Abstract
Granuloma formation is an inflammatory response of the host against invading pathogens or indigestible substances. We generated mesenteric oil granulomas by injecting pristane into the peritoneal cavity (PC) of mice, and compared oil granuloma formation in the C57BL/6J and BALB/cByJ strains of mice. The formation and kinetics of oil granulomas were distinct between the two strains. In C57BL/6J mice, injected pristane induced oil granuloma formation at both the mesenteric centers (MG) and margins (SG). MG was resolving by 11 weeks, and SG persisted. In BALB/cByJ mice, MG developed slower but persisted longer than in C57BL/6J mice, and SG resolved sooner than in C57BL/6J mice. Injection of India ink revealed that phagocytes were localised mainly to the SG in C57BL/6J mice, but were located diffusely in both MG and SG of BALB/cByJ mice. SG cells expressed more monocyte chemotactic protein-1 (MCP-1) mRNA than MG cells in C57BL/6J mice, but there was no difference in MCP-1 expression between the MG and SG in BALB/cByJ mice. These observations suggest that the recruitment of inflammatory leucocytes under the direction of chemokines differentiates the patterns of granuloma responses to pristane in C57BL/6J and BALB/cByJ mice.
Keywords: MCP-1, oil granuloma, pristane, strain
Granuloma formation constitutes a protective cellular reaction in response to persistent microbial antigens including Mycobacterium tuberculosis (Meyer et al. 1975), eggs of the blood fluke Schistosoma mansoni (Warren & Domingo 1970), and numerous pathogenic fungi (Hauser & Rothman 1950; Ley et al. 1951). The granuloma response sequesters foci of microbe pathogens, preventing their dissemination and restricting inflammation to protect surrounding tissue (Co et al. 2004). Granulomas can also be induced by foreign bodies resistant to catabolism, such as implanted biomaterials (Zeller 1983), sand particles (Ginsberg & Becker 1951), and coal dust (Kido et al. 1995).
The laboratories of Potter and Reeves have independently demonstrated that oil granulomas are readily induced in BALB/c mice by intraperitoneal injections of pristane, a naturally-occurring, saturated alkane (2,6,10,14-tetramethylpentadecane) (Potter & Maccardle 1964; Nacionales et al. 2006). The common history of pristane-induced granuloma responses in BALB/c mice has been well documented: a few days after injection, small volumes of pristane are phagocytosed by macrophages (Mφ) while larger volumes of pristane become surrounded by inflammatory leucocytes to form oil-cell aggregates that adhere to peritoneal surfaces, especially the mesentery (Potter & Maccardle 1964). Eventually, the mesothelium grows over the oil-cell aggregates to form oil granulomas that accumulate on mesenteric surfaces as long as free oil is available (Potter & Maccardle 1964).
In addition to Mφ, BALB/c pristane granulomas contain lymphocytes, neutrophils, and plasma cells (Potter & Maccardle 1964) that are recruited both from the PC and from the mesenteric blood supply (M. Potter, unpublished data).
Cellular responses to peritoneal pristane vary significantly between inbred mouse strains. For example, BALB/cJ but not C57BL/6J, develop arthritis (Wooley et al. 1989) and 50–60% of BALB/cAn mice develop peritoneal plasmacytomas (PCT) within a year of pristane injection (Potter 2003). In contrast, C57BL/6J do not develop arthritis (Wooley et al. 1989) and only 5% of C57BL/6J mice eventually develop PCT (Potter 2003). The genetic differences responsible for these disparate responses are poorly understood, in part because the induction and resolution of pristane granulomas in resistant strains has not been detailed.
Active granulomas are essential for BALB/cAn PCT induction and persistence, as most primary PCT do not survive when transplanted into normal, syngeneic hosts but do grow in pristane-conditioned recipients (Potter et al. 1972). Nordan et al. (1989) first noted exceptional amounts of interleukin 6 (IL-6) in BALB/cAn granuloma Mφ and subsequent studies in IL-6 knockout animals demonstrated resistance to PCT induction by pristane (Lattanzio et al. 1997). IL-6 is, therefore, a crucial factor in PCT transformation. In addition to IL-6, other factors control PCT induction by pristane, including constitutive expression of the anti-apoptotic factors Bcl2 and Bcl-xL (Potter 2003; Silva et al. 2003). Reciprocally, two unidentified loci on chromosome 4 have been shown to mediate the resistance of DBA/2 mice to PCT induction (Potter et al. 1994).
Mesenteric granulomas (MG) are considered to be the cellular and environmental source of pristane-induced PCT (Potter & Maccardle 1964). Whereas the formation of pristane granulomas has been detailed in BALB/c mice (Potter & Maccardle 1964), granuloma induction in PCT resistant strains, including C57BL/6J, is poorly understood. To better understand why C57BL/6J mice are resistant to PCT, we studied the evolution of pristane granulomas in C57BL/6J mice in comparison to the sensitive BALB/cByJ strain. We found that pristane induces vigorous granuloma responses in both mouse strains but that the types of granulomatous tissue formed in these mice are distinct. In C57BL/6J mice, pristane results in the accumulation of prominent serosal granulomas (SG) at the interface of the mesenteric margins and gut; in contrast, BALB/cByJ animals respond with a centripetal distribution of MG. Distinctive expression patterns of monocyte chemotactic protein-1 (MCP-1), a Mφ attracting chemokine (Mantovani et al. 1993), is suggested to account for the major differences in granuloma development in C57BL/6J and BALB/cByJ mice, and we propose that the distribution of pristane granulomas on the mesentery and/or within the PC directs the distinct pathologies induced by pristane in C57BL/6J and BALB/cByJ mice.
Materials and methods
Mice and treatments
Male and female C57BL/6J and female BALB/cByJ mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and housed at the Duke University Animal Care Facility with sterile bedding, water, and food. To minimise any effects of incidental antigen exposure on granuloma formation, all mice used in this study were maintained under specific pathogen free conditions. At 7–9 weeks of age, mice received a single intraperitoneal injection of pristane (100 or 300 μl; 2.8 × 10−4 or 8.3 × 10−4 mol respectively) (≥95% pure, Sigma-Aldrich, St Louis, MO, USA). Age-matched, untreated mice were used as controls.
In a separate study of India ink uptake by the mesentery, 0.5 ml of a 1/10 dilution of India ink in PBS was injected i.p. into mice that had been given 300 μl pristane 3 weeks earlier. Mice were sacrificed and various tissues recovered for analysis at 4 h, 1 day, 4 days, and 12 days after injection.
All experiments involving animals were reviewed and approved by the Duke University Institutional Animal Care and Use Committee.
Tissue isolation and cell collection
Whole mesenteric tissue (WMT), from the distal duodenum through terminal ileum, was removed intact by dissection from naïve or pristane-treated mice. WMT was spread out in ice-cold PBS and photographed with a Canon camera (EOS 20D) equipped with a macro-lens (EF-S 60 mm). Single cell suspensions were prepared by digesting recovered tissue in RPMI 1640 medium containing 0.5 mg/ml type I collagenase (Sigma-Aldrich), 0.5 mg/ml type IV collagenase (Sigma-Aldrich), 0.2 mg/ml deoxyribonuclease I (DNase I, Sigma-Aldrich), and 25 mM HEPES buffer in Erlenmeyer flasks with constant stirring. The tissue digestion was carried out at room temperature for 1 h; the resulting cell suspension was removed and filtered through fine nylon mesh (Denville Scientific Inc., Metuchen, NJ, USA). Cells were then washed and resuspended in buffer (HBSS+ 5% FBS) for flow cytometric analysis or chemokine expression analysis by quantitative PCR. In some experiments, 5 mm biopsy punches from the MG of pristane-treated mice or the corresponding area in naïve mice were excised. Individual SG in pristane-treated mice and the corresponding area in naïve mice were removed separately by a 2 mm punch. Cells in these tissues were extracted using collagenase/DNase I as for WMT.
Resident PC cells were collected by lavage with 10 ml of ice-cold RPMI supplemented with 5% FBS. After centrifugation, cell pellets were harvested for flow cytometric analysis. Cell samples were also obtained from the spleen and right femur for flow cytometric comparison to PC cell and population analyses.
Flow cytometry
Cells harvested from lymphoid tissues or the PC were incubated with ammonium chloride buffer for 1 min on ice to lyse red blood cells (RBCs) before immunolabelling. Typically, approximately 106 nucleated cells were incubated with FcR (CD16/32) blocking antibody for 20 min, washed, and then labelled (15 min/ice) with antibodies specific for B220 (PE-Cy7), TCRβ (APC), CD11c (PE), Gr-1 (FITC), and CD11b (APC-Cy7). For B-cell subset analysis, cells were stained with antibodies specific for B220 (PE-Cy7), CD93 (APC), and IgD (PE). B1 cells were identified by labelling cells with B220 (PE-Cy7), CD5 (PE), IgM (TxR), and CD11b (APC-Cy7). CD138 (PE) antibody was used to identify plasma cells in tissues. Propidium iodide (Sigma-Aldrich) was included to identify dead cells. Labelled cells were analysed in a FACSVantage with DIVA option. Flow cytometric data were analysed with FlowJo software (Tree Star, Ashland, OR, USA).
Quantitative PCR
Total RNA was extracted from approximately 106 cells in TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Messenger RNA was reverse transcribed (Superscript III; Invitrogen) with oligo (dT) primer for 1 h at 50 °C. Quantitative PCR was performed in an iCycler thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA) with SYBR® Green PCR core reagents (Applied Biosystems, Foster City, CA, USA) and primers for specific genes. Amplification conditions were as follows: denaturation at 94 °C/10 min; amplification at 94 °C/15 s, 60 °C/45 s, 40 cycles. These primers were used: β-actin, forward, 5′-AGCCATGTACGTAGCCATCC-3′, and reverse, 5′-CTCTCAGC TGTGGTGGTGAA-3′; MCP-1, forward, 5′-CTTCTGGG CCTGCTGTTCA-3′, and reverse, 5′-CCAGCCTACTCATTG GGATCA-3′; TNFα, forward, 5′-TCCCCAAAG GGATGAGAAGTTC-3′, and reverse, 5′-GGGAGTAGACAAGGTACAAC-3′; IL-6, forward, 5′-CCCAACAGACCTGTCTAT ACC-3′, and reverse, 5′-CAGCTTATCTGTTAGGAGAGC-3′. The relative levels of mRNA for specific target genes were calculated by the comparative Ct (threshold cycle) method recommended by the manufacturer (Applied Biosystems) normalised to β-actin message in the same sample. In brief, specific ΔCt values were determined as [ΔCt = (Ctβ-actin)–(Cttarget)]; relative expression levels were defined as: 2−ΔCt.
Immunohistochemistry and immunofluorescence staining
Granulomatous tissues were excised en bloc, rinsed in media, and snap frozen in OCT embedding compound. Cryostat sections were cut at 5 μm, fixed in acetone/methanol (1:1 vol.) for 10 min at −20 °C, and air dried. Slides were then washed in PBS buffer supplemented with 0.5% BSA and 0.1% Tween-20, then blocked with purified rat IgG and/or antibody to mouse CD16/32 (1:100, BD Pharmingen, San Jose, CA, USA) for 1 h at room temperature. For immunofluorescence staining, sections were then stained in humidified boxes with PE-conjugated CD138 antibody (1:400, BD Pharmingen) for 3.5 h at room temperature. DAPI (1 μg/ml, Fluka, Buche, Switzerland) was included to identify nuclei. Sections were washed and mounted in Fluoromount-G. In a separate experiment, sections were incubated with biotin-conjugated CD11b antibody, followed by streptavidin-conjugated horseradish peroxidase (HRP). For haematoxylin and eosin (H&E) staining, SG and MG were harvested as described for immunofluorescent staining, and fixed in Fekete's modification of Tellyesniczky's fixative [70% ethanol:formalin:glacial acetic acid (20:2:1 vol.)] overnight at 4 °C. Fixed tissue was embedded in paraffin, cut in 5 μm sections, and processed for H&E staining.
Statistics
Differences between paired groups were analysed using Student's t-test; P values ≤ 0.05 were considered significant.
Results
Distinct mesenteric responses to pristane in C57BL/6J and BALB/cByJ mice
To induce a granuloma response, 100 or 300 μl pristane was administered i.p. to C57BL/6J or BALB/cByJ mice, which were then sacrificed at various intervals (day 3–week 11). The gut-associated WMT, from distal duodenum to terminal ileum, was excised and photographed. Pristane induced the formation of two classes of granulomas on the mesentery: granulomas about the center of the mesenteric tissue and away from peripheral fat and blood vessels (MG); and individual SG that developed along the border of the mesentery and intestine. Granuloma responses to pristane were dose dependent; in mice treated with 100 μl pristane, granulomas resolved within 8–11 weeks, whereas injection of 300 μl pristane resulted in granulomas that persisted beyond 11 weeks (Figure S1). In all subsequent experiments, mice were injected with 300 μl pristane.
Following pristane injection, the clear mesentery in C57BL/6J mice turned milky, thickened as early as day 3, and became more dense by week 3 (Figures 1a and S1). MG began resolving at week 11, when the mesenteric tissue appeared much clearer and patchy. SG development began at week 2 after pristane (Figure S1), and was well-formed by week 3, with an average of 41(± 12) [ (± SD)] SG per mouse (Figure 1b). At week 3, SG were typically 1–2 mm in diameter but continued to grow in number until the margins of individual SG became indistinguishable from those of neighbouring SG (week 11). The number of SG at week 11 was 57(± 7) per mouse.
(± SD)] SG per mouse (Figure 1b). At week 3, SG were typically 1–2 mm in diameter but continued to grow in number until the margins of individual SG became indistinguishable from those of neighbouring SG (week 11). The number of SG at week 11 was 57(± 7) per mouse.
Figure 1.
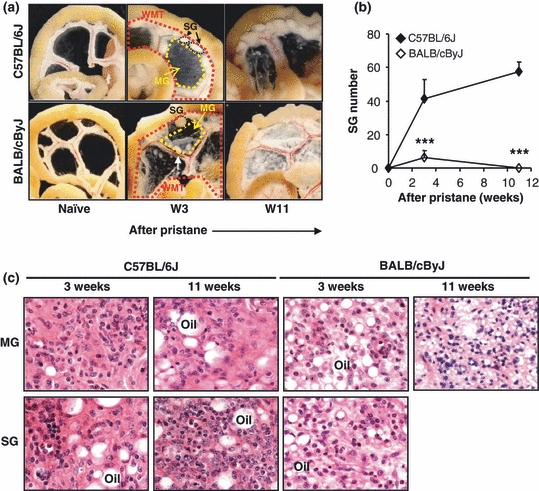
Differential response of the mesentery to pristane in C57BL/6J and BALB/cByJ mice. (a) After i.p. injection of 300 μl (8.31 × 10−4 mol) of pristane, C57BL/6J and BALB/cByJ mice were sacrificed at week 3 or week 11, and gut associated WMT from naïve or pristane-primed mice was photographed. A white arrow indicates a polyp on mesentery. (b) SG were numerated in C57BL/6J and BALB/cByJ mice. ***P < 0.001 shows the difference between C57BL/6J mice vs. BALB/cByJ mice. (c) An equivalent area of MG or strip of SG was excised from either C57BL/6J or BALB/cByJ mice at week 3 or 11 after pristane, and the tissue was fixed and stained with hematoxylin and eosin solution. Representative pictures are shown. Original magnification (objective): ×63.
Granulomas responses of BALB/cByJ mice to pristane followed closely the earlier description on BALB/cAn strain (Potter & Maccardle 1964) and the MG of BALB/cByJ mice exhibited distinctive structure and distributions in comparison to those in C57BL/6J mice, evidenced by focal leukocytic infiltrations. Similar to Dr. Potter's observations in BALB/cAn mice (Potter & Maccardle 1964), polyps were found on the mesentery of BALB/cByJ mice in response to pristane (Figure 1a). By week 11, the mesenteric deposition of pristane progressed dramatically, with numerous plaques of various size on the mesentery (Figure 1a). Polyps were not found at this time point. SG were rare in BALB/cByJ mice; only 6(± 3) SG per mouse were observed at week 3 and no SG were present at week 11 (Figure 1b). We conclude that the resolution of the granuloma response to pristane is distinct in these resistant and sensitive mouse strains.
Nonetheless by histology, the general structures of MG and SG were similar in both C57BL/6J and BALB/cByJ mice. Both MG and SG contained randomly distributed leucocytes. Typically, pristane was trapped in both MG and SG, appearing as oil droplets of various sizes (Figure 1c). It was our impression that the cellular density of granulomas in BALB/cByJ mice was somewhat lower than in C57BL/6J animals and that BALB/cByJ MG and SG contained more but smaller oil droplets (Figure 1c).
Comparable SG cellularity but distinct MG populations in C57BL/6J and BALB/cByJ mice
To quantify the cellularity of pristane-induced granulomas in C57BL/6J and BALB/cByJ mice, cells were isolated by collagenase digestion of WMT, which included both SG and MG, from naïve mice or pristane-treated mice. Cells were counted, and the types of leucocytes found in the WMT were identified by antibodies specific for B220, TCRβ, CD11c, CD11b, and Gr-1. Naïve C57BL/6J WMT contained few conventional B cells (B220+), T cells (TCRβ+), dendritic cells (DCs, CD11c+), neutrophils (CD11b+Gr-1hi) and Mφ (CD11b+Gr-1low) (Figure 2a).
Figure 2.
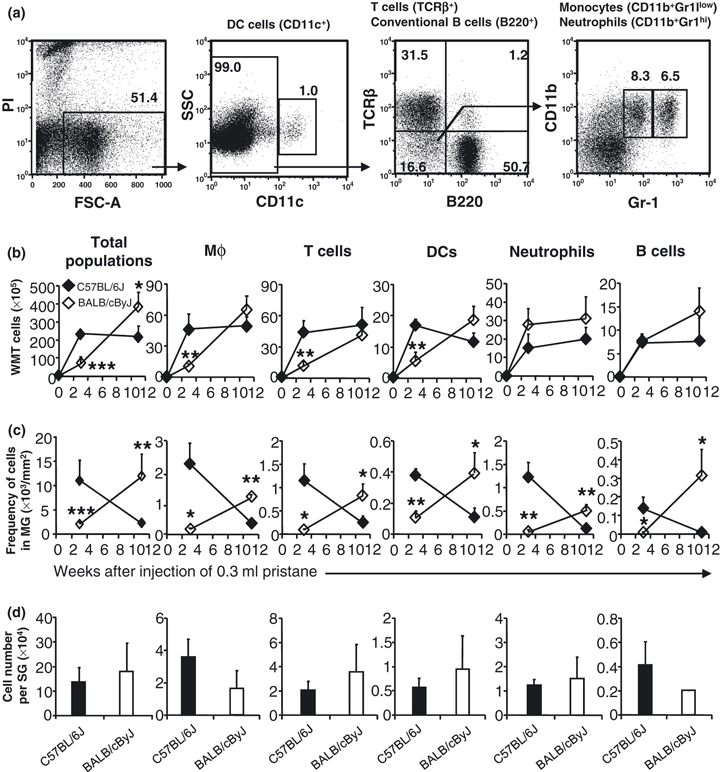
Leucocyte infiltration. (a) Identification of cell types by flow cytometry. Single cell suspensions prepared from spleen of pristane injected C57BL/6J mice (300 μl pristane, 11 weeks) were stained and analysed by flow cytometry as described in Materials and methods. Live cells were positively selected for analysis of CD11c expression, which results in identification of CD11c+ DCs and CD11c− fraction. Analysing expression of B220 and TCRβ of CD11c− cells reveals TCRβ+ fraction (T cells) and B220+ fraction (conventional B cells). The CD11c−B220−TCRβ− fraction was furthered fractionated by expression of CD11b and Gr-1 into CD11b+Gr-1low (monocytes) and CD11b+Gr-1hi (neutrophils). Monocytes, after migrating into tissues, are called Mφ. Mφ in mesenteric tissue were identified as CD11b+Gr-1low. (b) Cellularity of WMT was analysed by flow cytometry. (c) MG was punched out with a 5-mm punch and its cellularity was determined as for the cellularity of WMT. Data represent the number of total cells of each cell type per 1 mm2 of MG. (d) 4–8 Individual SG were isolated with a 2-mm punch at week 3 after injection of pristane, and digested with collagenase. Cells were enumerated. The SG cellularity is presented as cell number/SG. Black diamonds or columns represent C57BL/6J mice, and white diamonds or columns represent BALB/cByJ mice. *P < 0.05; **P < 0.01; ***P < 0.001 shows the difference between C57BL/6J mice vs. BALB/cByJ mice.
In normal mesenteric tissue, the combination of these leucocyte populations comprised <10% of the mesenteric tissue. After pristane, however, the mesentery was reshaped by a considerable influx of inflammatory leucocytes. The total number of cells in naïve C57BL/6J mesentery was 3.9(± 2.3) × 105 cells/mouse but 3 weeks after pristane injection, the total cell number increased 60-fold [2.4(± 0.2) × 107 cells/mouse, Figure 2b] and remained elevated through week 11 [2.2(± 0.6) × 107 cells, Figure 2b]. Whereas naïve BALB/cByJ and C57BL/6J WMT exhibited comparable leukocytic cellularities [3.7(± 1.9) × 105 cells], the influx of leucocytes in response to pristane was distinct. At week 3 after pristane, BALB/cByJ cellularity [6.9(± 3.7) × 106 cells] was only 25% of that in C57BL/6J mice (P < 0.001) but by week 11 BALB/cByJ WMT cellularity surpassed [3.9(± 0.8) × 107 cells], which was 75% greater than that in C57BL/6J WMT at the same time point (P < 0.05). The absolute numbers of Mφ, T cells, and DCs in BALB/cByJ WMT were <30% of those in C57BL/6J WMT at week 3 (P < 0.01), although neutrophil numbers were modestly but not significantly higher in BALB/cByJ WMT compared to C57BL/6J WMT (Figure 2b). Similarly, there was no difference in B-cell numbers between C57BL/6J and BALB/cByJ WMT at week 3 (Figure 2b). In C57BL/6J mice, the numbers of each leucocyte population in WMT stayed the same or decreased from weeks 3 to 11 after pristane injection (Figure 2b). In contrast, the numbers of each cell type in BALB/cByJ WMT continued to increase so that they were similar to, or even slightly greater than, that in C57BL/6J WMT by week 11 (Figure 2b). These data show that C57BL/6J and BALB/cByJ differ in their recruitment of leucocytes to the WMT during the pristane-induced granuloma response.
To determine if these differences in leucocyte recruitment were due to a decreased frequency of cells in the MG of BALB/cByJ mice, the MG or its corresponding areas in naïve mice were excised and digested with collagenase to extract cells from tissue. The frequency of cells in the C57BL/6J MG at week 3 was 1.1(± 0.4) × 104 cells/mm2 of mesentery (Figure 2c), 80-fold greater than that of the corresponding area of mesentery in naïve C57BL/6J mice [1.3(± 0.1) × 102 cells/mm2]. The cellularity of BALB/cByJ MG at week 3 after pristane was also elevated [2.0(± 0.5) × 103 cells/mm2] compared to the corresponding area in naïve BALB/cByJ mice [2.1(± 0.3) × 102 cells/mm2], but was < 20% of that of C57BL/6J MG at the same interval (panel far left, Figure 2c). The frequency of each leucocyte population was significantly less in BALB/cByJ MG than in C57BL/6J MG (Figure 2c). From weeks 3 to 11 after pristane injection, the dynamic of MG cellularity in C57BL/6J mice was reciprocal to that in BALB/cByJ mice. Over this period, the numbers of each cell type decreased in C57BL/6J mice, but increased in BALB/cByJ mice (Figure 2c) such that, by week 11 after pristane, the frequency of each cell type was significantly higher in BALB/cByJ MG than in C57BL/6J MG (Figure 2c).
We also analysed pristane-induced SG in C57BL/6J and BALB/cByJ mice. Three weeks after pristane, several individual SG or their corresponding areas in naïve mice were isolated from C57BL/6J and BALB/cByJ mice. The cellularity of SG was comparable between C57BL/6J [1.2(± 0.3) × 105 cells/SG] and BALB/cByJ mice [1.4(± 0.6) × 105 cells/SG] (Figure 2d) as were the numbers of each leucocyte type (Figure 2d). The cellularity of SG at week 11 after pristane could not be compared as no SG were observed in BALB/cByJ mice at this time point (Figure 1b). Collectively, these data demonstrate that the WMT cellularity attributable not only to the number of SGs but also to the frequency of cells in MG.
Pristane increases non-adherent cell numbers in the peritoneum
The development of pristane-induced granulomas requires the continuing adhesion of alkane micelles surrounded by organised collections of peritoneal cells (Potter & Maccardle 1964). The numbers of peritoneal cells available to enter these complexes may, therefore, affect granuloma development. To determine if the differences in WMT cellularity between C57BL/6J and BALB/cByJ mice could be attributable to differences in the availability of free peritoneal cells, non-adherent peritoneal cells were washed out after pristane injection and subsequently enumerated and characterised by flow cytometry. The number of free peritoneal cells increased dramatically 3 weeks after pristane injection into both C57BL/6J and BALB/cByJ mice compared to naïve mice.
By 3 weeks after injection into C57BL/6J mice, pristane induced striking influxes of neutrophils and Mφ (together constituting >80% of leucocytes) into the PC, and modest increases in the numbers of T cells and DCs (Figure 3a). BALB/cByJ exhibited similar increases in the numbers of PC neutrophils, Mφ, T cells and DCs (Figure 3a). The number of Conventional B cells in the peritoneal exudates of naïve C57BL/6J mice was approximately six times that in BALB/cByJ mice. Three weeks after pristane, B cell numbers were decreased in C57BL/6J mice, but were significantly elevated in BALB/cByJ mice (Figure 3a). By week 11 after pristane, the numbers of neutrophils, Mφ, DCs, T cells, and conventional B cells decreased to very low levels in C57BL/6J mice, but remained elevated in BALB/cByJ mice (Figure 3a). These results imply that generalised peritoneal inflammation continues in BALB/cByJ mice 11 weeks after pristane, but is resolved, along with the focal granuloma response (Figure 1a), in C57BL/6J mice.
Figure 3.
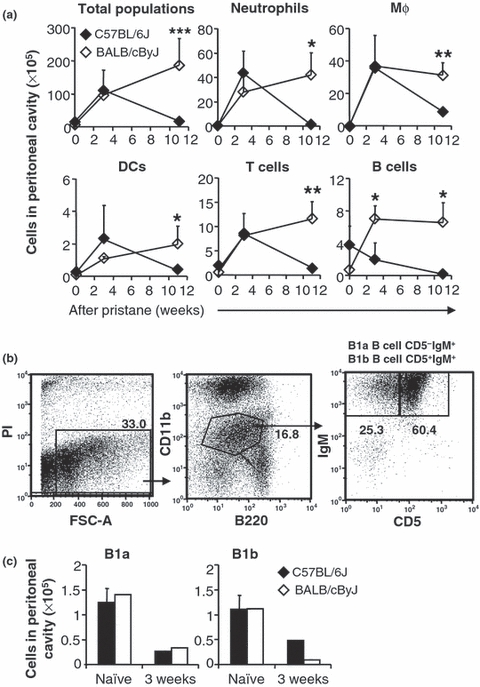
Influx of inflammatory leucocytes into the PC following pristane injection. (a) Peritoneal cells were harvested from naïve or pristane injected C57BL/6J (black diamonds) and BALB/cByJ mice (white diamonds) at 3 weeks or 11 weeks. Numbers of total cells or leucocytes were isolated from peritoneal lavage, stained and analysed by flow cytometry. (b) In a separate study, peritoneal cells harvested at week 3 were labelled with B220-PE-Cy7, CD11b-APC-Cy7, CD5-PE and IgM-TxR to identify B1a cells (B220lowCD5+IgMhiCD11blow) and B1b cells (B220lowCD5−IgMhiCD11blow). (c) The numbers of B1a and B1b in the PC are shown. Asterisks indicate significant differences between C57BL/6J and BALB/cByJ mice: *P < 0.05; **P < 0.01.
Characterisation of granuloma B cells
B1 cells, comprising B1a (B220lowCD5+IgMhiCD11blow) and B1b (B220lowCD5−IgMhiCD11blow) cells (Figure 3b), are a constitutive population of the PC and, presumably, the source of pristane-induced PCT (Potter 2003). Three weeks after pristane, there were significant reductions in the numbers of B1a cells (3-fold reduction) and B1b cells (2- to 4-fold reduction) in both C57BL/6J and BALB/cByJ mice (Figure 3c). To investigate whether free, PC B1 cells migrate into the pristine-induced granulomas, WMT cells were collected and labelled with antibodies specific for B1 cell surface markers, B220, CD5, IgM, and CD11b in preparation for flow cytometric analysis. There were virtually no B1 cells in the mesentery of naïve C57BL/6J or BALB/cByJ mice (data not shown). Their representation among WMT cells was <0.3% in both C57BL/6J and BALB/cByJ mice at weeks 3 and 11, indicating that there were few, if any, B1 cells in the granulomas. Therefore, these free B1 B cells may undergo apoptosis or leave the PC.
Inflammation mobilises lymphocytes, including immature B cells from the bone marrow (BM) to blood and the spleen (Ueda et al. 2004). To determine if immature, CD93+ B cell immigrants were present in MG and SG, we analysed the phenotypes of the B cells present in WMT by FACS. Virtually all B cells in both C57BL/6J and BALB/cByJ WMT at 3 and 11 weeks after pristane injection expressed high levels of B220 and IgD but no CD93, a surface phenotype indicative of full developmental maturity (Figure 4a). Presence of mature conventional B cells in the granuloma suggests their potential role in pristane-induced inflammation.
Figure 4.

Characterisation of B cells in oil granulomas. (a) B cells in oil granulomas exhibit mature phenotypes (B220hiCD93-). WMT cells were recovered from naïve mice or mice injected with 300 μl pristane for 3 or 11 weeks, labeled with B220-PE-Cy7, CD93-APC and IgD-PE, and analysed by flow cytometry. BM cells from naïve C57BL/6J mice were harvested and labeled as indicative for mature conventional B cells (B220hiCD93−IgD+) or immature B cells (B220lowCD93+IgD−). (b) Plasma cells were only present in BALB/cByJ MG at week 11. A piece of MG, or a peripheral strip of mesentery containing SG, was excised from pristane-treated C57BL/6J and/or BALB/ cByJ mice (week 11), and embedded in OCT compound. Sections of 5-μm were cut, and fixed with acetone/methanol (1:1). Sections were stained with CD138-PE antibody, and visualised by fluorescent microscopy. Original magnification (objective): ×20. Arrows indicate positive staining.
Plasma cells are recruited to mesenteric oil granulomas where pristane-induced PCT form when BALB/cAn mice are housed conventionally and exposed to incidental infection and antigens (Potter & Maccardle 1964). To determine whether plasma cells were also present in the pristine-induced granulomas in the C57BL/6J and BALB/cByJ mice maintained under specific-pathogen free conditions, WMT cells were isolated from these two strains given pristane 3 or 11 weeks earlier, and stained with PE-conjugated CD138 antibody for flow cytometry analysis. CD138 is commonly used as a marker to identify plasma cells (Rawstron et al. 1997). Interestingly, CD138+ cells were observed exclusively in the WMT and PC of BALB/cByJ at 11 weeks after pristane injection (data not shown). The presence of CD138+ cells in the BALB/cByJ WMT was confirmed in situ by immunofluorescence and once again, CD138+ cells were only present in BALB/cByJ MG sections at week 11 after pristane (Figure 4b). This finding correlates strikingly with the susceptibility of BALB/cByJ and resistance of C57BL/6J mice to pristane-induced PCT development (Potter 2003).
Organisational differences between MG and SG
BALB/cByJ mice developed lower numbers of SG at week 3 after pristane than did C57BL/6J mice (Figure 1b). These SG were absent in BALB/cByJ mice by week 11, while they persisted in C57BL/6J mice. On the other hand, BALB/cByJ MG became increasingly more cellular from weeks 3 to 11, but became less cellular in C57BL/6J mice at week 11 compared to week 3, suggesting that the granuloma response was resolving in C57BL/6J mice (Figures 1a and S1). Indeed, the cellularity of MG increased in BALB/cByJ mice from week 3 to week 11 after pristane, whereas it decreased in C57BL/6J mice (Figure 2c).
What accounts for these differences? One possibility is a differential distribution pattern of pristane in C57BL/6J and BALB/cByJ mice. Since pristane droplets have been shown to be surrounded by phagocytes (Potter & Maccardle 1964), we labelled phagocytes with India ink by injecting it intraperitoneally into mice at week 3 after pristane (Figure 5a). This allowed us to trace the kinetics of accumulation of pristane-phagocyte aggregates on the mesentery during later development of granuloma from 3 weeks to 11 weeks, and provides us with a tool to compare developmental kinetics of granuloma development in C57BL/6J and BALB/cByJ mice. Four hours after India ink injection, carbon particles were distributed evenly in both the MG and SG of BALB/cByJ mice, but were preferentially located in the SG of C57BL/6J mice (data not shown). The differences in India ink accumulation became more evident at day 12 after ink injection (Figure 5b) and were dependent on pristane-induced inflammation, as the patterns of ink distribution in naïve BALB/cByJ and C57BL/6J mice were similar. Histologically, carbon particles were absorbed on, or internalised into, CD11b+ cells (Figure 5c). In C57BL/6J mice, more CD11b+ cells were associated with carbon particles in SG than MG (Figure 5c). In contrast, the difference in distribution of CD11b+ cells containing carbon particles between SG and MG was not apparent in BALB/cByJ mice (Figure 5c). These data suggest that from week 3 after pristane, peritoneal inflammatory cells were preferentially recruited to and accumulated in the SG in C57BL/6J mice. This may explain why C57BL/6J mice preferentially maintained the SG structure and resolved MG. BALB/cByJ mice did not demonstrate any such preference. Continuous cell recruitment promoted the MG development in BALB/cByJ mice. SG eventually resolved, though they were still able to recruit cells, suggesting that other factors might affect the nodular structure of SG.
Figure 5.

India ink experiments. (a) C57BL/6J or BALB/cByJ mice were injected i.p. with 300 μl pristane, and 3 weeks later were injected i.p. with 0.5 ml of a 1/10 dilution of India ink in PBS. Twelve days after ink injection, the PC of mice was opened and gut-associated WMT was isolated and macro-photographed. Mice that were treated with only India ink were included as controls. (b) A representative picture of gut-associated WMT is shown. (c) Immunohistology of MG and SG. Sections were made from a block of peripheral mesentery containing SGs or an equivalent area of MG and were stained with biotin labelled CD11b, followed by incubation with streptavidin conjugated HRP. A piece of peripheral mesentery corresponding to SG (cSG) or a corresponding area of MG (cMG) in naïve mice was included as control. Original magnification (objective): ×20. (c) mRNA level of MCP-1 was determined in SG (Black columns) and MG (White columns) from C57BL/6J or BALB/cByJ mice. Asterisks indicate significant differences between SG and MG: *P < 0.05; **P < 0.01.
Leucocyte recruitment is regulated by their specific chemokines during inflammation (Douglas et al. 2002). Mφs are major CD11b+ expressing phagocytes in pristane-induced granulomas. To understand the preferential recruitment of Mφ to the mesentery in C57BL/6J, but not in BALB/cByJ mice, we analysed the expression of the Mφ chemokine MCP-1 by SG cells and MG cells at week 3 after pristane. Interestingly, C57BL/6J SG cells expressed more MCP-1 mRNA than C57BL/6J MG cells (Figure 5d). This may explain why from week 3 after pristane, Mφ were preferentially recruited to SG (Figure 5b), therefore leading to a greater amount of pristane brought to SGs through aggregates of pristane and Mφ or other leucocytes. However, in BALB/cByJ mice, there was no difference in MCP-1 expression between the two structures. This result suggests that from week 3, both SG and MG in BALB/cByJ mice were able to recruit Mφ or other inflammatory cells, correlating with the observation that MG and SG recruited CD11b+ cells equally (Figure 5b,c).
Collectively, the differential kinetics of MG development and resolution in BALB/cByJ and C57BL/6J mice, as well as the extended duration of SGs in B/6 mice, may be due to differences in the pattern of leucocyte recruitment that is directed by specific chemokines, such as MCP-1.
Discussion
Granulomateous inflammation acts as a protective response by walling off persistent living pathogens (Williams & Williams 1983; Co et al. 2004). However, granulomas induced by non-infectious agents such as suture material or pristane, cause immunopathology without any clear benefit to the host (Williams & Williams 1983; Tang & Eaton 1995). The present study applied a non-infectious model of the granuloma response by injecting pristane into the PC of mice. Whereas this model was developed almost 50 years ago (Potter & Maccardle 1964), for the first time we have detailed and compared the development of pristane granulomas in two mouse strains, BALB/cByJ and C57BL/6J, that support, or are resistant to inflammatory PCT (Potter 2003). In both strains, pristane induced recruitments of inflammatory leucocytes (Figure 2), resulting in the development of oil granulomas, that could be further divided into MG and SG (Figure 1a). Both MG and SG contained leucocyte populations, that were dominated by MΦ (approximately 20% of all the leucocytes), followed by T cells and neutrophils (approximately 15%, each), DCs (approximately 10%) and conventional B cells (approximately 3%). The cellular composition of pristane MG and SG is similar to that of tuberculous granulomas (Tsai et al. 2006).
General histological features were also similar between MG and SG in both strains. Like other causative agents in granuloma formation (Birkness et al. 2007), pristane was buried in the mesentery, surrounded by inflammatory leucocytes (Figure 1c). Pristane-induced granulomas had marked differences in organisation when compared to infectious granulomas. For example, during infection by tubercle bacillus, the invading pathogens are first surrounded by activated Mφ; this complex is encompassed by lymphocytes. Pristane granulomas, however, result from pristane being randomly surrounded by various types of cell populations, including Mφ, neutrophils and lymphocytes (Figure 1c). This difference might be due to, at least in part, differences between the nature of pristane and living pathogens. Deposition of collagen and fibrosis in pristane granulomas, commonly described in granuloma responses to other stimuli (Bowers et al. 1980; Restrepo et al. 2001), was confirmed histologically (Figure 1c).
The pattern of pristane-induced SG and MG development was quite distinct in the BALB/cByJ and C57BL/6J strains. BALB/cByJ mice in this study exhibited similar mesenteric responses to pristane as those previously described by Potter and Maccardle (1964), including the development of mesenteric polyps, the presence of Mφ, neutrophils and lymphocytes, plasma cells, and the appearance of rare oil granuloma nodules, or SG, along the junction of mesentery and gut. Briefly, in response to pristane, MG was the main form of oil granuloma in BALB/cByJ mice; whereas in C57BL/6J mice, SG was the typical form (Figure 1a).
Why does the granuloma response of BALB/cByJ mice preferentially take the form of MG, whereas the C57BL/6J mice response to pristane is dominated by SG? MG and SG contained common leucocytes (Figure 2c,d), and both began as pristane-associating leucocytes attached to mesenteric membranes. Injection of India ink to the pristane-treated mice revealed that phagocytes were diffusely located both at the SG and MG in BALB/cByJ mice, but phagocytes were mainly localised to the SG in C57BL/6J mice (Figure 5). These differences in the distribution of phagocytes became more prominent by day 12 after injection. This pattern was not apparent in naïve mice, and we conclude that inflammation induces the strain-specific patterns of pristane granulomas.
MCP-1 expression has been demonstrated to promote Mφ accumulation in granulomas in response to S. mansoni egg antigens (Chiu & Chensue 2002). To understand the reasons for the distinct pattern of granuloma responses in BALB/cByJ and C57BL/6J mice, we isolated MG and the rare SG of BALB/cByJ mice, and SG and the occasional MG of C57BL/6J mice 3 weeks after pristane. In BALB/cByJ mice, expression of messenger RNA of MCP-1 in MG was comparable to that in SG (Figure 5d), suggesting that Mφ-rich granulomas could develop either in the mesenteric margins or in mesenteric centres. In contrast, in C57BL/6J mice, MCP-1 messenger RNA expression level was 3-fold higher in SG than in MG (Figure 5d), suggesting that granuloma development is preferred at mesenteric margins. This bias may explain the dominance of SG in C57BL/6J mice (Figure 1a). Collectively, these data suggest that genes controlling expression of inflammatory chemokines affect the leucocyte recruitment and ultimately the pattern of the granuloma response to pristane.
Like inflammatory chemokines, inflammatory cytokines may also affect patterns of granuloma response to pristane. In the granuloma response to M. tuberculosis, the absence of tumor necrosis factor α (TNFα) results in poor granuloma formation and widespread dissemination of mycobacteria (Bean et al. 1999). We measured the expression of TNFα and IL-6 in the WMT (containing both MG and SG) from both BALB/cByJ and C57BL/6J mice at week 3 after pristane. TNFα messenger RNA expression in the WMT in C57BL/6J mice was 2-fold higher than that in BALB/cByJ mice (Figure S4). Not all inflammatory cytokines were higher in C57BL/6J than in BALB/cByJ mice. For example, comparable levels of IL-6 messenger RNA were detected in the WMT of both strains (Figure S4). These data suggest distinct roles for these cytokines in the granuloma response to pristane. These roles could be examined in the future by using mice deficient for each of these cytokines.
Additionally, other factors may affect granuloma development. For example, circulating leucocytes contribute to oil granuloma formation (unpublished data). The expression of adhesion molecules in the mesentery and the pool of circulating leucocytes may therefore have a role in regulating oil granuloma formation. Also, gender-specific differences in granuloma responses to pristane were obvious. Male C57BL/6J mice developed a less severe granuloma response than age-matched female C57BL/6J mice, as evidenced by less cellular MG and fewer SG (Figure S2a,b). This was confirmed by the fact that the frequency of cells in male C57BL/6J MG was <20% of that in female C57BL/6J MG (Figures S2c and S3a). There was no difference in the frequency of cells in SG between the two genders (Figure S2d and S3b). The cellularity of WMT in male C57BL/6J mice was <25% of that in female counterparts (Figures S2e and S3c). Recruitment of leucocytes to the WMT in male C57BL/6J mice was not as efficient as in age-matched female mice (Figure S3). The polyps that appeared on the male C57BL/6J mesentery were not observed in female C57BL/6J mice (Figure S2a). In the case of granulomas induced by infectious pathogens, inefficient sequestration of these microorganisms may permit higher incidence of escape, resulting in the progression of disease. A sexual bias is also evident in the epidemiological patterns of tuberculosis, where 70% more males than females receive smear-positive tuberculosis notifications (Diwan & Thorson 1999). Sex-based differences in pristane responses may reflect potential roles for sex-associated factors, including hormones (for example, oestrogen), or genes on the Y chromosome, in oil granuloma development.
The pristane-induced granuloma represents a non-infectious granuloma, and this study may provide implications for the granulomateous pathology induced by implantation of inert surgical biomaterials (Zeller 1983; Tang & Eaton 1995; Griffiths et al. 1996; Nicolau 2007). Dissecting the regulatory pathways and genes of granuloma responses to pristane may lead to a better understanding of this important mechanism of immunity.
Acknowledgments
We thank Dr. Michael Potter (Laboratory of Genetics, National Cancer Institute, Bethesda, Maryland) for valuable discussions and advice and for his critical review of the manuscript. We thank Pilar Snowden and Kathleen O’Hara for assistance in preparing the manuscript. This work was supported in part by NIH grants AI056363 and AI024335.
Disclosures
The authors declared they have neither financial nor personal conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Dose dependent response of mesentery to pristane. BL/6 mice were i.p. injected with 100 or 300 μl pristane and were harvested at several intervals thereafter. At each time point, three or more mice were killed and gut-associated with mesentery was macrophotographed. Representative photos were shown here. Untreated mice were used as controls.
Figure S2 Less responses of male BL/6 mice to pristane than female BL/6 mice. A: Male BL/6 (mBL/6) mice were given 300 μl pristane intraperitoneally and sacrificed at 3 weeks post pristane. Representative macrophotographs of gut-associated whole mesenteric tissue (WMT) from naive and pristane-administrated male BL/6 mice are shown. B: Number of serosal granulomas (SGs) developed in male mice were numerated, and number of SGs in female B / L6 (fBL/6) was included as a comparison. C: Fewer cells in male OG than in female OG. D: Frequency of cells in MG. It is shown as the number of cells in every 1 mm2 of mesentery. E: Frequency of cells in SG developed at week 3 after pristane, which was expressed as the number of cells per 1 individual SG. Values represent the mean and standard error of three or more mice per group. 6–8 individual SG from 3 mice were pooled together, which gave rise to the average and SD. Asterisks indicate significant differences between female (black columns) or male BL/6 mice (white columns): *, P < 0.05; **, P < 0.01; ***, P < 0.001
Figure S3 Cellularity of WMT, MG and SG in male and female BL/6 mice. A: Frequency of cells in MG. MG cells were collected from mice treated with pristane for 3 weeks, and were labeled with antibodies as described in material and methods. B: Frequency of cells in SG. 6-8 individual SGs were isolated from 3 mice at week 3 after pristane, digested with collagenases to release cells. C: Cellularity of WMT. WMT cells were harvested from naïve mice or mice injected with pristane for 3 weeks, and labeled with antibodies and analyzed as described in Material and Methods. Asterisks indicate significant differences between mBL/6 and fBL/6 mice: *, P < 0.05; **, P < 0.01, ***, P < 0.001.
Figure S4 Expression of cytokines by WMT cells. WMT cells were isolated from BL/6 (filled columns) and B/c (empty columns) mice treated without or with 300 μl pristane for 3 weeks. mRNA from these cells was quantified by quantative PCR with specific primers. Asterisks indicate significant differences between BL/6 and B/c mice: **, P < 0.01.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bean AG, Roach DR, Briscoe H, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- Birkness KA, Guarner J, Sable SB, et al. An in vitro model of the leukocyte interactions associated with granuloma formation in Mycobacterium tuberculosis infection. Immunol. Cell Biol. 2007;85:160–168. doi: 10.1038/sj.icb.7100019. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Houston F, Clinton R, Lewis M, Ballard R. A histological study of the carrageenan-induced granuloma in the rat lung. J. Pathol. 1980;132:243–253. doi: 10.1002/path.1711320306. [DOI] [PubMed] [Google Scholar]
- Chiu BC, Chensue SW. Chemokine responses in schistosomal antigen-elicited granuloma formation. Parasite Immunol. 2002;24:285–294. doi: 10.1046/j.1365-3024.2002.00466.x. [DOI] [PubMed] [Google Scholar]
- Co DO, Hogan LH, Il-Kim S, Sandor M. T cell contributions to the different phases of granuloma formation. Immunol. Lett. 2004;92:135–142. doi: 10.1016/j.imlet.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Diwan VK, Thorson A. Sex, gender, and tuberculosis. Lancet. 1999;353:1000–1001. doi: 10.1016/S0140-6736(99)01318-5. [DOI] [PubMed] [Google Scholar]
- Douglas MR, Morrison KE, Salmon M, Buckley CD. Why does inflammation persist: a dominant role for the stromal microenvironment? Expert Rev. Mol. Med. 2002;2002:1–18. doi: 10.1017/S1462399402005264. [DOI] [PubMed] [Google Scholar]
- Ginsberg JE, Becker LA. Silicon granuloma of skin due to traumatic sand inoculation. J. Am. Med. Assoc. 1951;147:751–753. doi: 10.1001/jama.1951.73670250003011a. [DOI] [PubMed] [Google Scholar]
- Griffiths MM, Langone JJ, Lightfoote MM. Biomaterials and Granulomas. Methods. 1996;9:295–304. doi: 10.1006/meth.1996.0034. [DOI] [PubMed] [Google Scholar]
- Hauser FV, Rothman S. Monilial granuloma; report of a case and review of the literature. Arch. Derm. Syphilol. 1950;61:297–310. doi: 10.1001/archderm.1950.01530090127012. [DOI] [PubMed] [Google Scholar]
- Kido M, Kajiki A, Nagata N, Manabe H, Iwata Y. A case of pulmonary hyalinizing granuloma with its occupational history of dust exposure. J. Uoeh. 1995;17:31–37. doi: 10.7888/juoeh.17.31. [DOI] [PubMed] [Google Scholar]
- Lattanzio G, Libert C, Aquilina M, et al. Defective development of pristane-oil-induced plasmacytomas in interleukin-6-deficient BALB/c mice. Am. J. Pathol. 1997;151:689–696. [PMC free article] [PubMed] [Google Scholar]
- Ley A, Jacas R, Oliveras C. Torula granuloma of the cervical spinal cord. J. Neurosurg. 1951;8:327–335. doi: 10.3171/jns.1951.8.3.0327. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Bottazzi B, et al. Monocyte chemotactic protein-1 (MCP-1): signal transduction and involvement in the regulation of macrophage traffic in normal and neoplastic tissues. Adv. Exp. Med. Biol. 1993;351:47–54. doi: 10.1007/978-1-4615-2952-1_6. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Ribi E, Azuma I. Biologically active components from mycobacterial cell walls. V. Granuloma formation in mouse lungs and guinea pig skin. Cell. Immunol. 1975;16:11–24. doi: 10.1016/0008-8749(75)90181-1. [DOI] [PubMed] [Google Scholar]
- Nacionales DC, Kelly KM, Lee PY, et al. Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane) Am. J. Pathol. 2006;168:1227–1240. doi: 10.2353/ajpath.2006.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau PJ. Long-lasting and permanent fillers: biomaterial influence over host tissue response. Plast. Reconstr. Surg. 2007;119:2271–2286. doi: 10.1097/01.prs.0000260710.30934.a1. [DOI] [PubMed] [Google Scholar]
- Nordan RP, Mock BA, Neckers LM, Rudikoff S. The role of plasmacytoma growth factor in the in vitro responses of murine plasmacytoma cells. Ann. N Y Acad. Sci. 1989;557:200–205. doi: 10.1111/j.1749-6632.1989.tb24013.x. [DOI] [PubMed] [Google Scholar]
- Potter M. Neoplastic development in plasma cells. Immunol. Rev. 2003;194:177–195. doi: 10.1034/j.1600-065x.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- Potter M, Maccardle RC. Histology of developing plasma cell neoplasia induced by mineral oil in Balb/C mice. J. Natl Cancer Inst. 1964;33:497–515. [PubMed] [Google Scholar]
- Potter M, Pumphrey JG, Walters JL. Growth of primary plasmacytomas in the mineral oil-conditioned peritoneal environment. J. Natl Cancer Inst. 1972;49:305–308. [PubMed] [Google Scholar]
- Potter M, Mushinski EB, Wax JS, Hartley J, Mock BA. Identification of two genes on chromosome 4 that determine resistance to plasmacytoma induction in mice. Cancer Res. 1994;54:969–975. [PubMed] [Google Scholar]
- Rawstron AC, Owen RG, Davies FE, et al. Circulating plasma cells in multiple myeloma: characterisation and correlation with disease stage. Br. J. Haematol. 1997;97:46–55. doi: 10.1046/j.1365-2141.1997.72653.x. [DOI] [PubMed] [Google Scholar]
- Restrepo BI, Alvarez JI, Castano JA, et al. Brain granulomas in neurocysticercosis patients are associated with a Th1 and Th2 profile. Infect. Immun. 2001;69:4554–4560. doi: 10.1128/IAI.69.7.4554-4560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S, Kovalchuk AL, Kim JS, Klein G, Janz S. BCL2 accelerates inflammation-induced BALB/c plasmacytomas and promotes novel tumors with coexisting T(12;15) and T(6;15) translocations. Cancer Res. 2003;63:8656–8663. [PubMed] [Google Scholar]
- Tang L, Eaton JW. Inflammatory responses to biomaterials. Am. J. Clin. Pathol. 1995;103:466–471. doi: 10.1093/ajcp/103.4.466. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Chakravarty S, Zhu G, et al. Characterisation of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KS, Domingo EO. Granuloma formation around Schistosoma mansoni, S. HAEMATOBIUM, AND S. Japonicum eggs. Size and rate of development, cellular composition, cross-sensitivity, and rate of egg destruction. Am. J. Trop. Med. Hyg. 1970;19:292–304. doi: 10.4269/ajtmh.1970.19.292. [DOI] [PubMed] [Google Scholar]
- Williams GT, Williams WJ. Granulomatous inflammation – a review. J. Clin. Pathol. 1983;36:723–733. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley PH, Seibold JR, Whalen JD, Chapdelaine JM. Pristane-induced arthritis. The immunologic and genetic features of an experimental murine model of autoimmune disease. Arthritis Rheum. 1989;32:1022–1030. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- Zeller JM. Surgical implants. Physiological response. AORN J. 1983;37:1284–1291. doi: 10.1016/s0001-2092(07)64976-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


