experiment was performed with the HCVpp system (Fig. 4, right). Interestingly, while intracellular E1 and E2 shared the same glycosylation profile between HCVcc and HCVpp systems, HCVpp-associated E2 displayed a majority of complex-type glycans, consistent with previous reports (21, 46). These data suggest that due to differences in the assembly process, the accessibility of HCV glycans to Golgi glycosidases and/or glycosyltransferases is different between HCVcc and HCVpp. Furthermore, our results also highlight the heterogeneity of the glycosylation pattern of HCV glycoprotein E2 in the context of the HCVcc system.
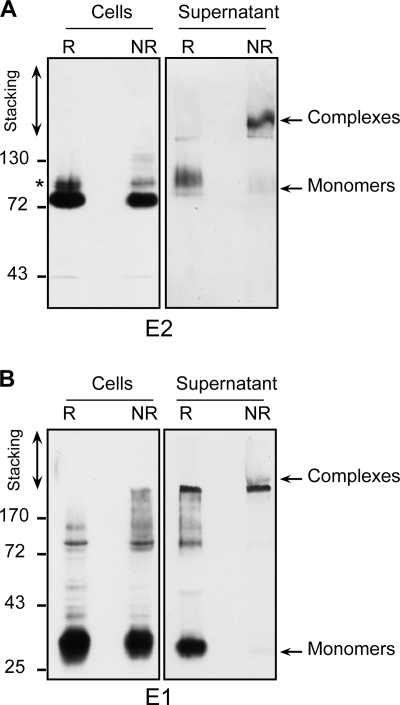
Virion-associated envelope glycoproteins are part of large disulfide-linked complexes.
It has been suggested that the functional complex for HCV entry consisted of a noncovalent E1E2 heterodimer (16, 46). In order to test whether this was the case for HCVcc-associated E1 and E2, we compared the migration profiles of HCV envelope glycoproteins in SDS-PAGE under reducing and nonreducing conditions (Fig. 5). In cell extracts, E1 and E2 migration profiles were barely affected by the addition of reducing agent. Together with the coprecipitation data (Fig. 3), this indicated that most intracellular E1 and E2 belonged to noncovalently linked complexes. In contrast, in the virion preparations, monomeric E1 and E2 were barely detected under nonreducing conditions. Instead, a high-molecular-mass complex could be detected by both anti-E1 and anti-E2 antibodies. These complexes were detected between the stacking and separating gels.
FIG. 5.
Oligomerization of envelope glycoproteins analyzed by SDS-PAGE. Glycoproteins from infected cell lysates or supernatants were separated by SDS-PAGE under reducing (R) or nonreducing (NR) conditions and analyzed by Western blotting with anti-E2 (A) or anti-E1 (B) antibodies. Molecular mass markers (in kDa) are indicated on the left. *, the slow-migrating band detected by the anti-E2 antibody in cell lysate might correspond to the E2-p7-NS2 precursor.
Importantly, similar results were obtained when samples were heated in Laemmli buffer at 37°C rather than 70°C or when glycoproteins were solubilized with n-dodecyl-β-d-maltopyranoside rather than Triton X-100. Likewise, a similar migration pattern was observed with freshly harvested infected cell culture supernatant after GNA pulldown of envelope glycoproteins (data not shown), indicating that the formation of large complexes was not an artifact due to virus treatment by ultracentrifugation or virus storage at −80°C.
To further characterize virion-associated envelope glycoproteins, we analyzed E1E2 complexes by sedimentation analysis in sucrose gradients under nondenaturing conditions. For such analyses, E1 and E2 from infected cells or concentrated supernatant were solubilized with n-dodecyl-β-d-maltopyranoside, a long-carbon-chain detergent, efficient at solubilizing membrane-inserted proteins while keeping their native conformation and properties. A control gradient with known protein standards was spun in parallel and served as a calibration. Fractions were harvested, and glycoproteins were precipitated with GNA to analyze their distribution in the gradient (Fig. 6A). Cell-extracted E1 and E2 were widespread along the gradient, although most glycoproteins sedimented in low-density fractions between the 66-kDa and 200-kDa marker proteins that might correspond to E1E2 heterodimers. In contrast, the sedimentation of virion-associated E1E2 complexes showed a completely different pattern. Indeed, the majority of virion-associated glycoproteins sedimented in the gradient pellet, corresponding to complexes larger than 440 kDa. Only a minority of virion-associated E1 and E2 proteins was found in lower-density fractions throughout the gradient. In conclusion, virion-associated envelope glycoproteins form high-molecular-weight disulfide bond complexes.
FIG. 6.
Separation of envelope glycoprotein complexes in sucrose density gradients. Glycoprotein complexes from infected cell lysates or supernatants were separated by sedimentation through a 5-to-20% sucrose gradient. Eleven 1-ml fractions and the gradient pellet (P) were harvested. After GNA pulldown (A) or CD81 pulldown (B), samples were analyzed for the presence of E1 and E2 glycoproteins by reducing SDS-PAGE and Western blotting. The sedimentation profiles of several standard proteins in a parallel gradient are indicated above. *, the slow-migrating band detected by the anti-E2 antibody in cell lysate might correspond to the E2-p7-NS2 precursor.
Virion-associated disulfide-linked E1E2 complexes are in a conformation competent for entry.
In various expression systems, HCV envelope glycoproteins have been shown to have a tendency to misfold and produce nonfunctional aggregates (18, 19, 21, 46). This could explain the presence of E1E2 covalent complexes at the surface of HCVcc. In this case, only a small proportion of HCV-associated E1 and E2 proteins would be functional for entry, with the following two possibilities: (i) the presence of two populations of viruses, a population of infectious particles harboring small noncovalent E1E2 complexes and a population of noninfectious particles harboring large covalent E1E2 complexes or (ii) the presence of a single population of viruses, harboring a mixture of noncovalent E1E2 complexes and large E1E2 complexes.
To discriminate between these possibilities, we first analyzed the distribution of noncovalent E1E2 complexes and large E1E2 complexes on virions separated in a density gradient. To this end, cell-cultured HCVs were separated in a sucrose gradient, as previously shown in Fig. 2. The fractions were analyzed for the presence of E1 and E2 by GNA pulldown and SDS-PAGE under reducing and nonreducing conditions. Interestingly, after particle sedimentation in a sucrose gradient, all E1E2-positive fractions of viral particles contained the same ratio of large disulfide-linked complexes and noncovalent E1 and E2 under nonreducing conditions (data not shown). Therefore, we could not detect different populations of E1E2-positive viruses based on their density.
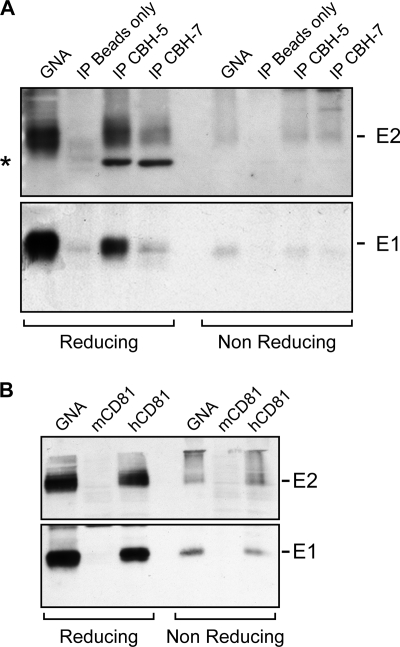
Next, to assess the functionality of E1E2 large disulfide-linked complexes, we studied their conformation using neutralizing MAbs or a recombinant form of CD81. E1 and E2 from concentrated infectious supernatant were efficiently immunoprecipitated with CBH-5 or CBH-7 antibodies, as seen by SDS-PAGE under reducing conditions (Fig. 7 A, left). However, by SDS-PAGE under nonreducing conditions, only a small fraction of immunoprecipitated E1 and E2 was detectable as monomeric species (Fig. 7A, right), indicating that most immunoprecipitated E1 and E2 were part of large disulfide-linked complexes. Similarly, E1 and E2 could be precipitated from concentrated supernatant by a recombinant form of CD81 (Fig. 3B and 7B, left), but under nonreducing conditions, the majority of immunoprecipitated E1 and E2 appeared to belong to large disulfide-linked complexes (Fig. 7B, right). Lastly, large covalent E1E2 complexes separated in sucrose gradients could also be precipitated by recombinant CD81 (Fig. 6B), confirming these results. We also observed that, in cells, part of glycoproteins consisted of E1 or E2 monomers (Fig. 6A and B, fraction 3), since E1 was not coprecipitated with recombinant CD81 in low-density fractions. However, most E1 and E2 did coprecipitate with recombinant CD81, indicating they were part of heterodimers (Fig. 6A and B, fractions 4 and 5) or larger complexes. All together, these data suggest that E1 and E2 incorporated into large covalent complexes exhibit a functional conformation.
FIG. 7.
Covalent E1E2 complexes display a functional conformation. (A) HCV glycoproteins from concentrated viral supernatant were immunoprecipitated (IP) with CBH-5 or CBH-7 MAbs or precipitated with GNA or protein-A Sepharose beads alone as positive and negative controls, respectively. Samples were analyzed by SDS-PAGE and Western blotting under reducing (left) or nonreducing (right) conditions. The band indicated by an asterisk corresponds to the heavy chain of the immunoglobulin used for immunoprecipitation. (B) HCV glycoproteins from concentrated viral supernatant were precipitated with GS4B beads incubated beforehand with a recombinant form of mouse CD81 protein (mCD81) or human CD81 protein (hCD81) or with GNA beads as positive control. Samples were analyzed by SDS-PAGE and Western blotting under reducing (left) or nonreducing (right) conditions.
DISCUSSION
The purpose of this study was to describe HCV glycoproteins associated with infectious viral particles. Indeed, E1 and E2 glycoproteins have been extensively described in heterologous expression systems, but until now, the lack of an efficient system to propagate the virus has hindered their characterization on the native HCV virion. Here, we compared the intracellular prebudding and secreted mature forms of E1 and E2 glycoproteins in terms of their functionality, maturation, and oligomerization. Importantly, besides maturation of some of their glycans, HCV envelope glycoproteins showed a dramatic change in their oligomeric state after incorporation into the viral particle. Indeed, virion-associated E1 and E2 envelope glycoproteins formed large covalent complexes stabilized by disulfide bridges, whereas the intracellular forms of these proteins assembled as noncovalent heterodimers.
Secreted E1 and E2 glycoproteins are associated with infectious virions.
A first concern when characterizing virion-associated envelope proteins was to make sure the glycoproteins detected in infected cell supernatant were genuinely associated with infectious virions rather than with defective particles or cell contaminants. Indeed, HCVcc have low specific infectivity, as measured by their RNA content (24, 39, 60, 62), and the heterogeneity of viral particles produced in cell culture or found in patient sera has already been described (24, 45). It was therefore crucial to analyze the distribution of envelope glycoproteins among the various populations of viral particles. In agreement with previous studies (24), we found two main populations of viral particles with different densities by analyzing infectivity and viral RNA. The first population (Fig. 2A, fractions 1 to 5) was the most infectious and had a density lower than 1.130 g/ml, with a peak of infectivity at 1.112 g/ml. Interestingly, most envelope proteins but little viral RNA content were found associated with this population. For instance, although it was the most infectious (∼25% of total infectivity), fraction 4 (Fig. 2A) hardly accounted for 5% of secreted viral RNA. A second population of viral particles (Fig. 2A, fractions 5 to 7) had an average density of 1.141 g/ml and corresponded to most secreted viral RNA and core proteins but was only poorly infectious. For instance, the fraction with the highest RNA content (fraction 6) accounted for 39% of viral RNA but only for 8.5% of the total infectivity. Because of its higher density and relative low abundance of envelope glycoproteins, it is likely that this population consists of a mixture of enveloped particles displaying no or few envelope glycoproteins and/or encapsidated but nonenveloped viral RNA. Therefore, the acquisition of a viral envelope seems to be a bottleneck in the secretion of cell-cultured infectious particles and could partly explain the low specific infectivity observed with HCVcc (about 2,000 genome equivalents per focus-forming unit in our case).
HCVcc travels through the Golgi apparatus, where it acquires complex glycans.
As already reported (25), HCV glycoproteins are heavily glycosylated, with a glycan shield accounting for nearly half of the glycoprotein mass. Intracellular envelope glycoproteins displayed mainly high-mannose-type glycans, consistently with their accumulation in the ER. However, complex-type glycans were found on the HCVcc-associated E2 glycoprotein. Such complex glycans are hallmarks of protein transit through the Golgi apparatus since they result from the maturation of high-mannose-type glycans by Golgi glycosidases and glycosyltransferases (28). E2 glycoforms obtained after Endo H digestion ranged from completely Endo H-resistant to Endo H-sensitive E2 proteins (Fig. 4), indicating that most E2 glycosylation sites can display either high-mannose-type glycans or modified glycans.
The incomplete maturation of E1 and E2 glycans indicated that some glycans remained shielded from Golgi enzymes. In contrast, HCVpp-associated glycans were more efficiently matured on the E2 glycoprotein. This underlined major differences in the trafficking of E1 and E2 glycoproteins, depending on the virus system used. These disparities are likely due to differences in the assembly process between HCVpp and HCVcc. Indeed, cell-cultured HCVs assemble in an ER-derived compartment (42), whereas HCVpp are assembled in a post-Golgi compartment (54). Therefore, HCV envelope glycoproteins incorporated into HCVpp travel through the secretory pathway independently of the other viral components and might be more easily accessible to Golgi enzymes, whereas they are supposed to travel through the secretory pathway in association with nascent viral particles in the context of the HCVcc system. In the latter case, they would probably be much less accessible to Golgi enzymes, especially if they belong to high-order complexes stabilized by disulfide bonds. Importantly, high-mannose-type glycans are potential targets for antivirals (for a review, see reference 1), and the high efficiency of cyanovirin-N in inhibiting HCVcc infectivity (29) coincides with the preponderance of high-mannose-type glycans on HCVcc envelope glycoproteins, for which cyanovirin-N is specific.
HCV glycoproteins do not seem to undergo proteolytic cleavage during their maturation.
There was no apparent cleavage of the E1 or E2 protein during virus secretion, since secreted glycoproteins were not smaller than their intracellular forms. However, we cannot rule out a cleavage occurring close to the protein ends. This distinguishes HCV from flaviviruses, for which the prM envelope companion protein becomes cleaved during virus maturation, yielding a mature protein about half the size of the precursor and inducing a rearrangement of the oligomeric form of the envelope protein (38).
Virion-associated E1 and E2 envelope glycoproteins form large covalent complexes stabilized by disulfide bridges.
In HCVpp-producing cells or in cells expressing recombinant forms of HCV glycoproteins, E1 and E2 proteins have been shown to form noncovalent heterodimers and large covalent complexes (46), with the latter corresponding to nonfunctional aggregates while the former was thought to be the functional entry complex (16, 18, 19, 21, 46). Surprisingly, in the HCVcc system, covalent complexes were preponderant on secreted virus but were the minority in infected cells. Therefore, it looks like E1E2 large covalent complexes are selectively incorporated into secreted virions and therefore do not correspond to aggregates that would likely be retained in the ER. However, in contrast to recombinant proteins, these complexes seem to maintain a native conformation, as they were able to bind conformation-sensitive neutralizing antibodies as well as a recombinant form of CD81.
The large molecular masses of virion-associated glycoprotein complexes suggests that these proteins might form an organized network at the virion surface. The presence of disulfide bridges between HCV envelope glycoproteins on the surface of the particle might reflect their role during the assembly process. Indeed, these interactions might be essential to facilitate the budding process. For some viruses, lateral interactions between envelope proteins are indeed known to play an active role during the budding process. This is the case for the alphaviruses (23) and the flaviviruses (20). However, for these viruses, interactions between the envelope proteins do not involve covalent interactions. In contrast, the involvement of disulfide bridges in protein-protein interactions has been proposed for the envelope proteins of hepatitis B virus (HBV) (and hepatitis D virus [HDV]) (9). Moreover, the HCV close relatives pestiviruses display envelope glycoproteins extensively linked by complex intermolecular disulfide bridges, although they form only homo- and heterodimers but no larger oligomers (58). In the case of the HCV, it is interesting to note that subviral particles can be produced when only the HCV envelope glycoproteins are expressed in lipoprotein-producing cell lines (33). Furthermore, such particles are functional in an in vitro fusion assay (49), suggesting that HCV envelope proteins also play an active role during the budding process. However, the oligomeric state of these recombinant envelope proteins has not been determined. Together with these observations, our data suggest that lateral protein-protein interactions assisted by disulfide-bond formation might play an active role in the budding process of the HCV particle. Furthermore, we cannot exclude that the entire virus is held together through disulfide linkages.
Based on comparison with class II fusion proteins and on the identification of intramolecular disulfide bonds in a recombinant form of the E2 glycoprotein ectodomain, a model of tertiary organization has been recently proposed for this protein (36). In this model, all the cysteine residues are involved in intramolecular disulfide bonds. However, domain II is suggested to be rather flexible and disordered. Furthermore, E1 might play a role in stabilizing this domain, which contains a putative fusion peptide. Due to the potential disordered structure of domain II, one possibility is that, after reshuffling, some cysteine residues in this domain might be involved in the intermolecular interactions observed at the surface of the virion. However, we cannot exclude the potential involvement of the cysteine residue(s) located in another domain. Interestingly, the E1 protein contains a conserved protein disulfide isomerase motif (15), which might play a role in disulfide bond reshuffling.
The presence of disulfide bonds in virion-associated glycoprotein complexes may also explain the lack of sensitivity of HCVcc to low-pH treatment (59). Enveloped viruses that are internalized by endocytosis are, in general, very sensitive to low-pH treatment. Indeed, exposure to acidic pH induces a conformational change in the envelope glycoproteins that prematurely exposes the fusion peptide and thereby diminishes the viral infectivity. However, like the related pestivirus bovine viral diarrhea virus (BVDV) (37), HCV requires an additional trigger to become acid sensitive. The presence of disulfide bridges between HCV envelope proteins might indeed block any conformation change in the presence of acidic pH.
To conclude, this study is the first one to describe envelope glycoproteins incorporated onto infectious HCV particles and uncovers unexpected features that should help orientate investigations into HCV assembly and entry.
Acknowledgments
We thank Sophana Ung for assistance with preparing the figures and Czeslaw Wychowski and Birke A. Tews for useful discussions. We are also grateful to T. Wakita, S. Foung, C. M. Rice, F. L. Cosset, and J. McKeating for providing reagents.
This study was supported by a grant from the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS). G.V. was supported by a fellowship from the French Ministry of Research and the Région Nord Pas-de-Calais, and X.T. was supported by a fellowship from the ANRS. J.D. is an international scholar of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 28 July 2010.
REFERENCES
- 1.Balzarini, J. 2007. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 5:583-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartenschlager, R. 2005. The hepatitis C virus replicon system: from basic research to clinical application. J. Hepatol. 43:210-216. [DOI] [PubMed] [Google Scholar]
- 3.Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003-41012. [DOI] [PubMed] [Google Scholar]
- 4.Barth, H., E. K. Schnober, F. Zhang, R. J. Linhardt, E. Depla, B. Boson, F. L. Cosset, A. H. Patel, H. E. Blum, and T. F. Baumert. 2006. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J. Virol. 80:10579-10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartosch, B., J. Bukh, J. C. Meunier, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. U. S. A. 100:14199-14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197:633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartosch, B., Y. Ciczora, C. Montigny, G. Verney, C. Wychowski, E. I. Pecheur, M. Le Maire, F. L. Cosset, J. Dubuisson, P. Falson, and F. Penin. 2007. E1 envelope glycoproteins from hepatitis C virus particles exists as a transmembrane domain-dependent trimer, abstr. 0-54. Abstr. 14th Int. Symp. Hepat. C Virus Relat. Viruses. International Symposium on Hepatitis C Virus and Related Viruses, Glasgow, United Kingdom.
- 8.Blanchard, E., S. Belouzard, L. Goueslain, T. Wakita, J. Dubuisson, C. Wychowski, and Y. Rouille. 2006. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 80:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruss, V. 2007. Hepatitis B virus morphogenesis. World J. Gastroenterol. 13:65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callens, N., Y. Ciczora, B. Bartosch, N. Vu-Dac, F. L. Cosset, J. M. Pawlotsky, F. Penin, and J. Dubuisson. 2005. Basic residues in hypervariable region 1 of hepatitis C virus envelope glycoprotein e2 contribute to virus entry. J. Virol. 79:15331-15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton, R. F., A. Owsianka, J. Aitken, S. Graham, D. Bhella, and A. H. Patel. 2002. Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles. J. Virol. 76:7672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocquerel, L., S. Duvet, J. C. Meunier, A. Pillez, R. Cacan, C. Wychowski, and J. Dubuisson. 1999. The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J. Virol. 73:2641-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocquerel, L., J. C. Meunier, A. Pillez, C. Wychowski, and J. Dubuisson. 1998. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J. Virol. 72:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocquerel, L., C. Wychowski, F. Minner, F. Penin, and J. Dubuisson. 2000. Charged residues in the transmembrane domains of hepatitis C virus glycoproteins play a major role in the processing, subcellular localization, and assembly of these envelope proteins. J. Virol. 74:3623-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean, J., I. Boo, G. Talbo, P. Poumbourios, and H. E. Drummer. 2009. Fusion activation triggers of hepatitis C virus envelope glycoproteins E1 and E2: potential role for thiol disulfide isomerisation, abstr. 0-10. Abstr. 16th Int. Symp. Hepat. C Virus Relat. Viruses. International Symposium on Hepatitis C Virus and Related Viruses, Nice, France.
- 16.Deleersnyder, V., A. Pillez, C. Wychowski, K. Blight, J. Xu, Y. S. Hahn, C. M. Rice, and J. Dubuisson. 1997. Formation of native hepatitis C virus glycoprotein complexes. J. Virol. 71:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgrange, D., A. Pillez, S. Castelain, L. Cocquerel, Y. Rouille, J. Dubuisson, T. Wakita, G. Duverlie, and C. Wychowski. 2007. Robust production of infectious viral particles in Huh-7 cells by introducing mutations in hepatitis C virus structural proteins. J. Gen. Virol. 88:2495-2503. [DOI] [PubMed] [Google Scholar]
- 18.Dubuisson, J., H. H. Hsu, R. C. Cheung, H. B. Greenberg, D. G. Russell, and C. M. Rice. 1994. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 68:6147-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubuisson, J., and C. M. Rice. 1996. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J. Virol. 70:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferlenghi, I., M. Clarke, T. Ruttan, S. L. Allison, J. Schalich, F. X. Heinz, S. C. Harrison, F. A. Rey, and S. D. Fuller. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593-602. [DOI] [PubMed] [Google Scholar]
- 21.Flint, M., C. Logvinoff, C. M. Rice, and J. A. McKeating. 2004. Characterization of infectious retroviral pseudotype particles bearing hepatitis C virus glycoproteins. J. Virol. 78:6875-6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flint, M., C. Maidens, L. D. Loomis-Price, C. Shotton, J. Dubuisson, P. Monk, A. Higginbottom, S. Levy, and J. A. McKeating. 1999. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 73:6235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forsell, K., L. Xing, T. Kozlovska, R. H. Cheng, and H. Garoff. 2000. Membrane proteins organize a symmetrical virus. EMBO J. 19:5081-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gastaminza, P., S. B. Kapadia, and F. V. Chisari. 2006. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J. Virol. 80:11074-11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goffard, A., N. Callens, B. Bartosch, C. Wychowski, F. L. Cosset, C. Montpellier, and J. Dubuisson. 2005. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J. Virol. 79:8400-8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goueslain, L., K. Alsaleh, P. Horellou, P. Roingeard, V. Descamps, G. Duverlie, Y. Ciczora, C. Wychowski, J. Dubuisson, and Y. Rouille. 2010. Identification of GBF1 as a cellular factor required for hepatitis C virus RNA replication. J. Virol. 84:773-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadlock, K. G., R. E. Lanford, S. Perkins, J. Rowe, Q. Yang, S. Levy, P. Pileri, S. Abrignani, and S. K. Foung. 2000. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J. Virol. 74:10407-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helenius, A., and M. Aebi. 2001. Intracellular functions of N-linked glycans. Science 291:2364-2369. [DOI] [PubMed] [Google Scholar]
- 29.Helle, F., C. Wychowski, N. Vu-Dac, K. R. Gustafson, C. Voisset, and J. Dubuisson. 2006. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J. Biol. Chem. 281:25177-25183. [DOI] [PubMed] [Google Scholar]
- 30.Higginbottom, A., E. R. Quinn, C. C. Kuo, M. Flint, L. H. Wilson, E. Bianchi, A. Nicosia, P. N. Monk, J. A. McKeating, and S. Levy. 2000. Identification of amino acid residues in CD81 critical for interaction with hepatitis C virus envelope glycoprotein E2. J. Virol. 74:3642-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoofnagle, J. H. 2002. Course and outcome of hepatitis C. Hepatology 36:S21-S29. [DOI] [PubMed] [Google Scholar]
- 32.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. U. S. A. 100:7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Icard, V., O. Diaz, C. Scholtes, L. Perrin-Cocon, C. Ramiere, R. Bartenschlager, F. Penin, V. Lotteau, and P. Andre. 2009. Secretion of hepatitis C virus envelope glycoproteins depends on assembly of apolipoprotein B positive lipoproteins. PLoS One 4:e4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato, T., A. Furusaka, M. Miyamoto, T. Date, K. Yasui, J. Hiramoto, K. Nagayama, T. Tanaka, and T. Wakita. 2001. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J. Med. Virol. 64:334-339. [DOI] [PubMed] [Google Scholar]
- 35.Keck, Z. Y., J. Xia, Z. Cai, T. K. Li, A. M. Owsianka, A. H. Patel, G. Luo, and S. K. Foung. 2007. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J. Virol. 81:1043-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krey, T., J. d'Alayer, C. M. Kikuti, A. Saulnier, L. Damier-Piolle, I. Petitpas, D. X. Johansson, R. G. Tawar, B. Baron, B. Robert, P. England, M. A. Persson, A. Martin, and F. A. Rey. 2010. The disulfide bonds in glycoprotein E2 of hepatitis C virus reveal the tertiary organization of the molecule. PLoS Pathog. 6:e1000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krey, T., H. J. Thiel, and T. Rumenapf. 2005. Acid-resistant bovine pestivirus requires activation for pH-triggered fusion during entry. J. Virol. 79:4191-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, L., S. M. Lok, I. M. Yu, Y. Zhang, R. J. Kuhn, J. Chen, and M. G. Rossmann. 2008. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science 319:1830-1834. [DOI] [PubMed] [Google Scholar]
- 39.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 40.Lindenbach, B. D., and C. M. Rice. 2005. Unravelling hepatitis C virus replication from genome to function. Nature 436:933-938. [DOI] [PubMed] [Google Scholar]
- 41.Meertens, L., C. Bertaux, and T. Dragic. 2006. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J. Virol. 80:11571-11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyanari, Y., K. Atsuzawa, N. Usuda, K. Watashi, T. Hishiki, M. Zayas, R. Bartenschlager, T. Wakita, M. Hijikata, and K. Shimotohno. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9:1089-1097. [DOI] [PubMed] [Google Scholar]
- 43.Moradpour, D., F. Penin, and C. M. Rice. 2007. Replication of hepatitis C virus. Nat. Rev. Microbiol. 5:453-463. [DOI] [PubMed] [Google Scholar]
- 44.Nakabayashi, H., K. Taketa, K. Miyano, T. Yamane, and J. Sato. 1982. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858-3863. [PubMed] [Google Scholar]
- 45.Nielsen, S. U., M. F. Bassendine, A. D. Burt, C. Martin, W. Pumeechockchai, and G. L. Toms. 2006. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J. Virol. 80:2418-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Op De Beeck, A., C. Voisset, B. Bartosch, Y. Ciczora, L. Cocquerel, Z. Keck, S. Foung, F. L. Cosset, and J. Dubuisson. 2004. Characterization of functional hepatitis C virus envelope glycoproteins. J. Virol. 78:2994-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owsianka, A., A. W. Tarr, V. S. Juttla, D. Lavillette, B. Bartosch, F. L. Cosset, J. K. Ball, and A. H. Patel. 2005. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J. Virol. 79:11095-11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owsianka, A. M., A. W. Tarr, Z. Y. Keck, T. K. Li, J. Witteveldt, R. Adair, S. K. Foung, J. K. Ball, and A. H. Patel. 2008. Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J. Gen. Virol. 89:653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pecheur, E. I., O. Diaz, J. Molle, V. Icard, P. Bonnafous, O. Lambert, and P. Andre. 15 June 2010. Morphological characterization and fusion properties of triglyceride-rich lipoproteins obtained from cells transduced with HCV glycoproteins. J. Biol. Chem. [Epub ahead of print.] doi: 10.1074/jbc.M110.131664. [DOI] [PMC free article] [PubMed]
- 50.Perotti, M., N. Mancini, R. A. Diotti, A. W. Tarr, J. K. Ball, A. Owsianka, R. Adair, A. H. Patel, M. Clementi, and R. Burioni. 2008. Identification of a broadly cross-reacting and neutralizing human monoclonal antibody directed against the hepatitis C virus E2 protein. J. Virol. 82:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 52.Ralston, R., K. Thudium, K. Berger, C. Kuo, B. Gervase, J. Hall, M. Selby, G. Kuo, M. Houghton, and Q. L. Choo. 1993. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J. Virol. 67:6753-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouille, Y., F. Helle, D. Delgrange, P. Roingeard, C. Voisset, E. Blanchard, S. Belouzard, J. McKeating, A. H. Patel, G. Maertens, T. Wakita, C. Wychowski, and J. Dubuisson. 2006. Subcellular localization of hepatitis C virus structural proteins in a cell culture system that efficiently replicates the virus. J. Virol. 80:2832-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandrin, V., P. Boulanger, F. Penin, C. Granier, F. L. Cosset, and B. Bartosch. 2005. Assembly of functional hepatitis C virus glycoproteins on infectious pseudoparticles occurs intracellularly and requires concomitant incorporation of E1 and E2 glycoproteins. J. Gen. Virol. 86:3189-3199. [DOI] [PubMed] [Google Scholar]
- 55.Shimoike, T., S. Mimori, H. Tani, Y. Matsuura, and T. Miyamura. 1999. Interaction of hepatitis C virus core protein with viral sense RNA and suppression of its translation. J. Virol. 73:9718-9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spillmann, D. 2001. Heparan sulfate: anchor for viral intruders? Biochimie 83:811-817. [DOI] [PubMed] [Google Scholar]
- 57.Steinmann, E., F. Penin, S. Kallis, A. H. Patel, R. Bartenschlager, and T. Pietschmann. 2007. Hepatitis C virus p7 protein is crucial for assembly and release of infectious virions. PLoS Pathog. 3:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thiel, H. J., R. Stark, E. Weiland, T. Rumenapf, and G. Meyers. 1991. Hog cholera virus: molecular composition of virions from a pestivirus. J. Virol. 65:4705-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tscherne, D. M., C. T. Jones, M. J. Evans, B. D. Lindenbach, J. A. McKeating, and C. M. Rice. 2006. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J. Virol. 80:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wessel, D., and U. I. Flugge. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138:141-143. [DOI] [PubMed] [Google Scholar]
- 62.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]