Abstract
Proteins found in the root exudates are thought to play a role in the interactions between plants and soil organisms. To gain a better understanding of protein secretion by roots, we conducted a systematic proteomic analysis of the root exudates of Arabidopsis thaliana at different plant developmental stages. In total, we identified 111 proteins secreted by roots, the majority of which were exuded constitutively during all stages of development. However, defense-related proteins such as chitinases, glucanases, myrosinases, and others showed enhanced secretion during flowering. Defense-impaired mutants npr1-1 and NahG showed lower levels of secretion of defense proteins at flowering compared with the wild type. The flowering-defective mutants fca-1, stm-4, and co-1 showed almost undetectable levels of defense proteins in their root exudates at similar time points. In contrast, root secretions of defense-enhanced cpr5-2 mutants showed higher levels of defense proteins. The proteomics data were positively correlated with enzymatic activity assays for defense proteins and with in silico gene expression analysis of genes specifically expressed in roots of Arabidopsis. In conclusion, our results show a clear correlation between defense-related proteins secreted by roots and flowering time.
Keywords: Bacteria, Development, Plant, Protein Secretion, Proteomics, Defense, Flowering, Roots
Introduction
The plant root system serves many roles, including anchorage and uptake of nutrients and water. The ability of roots to communicate with roots of neighboring plants and other organisms in the rhizosphere has been the focus of increasing attention (1, 2). Root secretions of secondary metabolites and volatile organic compounds have been shown to play offensive, defensive, and symbiotic roles (3–7). Likewise, the symbioses between Rhizobium species and various members of the legume family have been extensively studied and involve the initial secretion of specific flavonoids by plant roots (8–10). Similarly, a multitrophic interaction has been uncovered, in which volatile organic compounds released by Medicago truncatula were shown to attract soil nematodes that could in turn bring Rhizobia, used as food by the nematodes, to the roots and thus facilitate the symbiotic process (11). All these interactions involve carbon-containing compounds secreted in the root exudates.
Although numerous reports show changes in the protein profile of leaves and root tissues in response to wounding (12, 13), insect attack (14), fungal or oomycete infection (15–20), or the defense-related hormone jasmonic acid (21, 22), fewer reports have discussed the biological roles of proteins secreted by the roots (23–25). One recent study has shown that the composition of proteins secreted in the root exudates changes with the presence of a given microbial neighbor and that the exudation of proteins by a given bacterium is altered by the presence of a specific plant neighbor (26). These studies examined root secretion of proteins under inducible conditions, but there is no information available about the protein profiles in the root exudates under normal, nonstressed development. Such information is critical to determine the base line of protein secretion from roots and whether this process is developmentally controlled.
Some studies have shown that tobacco and Arabidopsis leaves have increased resistance to pathogens in the developmental transition from vegetative to flowering phase (27, 28). Furthermore, it has been shown in Celosia cristata leaves that the level of two antiviral proteins against a systemic host-virus is higher at the pre-flowering stage than at the post-flowering stage (29). However, there is no information in the literature about whether this induction of expression of defense-related genes is a systemic response throughout the plant from leaves to roots and into the rhizosphere.
Two-dimensional gel electrophoresis (2-DE)4 following different mass spectrometry methods has proven to be a useful tool for identification of proteins in the root exudates of Arabidopsis, pea, and alfalfa (24, 26, 30–32). In this study, we conducted a systematic proteomic analysis of root exudates of the model plant Arabidopsis thaliana to better understand the variation of protein secretion by roots throughout plant development.
EXPERIMENTAL PROCEDURES
Plant Material and Growth Conditions
A. thaliana ecotype Col-0 (WT) seeds were purchased from Lehle Seeds (Round Rock, TX). The transgenic line NahG, which lacks the ability to accumulate SA through the constitutive expression of a bacterial salicylic acid hydroxylase gene (33, 34), and the mutant line npr1-1, which does not express the positive regulator NPR1 involved in systemic acquired resistance (35, 36), were kind gifts from Dr. Xinnian Dong (Duke University). The following mutants were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus): cpr5-2, which accumulates large amounts of salicylic acid (37); co-1, which flowers later (day 28) than the WT (day 21) under long day conditions, possibly due to a mutation on the CO gene impairing a putative transcription factor required to promote flowering under long day conditions (38); fca-1, which exhibits a late flowering phenotype (around day 35) due to a mutation that disrupts the vernalization response (39), and stm-4, defective in the gene SHOOT MERISTEMLESS (STM) and thus impaired in the initiation and maintenance of the shoot apical meristem (40). The seeds were surface-sterilized with 3% (v/v) sodium hypochlorite for 2 min followed by three washes with sterile distilled water. The seeds were germinated on solidified MS medium (41) supplemented with 3% (w/v) sucrose in a growth chamber at 25 °C and 16/8-h day/night cycle at 55 μmol m−2 s−1. Replicates containing 10 7-day-old plants were transferred into each Magenta box containing 30 ml of liquid MS medium supplemented with 3% (w/v) sucrose and placed on a shaker set at 80 rpm, 24 + 2 °C under photoperiod of 16/8 h at 80 μmol m−2 s−1. Aseptic protocols were used, and no evidence of contamination in the media was observed throughout the experiment. In addition, we tested the culture media from WT, npr1-1, and NahG plants collected from different developmental time points, and we plated 100 μl of those exudates on Luria-Bertani (LB) and potato-dextrose agar (PDA) plates without supplementing antibiotics and incubated those plates at 28 ± 2 °C for 5 days to check for bacterial or fungal contamination. For each genotype, five Magenta boxes containing 10 plants each were used (n = 5), and each experiment was repeated three times.
Time Course Collection of Root Exudates
A. thaliana Col-0 (WT), npr1-1, NahG, cpr5-2, fca-1, stm-4, and co-1 plantlets were grown in Magenta boxes as described above. At 7-day intervals from transfer to Magenta boxes, the total root exudates of 10 plants from each box were collected and centrifuged at 8,000 × g for 15 min at 4 °C to remove the root sheathing. The supernatant was filtered through a 0.2-μm syringe filter, and the filtration was concentrated to 1 ml by passing it through Amicon ultracentrifugal filter devices (molecular mass cutoff of 5000 Da, Millipore) to remove the salts. It should be noted that small weight metabolites (≤5000 Da), such as sugars and secondary metabolites, were not retained in the protein solution. The protein concentration was determined as described by Bradford (42) using a protein assay kit (Bio-Rad) and bovine serum albumin (BSA) as a standard. Exudate protein samples were stored at −80 °C until further use.
2-DE Separations
Separation and quantification of secreted proteins sampled throughout the developmental stages of the WT and other genotypes used in this study were performed by two-dimensional SDS-PAGE (43). Two hundred and fifty micrograms of total root protein exudate from each mutant/genotype for each time point was analyzed by 2-DE using the protocol described by De-la-Peña et al. (26). Separated proteins were visualized using silver staining (44), and resultant gels were digitally imaged with a Bio-Rad FluorS equipped with a 12-bit camera. Protein spot detection, quantification, and comparative analyses were performed using Phoretix 2D Expression software (version 2005, Nonlinear Dynamics, Durham, NC). The reproducibility of each experiment was compared by running three different replicate gels (supplemental Fig. 1). Total spot volume was calculated, and each spot was assigned a normalized spot volume as a portion of this total value. Each individual protein spot was then matched with the identical protein spot from each replicate gel.
In-gel Trypsin Digestion
Qualitative identifications of 111 proteins were performed using in-gel digestion followed by high performance liquid chromatography tandem mass spectrometry (HPLC/MS/MS) and data base searching. The SDS-polyacrylamide gel plugs were de-stained (45) and dehydrated with 25 μl of acetonitrile for 15 min at room temperature. The gel spots were dried under vacuum and rehydrated in 20 μl of sequencing grade modified bovine trypsin (10 ng/μl in 25 mm ammonium biocarbonate, Roche Diagnostics). After rehydration for 30 min on ice, excess trypsin solution was removed, and 15 μl of 25 mm ammonium bicarbonate was added to each well to prevent dehydration during incubation. After protein hydrolysis, the supernatant was recovered, and the spots were extracted twice more with 25 μl of a 1:1 (v/v) solution of acetonitrile and 25 mm ammonium bicarbonate and once more with 25 μl of acetonitrile. All peptide extract fractions were pooled, concentrated to dryness, and resuspended in a 95% water, 5% acetonitrile solution containing a final concentration of 0.1% formic acid.
HPLC-Q-TOF MS/MS
Separations of the protein digests were achieved using the same method and specifications described by De-la-Peña et al. (26). The separated peptides were analyzed using tandem mass spectrometry and an ABI QSTAR Pulsar I hydrid Q-TOF mass spectrometer (Applied Biosystems) equipped with a nanoelectrospray ionization source (Protana) similar to that described Lei et al. (46).
Data Base Queries and Protein Identification
Protein identifications were determined through queries of the acquired mass spectral data against the NCBInr data base (nr = nonredundant) that was downloaded from the NCBI ftp site (ncbi.nih.gov), February 2007. Although the NCBInr data base contains a total of 1,415,660 sequences (455,667,871 residues), only the 38,475 A. thaliana annotated sequences were searched using a taxonomic filter in MASCOT Daemon (version 1.8.0, Matrix Science Ltd., London, UK) search engine (47, 48) and raw tandem mass spectral data (.wiff). The searches used a mass tolerance of 100 ppm and allowed for up to one trypsin miscleavage and variable amino acid modifications consisting of methionine oxidation and cysteine carbamidomethylation.
Enzymatic Assays
Enzymatic assays were performed to examine the biological activity of chitinases and β-1,3-glucanases present in the root total protein exudates collected from different mutants/genotypes. Chitinase activity was assayed as described by Gómez-Ramírez et al. (49), using chitinase azure as a substrate. β-1,3-Glucanase activity was assayed according to Abeles and Forrence (50), using laminarin from Laminaria digitata as substrate.
Antimicrobial Assays
The antimicrobial activity of total root exudate protein, isolated as explained above, was tested against Pseudomonas syringae pv. phaseolicola (Psph 3121) and P. syringae pv. lycopersici (DC3000) using the broth microdilution antimicrobial susceptibility test as described by Tilton et al. (51). Briefly, bacteria were grown overnight with 20 μg of protein collected from root exudates and plated with serial dilutions on LB agar plates supplemented with appropriate antibiotics to determine viable cell concentration as colony-forming units per ml to investigate the antibacterial activity.
Bioinformatics
We used four different programs to predict the subcellular localization of all proteins used in this study, which included The Proteome Analyst Specialized Subcellular Localization Server (PA-SUB) (52), WoLF PSORT (53), LocTREE (54), TargetP (55), and Multiloc (56). Both TargetP and Multiloc use the presence/absence of N-terminal targeting sequences to predict the multiple subcellular locations of proteins such as chloroplast, cytoplasm, endoplasmic reticulum, extracellular space, mitochondria lysosomes, Golgi apparatus, peroxisomes, plasma membrane, and vacuoles. In addition, we also analyzed the tissue-specific and development stage-specific expression patterns of the genes encoding 26 proteins identified in the exudates using GENEVESTIGATOR software (57).
RESULTS
Defense Protein Secretion in the Root Exudates Is Developmentally Regulated
Using 2-DE, a total of 111 proteins were resolved, at 7-day intervals, from day 7 (seedling stage) until day 49 (senescence stage) in WT root exudates (Fig. 1A; supplemental Table S1; supplemental Fig. 3). Under our experimental conditions, all plants except smt-4 flowered and produced siliques in the Magenta boxes (supplemental Fig. 2). Based on protein identifications and functional classifications, we observed that proteins belonging to two prominent groups were secreted by roots as follows: defense proteins, such as pathogenesis-related proteins (27%), and secretory proteins, such as isomerases (23%). In addition, we observed that proteins related to signaling (9%), energy (12%), secondary metabolism (3%), and proteins of unknown function (13%) composed a small percentage of the total secreted proteins (Fig. 1B).
FIGURE 1.
2-DE of total A. thaliana Col-0 (WT) root-secreted proteins. Two hundred and fifty micrograms of protein were isoelectrically focused and separated as described under “Experimental Procedures.” A, representative 2-DE gel from root-secreted proteins in WT at day 28 is shown. The molecular masses (kDa) of protein standards are indicated to the left of the gel, and the isoelectric point (pI) is indicated at the top of the gel. The arrows with numbers represent identified proteins that are listed in supplemental Table S1. The gray underlined numbers represent the proteins that are not present in the representative gel but are present in other samples. B, summary of the distribution of root secreted identified protein classes as determined using the protein function data base Pfam and classification schema previously reported for Arabidopsis (91).
Once the proteins were identified (supplemental Table S1), we focused our analysis on the proteins that showed both quantitative and qualitative changes during the root secretion time course of 49 days (Fig. 2). From all the 111 WT proteins, only 26 showed changes in their secretion patterns at different plant developmental stages (Fig. 2), and the other 85 proteins were secreted constitutively throughout the life of the plant (supplemental Table S1). Among the 26 proteins, several were related to defense responses and secreted in greatest quantities at days 7, 21 (flowering), and 28 (Fig. 2). The intensity of PR proteins, such as chitinases and glucanases (spots 1–5, 48, and 54) decreased or disappeared after day 28, and other proteins such as plant basic secretory protein (spot 6), lectins (spots 8 and 31), and thaumatin like-protein (spot 12) disappeared just before the onset of flowering (day 21) and appeared again after flowering (day 28). These results suggest that the overall pattern of protein secretion in the exudates is not predominantly developmentally dependent but that the secretion of particular PR proteins is correlated closely with plant developmental stage.
FIGURE 2.
Histograms of normalized volume values of the secreted proteins from A. thaliana WT that change throughout the whole 49-day temporal course. The normalized volumes were analyzed by Phoretix 2D Expression software. The spot numbers represent the identified proteins that are listed in supplemental Table S1. Each graph is a subset of proteins grouped together by class/isoforms. The error bars illustrate the S.E. values of three repetitions (n = 3).
Root Secretion of Defense Proteins in Defense-related Mutants
We tested the root secretions of defense-impaired and -enhanced Arabidopsis plants to identify candidate proteins that might be bioactive against pathogens. Defense-related proteins (spots 2, 3, 5, 11, 12, 17, 35, 48, 54, 82, and 106) exuded by wild type roots showed very dramatic changes, particularly in days 21 and 28 (Fig. 2; Table 1). We selected the defense-impaired mutants/genotypes npr1-1 and NahG and the defense-enhanced mutant cpr5-2. The proteins from these mutants were collected at flowering and post-flowering times (days 21 and 28) and analyzed by 2-DE (Fig. 3). We observed that the mutant cpr5-2, which has higher levels of endogenous salicylic acid than the WT, showed greater secretion levels of chitinases (spots 3, 5, 11, 48 and 54; Fig. 3) than WT at day 21, but NahG and npr1-1 (mutants that do not produce SA and lack the perception of SA, respectively), the chitinase (spot 5) was totally absent on day 21 and 28. Interestingly, myrosinases (spots 17 and 82), an enzyme involved in the hydrolysis of glucosinolates (defense-related secondary metabolites widely present in Brassicales), was only found in WT and cpr5-2 mutant but not (or almost undetectable) in the other defense-impaired mutants (Fig. 3). Other identified proteins such as endo-1,3-β-glucosidase (spot 1), isomerase (spot 15), lectin (spot 31), peroxidase (spot 52), and osmotin (spot 106) were secreted at low levels in defense-impaired mutants compared with the WT and the defense-enhanced mutant (Fig. 3).
TABLE 1.
List of the proteins identified in the root exudates of Arabidopsis that change during development
The abbreviations used are as follows: cTP, chloroplast transit peptide; mTP, mitochondrial targeting peptide; SP, secretory pathway signal peptide. Database used is NCBInr 042203 (1,415,660 sequences; 455,667,871 residues). Taxonomy used is A. thaliana (thale cress) (38,475 sequences). The NCBI nonredundant protein database (NCBInr) was downloaded on April 22, 2003. It contained a total of 1,415,660 sequences (455,667,871 residues) and the 38,475 sequences for taxonomy of A. thaliana against which the database was searched.
FIGURE 3.
Comparative histogram of normalized volume values from each of the 26 spots from 2-DE of A. thaliana WT and the defense-related mutants. The major differences between the root-secreted proteins of A. thaliana ecotype Col-0 (WT), impaired-defense mutant npr1-1, impaired-defense transgenic NahG, and the defense-related mutant cpr5-2 at days 21 and 28 are shown. Two hundred and fifty micrograms of protein for every genotype were analyzed by 2-DE as described under “Experimental Procedures.” The spot numbers represent the identified proteins that are listed in supplemental Table S1. The error bars illustrate the S.E. values of three repetitions (n = 3).
Root Secretion of Proteins in Flowering Mutants
The onset of flowering catalyzed significant changes in the root protein secretions, and these changes were observed pre- and post-flowering in WT as well as in the defense-impaired mutants npr1-1 and NahG and the defense-enhanced mutant cpr5-2 (days 21 and 28) (Figs. 2 and 3). The mutant stm-4 did not flower under our experimental conditions, but we collected their secretions at the same time as the wild type. To examine the mechanistic and biological relevance of flowering related to root secretion of defense proteins, we analyzed root-exuded proteins in three different flowering-related mutants (fca-1, stm-4, and co-1) (Fig. 4). We observed that at day 21 the WT secretes higher amounts of eight proteins (spot 1, 6, 9, 12, 14, 17, 82, and 106) compared with the three flowering-related mutants. From those eight proteins, four were only present in WT (spots 1, 17, 82, and 106) at day 21. It is worth mentioning that five proteins (spots 1, 12, 17, 82, and 106) that were absent or present in low concentrations in fca-1, stm-4, and co-1 compared with the WT at day 21 are related to defense; therefore, we suggest a direct link between flowering and defense. Interestingly, these five proteins (glucosidase, spot 1; thaumatin, spot 12; myrosinases, spots 17 and 82; and osmotin, spot 106) were highly accumulated in the defense-enhanced mutant cpr5-2 and absent or present at very low levels in the defense-impaired mutants npr1-1 and NahG (Fig. 3), which further emphasize our hypothesis that secretion of defense proteins and flowering are directly linked.
FIGURE 4.
Comparative histogram of normalized volume values from each of the 26 spots from 2-DE of A. thaliana WT and the flowering-related mutants. The major differences between the root-secreted proteins of A. thaliana ecotype Col-0 (WT), the mutant co-1, which flowers later (after day 28) than the WT (day 21) under long day conditions, the mutant fca-1, which has shown late flowering (around day 35), and the mutant stm-4 at days 21 and 28 are shown. Two hundred and fifty micrograms of protein for every genotype were analyzed by 2-DE as described under “Experimental Procedures.” The spot numbers represent the identified proteins that are listed in supplemental Table S1. The error bars illustrate the S.E. values of three repetitions (n = 3).
Bioactivity of Root-exuded Proteins
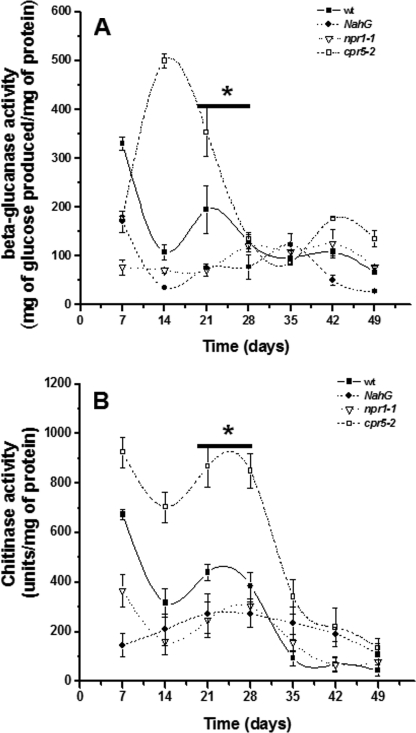
We performed β-1,3-glucanase and chitinase activity assays on the root exudates to investigate the enzymatic activity of two of the most abundant and variable proteins found in the root exudate proteome. The assay results showed variable trends dependent upon the plant type (WT and mutants) as well as the plant age (Figs. 5 and 6). For instance, β-1,3-glucanase activity in WT root exudates shows two increases, at day 7 and at day 21. The cpr5-2 mutant showed higher levels of β-glucanase activity (506 mg glucose/mg of total exudate protein) than the WT and the defense-impaired mutants at day 14 (Fig. 5A). Similarly, the WT showed higher levels of β-1,3-glucanase activity than the defense-impaired mutants (npr1-1, NahG) at all time points until the end of the flowering stage. We identified six potential glucanases (spots 1, 2, 4, 21, 53, and 95; supplemental Table S1), including the three that changed during the developmental time course (spots 1, 2, and 4; Table 1) that could contribute to the measured enzymatic activity.
FIGURE 5.
Enzymatic activity of β-1,3-glucanases, chitinases, and proteases in A. thaliana Col-0 (WT) and defense-related mutants. A, β-1,3-glucanase activity was quantified every 7 days in A. thaliana Col-0 (WT), the impaired defense mutant npr1-1, the impaired defense transgenic NahG, and the defense-related mutant cpr5-2 throughout the whole temporal study; for more details see under “Experimental Procedures.” B, enzymatic activity of chitinases. Chitinolytic activity in root exudates of A. thaliana Col-0 (WT), impaired defense mutant npr1-1, impaired defense transgenic NahG, and the defense-related mutant cpr5-2 was quantified using chitinase azure as a substrate and as described under “Experimental Procedures.” The error bars indicate the S.E. values. The bar with the asterisk represents the days when flowering was observed. The results represent experiments repeated three times with five replicates each.
FIGURE 6.
Enzymatic activity of β-1,3-glucanases, chitinases, and proteases in A. thaliana Col-0 (WT) and flowering-related mutants. A, β-1,3-glucanase activity was quantified every 7 days in A. thaliana Col-0 (WT), the mutant co-1, which flowers later (after day 28) than the WT (day 21) under long day conditions, the mutant fca-1, which has shown late flowering (around day 35), and the mutant stm-4 throughout the whole temporal study; for more details see under “Experimental Procedures.” B, enzymatic activity of chitinases. Chitinolytic activity in root exudates of A. thaliana Col-0 (WT), the mutant co-1, the mutant fca-1, and the mutant stm-4 was quantified using chitinase azure as a substrate and as described under “Experimental Procedures.” The error bars indicate the S.E. values. The results represent experiments repeated three times with five replicates each.
We also analyzed the chitinase activity in the exudates of the WT, defense-impaired, and defense-enhanced plants (Fig. 5B). It was observed that the WT chitinase activity followed the same pattern as the β-glucanase activity with two important increases, day 7 (674 units/mg of total exudate protein) and day 21 (440 units/mg of total exudate protein). The cpr5-2 mutant (defense-enhanced) showed 2-fold more chitinase activity (860 units/mg of total exudate protein) compared with the defense-impaired mutants (270 units/mg of total exudate protein) and the WT (400 units/mg of total exudate protein) in both time points (days 21 and 28) (Fig. 5B). We identified five possible chitinases in our proteomic analysis (spots 3, 5, 11, 48, and 54; supplemental Table S1) that could contribute to the activity, but only spots 48 and 54 (chitinases; At3g12500) presented the same accumulation patterns (Fig. 2) as the enzymatic activity reported for the WT. Spots 5 (chitinase; At2g43590), 11 (chitinase; At1g56680), 48 and 54 (chitinases; At3g12500) were highly accumulated in the root exudates of cpr5-2 at days 21 and 28 (Fig. 3), which could influence the high chitinase activity found in this mutant (Fig. 5B).
Furthermore, we analyzed the enzymatic activity of glucanases and chitinases in the root exudates of the flowering mutants (Fig. 6). For the glucanase activity (Fig. 6A), we did not find any correlation between enzymatic activity and the proteomic data (data not shown). However, there was a delay in the increase of chitinase activity that was correlated with flowering time for all the mutants except stm-4 (Fig. 6B). This increase shows a positive correlation with spots 48 and 54 (chitinases; At3g12500).
In Silico Expression Analyses of Genes Shows a Correlation with Secreted Proteins
To determine whether the higher secretion levels of these 26 proteins in the root exudates correlate to their gene expression in the plants, we performed in silico expression analyses of the corresponding genes using Gene Chronologer and Gene Atlas tools of the GENEVESTIGATOR data base (supplemental Table S2). It was found that most of the genes are highly expressed in roots compared with other tissues, and interestingly, the highest expression for most of the genes were found during flowering (<21.0–24.9 days) compared with other developmental stages of the plant (Table 1 and supplemental Table S2). Among the 20 genes that encoded the 26 proteins in the root exudates that changed throughout the developmental course of A. thaliana, 12 genes were highly expressed in roots and of those four are related to defense (Tables 1 and supplemental Table S2). Genes related to defense, such as chitinases (At3g12500 (spots 3, 48, and 54), At2g43590 (spot 5), and At1g56680 (spot 11)), showed higher expression before flowering than after flowering (supplemental Table S2). Similarly, the genes encoding jasmonate-inducible proteins (At1g52050 (spot 10), At1g52060 (spot 13), and At1g52070 (spot 14)) showed more than a 3-fold increase in expression before flowering than after flowering. Similar trends were found in genes of glucanase (At4g16260 (spots 1 and 4)), secretory protein (At2g15220 (spot 6)), lectin (At3g16420 (spot 8), and At3g16430 (spots 31 and 32)), myrosinases (At1g54010 (spot 17)), and osmotin (At4g11650 (spots 35 and 106)).
Antimicrobial Effect of Root-exuded Proteins
We found that most of the proteins in the root exudates of the WT that showed changes in their secretion levels at specific time points (days 21 and 28) are related to defense responses. To determine whether these proteins had antimicrobial activity, we tested the total proteins secreted from the WT and the mutants NahG, npr1-1, and cpr5-2 against P. syringae pv. lycopersici (DC3000), a compatible pathogen to Arabidopsis, and P. syringae pv. phaseolicola (Psph 3121), considered a non-pathogen to Arabidopsis (Fig. 7). We observed that the secreted proteins of cpr5-2 on day 21 and 28 inhibited significantly the growth of both nonpathogenic Pseudomonas strain Psph 3121 and the pathogenic strain DC3000 compared with the control (only the bacteria without the exudate proteins). In contrast, the proteins found in the root exudates of the WT (day 21) only inhibited the growth of the non-pathogen Psph 3121. The exuded proteins belonging to the defense-impaired mutants (npr1-1 and NahG) did not promote growth inhibition on either of the bacteria tested. On the contrary, the total proteins exuded from npr1-1 were found to induce the growth of pathogenic strain DC3000. It should be noted that this assay was performed with secreted proteins alone as explained under “Experimental Procedures,” and thus, no other metabolic components (i.e. carbohydrates, secondary metabolites, etc.) are likely to be responsible for the microbial inhibition or growth. Interestingly, the inhibitory effect found in the root exudates was correlated with high chitinase activity (Fig. 5B). Furthermore, we observed that high levels of spot 5 (a chitinase; At2g43590; Fig. 3) and spot 17 (a myrosinases; At1g54010; Fig. 3) in cpr5-2 seems to be correlated with the inhibitory effect against Psph3121. Both proteins (spots 5 and 17) were not present in the defense-impaired mutants npr1-1 and NahG and at low level in WT. The fact that multiple chitinases (i.e. proteins 5 (At2g43590), 11 (At1g56680), and 48 and 54 (At3g12500)) were secreted at higher levels by the defense-enhanced mutant cpr5-2 than the WT and the other mutants (NahG and npr1-1) suggests that growth of DC3000 was inhibited by the presence of the root-exuded proteins of cpr5-2 at day 21 (Fig. 7).
FIGURE 7.
Antibacterial assay from protein root exudates of A. thaliana Col-0 (WT) and the defense-related mutants. Antibacterial activity assays of protein root exudates from the WT, impaired-defense mutant npr1-1, impaired-defense transgenic NahG, and the defense-related mutant cpr5-2 at days 21 and 28. Twenty μg of protein exudates from every genotype were tested against P. syringae pv. lycopersici (DC3000) and P. syringae pv. phaseolicola (Psph 3121). The asterisk indicates the values that are statistically significant at p ≤ 0.05, n = 10. The error bars illustrate the S.E. values. The presented values are from two independent experiments with five biological replicates each.
DISCUSSION
We have found that protein root exudation is constitutive and developmentally dependent. Defense proteins are exuded in the greatest amounts just before flowering, despite the fact that root exudation represents a significant cost to the plant (58).
Root secretion of proteins has been previously studied by other researchers (24, 30, 31, 59), but the possible functional relationship of these secreted proteins with plant development is poorly understood. Previous studies analyzed the root secretion of proteins only at a specific stage of the plant development or in response to particular stress conditions (30, 31). Although Basu et al. (31) reported several extracytosolic proteins released from 10-day-old Arabidopsis roots, the temporal accumulation or possible regulation of secreted proteins or whether they are predisposed to be secreted in response to plant developmental changes were not clearly studied. Likewise, Charmont et al. (30) reported several proteins secreted from the roots of 14-day-old Arabidopsis etiolated seedlings but did not address the potential effect of etiolation on root secretion.
Some of the defense-related proteins that were identified in this study were also found by Charmont et al. (30), including chitinases (spots 3, 48, and 54) and thaumatin-like protein (spot 12) peroxidases (spots 18 and 52). They also found a putative lectin (spot 8) and a subtilisin-like protein (spot 16); however, the authors did not explain the possible role of these proteins in the rhizosphere. In this study, we analyzed the root exudation of proteins from plants growing under normal conditions (i.e. no visible stress) and found that root protein exudation is constitutive and varies with the developmental stage of the plant. We are confident that our experimental system remained sterile and was devoid of external microbial contamination (supplemental Fig. 4). However, it is worth considering that endophytes, originating from infected seed, colonized the roots of Arabidopsis contributing to root physiology changes. However, only a limited number of endophytic bacteria appear to be seed-borne (60) and, to our knowledge, none have been reported in Arabidopsis. To our knowledge, this is the first study describing how protein secretion in the absence of visible stress can change dramatically due to developmental conditions in WT (Fig. 2) and genotype-specific plants (Figs. 3 and 4). That flowering, an aboveground response, stimulates defense responses in the roots is an unprecedented finding.
We observed that 23% of the total proteins exuded by roots were defense-related and were exuded at specific developmental stages of the plant (Fig. 2 and Table S1). It has been reported that the induction of defense proteins within the plant varies with plant age (61), but their secretion into the rhizosphere was not addressed. In our study, we found that most of the defense-related genes expressed in roots were up-regulated during flowering (supplemental Table S2), a finding that correlated well with their root protein secretion.
We found myrosinases (spots 17 and 82; Table 1) in the root exudates, which are involved in defense against an extensive class of pathogens by employing a glucosinolate-myrosinase enzymatic system (62–64). Myrosinases have the catalytic capacity to degrade glucosinolates forming very toxic compounds, named isothiocyanates, that are active on herbivores and on a wide variety of bacteria (65–69), even in the rhizosphere (70). There is evidence that the volatile nature of some isothiocyanates (71) allows them to cover great distances; this property could have important implications in the rhizosphere. It has been reported that glucosinolates are found in roots during flowering (72–74), and our results suggest that it is possible that these compounds could be released into the rhizosphere. However, whether a myrosinase is able to cleave glucosinolates to produce isothiocyanates outside of the plant remains undetermined.
Induction of defense-related proteins in planta is considered a significant cost to the plant in the absence of stress (75, 76). De-la-Peña et al. (26) reported that root protein secretion could be triggered by specific microbes that come in contact with the plant. In this study, we identified six proteins that change upon developmental stage (spots 1 (β1,3-glucanase), 3 (chitinase), 6 (secretory protein), 48 (chitinase), 54 (chitinase), and 106 (osmotin); Fig. 2) that were highly accumulated in the interaction between A. thaliana-P. syringae DC3000 (26). Interestingly, we found that these proteins were also secreted at higher levels during flowering (Fig. 2) suggesting that the plant is taking measures to prevent pathogen attack during the most important stage of the developmental cycle. A previous report described that plants induce the production of proteins related to defense, such as chitinases, in leaves at flowering time (28). Furthermore, it has been demonstrated that the levels of SA can differentially induce the expression of chitinases in planta and regulate the flowering time in Arabidopsis (77, 78). Our results are in agreement with these studies as we found higher accumulation of chitinases at flowering time in the cpr5-2 compared with npr1-1 and NahG (Fig. 3 and Fig. 5B) which by definition have low or no SA, respectively.
It has been hypothesized that PR proteins such as chitinases (79, 80) and β-1,3-glucanases (81) might have a function in the sexual reproduction of higher plants. Similarly, it has been shown that the chitinase activity in tobacco roots is related to the developmental stage (80), and in Arabidopsis it is organ- and age-dependent (82). Overall, these data indicate that a number of defense-related proteins in roots are directly involved in the vegetative-to-floral transition and the subsequent sequential development of floral organs.
The composition and diversity of microbial communities in the rhizosphere is dependent on plant age (83), and root exudates are the driving factor for this selectivity (84). Our results showed that the proteins of the defense-enhanced mutant cpr5-2, secreted at flowering time, inhibited the growth of the compatible pathogen DC3000 and the non-pathogen Psph 3121 (Fig. 7). The root-exuded proteins of npr1-1, and those of the transgenic NahG, did not show any antimicrobial activity against either the nonpathogenic Psph 3121 and the pathogenic DC3000 strains. This lack of antimicrobial activity in npr1-1 and NahG mutants may be related to the fact that both mutants fail to induce systemic acquired resistance (33, 36, 85, 86) and, as a consequence, high enough levels of PR proteins are not secreted (86). However, the presence of defense proteins with both chitinase and glucanase activities in the root exudates of the defense-impaired genotypes indicates that an additional protein(s) is required for effective pathogen defense. According to Ludwig and Boller (87), fungi are temporarily inhibited by chitinases and glucanases, but they can adapt to these proteins very quickly. A similar adaptation by bacteria could explain our observation of more growth of Psph 3121 in the presence of the exudates of NahG (Fig. 7). It is conceivable that Psph 3121 hydrolyzed the protein exudates of the plant mutants/genotypes, suggesting that this pathogen is able to obtain amino acids from the proteins of npr-1 and NahG. It is likely that a mixture of different chitinases (At2g43590 (spot 5), At1g56680 (spot 11) and At3g12500 (spots 48 and 54)) plays a direct antimicrobial role against DC3000 (26). It should be noted that bacteria such as DC3000 do not have chitin in its cell walls, but chitinases could degrade peptidoglycans that are general components of the bacterial cell wall (88). Furthermore, it was reported that exochitinase activity in Arabidopsis leaves defend plants against DC3000 (89) and that chitinases can induce bacterial agglutination (90). In light of this evidence, the potential role of plant chitinases in defense against bacteria should be revisited.
In summary, our present findings suggest that protein secretion by Arabidopsis roots is developmentally dependent, and the secretion of defense-related proteins is particularly up-regulated at the onset of flowering. Further studies are necessary to determine how biotic and abiotic stresses interact with internal plant signals to produce changes in root exudation of proteins at different stages of development.
Supplementary Material
Acknowledgments
We are grateful to the Vivanco laboratory for commenting on the manuscript and Dr. Daniel K. Manter (United States Department of Agriculture-Agricultural Research Scientist) for technical advice. The ABI QSTAR Pulsar i was supported by the National Science Foundation Grant DBI-0109732 and The Samuel Roberts Noble Foundation.
This work was supported in part by National Science Foundation Grants MCB-0542642 and MCB-0950857 (to J. M. V.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. 1–4.
- 2-DE
- two-dimensional gel electrophoresis
- SA
- salicylic acid.
REFERENCES
- 1.Kaplan F., Badri D. V., Zachariah C., Ajredini R., Sandoval F. J., Roje S., Levine L. H., Zhang F., Robinette S. L., Alborn H. T., Zhao W., Stadler M., Nimalendran R., Dossey A. T., Brüschweiler R., Vivanco J. M., Edison A. S. (2009) J. Chem. Ecol. 35, 878–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanon A., Andrianjaka Z., Prin Y., Bally R., Thioulouse J., Comte G., Duponnois R. (2009) Plant. Soil 321, 259–278 [Google Scholar]
- 3.Baldwin I. T., Kessler A., Halitschke R. (2002) Curr. Opin. Plant Biol. 5, 351–354 [DOI] [PubMed] [Google Scholar]
- 4.Felton G. W., Tumlinson J. H. (2008) Curr. Opin. Plant Biol. 11, 457–463 [DOI] [PubMed] [Google Scholar]
- 5.Heil M., Lion U., Boland W. (2008) J. Chem. Ecol. 34, 601–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitner M., Kaiser R., Rasmussen M. O., Driguez H., Boland W., Mithöfer A. (2008) Phytochemistry 69, 2029–2040 [DOI] [PubMed] [Google Scholar]
- 7.Badri D. V., Vivanco J. M. (2009) Plant Cell Environ. 32, 666–681 [DOI] [PubMed] [Google Scholar]
- 8.Reddy P. M., Rendón-Anaya M., Soto del Río M. D., Khandual S. (2007) Dynamic Soil Dynamic Plant 1, 83–94 [Google Scholar]
- 9.Stacey G., Sanjuan J., Luka S., Dockendorff T., Carlson R. W. (1995) Soil Biol. Biochem. 27, 473–483 [Google Scholar]
- 10.Barnett M. J., Toman C. J., Fisher R. F., Long S. R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 16636–16641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horiuchi J., Prithiviraj B., Bais H. P., Kimball B. A., Vivanco J. M. (2005) Planta 222, 848–857 [DOI] [PubMed] [Google Scholar]
- 12.Kawaoka A., Kawamoto T., Ohta H., Sekine M., Takano M., Shinmyo A. (1994) Plant Cell Rep. 13, 149–154 [DOI] [PubMed] [Google Scholar]
- 13.Li Z. C., McClure J. W. (1990) J. Plant Physiol. 136, 398–403 [Google Scholar]
- 14.Van der Westhuizen A. J., Pretorius Z. (1996) J. Plant Physiol. 23, 645–648 [Google Scholar]
- 15.Hiilovaara-Teijo M., Hannukkala A., Griffith M., Yu X. M., Pihakaski-Maunsbach K. (1999) Plant Physiol. 121, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hugot K., Rivière M. P., Moreilhon C., Dayem M. A., Cozzitorto J., Arbiol G., Barbry P., Weiss C., Galiana E. (2004) Plant Physiol. 134, 858–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mithöfer A., Müller B., Wanner G., Eichacker L. A. (2002) Mol. Plant. Pathol. 3, 163–166 [DOI] [PubMed] [Google Scholar]
- 18.Olivieri F., Godoy A. V., Escande A., Casalongué C. A. (1998) Physiol. Plant. 104, 232–238 [Google Scholar]
- 19.Rep M., Dekker H. L., Vossen J. H., de Boer A. D., Houterman P. M., Speijer D., Back J. W., de Koster C. G., Cornelissen B. J. (2002) Plant Physiol. 130, 904–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce G., Moura D. S., Stratmann J., Ryan C. A., Jr. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12843–12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasternack C., Stenzel I., Hause B., Hause G., Kutter C., Maucher H., Neumerkel J., Feussner I., Miersch O. (2006) J. Plant Physiol. 163, 297–306 [DOI] [PubMed] [Google Scholar]
- 22.Grunwald I., Rupprecht I., Schuster G., Kloppstech K. (2003) Physiol. Plant. 119, 192–202 [Google Scholar]
- 23.Yu J. Q., Ye S. F., Zhang M. F., Hu W. H. (2003) Biochem. Syst. Ecol. 31, 129–139 [Google Scholar]
- 24.Wen F., VanEtten H. D., Tsaprailis G., Hawes M. C. (2007) Plant Physiol. 143, 773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nóbrega F. M., Santos I. S., Da Cunha M., Carvalho A. O., Gomes V. M. (2005) Plant Soil 272, 223–232 [Google Scholar]
- 26.De-la-Peña C., Lei Z., Watson B. S., Sumner L. W., Vivanco J. M. (2008) J. Biol. Chem. 283, 25247–25255 [DOI] [PubMed] [Google Scholar]
- 27.Hugot K., Aimé S., Conrod S., Poupet A., Galiana E. (1999) Plant J. 20, 163–170 [DOI] [PubMed] [Google Scholar]
- 28.Barto E. K., Cipollini D. (2005) Oecologia 146, 169–178 [DOI] [PubMed] [Google Scholar]
- 29.Balasubrahmanyam A., Baranwal V. K., Lodha M. L., Varma A., Kapoor H. C. (2000) Plant Sci. 154, 13–21 [DOI] [PubMed] [Google Scholar]
- 30.Charmont S., Jamet E., Pont-Lezica R., Canut H. (2005) Phytochemistry 66, 453–461 [DOI] [PubMed] [Google Scholar]
- 31.Basu U., Francis J. L., Whittal R. M., Stephens J. L., Wang Y., Zaiane O. R., Goebel R., Muench D. G., Good A. G., Taylor G. J. (2006) Plant Soil 286, 357–376 [Google Scholar]
- 32.Esaka M., Enoki K., Kouchi B., Sasaki T. (1990) Plant Physiol. 93, 1037–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedrich L., Vernooij B., Gaffney T., Morse A., Ryals J. (1995) Plant Mol. Biol. 29, 959–968 [DOI] [PubMed] [Google Scholar]
- 34.Delaney T. P., Uknes S., Vernooij B., Friedrich L., Weymann K., Negrotto D., Gaffney T., Gut-Rella M., Kessmann H., Ward E., Ryals J. (1994) Science 266, 1247–1250 [DOI] [PubMed] [Google Scholar]
- 35.Cao H., Glazebrook J., Clarke J. D., Volko S., Dong X. (1997) Cell 88, 57–63 [DOI] [PubMed] [Google Scholar]
- 36.Cao H., Bowling S. A., Gordon A. S., Dong X. (1994) Plant Cell 6, 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowling S. A., Guo A., Cao H., Gordon A. S., Klessig D. F., Dong X. (1994) Plant Cell 6, 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putterill J., Robson F., Lee K., Simon R., Coupland G. (1995) Cell 80, 847–857 [DOI] [PubMed] [Google Scholar]
- 39.Chandler J., Wilson A., Dean C. (1996) Plant J. 10, 637–644 [DOI] [PubMed] [Google Scholar]
- 40.Cole M., Nolte C., Werr W. (2006) Nucleic Acids Res. 34, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murashige T., Skoog F. (1962) Physiol. Plant. 15, 473–497 [Google Scholar]
- 42.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 43.O'Farrell P. H. (1975) J. Biol. Chem. 250, 4007–4021 [PMC free article] [PubMed] [Google Scholar]
- 44.Blum H., Beier H., Gross H. J. (1987) Electrophoresis 8, 93–99 [Google Scholar]
- 45.Sumner L. W., Wolf-Sumner B., White S. P., Asirvatham V. S. (2002) Rapid Commun. Mass Spectrom. 16, 160–168 [DOI] [PubMed] [Google Scholar]
- 46.Lei Z., Elmer A. M., Watson B. S., Dixon R. A., Mendes P. J., Sumner L. W. (2005) Mol. Cell. Proteomics 4, 1812–1825 [DOI] [PubMed] [Google Scholar]
- 47.Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 48.Creasy D. M., Cottrell J. S. (2002) Proteomics 2, 1426–1434 [DOI] [PubMed] [Google Scholar]
- 49.Gomez Ramirez M., Rojas Avelizapa L. I., Rojas Avelizapa N. G., Cruz Camarillo R. (2004) J. Microbiol. Met. 56, 213–219 [DOI] [PubMed] [Google Scholar]
- 50.Abeles F. B., Forrence L. E. (1970) Plant Physiol. 45, 395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tilton R. C., Lieberman L., Gerlach E. H. (1973) Appl. Environ. Microbiol. 26, 658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu Z., Szafron D., Greiner R., Lu P., Wishart D. S., Poulin B., Anvik J., Macdonell C., Eisner R. (2004) Bioinformatics 20, 547–556 [DOI] [PubMed] [Google Scholar]
- 53.Nakai K., Horton P. (1999) Trends Biochem. Sci. 24, 34–36 [DOI] [PubMed] [Google Scholar]
- 54.Nair R., Rost B. (2005) J. Mol. Biol. 348, 85–100 [DOI] [PubMed] [Google Scholar]
- 55.Emanuelsson O., Nielsen H., Brunak S., von Heijne G. (2000) J. Mol. Biol. 300, 1005–1016 [DOI] [PubMed] [Google Scholar]
- 56.Höglund A., Dönnes P., Blum T., Adolph H. W., Kohlbacher O. (2006) Bioinformatics 22, 1158–1165 [DOI] [PubMed] [Google Scholar]
- 57.Zimmermann P., Hirsch-Hoffmann M., Hennig L., Gruissem W. (2004) Plant Physiol. 136, 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shepherd T., Davies H. V. (1993) Ann. Bot. 72, 155–163 [Google Scholar]
- 59.Borisjuk N. V., Borisjuk L. G., Logendra S., Petersen F., Gleba Y., Raskin I. (1999) Nat. Biotechnol. 17, 466–469 [DOI] [PubMed] [Google Scholar]
- 60.Ryan R. P., Germaine K., Franks A., Ryan D. J., Dowling D. N. (2008) FEMS Microbiol. Lett. 278, 1–9 [DOI] [PubMed] [Google Scholar]
- 61.Cipollini D. F., Redman A. M. (1999) J. Chem. Ecol. 25, 271–281 [Google Scholar]
- 62.Rask L., Andréasson E., Ekbom B., Eriksson S., Pontoppidan B., Meijer J. (2000) Plant Mol. Biol. 42, 93–113 [PubMed] [Google Scholar]
- 63.Bones A. M., Rossiter J. T. (1996) Physiol. Plant. 97, 194–208 [Google Scholar]
- 64.Raybould A. F., Moyes C. L. (2001) Heredity 87, 383–391 [DOI] [PubMed] [Google Scholar]
- 65.Textor S., Gershenzon J. (2009) Phytochem. Rev. 8, 149–170 [Google Scholar]
- 66.Agerbirk N., De Vos M., Kim J., Jander G. (2009) Phytochem. Rev. 8, 101–120 [Google Scholar]
- 67.Halkier B. A., Gershenzon J. (2006) Annu. Rev. Plant Biol. 57, 303–333 [DOI] [PubMed] [Google Scholar]
- 68.Mishina T. E., Zeier J. (2007) Physiol. Plant. 131, 448–461 [DOI] [PubMed] [Google Scholar]
- 69.Aires A., Mota V. R., Saavedra M. J., Monteiro A. A., Simões M., Rosa E. A., Bennett R. N. (2009) J. Appl. Microbiol. 106, 2096–2105 [DOI] [PubMed] [Google Scholar]
- 70.Bressan M., Roncato M. A., Bellvert F., Comte G., Haichar F. Z., Achouak W., Berge O. (2009) ISME J. 3, 1243–1257 [DOI] [PubMed] [Google Scholar]
- 71.Hashem F. A., Wahba H. E. (2000) Phytother. Res. 14, 284–287 [DOI] [PubMed] [Google Scholar]
- 72.Zeng R. S., Mallik A. U., Setliff E. (2003) J. Chem. Ecol. 29, 1337–1355 [DOI] [PubMed] [Google Scholar]
- 73.Pongrac P., Vogel-Mikus K., Regvar M., Tolrà R., Poschenrieder C., Barceló J. (2008) J. Chem. Ecol. 34, 1038–1044 [DOI] [PubMed] [Google Scholar]
- 74.Brown P. D., Tokuhisa J. G., Reichelt M., Gershenzon J. (2003) Phytochemistry 62, 471–481 [DOI] [PubMed] [Google Scholar]
- 75.Cipollini D., Enright S., Traw M. B., Bergelson J. (2004) Mol. Ecol. 13, 1643–1653 [DOI] [PubMed] [Google Scholar]
- 76.Truman W., Bennett M. H., Kubigsteltig I., Turnbull C., Grant M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martínez C., Pons E., Prats G., León J. (2004) Plant J. 37, 209–217 [DOI] [PubMed] [Google Scholar]
- 78.Davis J. M., Wu H., Cooke J. E., Reed J. M., Luce K. S., Michler C. H. (2002) Mol. Plant Microbe Interact. 15, 380–387 [DOI] [PubMed] [Google Scholar]
- 79.Leung D. W. (1992) Phytochemistry 31, 1899–1900 [Google Scholar]
- 80.Neale A. D., Wahleithner J. A., Lund M., Bonnett H. T., Kelly A., Meeks-Wagner D. R., Peacock W. J., Dennis E. S. (1990) Plant Cell 2, 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lotan T., Ori N., Fluhr R. (1989) Plant Cell 1, 881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samac D. A., Hironaka C. M., Yallaly P. E., Shah D. M. (1990) Plant Physiol. 93, 907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Micallef S. A., Channer S., Shiaris M. P., Colon-Carmona A. (2009) Plant Sign. Beh. 4, 777–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broeckling C. D., Broz A. K., Bergelson J., Manter D. K., Vivanco J. M. (2008) Appl. Environ. Microbiol. 74, 738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stintzi A., Heitz T., Prasad V., Wiedemann-Merdinoglu S., Kauffmann S., Geoffroy P., Legrand M., Fritig B. (1993) Biochimie 75, 687–706 [DOI] [PubMed] [Google Scholar]
- 86.Wang D., Weaver N. D., Kesarwani M., Dong X. (2005) Science 308, 1036–1040 [DOI] [PubMed] [Google Scholar]
- 87.Ludwig A., Boller T. (1990) FEMS Microbiol. Lett. 69, 61–66 [DOI] [PubMed] [Google Scholar]
- 88.Collinge D. B., Kragh K. M., Mikkelsen J. D., Nielsen K. K., Rasmussen U., Vad K. (1993) Plant J. 3, 31–40 [DOI] [PubMed] [Google Scholar]
- 89.Traw M. B., Kim J., Enright S., Cipollini D. F., Bergelson J. (2003) Mol. Ecol. 12, 1125–1135 [DOI] [PubMed] [Google Scholar]
- 90.Guan Y., Ramalingam S., Nagegowda D., Taylor P. W., Chye M. L. (2008) J. Exp. Bot. 59, 3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bevan M., Bancroft I., Bent E., Love K., Goodman H., Dean C., Bergkamp R., Dirkse W., Van Staveren M., Stiekema W., Drost L., Ridley P., Hudson S. A., Patel K., Murphy G., Piffanelli P., Wedler H., Wedler E., Wambutt R., Weitzenegger T., Pohl T. M., Terryn N., Gielen J., Villarroel R., De Clerck R., Van Montagu M., Lecharny A., Auborg S., Gy I., Kreis M., Lao N., Kavanagh T., Hempel S., Kotter P., Entian K. D., Rieger M., Schaeffer M., Funk B., Mueller-Auer S., Silvey M., James R., Montfort A., Pons A., Puigdomenech P., Douka A., Voukelatou E., Milioni D., Hatzopoulos P., Piravandi E., Obermaier B., Hilbert H., Düsterhöft A., Moores T., Jones J. D., Eneva T., Palme K., Benes V., Rechman S., Ansorge W., Cooke R., Berger C., Delseny M., Voet M., Volckaert G., Mewes H. W., Klosterman S., Schueller C., Chalwatzis N. (1998) Nature 391, 485–488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.