Abstract
Background
Treatment of cancer is increasingly effective but associated with short and long term side effects. Oral side effects, including oral mucositis (mouth ulceration), remain a major source of illness despite the use of a variety of agents to treat them.
Objectives
To assess the effectiveness of interventions for treating oral mucositis or its associated pain in patients with cancer receiving chemotherapy or radiotherapy or both.
Search methods
Electronic searches of Cochrane Oral Health Group and PaPaS Trials Registers (to 1 June 2010), CENTRAL via The Cochrane Library (to Issue 2, 2010), MEDLINE via OVID (1950 to 1 June 2010), EMBASE via OVID (1980 to 1 June 2010), CINAHL via EBSCO (1980 to 1 June 2010), CANCERLIT via PubMed (1950 to 1 June 2010), OpenSIGLE (1980 to 1 June 2010) and LILACS via the Virtual Health Library (1980 to 1 June 2010) were undertaken. Reference lists from relevant articles were searched and the authors of eligible trials were contacted to identify trials and obtain additional information.
Selection criteria
All randomised controlled trials comparing agents prescribed to treat oral mucositis in people receiving chemotherapy or radiotherapy or both. Outcomes were oral mucositis, time to heal mucositis, oral pain, duration of pain control, dysphagia, systemic infection, amount of analgesia, length of hospitalisation, cost and quality of life.
Data collection and analysis
Data were independently extracted, in duplicate, by two review authors. Authors were contacted for details of randomisation, blindness and withdrawals. Risk of bias assessment was carried out on six domains. The Cochrane Collaboration statistical guidelines were followed and risk ratio (RR) values calculated using fixed‐effect models (less than 3 trials in each meta‐analysis).
Main results
Thirty‐two trials involving 1505 patients satisfied the inclusion criteria. Three comparisons for mucositis treatment including two or more trials were: benzydamine HCl versus placebo, sucralfate versus placebo and low level laser versus sham procedure. Only the low level laser showed a reduction in severe mucositis when compared with the sham procedure, RR 5.28 (95% confidence interval (CI) 2.30 to 12.13).
Only 3 comparisons included more than one trial for pain control: patient controlled analgesia (PCA) compared to the continuous infusion method, therapist versus control, cognitive behaviour therapy versus control. There was no evidence of a difference in mean pain score between PCA and continuous infusion, however, less opiate was used per hour for PCA, mean difference 0.65 mg/hour (95% CI 0.09 to 1.20), and the duration of pain was less 1.9 days (95% CI 0.3 to 3.5).
Authors' conclusions
There is limited evidence from two small trials that low level laser treatment reduces the severity of the mucositis. Less opiate is used for PCA versus continuous infusion. Further, well designed, placebo or no treatment controlled trials assessing the effectiveness of interventions investigated in this review and new interventions for treating mucositis are needed.
Keywords: Humans, Analgesics, Analgesics/therapeutic use, Anti‐Ulcer Agents, Anti‐Ulcer Agents/therapeutic use, Low‐Level Light Therapy, Low‐Level Light Therapy/methods, Mouth Diseases, Mouth Diseases/etiology, Mouth Diseases/therapy, Neoplasms, Neoplasms/drug therapy, Neoplasms/radiotherapy, Oral Ulcer, Oral Ulcer/etiology, Oral Ulcer/therapy, Pain, Pain/etiology, Pain Management, Randomized Controlled Trials as Topic, Stomatitis, Stomatitis/etiology, Stomatitis/therapy
Interventions for treating oral mucositis for patients with cancer receiving treatment
Using a low level laser may reduce the severity of ulcers caused by cancer treatment. Treatments for cancer can cause severe ulcers (sores) in the mouth. These can be painful and slow to heal. The review found some evidence that using a laser relieves or cures the ulcers. Morphine can control the pain. Although using morphine automatically on a constant drip, or self controlled use, provide similar relief, people use less morphine when they are controlling it themselves.
Background
Treatment of malignant diseases with cytotoxic chemotherapy or radiotherapy or both, is becoming increasingly more effective but it is associated with short and long term side effects. Among the clinically important acute side effects is disruption in the function and integrity of the oral mucosa. The consequences of this include severe ulceration (oral mucositis) and fungal infection of the mouth (oral candidiasis). These disease and treatment induced complications may also produce oral discomfort and pain, poor nutrition, delays in administration of chemotherapy or radiotherapy, reductions in the doses of chemotherapy drugs, increased length of inpatient stays and associated economic costs and in some patients life threatening infection (septicaemia in neutropenic patients).
Oral complications remain a major source of illness despite the use of a variety of agents to prevent them. There are variations in usage between cancer centres in terms of the mouthcare regimen used. Compliance with recommended use of product is variable and there are conflicting reports of the effectiveness of prophylactic agents. The qualitative and quantitative benefits, side effects and costs of oral therapies are of importance to the cancer teams responsible for the treatment of patients.
There have been several traditional reviews published and most of these present a general discussion for both chemotherapy and radiotherapy induced oral side effects (De Pauw 1997; Denning 1992; Knox 2000; Lortholary 1997; Plevova 1999; Shaw 2000; Stevens 1995; Symonds 1998; Verdi 1993; White 1993). The conclusions drawn and recommendations made vary from advocating a particular therapy to recommending oral care procedures that have not been systematically investigated. Five systematic reviews, which were not Cochrane reviews, have focused on the prevention and treatment of oral mucositis in patients with cancer. One older review published in 1998 concluded that for most strategies reviewed there is insufficient evidence to draw any conclusions regarding their effectiveness (Kowanko 1998). The other three more recent reviews focused on patients with head and neck cancer only (Shih 2002; Sutherland 2001; Trotti 2003), and two were unable to draw definite conclusions regarding the effectiveness of any of the agents tested, however in the Sutherland 2001 review the main analysis combined all the interventions in one meta‐analysis and found a beneficial effect of prophylactic interventions. This pooling of interventions is impossible to interpret and therefore not helpful.
A systematic review looking at antimicrobial therapy to prevent or treat mucositis identified 31 prospective trials (not just randomised trials) of which 13 reported some benefit. The review concludes that there is no clear pattern emerging regarding the benefit or otherwise of antimicrobial use to manage mucositis, and there is limited evidence supporting the use of antimicrobial agents for reducing oral mucositis (Donnelly 2003).
Another review looked at granulocyte macrophage‐colony stimulating factor (GM‐CSF) for the prevention and treatment of oral mucositis (Fung 2002). This review included studies of different types including some with historical controls. The authors conclude that although there are published studies, these studies are limited and the use of systemic or topical GM‐CSF cannot be recommended for prevention or treatment of mucositis.
This review is part of a series of four Cochrane reviews looking at the prevention and treatment of oral mucositis and oral candidiasis in patients with cancer receiving treatment (Worthington 2007a; Clarkson 2007; Worthington 2007b). The review for the prevention of oral mucositis (Worthington 2007b) found 11 out of the 33 interventions investigated showed some evidence of a benefit (albeit sometimes weak) for either preventing or reducing the severity of mucositis. Interventions with more than one trial were amifostine, Chinese medicine, hydrolytic enzymes and ice chips (cryotherapy). Interventions with only one study were: benzydamine, calcium phosphate, etoposide bolus, honey, iseganan, oral care and zinc sulphate. This review is currently being updated and will be published in 2010.
Objectives
To assess the effectiveness of interventions (which may include placebo or no treatment) for the treatment of oral mucositis or its associated pain for patients with cancer receiving chemotherapy or radiotherapy or both. The following null hypotheses were tested, against alternative hypotheses of a difference, for comparisons between groups treated for mucositis.
There is no difference in the proportion of patients with improvement in mucositis after treatment for mucositis.
There is no difference in the proportion of patients with mucositis eradicated after treatment for mucositis.
There is no difference in the proportion of patients with severe mucositis (≥grade 3) after treatment for mucositis
There is no difference in the mean number of days taken to heal.
There is no difference in the mean pain scores after treatment or analgesia for mucositis.
The review is divided into two parts, one concerning interventions for the treatment of mucositis and one concerning the control of the pain in patients with mucositis. In this review we also addressed the hypothesis of no difference between groups treated for mucositis for the following outcomes if data were available from studies which included a primary outcome:
relief of dysphagia (problems during eating);
incidence of systemic infection;
amount of analgesia;
days stay in hospital;
cost of oral care;
patient quality of life.
The following subgroup analyses were proposed a priori:
cancer type (head and neck, other solid tumours, leukaemia, and mixed);
cancer treatment type;
age group (children, adults, children and adults).
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) were eligible for inclusion in this review.
Types of participants
Anyone with cancer who is receiving chemotherapy or radiotherapy or both and has oral mucositis.
Types of interventions
Active agents: any intervention for the treatment of oral mucositis or its associated pain. Control: may be placebo, no treatment, or another active intervention.
Types of outcome measures
The following outcomes were considered in this review:
Mucositis at different levels of severity
Days to heal (mean)
Oral pain scores or categories
Relief of dysphagia
Incidence of systemic infection
Amount of analgesia
Days stay in hospital
Cost of oral care
Patient quality of life.
Search methods for identification of studies
This review is part of a series of four reviews on the prevention and treatment of oral candidiasis and oral mucositis in patients with cancer, and the same search strategies were used for all four reviews. The searches attempted to identify all relevant trials irrespective of language. Papers not in English were translated by members of The Cochrane Collaboration. Sensitive search strategies were developed for each database using a combination of free text and MeSH terms. The MEDLINE and CANCERLIT searches combined the subject search with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in boxes 6.4a and 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] (Higgins 2009). The EMBASE and CINAHL searches were combined with sensitive search strategies developed by the Cochrane Oral Health Group for identifying RCTs. The LILACs subject search was combined with the Brazilian Cochrane Centre search strategy for identifying RCTs in LILACS.
Electronic searching ‐ the databases searched were: Cochrane Oral Health Group Trials Register (to 1 June 2010) (see Appendix 1) Cochrane Pain, Palliative and Supportive Care (PaPaS) Group Trials Register (to 1 June 2010) (see Appendix 1) Cochrane Central Register of Controlled Trials (CENTRAL) (via The Cochrane Library 2010, Issue 2, 1 June 2010) (see Appendix 2) MEDLINE and MEDLINE Pre‐indexed via OVID (1950 to 1 June 2010) (see Appendix 3) EMBASE via OVID (1980 to 1 June 2010) (see Appendix 4) CINAHL via EBSCO (1980 to 1 June 2010) (see Appendix 5) CANCERLIT via PubMed (1950 to 1 June 2010) (see Appendix 6) OpenSIGLE (1980 to 1 June 2010) (see Appendix 7) LILACS via The Virtual Health Library (see Appendix 8).
Only handsearching carried out by The Cochrane Collaboration was included in the search (see master list www.cochrane.org).
The controlled trials database (www.controlled‐trials.com) was also searched to identify ongoing and completed trials and to contact trialists for further information about these trials.
The reference list of related review articles and all articles obtained were checked for further trials. Authors of trial reports and specialists in the field known to the review authors were written to concerning further published and unpublished trials.
The review will be updated every 2 years using the Cochrane Oral Health Group Trials Register, CENTRAL, MEDLINE, EMBASE, CINAHL, CANCERLIT and LILACS. The search of OpenSIGLE was discontinued as this database ceased being updated in 2005.
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the searches were scanned by two review authors. Full reports were obtained for trials appearing to meet the inclusion criteria or for which there was insufficient information in the title and abstract to make a clear decision. The full reports obtained from all the electronic and other methods of searching were assessed independently, in duplicate, by two review authors to establish whether the trials met the inclusion criteria or not. Disagreements were resolved by discussion.
Data extraction and management
Data were extracted by two review authors independently using specially designed data extraction forms. The characteristics of the trial participants, interventions and outcomes for the included trials are presented in the study tables. Mucositis may be dichotomised at different levels of severity. In order to maximise the availability of similar outcome data, we recorded the number of patients in each category of mucositis usually on a 0 to 4 scale, and used the following dichotomies: 0 versus 1+, 0‐1 versus 2+, 0‐2 versus 3+. Pain was assessed on visual analogue scales (0 to 100), the means and standard deviations for each group were recorded. The duration of trials and timing of assessments were recorded in order to make a decision about which to include for commonality. We also recorded the country where the trial was conducted and whether a dentist was involved in the investigation. Authors of full study reports and abstracts identified were contacted for clarification or for further information.
Assessment of risk of bias in included studies
This was conducted using the recommended approach for assessing risk of bias in studies included in Cochrane reviews (Higgins 2009). It is a two‐part tool, addressing the six specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and ‘other issues’). Each domain includes one or more specific entries in a ‘Risk of bias’ table. Within each entry, the first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry. This is achieved by answering a pre‐specified question about the adequacy of the study in relation to the entry, such that a judgement of ‘Yes’ indicates low risk of bias, ‘No’ indicates high risk of bias, and ‘Unclear’ indicates unclear or unknown risk of bias.
The domains of sequence generation, allocation concealment, incomplete outcome data and selective outcome reporting are each addressed in the tool by a single entry for each study. For blinding two entries were used because assessments need to be made separately for a) patients and carers and b) outcome assessor. The final domain (‘other sources of bias’) was assessed as a single entry for studies as a whole.
The risk of bias assessment was undertaken independently and in duplicate by two review authors as part of the data extraction process.
Summarising risk of bias for a study:
After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories. We assumed that the risk of bias was the same for all outcomes and each study was assessed as follows:
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias. | Plausible bias unlikely to seriously alter the results. | Low risk of bias for all key domains. | Most information is from studies at low risk of bias. |
| Unclear risk of bias. | Plausible bias that raises some doubt about the results. | Unclear risk of bias for one or more key domains. | Most information is from studies at low or unclear risk of bias. |
| High risk of bias. | Plausible bias that seriously weakens confidence in the results. | High risk of bias for one or more key domains. | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results. |
A risk of bias table was completed for each included study (Risk of bias in included studies). Results are presented graphically by study (see Figure 1) and by domain over all studies (Figure 2) .
Figure 1.
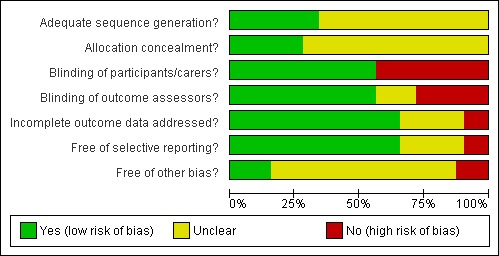
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Figure 2.
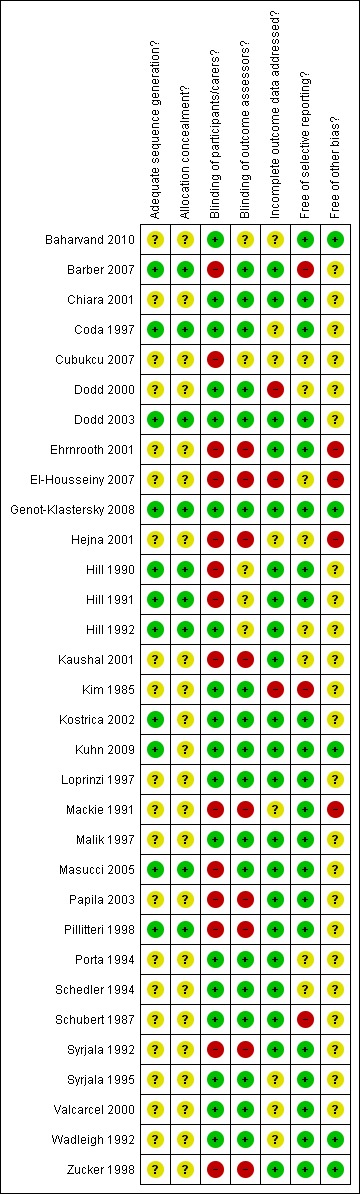
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measure of treatment effect
For dichotomous outcomes, the estimates of effect of an intervention were expressed as risk ratios together with 95% confidence intervals. For continuous outcomes mean differences together with 95% confidence intervals were used.
Dealing with missing data
Intention‐to‐treat analyses were conducted if possible. Methods outlined the handbook (Higgins 2009) were used to impute missing standard deviations if these could not be obtained from authors.
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects from the different trials was assessed by means of Cochran's test for heterogeneity and quantified by I2 statistics. Heterogeneity was considered statistically significant if P value was < 0.1. A rough guide to the interpretation of I2 given in the Handbook is: 0 to 40% might not be important, 30 to 60% may represent moderate heterogeneity, 50 to 90% may represent substantial heterogeneity, 75 to 100% considerable heterogeneity (Higgins 2009).
Assessment of reporting biases
We tabulated all the outcomes considered here.
Data synthesis
Meta‐analyses were done only with studies of similar comparisons. Risk ratios were combined for dichotomous data using random‐effects models (fixed‐effect models used if less than 3 studies in meta‐analysis).
It was planned to undertake a sensitivity analysis to examine the effect of concealed allocation and blind outcome assessment on the overall estimates of effect. However there were insufficient trials to undertake this.
Subgroup analysis and investigation of heterogeneity
We proposed a priori to conduct subgroup analyses for different cancer types (head and neck, other solid tumours, haematological and mixed), cancer treatment (chemotherapy, radiotherapy) and age groups (children, adults and mixed).
We planned to investigate clinical heterogeneity by examining the different cancer types and age groups.
Results
Description of studies
SeeCharacteristics of included studies table. SeeCharacteristics of excluded studies table.
Electronic searches identified over 6000 titles and abstracts and from this we obtained over 600 full reports. Ninety‐five studies were considered eligible according to the defined criteria for trial design, participants, interventions and outcomes.
Characteristics of the trial setting and investigators
Of the 95 eligible studies, 64 were subsequently excluded for the following reasons:
not randomised controlled trial (RCT) (12 studies);
abstract only (27 studies: where possible authors were contacted for further information but no replies were received);
unsuitable design (14 studies: reasons include: trial stopped when obtained result (unplanned), all patients allocated but only given intervention if they had mucositis, cross‐over but unsure if mucositis at second period);
protocol violation (1 study: recruitment was halted early due to ethical concerns relating to rinse);
no useable data (6 studies: progression of mucositis, number of ulcers, area covered, means);
no relevant outcomes (3 studies);
unclear information on number of withdrawals (1 study).
Of the 32 included trials, 13 were conducted in USA (Coda 1997; Dodd 2000; Dodd 2003; Hill 1990; Hill 1991; Hill 1992; Kim 1985; Loprinzi 1997; Mackie 1991; Schubert 1987; Syrjala 1992; Syrjala 1995; Wadleigh 1992), two in Germany (Schedler 1994; Zucker 1998), two in Italy (Chiara 2001; Porta 1994), two in the UK (Barber 2007; Pillitteri 1998), two in Turkey (Cubukcu 2007; Papila 2003), and one each in Pakistan (Malik 1997), Denmark (Ehrnrooth 2001), Austria (Hejna 2001), India (Kaushal 2001), Iran (Baharvand 2010), Czech Republic (Kostrica 2002), Sweden (Masucci 2005), Egypt (El‐Housseiny 2007), Belgium (Genot‐Klastersky 2008), Brazil (Kuhn 2009) and Spain (Valcarcel 2000). Six of the trials were multicentre studies (Baharvand 2010; Barber 2007; Dodd 2003; Loprinzi 1997; Masucci 2005; Schubert 1987). Twenty of the trials received external funding, 10 obtained government funding, nine acknowledged assistance from the pharmaceutical industry (Dodd 2003; Hill 1990; Hill 1991; Kim 1985; Mackie 1991; Malik 1997; Masucci 2005; Schubert 1987; Valcarcel 2000). The providers and assessors of the treatments were mainly medical staff although seven of the trials involved a dentist (Cubukcu 2007; Dodd 2000; Dodd 2003; Kuhn 2009; Masucci 2005; Schubert 1987; Wadleigh 1992), four a hygienist (Coda 1997; Hill 1990; Hill 1991; Hill 1992) and in two trials only patients were involved in the mucositis assessment (Dodd 2000; Loprinzi 1997).
Characteristics of the participants
Twenty‐eight of the 32 included trials recruited only adult patients with cancer, four included only children (Cubukcu 2007; El‐Housseiny 2007; Kuhn 2009; Mackie 1991). Fourteen trials included patients treated for a combination of leukaemia and solid tumours, eight trials included patients with head and neck cancer (Barber 2007; Dodd 2003; Ehrnrooth 2001; Kaushal 2001; Kim 1985; Kostrica 2002; Masucci 2005; Schedler 1994), a further six trials only treated patients with solid cancers (Chiara 2001; Cubukcu 2007; Dodd 2000; Hejna 2001; Papila 2003; Porta 1994) and two trials included patients with leukaemia only (Genot‐Klastersky 2008; Valcarcel 2000). The cancer type was unclear in two trials (Baharvand 2010; El‐Housseiny 2007). The patients in 11 trials received bone marrow transplants and stem cell transplants (Coda 1997; Genot‐Klastersky 2008; Hill 1990; Hill 1991; Hill 1992; Mackie 1991; Pillitteri 1998; Syrjala 1992; Syrjala 1995; Valcarcel 2000; Zucker 1998). The patients in 22 trials received chemotherapy only (Baharvand 2010; Chiara 2001; Coda 1997; Cubukcu 2007; Dodd 2000; El‐Housseiny 2007; Hejna 2001; Hill 1990; Hill 1991; Hill 1992; Kuhn 2009; Loprinzi 1997; Mackie 1991; Malik 1997; Papila 2003; Pillitteri 1998; Porta 1994; Syrjala 1992; Syrjala 1995; Valcarcel 2000; Wadleigh 1992; Zucker 1998), in eight trials the patient received only radiotherapy (Barber 2007; Dodd 2003; Ehrnrooth 2001; Kaushal 2001; Kim 1985; Kostrica 2002; Masucci 2005; Schedler 1994) and in two trials the patient received both chemotherapy and radiotherapy (Genot‐Klastersky 2008; Schubert 1987).
Although the reporting of the reasons for withdrawal by study group was unclear in nine trials, the percentage of withdrawals was clear in all trials apart from two and this ranged from 0% to 60% with a median of 9%.
Characteristics of the interventions
Treatment of mucositis
Twenty‐one trials looked at the effectiveness of 15 agents treating clinical signs of mucositis. Ten of these were placebo controlled trials and a further 11 trials had other comparisons as shown below:
allopurinol versus placebo (Porta 1994);
benzydamine HCl versus placebo (Kim 1985; Schubert 1987);
chlorhexidine versus salt and soda (Dodd 2000);
debridement versus no debridement (Cubukcu 2007);
Gelcaire versus sucralfate and mucaine (Barber 2007);
GM‐CSF (granulocyte macrophage‐colony stimulating factor) versus no treatment (Masucci 2005);
GM‐CSF versus placebo (Valcarcel 2000);
GM‐CSF versus povidone iodine (Hejna 2001);
GM‐CSF versus antimycotic mouthrinse (Papila 2003);
human placental extract versus Disprin™ (Kaushal 2001);
laser versus sham treatment (Genot‐Klastersky 2008;Kuhn 2009);
'magic' (lidocaine solution, diphenhydramine hydrochloride and aluminium hydroxide suspension) versus salt and soda (Dodd 2000);
phenytoin mouthrinse versus placebo (Baharvand 2010);
polyvariant intramuscular immunoglobulin versus placebo (Schedler 1994);
sucralfate versus placebo (Chiara 2001; Loprinzi 1997);
sucralfate versus salt and soda (Dodd 2003);
tetrachlorodecaoxide versus placebo (Malik 1997);
vitamin E versus placebo (Wadleigh 1992);
vitamin E (topical) versus vitamin E (swallowed) (El‐Housseiny 2007).
Control of mucositis pain
Fourteen trials examined the effectiveness of pain control in patients with mucositis (Baharvand 2010; Coda 1997; Dodd 2000; Dodd 2003; Ehrnrooth 2001; Hill 1990; Hill 1991; Hill 1992; Kostrica 2002; Mackie 1991; Pillitteri 1998; Syrjala 1992; Syrjala 1995; Zucker 1998). Fourteen different agents were assessed, trials frequently looking at different methods of delivery of the same agent. Seven trials included a group receiving morphine and four trials compared patient controlled versus continuous infusion of pain control. All the comparisons are shown below:
alfentanil versus morphine (Hill 1992);
hydromorphone versus morphine (Coda 1997);
sufentanil versus morphine (Coda 1997);
morphine versus tricyclic antidepressant (Ehrnrooth 2001);
sufentanil versus hydromorphone (Coda 1997);
patient controlled analgesia (PCA) versus continuous infusion delivery of morphine (Hill 1990; Mackie 1991; Pillitteri 1998);
pharmacokinetically based patient controlled analgesic infusion system (PKPCA) versus PCA for delivery of morphine (Hill 1991);
PCA versus staff controlled delivery of pethidine (Zucker 1998);
diclofenac versus placebo (Kostrica 2002);
therapist versus control (Syrjala 1992; Syrjala 1995);
relaxation and imagery therapy versus control (Syrjala 1992; Syrjala 1995);
cognitive behaviour versus control (Syrjala 1992; Syrjala 1995);
hypnosis versus control (Syrjala 1992; Syrjala 1995);
chlorhexidine versus salt and soda (Dodd 2000);
'magic' (lidocaine solution, diphenhydramine hydrochloride and aluminium hydroxide suspension) versus salt and soda (Dodd 2000);
sucralfate versus salt and soda (Dodd 2003);
phenytoin mouthrinse versus placebo (Baharvand 2010).
Characteristics of outcome measures
Treatment of mucositis
Nine trials looked at whether the mucositis had improved (Chiara 2001; El‐Housseiny 2007; Kaushal 2001; Kim 1985; Malik 1997; Masucci 2005; Porta 1994; Schedler 1994; Schubert 1987) and seven trials reported whether the mucositis was eradicated completely (Baharvand 2010; Chiara 2001; Dodd 2000; El‐Housseiny 2007; Loprinzi 1997; Porta 1994; Wadleigh 1992). Three trials looked at mild to moderate (rather than severe) mucositis (Barber 2007; Genot‐Klastersky 2008; Kuhn 2009). Six trials reported the mean number of days to heal the mucositis (Dodd 2000; Dodd 2003; Hejna 2001; Papila 2003; Porta 1994; Valcarcel 2000). Six trials used WHO criteria for mucositis on a 0 to 4 scale, where 0 = none, 1 = erythema/soreness, 2 = ulcer and able to eat, 3 = ulcer and limited eating, 4 = ulcer with haemorrhage and necrosis (Cubukcu 2007; El‐Housseiny 2007; Loprinzi 1997; Malik 1997; Porta 1994; Wadleigh 1992). Two trials used NCI Common Toxicity Criteria (Baharvand 2010; Barber 2007), and a further trial the EORTC scale (Genot‐Klastersky 2008). Two trials used a scale with no specific criteria as follows: none, slight, moderate and severe mucositis (Kim 1985; Kostrica 2002). A final trial reported the proportion of patients with an improvement in mucositis pain (Schubert 1987). This trial was included with other trials which dichotomised mucositis as improved or not.
Control of mucositis pain
Twelve of the 14 trials on pain control (Baharvand 2010; Coda 1997; Ehrnrooth 2001; Hill 1990; Hill 1991; Hill 1992; Kostrica 2002; Mackie 1991; Pillitteri 1998; Syrjala 1992; Syrjala 1995; Zucker 1998) used a patient scored visual analogue scale, with Dodd 2000 using a 0 (no soreness) to 10 (very sore) scale which could be converted into a 0 to 100 scale, and Dodd 2003 a 0 to 3 scale.
The mean and standard deviations for mucositis pain were presented at different time points: average over 2 to 5 days (Coda 1997); daily up to 7 days (Dodd 2000); average for worst mucositis (Dodd 2003); 7 and 14 days post radiotherapy (Ehrnrooth 2001); 7 and 14 days post start of treatment (Baharvand 2010); daily up to day 9 (Hill 1990; Hill 1991; Hill 1992); daily up to day 28 (Kostrica 2002); daily up to day 18 (Mackie 1991); daily up to day 14 (Pillitteri 1998); average over 3 weeks (Syrjala 1992); average from days 6 to day 16 (Syrjala 1995); mean pain score over treatment (Zucker 1998). We decided to use the time point 7 days after the start of treatment for mucositis as these data was available for most trials, otherwise we used the nearest time point reported. Four trials presented data on mean morphine utilisation (mg/hour) for day 7 (Hill 1990; Hill 1991; Hill 1992; Pillitteri 1998). Two trials presented the data in a different form, one as mean mg of morphine/day averaged over each week of the trial (Syrjala 1992) and a further trial presented the data as mg of morphine/kg/hour (Mackie 1991).
Three trials reported the duration of the pain control therapy (Hill 1990; Mackie 1991; Pillitteri 1998). Only two trials reported other outcome measures which were related to oral function and ability to eat (Kim 1985; Malik 1997).
Risk of bias in included studies
A summary of the risk of bias assessments appears in Figure 1 and Figure 2. The concealment of allocation was adequate for nine (28%) of the 32 trials and it was unclear for the remaining 23; in no trials was this considered inadequate. In seven trials assessing pain the patient could not be blinded to the intervention. The outcome assessor was blinded for 18 of the remaining 24 trials. Twenty‐one trials gave a clear description of withdrawals by trial group, this being unclear in the remaining trials. Letters were sent to authors of the trials where clarification was needed and seven replies were received (Dodd 2003; Hejna 2001; Hill 1990; Loprinzi 1997; Masucci 2005; Pillitteri 1998; Valcarcel 2000), with the information supplied changing the assessment of concealed randomisation from unclear to adequate in five studies (Dodd 2003; Genot‐Klastersky 2008; Hill 1990; Hill 1991; Hill 1992).
The validity of each study was assessed as at low, unclear or high risk of bias. Four studies were rated as at low risk of bias (Coda 1997; Dodd 2003; Genot‐Klastersky 2008;Hill 1992), six assessed as unclear (Barber 2007; Hill 1990;Hill 1991;Kuhn 2009; Masucci 2005; Pillitteri 1998) and the remaining 22 trials at high risk of bias.
Effects of interventions
For the 32 trials included in the review 1505 patients were randomised and provide data for this review. This comprised 1023 patients participating in the 20 trials investigating the effectiveness of agents to treat mucositis and 718 in the 14 trials evaluating pain relief, with some trials providing data for more than one of these outcomes. The number of patients ranged from 6 to 71 per treatment or control group.
Treatment of mucositis
The following comparisons only included one trial for one or more of the mucositis outcomes. As this review is concerned with the meta‐analysis of trials we have summarised the data from single trials below by indicating any which showed a significant benefit for the active intervention (further data is given in Table 7):
Table 1.
Data from comparisons and outcomes with single trials
| Experimental | Control | RR/MD (95%CI) | P value | ||||
| Events/ Mean (SD) | Total | Events/Mean (SD) | Total | ||||
| Allopurinol mouthwash versus placebo (Porta 1994) |
Improvement in mucositis |
19 | 22 | 3 | 22 | RR 6.33 (2.18, 18.37) | 0.0007 |
| Mucositis eradicated |
9 | 22 | 0 | 22 | RR 19.00 (1.17, 307.63) | 0.04 | |
| Time to heal mucositis (days) |
4 (1.16) | 22 | 8.5 (2.82) | 22 | MD ‐4.50 (‐5.77, ‐3.23) | < 0.001 | |
| Chlorhexidine versus salt and soda (Dodd 2000) |
Mucositis eradicated |
51 | 67 | 49 | 71 | RR 1.10 (0.90, 1.35) | 0.35 |
| Time to heal mucositis (days) |
6.6 (2.57) | 51 | 7.0 (2.99) | 49 | MD ‐0.40 [‐1.49, 0.69] | 0.47 | |
| Average pain scores |
11.3 (20.1) | 32 | 14.8 (19.8) | 31 | MD ‐3.50 [‐13.35, 6.35] | 0.49 | |
| Gelclair versus sucralfate and mucaine (Barber 2007) |
Mild to moderate mucositis |
3 | 10 | 6 | 10 | RR 0.50 [0.17, 1.46] | 0.21 |
| GM‐CSF versus no treatment (Mascucci 2005) |
Improvement in mucositis at 14 days |
11 | 29 | 4 | 22 | RR 2.09 [0.77, 5.68] |
0.15 |
| Improvement in mucositis at end of radiotherapy | 14 | 32 | 3 | 29 | RR 4.23 (1.35, 13.24) | 0.01 | |
| GM‐CSF versus placebo (Valcarcel 2000) |
Time to heal mucositis (days) |
11.4 (4.00) | 16 | 12.5 (3.4) | 19 | MD ‐1.10 (‐3.59, 1.39) | 0.39 |
| GM‐CSF versus povidone iodine (Henja 2001) |
Time to heal mucositis (days) |
2.8 (0.7) | 15 | 6.3 (1.1) | 16 | MD ‐3.50 [‐4.14, ‐2.86] | < 0.001 |
| GM‐CSF versus antimycotic mouthwash (Papila 2003) |
Time to heal mucositis (days) |
3.95 (2.01) | 20 | 6.35 (3.44) | 20 | MD ‐2.40 [‐4.15, ‐0.65] |
0.007 |
| Human placental extract versus DisprinTM (Kaushal 2001) |
Improvement in mucositis | 36 | 60 | 8 | 60 | RR 4.50 [2.29, 8.86] | < 0.001 |
| ‘Magic’ versus salt and soda (Dodd 2000) |
Mucositis eradicated |
42 | 62 | 49 | 71 | RR 0.98 [0.78, 1.24] |
0.88 |
| Time to heal mucositis (days) |
7.17 (2.57) | 42 | 7.00 (2.99) | 49 | MD 0.17 [‐0.97, 1.31] | 0.77 | |
| Average pain scores |
14.2 (19.8) |
26 | 14.8 (19.8) | 31 | MD ‐0.60 [‐10.92, 9.72] | 0.91 | |
| Phenytoin mouthrinse versus placebo (Baharvand 2010) | Average pain scores | 1.5 (2.4) | 6 | 3.2 (1.2) | 6 | MD ‐1.70 [‐3.85, 0.45] | 0.12 |
| Quality of Life | 51.7 (4.8) | 6 | 66.8 (12.8) | 6 | MD ‐15.10 [‐26.04,‐4.16] | 0.007 | |
| Polyvariant intramuscular immunoglobulin versus placebo (Schedler 1994) |
Improvement in mucositis | 31 | 39 | 18 | 41 | RR 1.81 [1.24, 2.65] |
0.002 |
| Sucralfate versus placebo (Chiara 2001) |
Improvement in mucositis | 14 | 17 | 15 | 17 | RR 0.93 [0.71, 1.24] |
0.63 |
| Sucralfate versus salt and soda (Dodd 2003) |
Time to heal mucositis (days) | 70.8 (28.9) | 13 | 57.7 (22.5) | 15 | MD 13.10 [‐6.30, 32.50] | 0.19 |
| Average pain intensity | 2.1 (1.1) | 14 | 2.4 (0.9) | 15 | MD ‐0.30 [‐1.03, 0.43] | 0.42 | |
| Tetrachlorodecaoxide versus placebo (Malik 1997) |
Improvement in mucositis | 29 | 32 | 22 | 30 | RR 1.24 [0.97, 1.58] |
0.09 |
| Vitamin E versus (not true) placebo (Wadleigh 1992) |
Mucositis eradicated |
6 | 9 | 1 | 9 | RR 6.00 [0.89, 40.31] |
0.07 |
| Vitamin E (topical) versus vitamin E (swallowed) at 5 days (El‐Housseiny 2007) | Improvement in mucositis | 28 | 30 | 2 | 33 | RR 15.40 [4.01, 59.21] |
< 0.001 |
| Mucositis eradicated |
24 | 30 | 0 | 33 | RR 53.74 [3.41, 846.84] | 0.005 | |
| Alfentanil (PKPCA) versus morphine (PKPCA) (Hill 1992) |
Average pain scores |
48.0 (52.0) | 12 | 48.0 (20.0) | 16 | MD 0.00 [‐31.01, 31.01] | 1.00 |
| Daily mean opiate intake per hour |
2.3 (2.8) | 12 | 6.2 (4.0) | 16 | MD ‐3.90 [‐6.42, ‐1.38] |
0.002 | |
| Hydromorphone (PCA) versus morphine (PCA)) (Coda 1997) |
Average pain scores |
48.9 (19.9) | 27 | 48.3 (17.5) | 29 | MD 0.60 (‐9.24, 10.44) | 0.90 |
| Sufentanil (PCA) versus morphine (PCA) (Coda 1997) |
Average pain scores |
53.7 (16.0) | 31 | 48.3 (17.5) | 29 | MD 5.40 [‐3.10, 13.90) | 0.21 |
| Opioid versus antidepressant (Ehrnrooth 2001) |
Average pain scores |
33.5 (17.7) |
20 | 52.6 (20.1) | 19 | MD ‐19.10 [‐31.01, ‐7.19] | 0.002 |
| Morphine (PKPCA) versus morphine (PCA) (Hill 1991) |
Average pain scores |
48.0 (19.0) | 15 | 66.0 (22.0) | 20 | MD ‐18.00 [‐31.62, ‐4.38] | 0.01 |
| Daily mean opiate intake per hour |
6.4 (3.9) | 15 | 2.8 (1.8) | 20 | MD 3.60 [1.47, 5.73) | 0.0009 | |
| PCA versus staff controlled (pethidine) (Zucker 1998) |
Average pain scores |
41.0 (24.0) |
10 | 62.032.0 | 10 | MD ‐21.00 [‐45.79, 3.79] | 0.10 |
| Daily mean opiate intake per hour |
17.0 (7.0) | 10 | 21.0 (7.0) | 10 | MD ‐4.00 [‐10.14, 2.14) | 0.20 | |
| Diclofenic versus placebo (Kostrica 2002) |
Average pain scores |
0.75 (1.17) | 32 | 1.05 (1.17) | 34 | MD ‐0.30 [‐0.86, 0.26] | 0.30 |
| Therapist versus control (Syrjala 1992) |
Daily mean opiate intake per hour |
1.86 (5.0) | 12 | 1.66 (5.4) |
10 | MD 0.20 [‐4.18, 4.58 | 0.93 |
| Relaxation and imagery versus control (Syrjala 1995) |
Average pain scores |
45.56 (23.7) | 23 | 53.78 (23.25) | 23 | MD ‐8.22 [‐21.79, 5.35] | 0.24 |
| Cognitive behaviour versus control (Syrjala 1992) |
Daily mean opiate intake per hour | 1.46 (3.98) | 11 | 1.66 (5.4) | 10 | MD ‐0.20 [‐4.29, 3.89] | 0.92 |
| Hypnosis versus control (Syrjala 1992) |
Average pain scores |
23.0 (31.1) | 12 | 33.5 (29.9) | 10 | MD ‐10.50 [‐36.05, 15.05] | 0.42 |
| Daily mean opiate intake per hour | 1.21 (3.78) | 12 | 1.66 (5.4) | 10 | MD ‐0.45 [‐4.42, 3.52] | 0.82 | |
allopurinol versus placebo (Porta 1994: statistically significant benefit in favour of allopurinol for improvement in mucositis, eradication and time to heal);
chlorhexidine versus salt and soda (Dodd 2000: no statistically significant difference);
debridement versus no debridement (Cubukcu 2007: statistically significant benefit for debridement for days to clinical resolution and decreased severity);
Gelcaire versus sucralfate and mucaine (Barber 2007: no statistically significant difference);
GM‐CSF (granulocyte macrophage‐colony stimulating factor) versus no treatment (Masucci 2005: statistically significant benefit for improvement in mucositis at end of radiotherapy);
GM‐CSF versus placebo (Valcarcel 2000: no statistically significant difference);
GM‐CSF versus povidone iodine (Hejna 2001: statistically significant benefit for GM‐CSF for time to heal);
GM‐CSF versus antimycotic mouthrinse (Papila 2003: statistically significant benefit for GM‐CSF for time to heal);
human placental extract versus Disprin™ (Kaushal 2001: statistically significant benefit for human placental extract for improvement in mucositis);
'magic' (lidocaine solution, diphenhydramine hydrochloride and aluminium hydroxide suspension) versus salt and soda (Dodd 2000: no statistically significant differences);
phenytoin mouthrinse versus placebo (Baharvand 2010: statistically significant difference in quality of life at 7 days favouring phenytoin);
polyvariant intramuscular immunoglobulin versus placebo (Schedler 1994: statistically significant benefit for the immunoglobulin for improvement of mucositis);
sucralfate versus salt and soda (Dodd 2003: no statistically significant difference);
tetrachlorodecaoxide versus placebo (Malik 1997: no statistically significant difference);
vitamin E versus placebo (Wadleigh 1992: no statistically significant difference);
vitamin E (topical) versus vitamin E (swallowed) (El‐Housseiny 2007: statistically significant benefit for the topical vitamin E for improvement of mucositis).
The comparisons below include more than one trial for some of the outcomes measured and the results of the meta‐analysis are presented:
Benzydamine HCl versus placebo
Two trials (Kim 1985; Schubert 1987) provided data (Analysis 1.1) for improvement in mucositis and there was no statistically significant difference between benzydamine and placebo with risk ratio (RR) 1.22 (95% confidence interval (CI) 0.94 to 1.60). Both trials were assessed as at high risk of bias. Schubert 1987 noted a lack of power in this study.
Analysis 1.1.
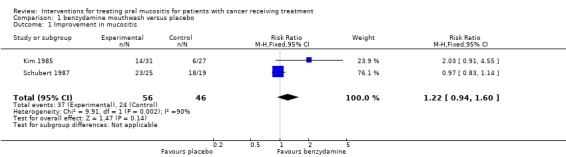
Comparison 1 benzydamine mouthwash versus placebo, Outcome 1 Improvement in mucositis.
Sucralfate versus placebo
Two trials (Chiara 2001; Loprinzi 1997) provided data for eradication of mucositis (Analysis 3.1) and there was no statistically significant difference between sucralfate and placebo with RR 1.13 (95% CI 0.66 to 1.94). One trial was assessed as at unclear risk of bias, the other at high risk of bias.
Analysis 3.1.
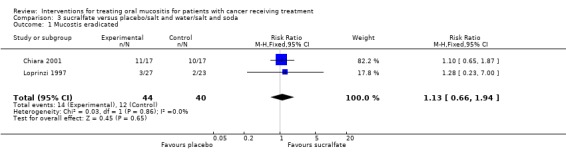
Comparison 3 sucralfate versus placebo/salt and water/salt and soda, Outcome 1 Mucostis eradicated.
Laser versus sham treatment
Two trials (Genot‐Klastersky 2008;Kuhn 2009) provided data (Analysis 2.1) for the outcome of mild to moderate mucositis and there was a statistically significant benefit for the laser with RR 5.28 (95% CI 2.30 to 12.13). One trial was assessed as at low risk of bias the other as at unclear risk of bias.
Analysis 2.1.
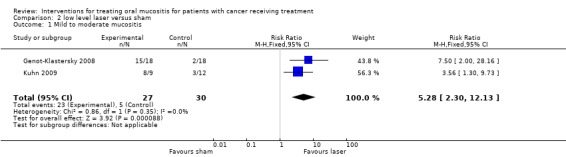
Comparison 2 low level laser versus sham, Outcome 1 Mild to moderate mucositis.
Control of mucositis pain
As above the comparisons below only included one trial for the pain outcomes (further data given in Table 7):
alfentanil versus morphine (Hill 1992: statistically significant difference in favour of alfentanil for daily mean opiate intake);
hydromorphone versus morphine (Coda 1997: no statistically significant difference);
sufentanil versus morphine (Coda 1997: no statistically significant difference);
morphine versus tricyclic antidepressant (Ehrnrooth 2001: statistically significant difference in favour of morphine for less pain);
sufentanil versus hydromorphone (Coda 1997: no statistically significant difference);
pharmacokinetically based patient controlled analgesic infusion system (PKPCA) versus PCA for delivery of morphine (Hill 1991: statistically significant difference in favour of PKPCA morphine for less pain but more opiate per hour);
PCA versus staff controlled delivery of pethidine (Zucker 1998: no statistically significant differences);
phenytoin mouthrinse versus placebo (Baharvand 2010: no statistically significant difference);
diclofenac versus placebo (Kostrica 2002: no statistically significant difference);
relaxation and imagery therapy versus control (Syrjala 1995: no statistically significant difference);
hypnosis versus control (Syrjala 1992: no statistically significant differences);
chlorhexidine versus salt and soda (Dodd 2000: no statistically significant difference);
'magic' (lidocaine solution, diphenhydramine hydrochloride and aluminium hydroxide suspension) versus salt and soda (Dodd 2000: no statistically significant difference);
sucralfate verus salt and soda (Dodd 2003: no statistically significant difference).
The comparisons below include more than one trial for some of the outcomes measured and the results of the meta‐analysis are given below:
Patient controlled analgesia (PCA) versus continuous infusion delivery of morphine
Three trials (Hill 1990; Mackie 1991; Pillitteri 1998) provide data for mean pain score (7 day data used for Pillitteri 1998) (Analysis 4.1; Analysis 4.2; Analysis 4.3). The meta analysis showed no statistically significant difference in mean pain score with a mean difference (on 0 to 100 scale) of ‐2.49 (95% CI ‐12.28 to 7.29). The three trials also provided data for daily mean opiate intake mg per hour and this outcome showed a statistically significant reduction in mean opiate intake favouring the PCA morphine group, with a mean difference (reduction) of 0.65 mg/hour (95% CI 0.09 to 1.20) (P = 0.02). The trials also provided data on the duration of pain control (days) and the meta‐analysis showed a statistically significant reduction in days of pain favouring PCA, with a mean difference of ‐1.87 (95% CI ‐3.49 to ‐0.25) (P = 0.02). There was no evidence of heterogeneity between the effects for the three studies for either mean opiate intake or duration (P > 0.50, I2 = 0). However two of the three trials were assessed as unclear risk of bias and one as at high risk of bias (Mackie 1991).
Analysis 4.1.
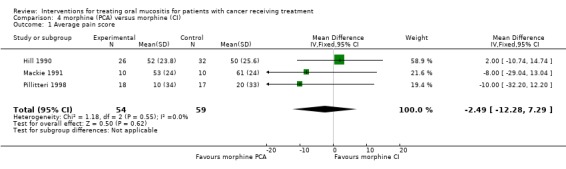
Comparison 4 morphine (PCA) versus morphine (CI), Outcome 1 Average pain score.
Analysis 4.2.
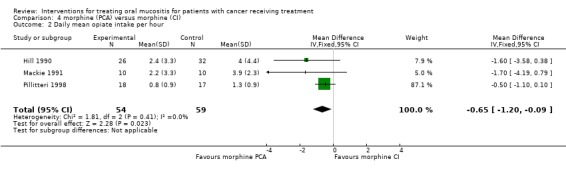
Comparison 4 morphine (PCA) versus morphine (CI), Outcome 2 Daily mean opiate intake per hour.
Analysis 4.3.
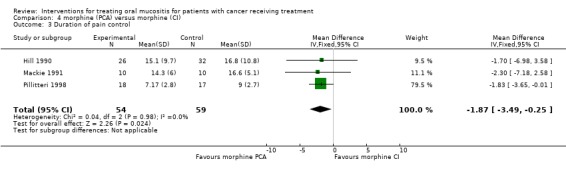
Comparison 4 morphine (PCA) versus morphine (CI), Outcome 3 Duration of pain control.
Therapist versus control
Two trials (Syrjala 1992; Syrjala 1995) provided data (Analysis 5.1) for average pain score but the meta analysis showed no statistically significant difference between the group who received therapist visits and the control group who received treatment as usual, with mean difference (on 0 to 100 scale) of ‐5.61 (95% CI ‐17.25 to 6.02). Both trials were assessed as at high risk of bias.
Analysis 5.1.
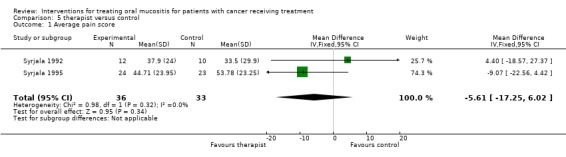
Comparison 5 therapist versus control, Outcome 1 Average pain score.
Cognitive behaviour therapy versus control
Two trials (Syrjala 1992; Syrjala 1995) provided data (Analysis 6.1) for average pain score and the meta analysis showed no statistically significant difference between the group who received cognitive behaviour therapy and those who received treatment as usual (control group) with mean difference (on 0 to 100 scale) of ‐7.29 (95% CI ‐17.40 to 2.83). Both trials were assessed as at high risk of bias.
Analysis 6.1.
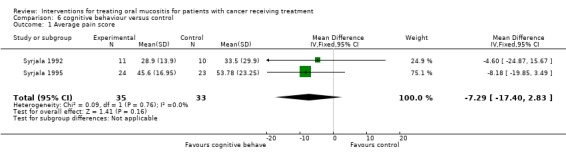
Comparison 6 cognitive behaviour versus control, Outcome 1 Average pain score.
Discussion
Oral mucositis is a common and painful complication of cancer chemotherapy and radiotherapy. It can limit the tolerability of therapy, leading to reductions in treatment and, therefore, potentially limiting treatment efficacy (Kowanko 1998). This review updates the evidence for the efficacy of interventions to treat mucositis and another Cochrane review looks at the evidence for preventing mucositis (Worthington 2007b).
Since our original reviews there has been an expansion of evidence in this area of cancer care. The number and range of interventions have increased. In addition, the last 5 years have seen increasingly frequent reporting of outcomes other than the presence or absence of mucositis. As a result, two new outcomes were included in our previous update (2005) of this review: time taken to heal mucositis and duration of pain control therapy. Our current update includes a further outcome: the severity of mucositis. To reflect the increased number and range of studies the review has been split into two parts, one evaluating the effectiveness of agents to treat mucositis and the other the management of pain associated with the condition. There has been increasing recognition that other endpoints, such as oral intake (ability to take fluids or solid food by mouth), quality of life and duration of hospital stay, may be clinically more relevant and more important to patients (Bellm 2002). Unfortunately, there was insufficient data to include these more patient‐oriented endpoints in this review nor was there data on systemic infection and use of antibiotics. Whilst this latter outcome is often cited as a possible consequence of mucositis it may be due to other causes.
The broad scope of interventions included in this review indicate the importance of this condition to clinicians and the uncertainty of how to manage it optimally. The 32 trials included have recruited 1505 patients and evaluated 27 different interventions. Of all the interventions examined in this review only three mucositis treatments were investigated by more than one trial and only one comparison was significant for one outcome: low level laser treatment was found to reduce the severity of mucositis. There were only three comparisons for pain control which included more than one trial, only one of which showed a statistically significant effect. There was no evidence to suggest that there was a difference in pain control between continuous infusion and patient controlled analgesia (PCA). However, the PCA group required less morphine than the continuous infusion group, and the pain lasted for 2 days less.
For the remainder of the comparisons of treatment and pain control examined in this review, a lack of duplication of studies by independent groups investigating the same interventions limits the strength of evidence and generalisability of the results. Our review agrees with two systematic reviews looking at antimicrobial therapy (Donnelly 2003) and GM‐CSF (Fung 2002) for the prevention and treatment of oral mucositis. There is no clear pattern emerging regarding the benefit or otherwise of antimicrobial use to manage mucositis. Studies looking at GM‐CSF are limited and the use of GM‐CSF cannot currently be recommended for the treatment of mucositis.
Several issues identified in this review may have affected the validity of the results. The setting of the included trials varied with the majority being conducted by medical teams who did not report any involvement with a dentist or hygienist (68%). None of the trials assessed the reliability of the outcome measures used, particularly with regard to the presence or absence of oral candidiasis. The appearance of mucositis and oral candidiasis can be similar; leading to potential mis‐diagnosis if the assessors were not sufficiently trained or experienced in the diagnosis of these oral lesions. Several different scoring systems were used to assess mucositis severity and in some studies the scoring systems were not defined. This variability may have led to discrepancies between studies. Accepting that caveat, there was consistency in the number of categories used and in every case the lowest score indicated no mucositis. Only four of the included studies reported power calculations a priori (Genot‐Klastersky 2008; Loprinzi 1997; Masucci 2005) and one trial reported a post hoc power calculation as an explanation for their findings (Schubert 1987). It is possible that some studies which found no difference between treatments compared were underpowered. With respect to publication bias, several negative studies for mucositis have been reported and we congratulate the authors and editors for doing so. It was not possible to detect any existing publication bias, as there were insufficient studies in each meta‐analysis investigating the same interventions.
The country of conduct, financial support and the design of trials varied greatly between studies. It was especially unfortunate that six studies presented data in an unusable form. We feel that the use of structured abstracts and adherence to the Consolidated Standards of Reporting Trials (CONSORT) guidelines will greatly improve the reporting and hopefully the conduct of randomised controlled trials (RCTs) (Begg 1996; Moher 2001).
For patients being treated for cancer the decision‐making around giving potentially toxic therapy to prevent mucositis versus treating mucositis once it is established can be a clinical dilemma. Our recent review of interventions to prevent mucositis identified a small number of interventions with weak, unreliable evidence of benefit. This review of therapies for established mucositis shows limited evidence, from two small trials including a total of 57 participants, that low level laser treatment reduced mucositis severity, and unreliable evidence that opiate delivered by PCA results in a lower total dose of opiate, and improved duration of pain control compared to delivery by a continuous infusion. Given the pain and inconvenience that mucositis causes to a population of patients receiving treatment for cancer it is important that further, well designed, RCTs should be conducted investigating new treatments for the management of mucositis and new ways of controlling pain. Future studies should include more patient‐oriented outcomes in addition to measures of mucositis severity.
Authors' conclusions
There is limited evidence that low level laser treatment is beneficial in reducing the severity of mucositis. There is no evidence that patient controlled analgesia is better than continuous infusion method for controlling pain. However there is unreliable evidence that less opiate is used per hour, and the duration of pain is slightly reduced with patient controlled analgesia.
There is a need for further, well designed trials, preferably including a placebo or no treatment control, assessing the effectiveness of interventions considered in this review and new interventions for managing oral mucositis. These should be reported according to the CONSORT guidelines. Consideration should be given to the adoption of standard clinical outcome measures, the development of patient based outcome measures and inclusion of the cost of the interventions.
Feedback
Low level laser, 9 August 2010
Summary
"I ask you to file and publish the following comment:
There seems to be an imbalance in the way that low level laser therapy (LLLT) is handled in the risk of bias assessment and the review conclusion. The two LLLT‐studies receive the highest method scores of the review with 7/7 and 6/7 points, respectively, and no red circles for high bias risk in the assessments. Still in the results section, the Kuhn (6/7) LLLT study is classified as having high risk of bias. Although the scientific evidence may be classified as limited for LLLT in oral mucositis, I cannot see that the published conclusion of weak and unreliable evidence is justified for studies receiving such extraordinarily high method scores. Another matter is that the review only includes less than half of the published LLLT trials in cancer therapy‐induced oral mucositis."
Reply
The Kuhn 2009 study was correctly assessed as being at unclear risk of bias under the heading Effects of Interventions‐ Laser versus sham treatment, but was not included in the group at unclear risk of bias, under the heading Risk of Bias. This error has been corrected.
We have amended the text in the Abstract, Results & Discussion sections concerning low level laser therapy, deleting the word unreliable and describing the evidence as limited. The term 'unreliable' has been added to the description of the evidence for patient controlled analgesia as these trials are at either unclear or high risk of bias.
We believe that we have included all of the trials of low level laser therapy for the treatment of oral mucositis that meet the inclusion criteria for this review. An update of a second systematic review of interventions for the prevention of oral mucositis is nearing completion and is due to be published later this year. We would be grateful if Professor Bjordal could send us information about other trials that he believes should be included in these review(s).
We would like to thank Professor Bjordal for taking the time to bring these matters to our attention.
Contributors
Professor Jan Bjordal, Centre for Evidence‐based Practice, Bergen University College, Norway
Acknowledgements
Thanks go to Anne Littlewood, Trials Search Co‐ordinator for the Cochrane Oral Health Group for carrying out the searches for the review, Luisa Fernandez Mauleffinch, Managing Editor of the Cochrane Oral Health Group for her help with the administration of the review and Phil Riley for his help in locating all the articles for the review. Thanks also go to Tim Eden (OE) who provided advice on cancer, its treatment and the interventions included in past versions of the review. Thanks also go to Tatiana Macfarlane, Marco Esposito, Mikako Hayashi and Angelika Schreiber for translating papers from Russian, Italian, Japanese and German. The review authors would like to thank the following trialists for responding to letters requesting information: Dr Giuseppe Masucci, Dr MUR Naidu, Dr Charles Loprinzi, Dr Richard Chapman, Dr Ruby Meredith, Dr Richard Clark, Professor Michael Hejna, Dr Anna Sureda, Professor Marylin Dodd. Finally the review authors are grateful to the following peer reviewers for their very helpful comments which greatly improved this update of the review: Douglas Peterson, Alexander Molassiotis, Marco Esposito, Ian Needleman, David Moles, Lee Hooper, Marylin Dodd, Amid Ismail and Anne‐Marie Glenny.
Appendices
Appendix 1. Cochrane Oral Health Group's Trials Register; Cochrane Pain, Palliative & Supportive Care Group's Trials Register search strategy
((neoplasm* OR leukemia OR leukaemia OR leukaemia OR lymphoma* OR plasmacytoma OR "histiocytosis malignant" OR reticuloendotheliosis OR "sarcoma mast cell" OR "Letterer Siwe disease" OR "immunoproliferative small intestine disease" OR "Hodgkin disease" OR "histiocytosis malignant" OR "bone marrow transplant*" OR cancer* Or tumor* OR tumour* OR malignan* OR neutropeni* OR carcino* OR adenocarcinoma* OR radioth* OR radiat* OR radiochemo* OR irradiat* OR chemo*) AND (stomatitis OR "Stevens Johnson syndrome" OR "candidiasis oral" OR mucositis OR (oral AND (cand* OR mucos* OR fung*)) OR mycosis OR mycotic OR thrush))
Appendix 2. The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
Search strategy for the Cochrane Library
Exp NEOPLASMS
Exp LEUKEMIA
Exp LYMPHOMA
Exp RADIOTHERAPY
Exp BONE MARROW TRANSPLANTATION
neoplasm* or cancer* or carcino* or malignan*
leukemi* or leukaemia*
tumour* or tumor*
neutropeni*
adenocarcinoma*
lymphoma*
(radioth* or radiat* or irradiat* or radiochemo*)
(bone next marrow next transplant*)
chemo* or radiochemo*
(#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14)
Exp STOMATITIS
MUCOSITIS
CANDIDIASIS ORAL
stomatitis
(stevens next johnson next syndrome)
mucositis
oral near cand*
mouth near cand*
oral and fung*
mouth and fung*
(mycosis or mycotic or thrush)
#16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#15 AND #27
Appendix 3. MEDLINE via OVID search strategy (including MEDLINE Pre‐Indexed)
1. exp NEOPLASMS/ 2. exp LEUKEMIA/ 3. exp LYMPHOMA/ 4. exp RADIOTHERAPY/ 5. Bone Marrow Transplantation/ 6. neoplasm$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 7. cancer$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 8. (leukaemi$ or leukemi$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 9. (tumour$ or tumor$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 10. malignan$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 11. neutropeni$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 12. carcino$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 13. adenocarcinoma$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 14. lymphoma$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 15. (radioth$ or radiat$ or irradiat$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 16. (bone adj marrow adj5 transplant$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 17. chemo$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 18. or/1‐17 19. exp STOMATITIS/ 20. Candidiasis, Oral/ 21. stomatitis.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 22. mucositis.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 23. (oral and cand$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 24. (oral adj6 mucos$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 25. (oral and fung$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 26. (mycosis or mycotic).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name, device trade name] 27. or/19‐26 28. 18 and 27
The above subject search was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (September 2009 revision) as referenced in Chapter 6 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009].
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. exp animals/ not humans.sh.
11. 9 not 10
Appendix 4. EMBASE via OVID search strategy
1. exp NEOPLASM/ 2. exp LEUKEMIA/ 3. exp LYMPHOMA/ 4. exp RADIOTHERAPY/ 5. exp bone marrow transplantation/ 6. (neoplasm$ or cancer$ or leukemi$ or leukaemi$ or tumour$ or tumor$ or malignan$ or neutropeni$ or carcino$ or adenocarcinoma$ or lymphoma$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] 7. (radioth$ or radiat$ or irradiat$ or radiochemo$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] 8. (bone marrow adj3 transplant$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] 9. chemo$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] 10. or/1‐9 11. exp Stomatitis/ 12. Thrush/ 13. (stomatitis or mucositis or (oral and candid$) or (oral adj4 mucositis) or (oral and fung$) or mycosis or mycotic or thrush).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] 14. or/11‐13 15. 10 and 14
The above subject search was run with the Cochrane Oral Health Group sensitive search strategy for identifying randomized controlled trials in EMBASE:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 5. CINAHLvia EBSCO search strategy
S1 (MH "Neoplasms+")
S2 (MH "Leukemia+")
S3 (MH "Lymphoma+")
S4 (MH "Radiotherapy+")
S5 (MH "Bone Marrow Transplantation")
S6 neoplasm*
S7 cancer*
S8 (leukemi* or leukaemi*)
S9 (tumour* or tumor*)
S10 malignan*
S11 neutropeni*
S12 carcino*
S13 adenocarcinoma*
S14 lymphoma*
S15 (radioth* or radiat* or irradiat*)
S16 (bone N1 marrow N5 transplant*)
S17 chemo*
S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or
S12 or S13 or S14 or S15 or S16 or S17
S19 MH "Stomatitis+"
S20 MH "Candidiasis, Oral"
S21 stomatitis
S22 mucositis
S23 (oral and cand*)
S24 (oral N6 mucos*)
S25 (oral and fung*)
S26 (mycosis or mycotic)
S27 S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26
S28 S18 AND S27
The above subject search was run with the Cochrane Oral Health Group sensitive search strategy for identifying randomized controlled trials in CINAHL:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design
S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study")
S3 TI random* or AB random*
S4 AB "latin square" or TI "latin square"
S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over)
S6 MH Placebos
S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*)
S8 TI blind* or AB mask* or AB blind* or TI mask*
S9 S7 and S8
S10 TI Placebo* or AB Placebo* or SU Placebo*
S11 MH Clinical Trials
S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial)
S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. CANCERLIT (PubMed Cancer Subset) search strategy
((neoplasm* OR leukemia OR leukaemia OR leukaemia OR lymphoma* OR plasmacytoma OR "histiocytosis malignant" OR reticuloendotheliosis OR "sarcoma mast cell" OR "Letterer Siwe disease" OR "immunoproliferative small intestine disease" OR "Hodgkin disease" OR "histiocytosis malignant" OR "bone marrow transplant*" OR cancer* Or tumor* OR tumour* OR malignan* OR neutropeni* OR carcino* OR adenocarcinoma* OR radioth* OR radiat* OR radiochemo* OR irradiat* OR chemotherap*) AND (stomatitis OR "Stevens Johnson syndrome" OR "candidiasis oral" OR mucositis OR (oral AND (candid* OR mucos* OR fung*)) OR mycosis OR mycotic OR thrush))
The above subject search was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (September 2009 revision) as referenced in Chapter 6 and detailed in box 6.4.a of The Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]
#1 randomized controlled trial [pt] #2 controlled clinical trial [pt] #3 randomized [tiab] #4 placebo [tiab] #5 drug therapy [sh] #6 randomly [tiab] #7 trial [tiab] #8 groups [tiab] #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10 animals [mh] NOT humans [mh] #11 #9 NOT #10
Appendix 7. OpenSIGLE search strategy
SIGLE no longer supports complex searching, so a series of keyword searches was performed as below:
cancer AND mucositis AND oral
leukemia AND mucositis AND oral
leukaemia AND mucositis AND oral
carcinoma AND mucositis AND oral
lymphoma AND mucositis AND oral
tumour AND mucositis AND oral
tumor AND mucositis AND oral
cancer AND candidiasis AND oral
leukemia AND candidiasis AND oral
leukaemia AND candidiasis AND oral
carcinoma AND candidiasis AND oral
lymphoma AND candidiasis AND oral
tumour AND candidiasis AND oral
tumor AND candidiasis AND oral
Appendix 8. LILACS via the Virtual Health Library search strategy
Mh NEOPLASMS OR Tw neoplasm$ OR Tw cancer$ OR Tw carcinoma$ OR Tw tumour$ OR Tw tumor$ OR Tw malignan$ OR Tw carcino$ OR Tw nuetropeni$ OR Tw adenocarcinoma$ OR Mh leukemia OR Tw leukaemia$ OR Tw leukemi$ OR Tw lymphoma$ OR Tw "bone marrow transplantation" OR Tw "bone marrow transplant$" OR Tw radiotherapy OR Tw radioth$ OR Tw radiat$ OR Tw irradiat$ OR Tw radiochemo$ OR Tw chemo$
AND
Mh stomatitis OR Tw stomatitis OR Mh Candidiasis‐Oral OR Tw "oral candidiasis" OR (Tw candida$ AND (Tw mouth OR Tw oral)) OR Tw mucositis OR ((Tw oral OR mouth) AND Tw fung$) OR (Tw oral AND Tw candidiasis$)
The above subject search was run with the Brazilian Cochrane Centre highly sensitive search strategy for identifying randomized controlled trials in LILACS:
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animals AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animals AND NOT (Ct human and Ct animals)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animals AND NOT (Ct human and Ct animals)))
Data and analyses
Comparison 1.
benzydamine mouthwash versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in mucositis | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.94, 1.60] |
Comparison 2.
low level laser versus sham
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mild to moderate mucositis | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [2.30, 12.13] |
Comparison 3.
sucralfate versus placebo/salt and water/salt and soda
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mucostis eradicated | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.66, 1.94] |
Comparison 4.
morphine (PCA) versus morphine (CI)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average pain score | 3 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐12.28, 7.29] |
| 2 Daily mean opiate intake per hour | 3 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.20, ‐0.09] |
| 3 Duration of pain control | 3 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐1.87 [‐3.49, ‐0.25] |
Comparison 5.
therapist versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average pain score | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐5.61 [‐17.25, 6.02] |
Comparison 6.
cognitive behaviour versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average pain score | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐7.29 [‐17.40, 2.83] |
What's new
| Date | Event | Description |
|---|---|---|
| 24 August 2010 | Feedback has been incorporated | Error concerning overall risk of bias categorisation for Kuhn 2009 corrected. Wording changed in results and discussion sections concerning low level laser treatment, in response to feedback received. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 6 July 2010 | New search has been performed | New searches up to 01 June 2010. |
| 6 July 2010 | New citation required and conclusions have changed | 5 new included studies, 8 new excluded studies. Risk of bias assessments completed on all included studies. Added new outcome: proportion of patients with mild/moderate mucositis. Review restructured to reflect many of comparisons have only one trial and to downgrade this. 4 new authors. |
| 17 June 2008 | Amended | Converted to new review format. |
| 16 February 2007 | New citation required and conclusions have changed | Substantive amendment. In this update we have added 1 included and 28 excluded studies. |
Differences between protocol and review
New outcome added for 2010 update: proportion of patients with severe mucositis.
Two new outcomes added for 2005 update: time taken to heal mucositis; duration of pain control therapy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Location: Iran. Number of centres: Multicentre. Funding source: Unclear. Recruitment period: December 2006 to May 2007. |
|
| Participants | Inclusion: Adults undergoing chemotherapy who developed oral mucositis Gr 2 or worse, with no other systemic disease, life expectancy at least 6 months. Exclusion: Other concomitant disease, heavy smoker, severe psychological disorder. 14 randomised, 12 completed. |
|
| Interventions | GpA (6) Phenytoin, 0.5% (50:50 mixture sodium phenytoin and phenytoin powder), 10 ml given 4 times daily, swished around mouth and spat out. GpB (6) Placebo 10 ml 4 times daily, swished around mouth and spat out. |
|
| Outcomes | Assessed at baseline, 1 week and 2 weeks after start of treatment. VAS scores for pain, Mucositis score NCI Common Toxicity Criteria (0 to 4 scale), duration of mucositis (not used as data presented in a format not suitable for pooling). |
|
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Patients were assigned to case and control groups randomly and separately at each department. Sampling method was performed on a multi‐central, non‐probable (easy to access) basis.' |
| Allocation concealment? | Unclear risk | Not mentioned. |
| Blinding of participants/carers? | Low risk | Patients and nurses blinded to treatment group. |
| Blinding of outcome assessors? | Unclear risk | Ppatients self assessed pain and QoL, unclear who conducted the mucositis assessments. |
| Incomplete outcome data addressed? | Unclear risk | 2/14 randomised were subsequently excluded because they were 'uncooperative' ‐ no details given. It is not stated which group these were from, but if both exclusions were from the same group, this would represent 25% drop out from that group and a significant risk of bias. |
| Free of selective reporting? | Low risk | Reported mucositis scores, duration of mucositis, pain and quality of life. |
| Free of other bias? | Low risk | |
| Methods | Location: United Kingdom. Number of centres: Two. Funding source: Not stated. Recruitment period: September 2004 to April 2005. |
|
| Participants | Adults aged 18 years and over, with at least Grade 1 OM and when they felt they were no longer receiving adequate pain control via simple analgesic, receiving daily doses of radiotherapy to the head and neck. 20 eligible, 20 randomised. |
|
| Interventions | GpA (10) Gelclair, 4x in the 24 hour study (30 min to 1 hour before eating/drinking and before bedtime, swished around mouth and spat out). GpB (10) Standard care 10 ml 4x in 24 hour study (30 min to 1 hour before eating/drinking sucralfate and Mucaine swished around mouth and swallowed) (30 min to 1 hour before eating/drinking). |
|
| Outcomes | Assessed at baseline, 1 hour, 3 hours and 24 hours post treatment. VAS scores for pain (not used as only 24 hour assessment), Pain on speaking, Mucositis score NCI Common Toxicity Criteria (0‐4 scale). Secondary outcome ‐ extent to which ability to swallow is influenced by pain (odynophagia). |
|
| Notes | Outcomes assessed by nurse specialists blinded to group allocation. No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "computer generated random allocation sequence for the 2 centres was prepared by Exeter clinical research radiographer". |
| Allocation concealment? | Low risk | Quote: "radiographer accessed the randomisation list by telephone to determine the patients group allocation". |
| Blinding of participants/carers? | High risk | Comment: Not possible to blind patients and carers because one product 'swish and spit' and the other 'swish and swallow'. |
| Blinding of outcome assessors? | Low risk | Quote: "single blind trial with the administering nurse specialist being unaware as to what medication had been issued". |
| Incomplete outcome data addressed? | Low risk | Quote: "one patient despite their eagerness to participate fell asleep during the course of the trial and did not take their Sucralfate and Mucaine before the 24‐h assessment" "The missing 24‐h scores were substituted with 3‐h assessment scores". Comment: Unlikely to influence results. |
| Free of selective reporting? | High risk | Comment: Despite intention to report mucositis it was not reported apart from at baseline. |
| Free of other bias? | Unclear risk | Small pilot study which lacks power. At baseline there are differences between the groups that may have influenced the results. Intervention group had more severe OM (6/10 vs 3/10), and included all patients with enteral feeding and support. 3/10 in control group and 1/10 of intervention group had concomitant chemotherapy during trial. Funding unclear. |
| Methods | Location: Italy. Number of centres: One. Funding source: Not stated. Recruitment period: Not stated. |
|
| Participants | Inclusion: Adults with histopathologically confirmed diagnosis of malignancy, WHO performance status </=2 chemotherapy induced stomatitis at any time during chemotherapy treatment with any antiblastic drug alone, or in combination. Exclusion: Previous or concomitant radiotherapy to oral mucosa. 40 enrolled and randomised, 34 completed. |
|
| Interventions | GrA (n = 20) sucralfate gel (1 gr) (5 ml) applied over mucosa, 3 times daily. Evaluated after 1 or 2 weeks and at time of next course of chemotherapy. GrB (n = 20) placebo (in identical sachets with identical taste, colour and consistency to sucralfate) (5 ml) applied over mucosa, 3 times daily. Evaluated after 1 or 2 weeks and at time of next course of chemotherapy. |
|
| Outcomes | Complete response to treatment, VAS scores for pain but data not reported. Mucositis not resolved at 14 days is used. | |
| Notes | "No sample size was calculated". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomised' no further details given. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding of participants/carers? | Low risk | Quote: "....double blind placebo controlled...." Quote: "Drug or placebo were provided in individual sachets of 5 ml with identical taste, colour and consistency". |
| Blinding of outcome assessors? | Low risk | Quote: "....double blind placebo controlled...." |
| Incomplete outcome data addressed? | Low risk | Withdrawals and exclusions clearly described and balanced in each group (2 in each group non‐compliant and 1 in each group withdrew due to taste disturbances). 15% lost to follow up. |
| Free of selective reporting? | Low risk | Eradication and improvement of mucositis reported. |
| Free of other bias? | Unclear risk | No information on funding. |
| Methods | Location: Seattle, USA. Number of centres: One. Funding source: Not stated. Recruitment period: March 1991 to July 1993. Eligible patients had oral mucositis and had been on opioids for at least 2 days. |
|
| Participants | Inclusion: Patients undergoing bone marrow transplant for haematological malignancies or breast cancer, who were treated with total body irradiation or busulphan, who developed oral mucositis and stayed on study for at least 2 days. Exclusion: History of adverse reaction to opioid analgesics, moderate to severe hepatic, renal or pulmonary diseases, those currently taking opioids for other purposes, history of opioid, alcohol or cocaine abuse. 119 enrolled and randomised, 97 completed at least 2 days of treatment. |
|
| Interventions | Gr A (n = 39) Patient controlled analgesia (PCA) morphine 5 mg/ml for at least 2 days. Gr B (n = 40) PCA hydromorphone 1 mg/ml for at least 2 days. Gr C (n = 40) PCA sufentanil 5 μg/ml for at least 2 days. In all groups PCA was set to deliver bolus doses equivalent to 15 μg/kg morphine sulphate, with a 10 min lockout period. If increasing the demand for bolus doses was not sufficient to control pain, a baseline continuous infusion (CI) was added at a rate of approx 50% of the average rate during the previous 24 hour period. Patients were given the option of a higher CI rate during the night in order to minimise the demand for bolus doses during sleep. |
|
| Outcomes | Average pain VAS score (0 to 100) for days 2 to 5. Number who discontinued as drug not working. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "....randomised using a computer generated randomisation scheme". |
| Allocation concealment? | Low risk | Not reported. |
| Blinding of participants/carers? | Low risk | Quote: "....randomised double blind study". Quote: "study opioids were supplied by the pharmacy service and were coded 30 ml PCA vials that were labelled 5mg/ml morphine sulphate". |
| Blinding of outcome assessors? | Low risk | Quote: "....randomised double blind study". |
| Incomplete outcome data addressed? | Unclear risk | 19/119 (16%) patients randomised had < 2 days of treatment and were therefore not considered evaluable (9, 6 & 4 in groups A, B & C respectively). See below. Of the 100 patients who had at least 2 days of treatment, 69/100 completed the study and a further 31/97 were excluded ‐ 11 due to side effects of treatment (2, 6 & 3 in groups A, B & C respectively), 8 due to inadequate pain relief (1, 0 & 7 respectively) and 12 due to BMT complications (1, 5 & 6 respectively) for total withdrawal/exclusion rates of randomised participants of 13/39 (33%), 17/40 (43%) and 20/40 (50%) in groups A, B & C respectively. Results are reported for the 'evaluable' patients (30, 34 & 36 in groups A, B & C respectively). |
| Free of selective reporting? | Low risk | Many outcomes described and reported. |
| Free of other bias? | Unclear risk | Baseline characteristics given for 'evaluable' patients only. Some randomised patients had less than 2 days treatment and were excluded because they did not develop mucositis (4, 1 & 2 in groups A, B & C), stopped treatment due to side effects (4, 1 & 2 in groups A, B & C), protocol violation (1, 3 in groups A & B) or died (1 in Gr B). Likely that prognostic factors were distributed differently in these patients. Funding unclear. |
| Methods | Location: Turkey. Number of centres: One. Funding source: Not stated. Recruitment period: February 2002 to September 2005. |
|
| Participants | Children aged 1 to 14 years with solid tumours, hospitalised for induction chemotherapy, who had large contiguous oral mucositis lesions, neutrophil count </= 1000/mm3, were taking systemic opioids and prophylactic antibiotics. 40 eligible, 40 randomised. |
|
| Interventions | Gp A (n = 20) Debridement of oral lesions + standard care. Gp B (n = 20) Standard care only. |
|
| Outcomes | Time to complete healing of lesions, mean mucositis scores at 6 days using WHO scale. 0 to 3 scale for pain included opiate use and presence of ulcers so not used in meta analysis. No specific information given on withdrawals or losses to follow‐up. Oral assessments conducted by two trained calibrated dentists. Can only report results without using the data. | |
| Notes | Outcome data collected by oral examinations performed by 'two calibrated dentists' three times per week. Total number of oral examination performed was 960 in Gp A and 980 in Gp B. No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Children were randomly assigned...." Quote: "....were assigned one by one into two groups according to the intervention administered". Comment: Unclear as to method of sequence generation ‐ possibly alternation? |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding of participants/carers? | High risk | Not really possible to blind patients or carers. |
| Blinding of outcome assessors? | Unclear risk | Not mentioned. |
| Incomplete outcome data addressed? | Unclear risk | No information given. |
| Free of selective reporting? | Unclear risk | No information given. |
| Free of other bias? | Unclear risk | Groups well balanced at baseline. Unclear funding. |
| Methods | Location: San Francisco, USA. Number of centres: Not stated (23 referral centres). Funding source: Chlorhexidine mouthwash provided by Proctor and Gamble. Recruitment period: Not stated. Patient had mucositis confirmed by clinician. |
|
| Participants | Inclusion: Adults over 18 years, undergoing stomatotoxic chemotherapy, who could read & write English, were mentally capable of understanding research protocol, had Karnofsky Performance Status >/= 60%. Eilers Oral Assessment Guide score >/=10, and physician assessed & documented oral mucositis. Exclusion: Concurrent RT to head & neck, diagnosed with AIDS or leukaemia, undergoing bone marrow transplant. 299 eligible, 200 randomised, 142 completed. |
|
| Interventions | Gr A Chlorhexidine mouthwash 0.12% 20 ml in mouth, swish for 20 seconds, 4 times/day for 12 days. Gr B Salt & Soda mouthwash (1 teaspoon each in 1 pint water) 20 ml in mouth, swish for 20 seconds, 4 times/day for 12 days. Gr C Magic mouthwash (lidocaine 0.5%, diphenhydramine HCL 0.25 ml, Aluminium hydroxide 14.75 ml) 20 ml in mouth, swish for 20 seconds, 4 times/day for 12 days. All patients were instructed in PROSELF Mouth Aware (PSMA) programme of oral hygiene ‐ new toothbrush for each CT cycle, regular & thorough brushing, daily flossing, regular oral assessment using Oral Assessment Guide, training to recognise mouth lesions requiring nurse assessment. |
|
| Outcomes | Patient soreness rating on 0 (no soreness) to 10 (very sore) scale for 7 days, day 7 used. Mucositis oral assessment guide used by patients dichotomised as eradicated or not. The mean time to cessation of symptoms (SD) is also used. | |
| Notes | 0 to 10 scale converted VAS scale to 0 to 100. No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: 'randomised double blind clinical trial". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | Low risk | Quote: 'double blind'. Quote: "Intervention nurses who performed initial oral assessments and the telephone contacts with the patients were blind to the group assignments". Comment: Although one mouthwash contained a suspension and caused oral numbness, one caused discolouration of teeth & gums and one clear solution we consider it to be blind. |
| Blinding of outcome assessors? | Low risk | Quote: 'double blind'. Comment: Outcomes assessed by patients. |
| Incomplete outcome data addressed? | High risk | 58/200 (29%) of those randomised were excluded. 47 (24%) because they were hospitalised, too nauseated, too ill, or didn't like taste or numbness caused by mouthwash (likely to be more unwell that those included). A further 11 patients took more than 12 days to report cessation of OM symptoms. The treatment group allocation of these 58 is not given but "no significant difference in demographic characteristics between 142 patients who reported cessation of signs & symptoms of mucositis within 12 days and those who didn't .... and proportion similar in 3 mouthwash groups (P = 0.52). |
| Free of selective reporting? | Unclear risk | Comment: Pain can only be measured by the patient which is subjective. No other outcomes reported. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: San Francisco, USA. Number of centres: Two. Funding source: Oncology Nursing Foundation, Sigma theta Tau International, Reserach Centre for Symptom Management at UCSF, School of Nursing. Recruitment period: Unknown. |
|
| Participants | Adults who were initiating radiotherapy for head and neck cancer were invited to participate and were randomised when mucositis developed. Inclusion: > 18 years, able to read and understand English, Karnofsky Performance Status >/= 60%, mentally capable of giving informed consent 35 eligible, 33 enrolled, 30 completed. Excluded: AIDS, previous radiotherapy to head & neck, previous chemotherapy. |
|
| Interventions | Gr A (n = 14) micronised sucralfate, 1 g/15 ml mouthwash. Patients instructed to swish and spit 4 times daily after meals and at bedtime. Gr B (n = 16) Salt and soda Mouthwash (1 teaspoon each of salt and sodium bicarbonate to a pint of water). Patients instructed to swish and spit 4 times daily after meals and at bedtime. |
|
| Outcomes | Mean healing time in days is used. Pain (swallowing) at end of RT and at 1 month, and pain intensity (0 to 3 scale) at time of worst mucositis which was used. Breaks in RT. | |
| Notes | MacDibbs score for mucositis (mean and SD). Breaks in RT. No sample size calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Comment: No details given in paper. Correspondence from author that randomisation was by computer generated random numbers. |
| Allocation concealment? | Low risk | Comment: Correspondence from author ‐ allocation was concealed in opaque sealed envelopes. Mouthwashes packaged in opaque bottles. |
| Blinding of participants/carers? | Low risk | Quote: "Double blind". |
| Blinding of outcome assessors? | Low risk | Quote: "Double blind". |
| Incomplete outcome data addressed? | Low risk | 3/33 (9%) dropped out ‐ not stated which group these patients were in, but is unlikely to affect results. |
| Free of selective reporting? | Low risk | Planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: Denmark. Number of centres: One. Funding source: Not stated. Recruitment period: January 1995 to January 1999. |
|
| Participants | Inclusion: Adults undergoing radiotherapy with curative intent for head & neck cancer (biopsy verified squamous cell carcinoma of larynx, pharynx or oral cavity) with radiation induced pain not managed with acetaminophen. Exclusion: Cancer related pain before the initiation of radiotherapy, ongoing treatment with opioids, tricyclics, or non‐steroidal anti‐inflammatory drug, or known contraindications to tricyclics. 86 eligible, 43 enrolled and randomised and 39 completed. |
|
| Interventions | Gr A (n = 22) oral morphine chloride (5 mg x 6) additional doses of 5 mg each, plus stimulant laxative. Gr B (n = 21) oral nortriptyline (tricyclic antidepressant) (25 mg x 2). Patients on nortriptyline had the dose increased by 25 mg every second day, until pain relief or intolerable side effects were experienced (up to max dose of 150 mg/day). Acetaminophen was used for breakthrough pain in nortriptyline group. Patients with insufficient pain relief were offered supplementary medication from the opposite treatment arm. |
|
| Outcomes | VAS pain scores at 1, 2 weeks after randomisation and 2 weeks after end of radiotherapy. The 7 day scores are used. | |
| Notes | Danish version of McGill Pain Questionnaire. Sample size calculation reported: to detect a 10 mm difference in VAS score for pain (smallest clinically significant difference) assuming a standard deviation of 0.2 in VAS score, with α = 0.05 and β = 0.1 a total of 43 patients was required to detect this difference. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "'in a randomised design". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | High risk | Different frequency for interventions. |
| Blinding of outcome assessors? | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | Post randomisation exclusions and withdrawals described and reasons given. Equal numbers in both groups. Two patient in each arm considered in evaluable. 9% lost to follow‐up. |
| Free of selective reporting? | Low risk | Primary and secondary outcomes described and reported. |
| Free of other bias? | High risk | Patients with insufficient pain relief were offered supplementary medication from the other treatment arm. 11/21 (52%) of patients in Gr2 had morphine as well as nortriptyline (mean time to morphine 13.4 +/‐ 8.4 days). None of the morphine group took nortriptyline. |
| Methods | Location: Egypt. Number of centres: One (one treatment centre but two recruitment centres). Funding source: Not stated. Recruitment period: Not stated. |
|
| Participants | Children under 12 years with mucositis, cancer type unclear but receiving chemotherapy. 80 eligible, 80 randomised (40 to each group), 63 completed. | |
| Interventions | Gp A 100 IU Vitamin E capsule either emptied into mouth or chewed, twice daily. Gp B 100 IU Vitamin E capsule swallowed twice daily. |
|
| Outcomes | Mucositis severity scores by WHO criteria, improvement of mucositis at 5 days. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "patients were randomly assigned". |
| Allocation concealment? | Unclear risk | No mention of allocation concealment. |
| Blinding of participants/carers? | High risk | One group were instructed to swallow capsule, the other group instructed to either chew it or have contents emptied into mouth by carer. |
| Blinding of outcome assessors? | High risk | Not mentioned. |
| Incomplete outcome data addressed? | High risk | In Gp A 3 patients non compliant with treatment, 6 died & 3 lost to follow‐up (30% excluded from analysis), in Gp B 2 patients non compliant with treatment (analysed in Gp A), 2 died & 3 lost to follow‐up (18% excl from analysis). Data analysed on 28/40 (70%) patients randomised to Gp A plus the 2 from Gp B, and 33/40 (83%) randomised to Gp B. Possible that incomplete outcome data may have influenced results. |
| Free of selective reporting? | Unclear risk | Comment: Planned to follow patients followed for 5 days. Comment in discussion that some of the Gp B patients 'improved later', suggesting that the relative effects may be different after longer follow‐up. |
| Free of other bias? | High risk | Comment: Differences between groups at baseline. Severity of mucositis (Gp A median severity score 3, Gp B median severity score 2). Distribution of mucositis sites different in each group. "On treatment" analysis, more patients excluded from analysis in Gp A, and non compliant patients in Gp B added to Gp A. |
| Methods | Location: Belgium. Number of centres: One. Funding source: Not stated. Recruitment period: Not stated. Two studies reported, one prevention and one treatment of OM. |
|
| Participants | Adults with haematological malignancies and grade 1 or 2 OM induced by chemotherapy +/‐ radiotherapy prior to stem cell transplantation. 37 patients seemed eligible, 36 randomised, 18 to each group. | |
| Interventions | GpA (18) low energy laser (LEL) on alternate working days (approx 3 sessions of 6 mins per week delivering 2 J/cm2 to each lesion) GpB (18) sham illumination (laser switched off) on alternate working days (approx 3 sessions of 6 mins per week) |
|
| Outcomes | Primary ‐ time to development of grade 3 OM using EORTC scale. Secondary ‐ success of treatment (no lesions >/= Gr 3). Number with grade 3 OM after 7 days. |
|
| Notes | 3 additional outcome measures added retrospectively: development of oesophageal mucositis, presence of diarrhoea, development of septicaemia. Sample size calculation for expected rates of OM at 7 days of 10% intervention and 60% control, 20 patients per group will give 90% power for 2‐sided log rank test and significance level of 5%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomised". Clarified by e‐mail from authors. |
| Allocation concealment? | Low risk | Not mentioned but clarified as sealed envelopes in e‐mail from authors. |
| Blinding of participants/carers? | Low risk | Therapists and nurses doing pretreatment assessments not blinded (for safety/ethical reasons), but patients were blinded. |
| Blinding of outcome assessors? | Low risk | Quote: "independent qualified healthcare professional observer (blinded to the treatment administered)". |
| Incomplete outcome data addressed? | Low risk | Outcome data complete for primary and secondary outcomes. 1 patient missing from retrospective outcome data in each group. 6% loss to follow‐up. |
| Free of selective reporting? | Low risk | Planned primary and secondary outcomes reported as well as additional retrospectively added outcomes. Acknowledged the 'methodological limitation not to have planned this data collection and analysis at the beginning of the trial'. |
| Free of other bias? | Low risk | Comment: All patients analysed in the groups to which they were randomised (ITT) and groups were comparable at baseline ‐ so probably. |
| Methods | Location: Vienna, Austria. Number of centres: One. Funding source: Not stated. Recruitment period: March 1998 to June 1999. |
|
| Participants | Inclusion: Patients with WHO grade 1 to 3 oral mucositis following 5 FU based chemotherapy. Patients were > 19 years of age, could read & understand German, had WHO Performance Status < 3. Exclusion: History of adverse reactions to G‐CSF, or GM‐CSF, or iodine, severe concomitant diseases including hyperthyroidism, dermatitis herpetiformis or need for systemic G‐CSF or GM‐CSF for neutropenia. 31 recruited and randomised and completed. |
|
| Interventions | Gr A (n = 15) GM‐CSF (Malgramostim) mouthwash three times daily (400 ug molgramostim dissolved in 250 ml water. Patients were instructed to wash mouths with 25 ml and keep in mouth for 3 min, then rinse and repeat 10 times within 30 mins). Gr B (n = 16) Antiseptic mouthwash (Povidone iodine) 4 ml povidone‐iodine in 125 ml water was used 6 times daily. This was kept in mouth for 3 mins. Patients received 4 daily tablets of amphotericin B, 10 mg (AA regimen). All patients with oral mucositis exceeding grade 2 were offered topical lidocaine 6 times daily. |
|
| Outcomes | Mean duration of therapy until complete remission. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Randomisation performed without stratification according to a balanced block randomisation procedure carried out by a third investigator". |
| Allocation concealment? | Unclear risk | No details given. |
| Blinding of participants/carers? | High risk | Quote " Physicians were not blinded but the patients were as to the efficacy of any of the chosen regimes". Comment: Different mouthwashes for different frequencies plus only one group received amphotericin B tablets. |
| Blinding of outcome assessors? | High risk | Quote " Physicians were not blinded but the patients were as to the efficacy of any of the chosen regimes". |
| Incomplete outcome data addressed? | Unclear risk | All patients completed. 2/16 (12%) patients in control group did not provide final OM score. |
| Free of selective reporting? | Unclear risk | Unclear. |
| Free of other bias? | High risk | Significant differences between groups at baseline for the following prognostic factors age group, WHO performance status, smoking status, oral hygiene. May have influenced results. 4 patients in control arm did not receive treatment but ITT analysis conducted. |
| Methods | Location: Seattle, USA. Number of centres: One. Funding source: Pogramme project grant for National Cancer Institute and assistance from Abbott Laboratories. Recruitment period: November 1985 to January 1987. |
|
| Participants | Inclusion: Adults aged 18 to 50 years with acute lymphhatic leukaemia, acute non‐lymphatic leukaemia, chronic myeloid leukaemia, Hodgkins or non‐Hodgkins lymphoma or pre‐leukaemic syndrome undergoing high dose chemotherapy and total body irradiation prior to bone marrow transplant. Patients had severe oral mucositis and remained on study for a minimum of 7 days. Exclusion: History of drug abuse, unable to understand English, life threatening cardiac, hepatic or renal disease. 84 randomised, 58 completed. |
|
| Interventions | Gr A Patient Controlled analgesia ‐ morphine (PCA) 2 to 5 mg loading dose with 1 mg bolus & lockout of 10 min. Bolus dose adjustment up to 5 mg available, & lockout could be reduced to 5 minutes as treatment progressed. maximum allowable dose 60 mg/hour. At night PCA patients had a continuous infusion set at the mean hourly rate of morphine for the previous day shifts (16 hours). Minimum 7 days of treatment. Gr B Continuous infusion (CI) morphine ‐ adjusted by nurses at patient's request. Minimum 7 days of treatment. Minimum of 9 days. |
|
| Outcomes | Average pain VAS score (0 to 100) for day 7. Daily mean morphine intake day 7. The mean duration of morphine (days) ‐ we are assuming that the standard errors not the standard deviations are presented here. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Assigned randomly' is stated in paper. Personal communication from study investigator states that randomisation was computer generated by the Statistics department. |
| Allocation concealment? | Low risk | Randomisation generated by third party ‐ probably adequate. Confirmed by author. |
| Blinding of participants/carers? | High risk | Comment: Interventions given differently. |
| Blinding of outcome assessors? | Unclear risk | Comment: Patients self assessed pain; hygienist completed mucositis ratings unclear if blinded. |
| Incomplete outcome data addressed? | Low risk | 14/40 and 12/40 excluded from PCA and CI groups respectively, because they were on study for less than 7 days. Reasons given and balanced in both groups 31 % follow‐up. |
| Free of selective reporting? | Low risk | Comprehensive. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: Seattle, USA. Number of centres: One. Funding source: National Cancer Instiute grant (CA38552) and National Institute on Drug Abuse grant (DA 05513). Abbott Laboratories supplied the PCA infusers and drug supplies. Recruitment period: Not stated. Randomised but not blind. Eligible patients had severe oral mucositis pain. Clear information on withdrawals. 35% lost to follow‐up. |
|
| Participants | Inclusion: Adults with acute lymphocytic leukemia, acute non‐lymphocytic leukemia, chronic myelogenous leukemia, Hogkin's and non‐Hodgkin's lymphoma or preleukemic syndrome planned to undergo bone marrow transplant. 54 eligible, 54 enrolled, 35 completed. | |
| Interventions | Gr A (n = 31) PCA Morphine bolus 1 to 2 mg morphine sulphate as bolus, with 10 min lockout. Gr B (n = 23) PKPCA (pharmacokinetically based patient controlled analgesic infusion system). Individual pharmacokinetic profiles for morphine were determined on the day prior to bone marrow transplant. 75 μg/kg dose was administered and venous blood samples taken over following 8 hours to determine PK profile, and this information was used to tailor the individual dose for the PKPCA intervention. |
|
| Outcomes | Average pain VAS score (0 to 100) for each study day, day 7 used. Mean opiate use mg/hour for day 7. Severity of mucositis assessed by dental hygienist. | |
| Notes | Data obtained from graphs. No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "randomly assigned". Author clarified this. |
| Allocation concealment? | Low risk | No information given but clarified by correspondence with author. |
| Blinding of participants/carers? | High risk | Comment: Unblinded patients self assessed pain; nurse assessment of amount morphine, hygienist completed mucositis ratings unclear if blinded. |
| Blinding of outcome assessors? | Unclear risk | Comment: Unblinded patients self assessed pain; nurse assessment of amount morphine, hygienist completed mucositis ratings unclear if blinded. |
| Incomplete outcome data addressed? | Low risk | 19/54 (35%) of those randomised were excluded, 11/31 (35%) from Gr A and 8/23 (35%) from Gr B. Reasons for each withdrawal given in table 2. |
| Free of selective reporting? | Low risk | Planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: Seattle, USA. Number of centres: One. Funding source: Not stated. Recruitment period: Not stated. |
|
| Participants | Inclusion: Adult patients undergoing bone marrow transplant for haematological malignancies, who required treatment for oral mucositis pain. 28 completed at least 4 days. |
|
| Interventions | Gr A Morphine by Pharmacokinetically based patient controlled analgesic infusion system (PKPCA). Gr B Alfentanil (PKPCA). In both groups patients had individual pharmacokinetic profile for the assigned opioid determined prior to start of the study. A bolus dose of either 75 μg/kg morphine or 15 μg/kg alfentanil was given followed by venous blood sampling over the following 8 hours, to measure plasma drug concentrations. These data were then fitted to tri or bi exponential functions and the function providing the best fit for each patient was used in the computer program for calculations of infusion rates for that patient during therapy, providing a dose tailored to give optimum plasma concentrations of opioid for each individual. |
|
| Outcomes | Average pain VAS score (0 to 100) for days 1 to 14, day 7 taken. Mean opiate use mg/hour for day 7. | |
| Notes | Standard deviations calculated from graphs. No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote "random assignment patient blind assessment". |
| Allocation concealment? | Low risk | No information given but clarified by correspondence with author. |
| Blinding of participants/carers? | Low risk | Quote "random assignment patient blind assessment". Provider not blinded. |
| Blinding of outcome assessors? | Unclear risk | Patients self reported pain. Mean opiate use calculated by computer. |
| Incomplete outcome data addressed? | Low risk | All patients included in pain scores and opioid consumption. 0% drop‐outs. |
| Free of selective reporting? | Unclear risk | Unclear. |
| Free of other bias? | Unclear risk | Quote "Although significant bias was evident in several patients in group 5/16 for morphine and 8/12 for Alfentanil...." |
| Methods | Location: India. Number of centres: One. Funding source: Not stated. Recruitment period: August 1997 to March 1999. |
|
| Participants | Inclusion: Adults who developed oral mucositis after radiotherapy for histologically proven squamous cell carcinoma of head and neck. Exclusion: Patients with early head and neck cancer suitable for brachytherapy, those on palliative radiotherapy, had previous radiotherapy of chemotherapy, those with concurrent chemotherapy or those with distant metastases. 120 randomised and completed. |
|
| Interventions | Gr A (n = 60) Human placental extract (Placentrex) 2 ml IM, 5 times weekly for 3 weeks. Gr B (n = 60) Disprin™ gargle, 1 tablet in a cup of water, 3 times daily 0.5 mg/ml betamethasone oral drops, 8 drops 3 times daily. Both groups received Betadine™ antiseptic mouthwashes three times daily. |
|
| Outcomes | Progression of mucositis to grade 3, improvement in dysphasia, decrease in pain (data not used). All evaluated 2 times weekly, but unclear over what time period. Interruption of radiotherapy due to severe radiation reactions. To include the data we included the patients who did not get worse with the mucositis improved outcome. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "randomised" ‐ no details given. |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | High risk | Comment: Interventions given differently. |
| Blinding of outcome assessors? | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | All randomised patients included in analysis. 0% drop‐outs. |
| Free of selective reporting? | Unclear risk | Planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Placentrex given for 3 weeks ‐ ?duration of control group treatment. |
| Methods | Location: New York, USA. Number of centres: One. Funding source: DuPont Pharmaceuticals provided the medications used in the trial. Recruitment period: Not stated. Randomised, double blind. Patients who complained of mouth or throat pain were eligible. Unclear information on withdrawals. 60% lost to follow‐up. |
|
| Participants | Adults with head and neck cancer receiving radiation therapy to oral pharyngeal regions, complaining of throat or mouth pain. 67 randomised, 37 received benzydamine and 30 placebo. Results for mucositis given for days 2/3, for 33 in benzydamine and 28 in placebo groups, with loss to follow‐up of 11% and 7% respectively. | |
| Interventions | Gr A Benzydamine chloride rinse/gargle 1.5 mg/ml used every 3 hours during the day. Gr B Placebo. |
|
| Outcomes | Improvement in mucositis up 2/3 days, assessed by clinician. Specific criteria not given but mucositis is presented on 1 to 4 scale with 1 = none, 2 = slight; 3 = moderate and 4 = severe. At baseline there were 2 patients in the benzydamine group and 1 patient in the placebo group with no mucositis and these patients have been excluded from our analysis. We dichotomised 4 point scale as mucositis not improved by day 2/3. Relief from pain on swallowing also reported. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "randomised". No details given. |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | Low risk | Quote: "double blind". |
| Blinding of outcome assessors? | Low risk | Quote: "double blind". |
| Incomplete outcome data addressed? | High risk | Less than half of those randomised were evaluated for some outcomes on day 2. No explanation given. 60% lost to follow‐up for some outcomes. drop‐outs for mucositis 9%. |
| Free of selective reporting? | High risk | Only some of planned outcomes reported for some of the participants. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: Czech Republic. Number of centres: One. Funding source: Not stated. Recruitment period: March 2000 to March 2001. |
|
| Participants | Inclusion: Adults over 18 years with head and neck cancer receiving radiation treatment with minimum dose of 40 Gy, who develop oral mucositis, are on routine supportive treatment for OM, are likely to return for assessment, have a life expectancy > 6 months, not pregnant, on contraception (as appropriate). Eligible patients had mucositis in large parts of mouth. Exclusion: Those who have participated in clinical trial in previous 4 weeks, a history of liver or kidney disease, gastrointestinal ulcers, are 'non‐compliant', intolerant of diclofenac, have inadequate oral hygiene. 77 randomised, 69 included in efficacy analysis by clinician, 66 in efficacy analysis assessment by patient. |
|
| Interventions | Gr A (n = 39) Diclofenac (free acid 0.074%) mouthwash, 15 ml, three times daily for 2 to 6 weeks. Gr B (n = 38) placebo mouthwash, 15 ml, three times daily for 2 to 6 weeks. |
|
| Outcomes | Patient assessment for pain ‐ 0 absent; 1 slight; 2 moderate; 3 severe. Dysphagia has 5 point scale. Assessments for both presented in figures giving mean values for each day from day 0 to 28, and end of treatment. Day 7 used in the analysis. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "randomised using randomisation numbers". |
| Allocation concealment? | Unclear risk | No information reported. |
| Blinding of participants/carers? | Low risk | Quote: "double blind". |
| Blinding of outcome assessors? | Low risk | Quote: "double blind". |
| Incomplete outcome data addressed? | Low risk | Post randomisation exclusions clearly described for each group.10% lost to follow‐up. |
| Free of selective reporting? | Low risk | Planned outcome measure described and reported. |
| Free of other bias? | Unclear risk | No information on funding. |
| Methods | Location: Brazil. Number of centres: One. Funding source: Supported by Cancer Institute in Brazil. Recruitment period: October 2005 to May 2006. |
|
| Participants | Incusion: Children over 3 years with mixed cancer. All completed study 9 randomised to laser and 12 to sham. |
|
| Interventions | low level laser GaAlAs instrument by photon laser II with continuous 830 nm wavelength, 100 mW power versus sham. Treatment time = energy x surface area/power. Energy density of 4 J/cm2 was delivered to the mucositis lesions. Duration 7 days. Dentist who applied the laser did not participate in the evaluation. Patients received pain control and symptomatic treatment according to severity of OM. |
|
| Outcomes | OM by a dentist blinded to the intervention. OM was measured 0 to 4 scale (National Cancer Institute Common Toxicity Criteria version 2 at baseline and every day until complete healing). | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "placebo controlled randomised trial". Quote: "patients were randomised by computer code generation in two groups". |
| Allocation concealment? | Unclear risk | No information. |
| Blinding of participants/carers? | Low risk | Participants were, carers were not. |
| Blinding of outcome assessors? | Low risk | Comment: Different to laser provider. |
| Incomplete outcome data addressed? | Low risk | No drop outs ‐ 0%. |
| Free of selective reporting? | Low risk | Very clear outcome outcome reporting. |
| Free of other bias? | Low risk | Comment: Probably. |
| Methods | Location: USA. Number of centres: Multicentre (actual number unclear). Funding source: Grants from the US Public Health Service and the National Cancer Institute. Recruitment period: January to December 1994. |
|
| Participants | Adults with mixed cancer receiving first cycle 5FU based chemotherapy. Patients who complained of mouth or throat pain were eligible. 135 eligible, 50 enrolled, 50 completed. |
|
| Interventions | Placebo versus sucralfate solution 1 g/30 ml. Rinse 4 times/day for median of 7 days. | |
| Outcomes | Daily patient reported maximum over 7 days (0 to 4 scale). Clinical evaluation of mucositis by healthcare provider ("in a manner commonly used in cancer trials usually judged by historical means") 4/5 weeks after chemotherapy on WHO 0 to 4 scale. Mucositis not resolved used. | |
| Notes | All patients had ice chips during chemotherapy. Power calculation reported: Anticipated that 50% of those recruited would report stomatitis. Actual % less, (38%) so accrual continued till 50 patients reported stomatitis providing a 73% power to detect a 1‐grade decrease in stomatitis, 85% power to detect a 1.5‐grade decrease & 95% power to detect a 2‐grade decrease, via a one‐sided Wilcoxon test with a 2.5% error rate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Patients were stratified and randomised...." |
| Allocation concealment? | Unclear risk | No details given. |
| Blinding of participants/carers? | Low risk | Quote: "Double blind". |
| Blinding of outcome assessors? | Low risk | Quote: "Double blind". |
| Incomplete outcome data addressed? | Low risk | 131 patients randomised but only 50/131 developed stomatitis and underwent therapy. However all randomised patients who developed stomatitis included in analysis. None lost to follow‐up 0%. |
| Free of selective reporting? | Low risk | Planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: Seattle, USA. Number of centres: One. Funding source: Grants from National Cancer Institute (CA38552), National Institute on Drug Abuse (DA 05513) and Abbott Laboratories who supplied the PCA pumps. Recruitment period: Not stated. Randomised not blind. Eligible patients had severe oral mucositis pain. Unclear information on withdrawals. 10% lost to follow‐up. |
|
| Participants | Inclusion: Adolescents aged 12 to 18 years who were presenting for bone marrow transplant for treatment of leukaemia or lymphoma. 35 randomised, 20 completed. |
|
| Interventions | Gr A PCA 15 μg/kg morphine & 10 min lockout. At night patients had continuous infusion at rate equal to previous 16 hour mean hourly rate to facilitate sleep. Gr B CI 15 μg/kg morphine after a loading bolus dose of 45 μg/kg. Any increase in rate was accompanied by a bolus of 3 times the rate change in μg/hr. |
|
| Outcomes | Average pain VAS score (0 to 100) for days 0 to 18, day 7 taken. Mean opiate use mg/kg/hour for day 7, converted to mg/hr assuming mean weight given in Table 1. The mean duration of morphine (days) ‐ we are assuming that standard errors not standard deviations are presented here. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: 'randomised' ‐ no details given. |
| Allocation concealment? | Unclear risk | Nno information given. |
| Blinding of participants/carers? | High risk | Interventions given differently. |
| Blinding of outcome assessors? | High risk | Self assessment. |
| Incomplete outcome data addressed? | Unclear risk | 10/15 patients who required less than 7 days treatment are accounted for and their allocated treatment given. However 43% of randomised patients are not included in the outcome assessment which may cause a bias. |
| Free of selective reporting? | Low risk | Yes. |
| Free of other bias? | High risk | Comment: Patients who experienced adverse events such as nausea were excluded. |
| Methods | Location: Pakistan. Number of centres: One. Funding source: Brooks Pharmaceutical co supplied the TCDO and placebo. Recruitment period: Not stated. Randomised, double blind. Eligible patients had mucositis on entry. None lost to follow‐up. |
|
| Participants | Adults with mixed cancer, with oral mucositis WHO grades 2 to 4, induced by chemotherapy in preceding 2 weeks, including those given prophylactic nystatin or antiseptic mouthwash. 62 eligible, 62 randomised , 62 completed. |
|
| Interventions | GrA (n = 32) TCDO (tetrachlorodecaoxide). 10 ml twice daily, swish then swallow. Maximum 7 days. GrB (n = 30) placebo 10 ml twice daily, swish then swallow. Maximum 7 days |
|
| Outcomes | Objective clinical mucositis improvement by day 3 using WHO criteria on 0 to 4 scale, mucositis not improved by day 3 used. Symptomatic improvement by patient of oral pain and ability to eat by day 3. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "double blind placebo controlled randomised trial". |
| Allocation concealment? | Unclear risk | No information. |
| Blinding of participants/carers? | Low risk | Quote: "Double blind". Comment: TCDO and placebo supplied in identical containers. |
| Blinding of outcome assessors? | Low risk | Quote: "Double blind". |
| Incomplete outcome data addressed? | Low risk | All randomised patients included in analysis. |
| Free of selective reporting? | Low risk | Planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Comment: Some pharmaceutical funding. |
| Methods | Location: Sweden. Number of centres: Five. Funding source: Unrestricted grant from Schering Plough AB, Stockholm. Duration: End of radiotherapy. |
|
| Participants | Adults with recently detected head and neck cancer, who were about to undergo radiotherapy. 92 patients were entered onto database and randomised. Only 62 developed mucositis scores > 1.5. 61 included an end point. 10 patients had only 1 evaluation. 51 analysed for mucositis outcome. | |
| Interventions | GrA: Recombinant human granulocyte monocyte‐colony stimulating factor GM‐CSF (Molgramostim, Leucomax, Schering‐Plough, Sweden) 4 g/kg/d sc. By injection every day (including radiotherapy free days) from when oral mucositis scored reached 1.5 until full radiotherapy dose was given. 'Conventional care' as well. GrB: 'Conventional care' ‐ details not provided. |
|
| Outcomes | Improvement in mucositis scores assessed as change of 1 unit from baseline (this was a mean value over several sites). Assessment at 1, 2 and end of radiotherapy. Additional side effects: nutritional status, weight loss. Pain and difficulty eating were assessed but data not presented (pain and difficulty eating incorporated in mucositis scores). | |
| Notes | Power calculation "Assuming a binomial distribution 2x39 patients were needed to achieve a power of 80%, or 2 x 52 for a power of 90%, to reject the hypothesis of equal success probabilities, using a 2 sided test at the significance level of 5% (normal approximation)". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation performed centrally at start of radiotherapy 'according to a computer generated code in blocks of 10.' Only those who subsequently developed OM score greater than 1.5 entered the study. |
| Allocation concealment? | Low risk | 'Treatment allocation at centres performed using sealed envelopes'. |
| Blinding of participants/carers? | High risk | Quote: "multicentre open randomised phase III study". |
| Blinding of outcome assessors? | Low risk | Outcome assessment conducted by a physician or dentist blinded to treatment allocation. |
| Incomplete outcome data addressed? | Low risk | Comment: Randomised patients accounted for: 21/92 (11 and 10 in GrA & Gr B respectively) patients did not enter treatment because they did not develop OM score > 1.5. A further 5 patients in each group excluded from analysis due to insufficient documentation, leaving 61/71 evaluable patients. 51 patients included in primary outcome. 28% drop‐outs (based on those who were eligible). 3 and 7 patients from GM‐CSF & control groups respectively did not have data for primary endpoint (after 2 weeks). |
| Free of selective reporting? | Low risk | Planned outcomes reported. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: Turkey. Number of centres: One. Funding source: Not stated. Recruitment period: Not stated. |
|
| Participants | Adults with solid cancer (head, neck, lung) receiving chemotherapy, with oral mucositis. 40 patients eligible, enrolled and completed. | |
| Interventions | GM‐CSF 400 μg in 100 ml saline 4x daily versus nystatin 1 ml 4x daily. | |
| Outcomes | Mean time to heal (days) and standard deviations are given. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: 'randomly assigned' ‐ no further information given. |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | High risk | Not mentioned. |
| Blinding of outcome assessors? | High risk | Not mentioned. |
| Incomplete outcome data addressed? | Low risk | All randomised patients included in outcome assessment, 0% drop‐out. |
| Free of selective reporting? | Low risk | Planned outcomes reported. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: Liverpoool, United Kingdom. Number of centres: One. Funding source: Not stated. Recruitment period: February 1995 to August 1997. |
|
| Participants | Inclusion: Patients undergoing conditioning chemotherapy in preparation for bone marrow transplant or autologous transplant of peripheral blood stem cells (cyclophosphamide/TBI, high dose melphalan or BEAM). 81 screened, 65 randomised, 43 sought treatment for severe mucositis pain when non‐opioids were ineffective, 35 completed. |
|
| Interventions | GrA (n = 33) Diamorphine by PCA 0.8 mg bolus + 2 mg/hour (max) to max of 96 mg/24 hour for 14 days. GrB (n = 32) Diamorphine by continuous infusion, 24 mg in 24 hour (with requested doses increases up to a max dose of 96 mg/24 hour) for up to 14 days. |
|
| Outcomes | Average pain VAS score (0 to 100) day 7 taken. Mean opiate use mg per hour for day 7. The mean duration of morphine (days) ‐ we have converted the standard errors to standard deviations. | |
| Notes | All patients, on 2 occasions, received prophylactic amphotericin lozenges, nystatin, chlorhexidine, oral acyclovir, oral ciprofloxacin and colistin. No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "serial sealed envelopes containing computer generated random treatment allocation". |
| Allocation concealment? | Low risk | Quote: "serial sealed envelopes containing computer generated random treatment allocation". |
| Blinding of participants/carers? | High risk | Interventions given differently. |
| Blinding of outcome assessors? | High risk | Self assessment. |
| Incomplete outcome data addressed? | Low risk | Comment: only 45/65 (66%) of those randomised required treatment for mucositis. 8 patients who received treatment were subsequently excluded from analysis (4 in Gr A failed attempt at PCA and 1 needed diamorphine for 'other pain', and 3 in Gr B needed diamorphine for 'other pain'). 18% drop‐outs. |
| Free of selective reporting? | Low risk | Planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Not all randomised participants required opioid treatment for severe mucositis pain. No funding mentioned. |
| Methods | Location: Italy. Number of centres: One. Funding source: Not stated. Recruitment period: Not stated. |
|
| Participants | Inclusion: Adults with advanced malignancies (mostly gastrointestinal) treated with 5FU and folinic acid or fluorouracil. Eligible patients had oral mucositis grade 2 or 3. 44 enrolled, 44 completed. |
|
| Interventions | Gr A (n = 22) Allopurinol dispersion mouthwash 300 mg dissolved in water, rinse for 1 min 4 to 6 times/day for at least 7 days. GrB (n = 22) Placebo mouthwash, rinse for 1 min 4 to 6 times/day for at least 7 days. |
|
| Outcomes | Clinical evaluation of mucositis resolved, or improved at 5 days using WHO criteria (0 to 4 scale), by physician. Mean duration of mucositis (days used). | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "patients were randomised to received in a double blind manner". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | Low risk | Double blind. |
| Blinding of outcome assessors? | Low risk | Double blind. |
| Incomplete outcome data addressed? | Low risk | All randomised patients included in outcome assessments. |
| Free of selective reporting? | Unclear risk | 5 day treatment cycle unclear. |
| Free of other bias? | Unclear risk | Funding unclear. |
| Methods | Location: Germany. Number of centres: One. Funding source: Unclear. Recruitment period: Unclear. |
|
| Participants | Inclusion: Adults with Grade 1 or 2 mucositis after undergoing radiotherapy for head and neck cancer who had Karnofsky Performance Status > 50%. Exclusion: Previous radiotherapy, pervious therapy for oral mucositis, recent chemotherapy, skin or mucosal defects. |
|
| Interventions | Gr B (n = 39) Polyvalent immunoglobulin (160 mg/ml) 10 ml IM on day ), then 5 ml on days 2 & 4. Gr B (n = 42) Placebo (10% human albumin) 10 ml IM on day ), then 5 ml on days 2 & 4. |
|
| Outcomes | EORTC grade did not reduce to 0 or 1 used in review. | |
| Notes | Double blind study up to day 7. Both groups received nystatin. Power calculation ‐ not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "A randomised double blind study". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | Low risk | Double blind. |
| Blinding of outcome assessors? | Low risk | Double blind. |
| Incomplete outcome data addressed? | Low risk | Only one dropout from placebo group due to poor general health 1% drop out. |
| Free of selective reporting? | Unclear risk | Unclear. |
| Free of other bias? | Unclear risk | Unclear funding. |
| Methods | Location: USA. Number of centres: Multicentre. Funding source: Inter America Pharmaceuticals. Recruitment period: Not stated. |
|
| Participants | Inclusion: Adults with moderate oral mucositis pain after chemotherapy of chemoradiotherapy with clinically obvious mucosal changes consistent with oral mucositis. Exclusion: Patients taking systemic analgesics or anti‐inflammatory drugs or those using topical anaesthetics. 44 randomised, 44 completed. |
|
| Interventions | Gr A (n = 25): Benzydamine 0.15% in mouthwash base. Swish and hold 15 ml of solution for 1 min every 2 hours. Minimum of 5 doses per day. Gr B (n = 19): Placebo. Swish and hold 15 ml of solution for 1 min every 2 hours. Minimum of 5 doses per day. Study length min 9 hours, max 10 days. |
|
| Outcomes | Patients assessment of pain relief at day 1. We dichotomised this as poor versus fair to excellent and used this as an outcome for whether mucositis had improved or not. | |
| Notes | Sample size calculation reported (post hoc): to have a 90% chance of detecting a significant difference between placebo and benzydamine solutions (assuming placebo 45% effective and benzydamine 65%) would require 105 subjects per arm, if difference in effectiveness between the groups is increased to 25% (45% and 70%) then would need 75 subjects per group. Study planned to accrue 214 subjects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: 'A multicentre double blind controlled trial was undertaken". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | Low risk | Double blind. |
| Blinding of outcome assessors? | Low risk | Double blind. |
| Incomplete outcome data addressed? | Low risk | All randomised patients included in outcome assessment. |
| Free of selective reporting? | High risk | Preliminary results reported, for pain at day 1 and duration of treatment. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: USA. Number of centres: One. Funding source: Grants from National Cancer Institute (CA 38522) American Cancer Society (Institutional grant IN‐24), Biomedical Research Support Grant (RR‐05346). Recruitment period: Not stated. Randomised patients and assessor not blinded. |
|
| Participants | Inclusion: Adults with haematological malignancy or lymphoma having BMT, who spoke English and were aged > 18 years. Patients had oral mucositis with pain. 67 enrolled, 45 completed. |
|
| Interventions | Gr A (n = 18) HYP Hypnosis ‐ 2 training sessions prior to treatment twice weekly sessions for 5 weeks. Gr B (n = 17) CB Cognitive Behavioural coping skills,cognitive restructuring, & relaxation ‐ 2 training sessions prior to treatment twice weekly sessions for 5 weeks. Gr C (n = 16) TCC Therapist contact control group ‐ therapist met with patients for a chat but no new coping skills were taught ‐ 2 training sessions prior to treatment twice weekly sessions for 5 weeks. Gr D (n = 16) TAU Treatment as usual group ‐ received 'standard interventions for nausea, pain and emesis. |
|
| Outcomes | Average pain VAS score mean over week 3. Average mean opiate use per day for week 3. | |
| Notes | Average mean opiate use per day was converted to hourly rate by dividing by 24. No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Patients were randomised to one of 4 intervention groups". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | High risk | Interventions delivered differently. |
| Blinding of outcome assessors? | High risk | Self assessment. |
| Incomplete outcome data addressed? | Low risk | Reasons for post randomisation withdrawal and exclusions given for each group and balanced across groups. (17 patients too ill to continue & 5 had missing data (HYP 6, CB 6, TCC 4, TAU 6 respectively). 33% withdrew. |
| Free of selective reporting? | Low risk | planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Quote: "During random assignment a marked sex difference occurred between groups". |
| Methods | Location: Seattle USA. Number of centres: One. Funding source: NCI Grants (38552 & 57807). Recruitment period: Not stated. |
|
| Participants | Inclusion: English speaking adults undergoing bone marrow transplant, aged > 18 years, with leukaemia, myelodysplasia or lymphoma. Had to receive 2 outpatient training sessions and remain well enough to participate for 1 month after their bone marrow transplant. Exclusion: Those actively practising imagery. 161 randomised, 94 completed. |
|
| Interventions | Gr A: R&I relaxation & imagery training. Gr B: CB cognitive /behavioural coping skills training. Gr C: TS therapist support. Gr D: TAU treatment as usual (no training). 2 training sessions prior to treatment, then twice weekly sessions for 5 weeks. All patients received standard interventions for pain, nausea and emesis. |
|
| Outcomes | Average pain VAS score mean day 6 to 26. | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Randomised patients to one of four intervention conditions. Randomisation was stratified for conditioning regimen (CT), whether patients had total body irradiation, and gender". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | Low risk | Quote: "Patients blinded to content of other intervention groups. They were told the content only for the intervention they received". |
| Blinding of outcome assessors? | Low risk | Quote: "These research assistants were blind to the randomisation of the patients". |
| Incomplete outcome data addressed? | Unclear risk | 67/161 = 42% did not complete the study. Details reasons given in Table 2 and reasons and numbers similar in each group. However authors acknowledge that this introduces a risk of bias and have attempted to deal with this in the analysis. |
| Free of selective reporting? | Low risk | Planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Baseline characteristics not given by allocated treatment group ‐ uncertainty as to whether randomisation resulted in balanced groups and/or high proportion of non completers caused bias. |
| Methods | Location: Barcelona, Spain. Number of centres: One. Funding source: rhGM‐CSF (Molgramastin) supplied by Schering Plough. Recruitment period: October 1998 to March 2001. |
|
| Participants | Inclusion: Adults with WHO grade 3 to 4 oral mucositis who had haematological malignancies undergoing stem cell transplantation. 41 patients recruited, 35 completed. |
|
| Interventions | GrA (n = 18) rhGM‐CSF 400 ug of rhGM‐CSF dissolved in 200 ml saline solution, mouthrinse to be used for 30 mins, three times daily, for 5 days. Swish and spit. GrB (n = 23) placebo (saline solution only), mouthrinse to be used for 30 mins, three times daily, for 5 days. Swish and spit. |
|
| Outcomes | Overall duration of mucositis (days) is used. Reduction in at least 1 grade of mucositis, need for PCA morphine (however there were significantly more already needing this in the placebo group at the beginning of the treatment protocol). | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Double blind randomised controlled trial". Quote: "Both patient randomisation and drug prepartion were performed in the pharmacy department". |
| Allocation concealment? | Unclear risk | No further details given. |
| Blinding of participants/carers? | Low risk | Double blind. Quote: "Active and placebo mouthrinses looked and tasted identical". |
| Blinding of outcome assessors? | Low risk | Double blind. |
| Incomplete outcome data addressed? | Unclear risk | 6/41 (15%) excluded post randomisation, reasons (3 died, 2 refused, 1 required ventilation). 16/18 in Gr A & 19/23 Gr B completed treatment SF. |
| Free of selective reporting? | Low risk | Planned outcomes described and reported. |
| Free of other bias? | Unclear risk | Unclear. |
| Methods | Location: USA. Number of centres: One. Funding source: Not stated. Recruitment period: Not stated. |
|
| Participants | Inclusion: Adult cancer patients who had undergone chemotherapy with no prior treatment for oral mucositis, no oral yeast infection or oral herpes infection. All had oral mucositis grades 2 to 4 (WHO criteria). "24 were initially evaluated but 6 were subsequently excluded". Unclear when randomisation took place. |
|
| Interventions | Gr A (n = 9) Vitamin E oil 400 mg/ml 1 ml applied topically to lesions twice daily for 5 days. Gr B (n = 9) Placebo (coconut and soyabean oil) 1 ml applied topically to lesions twice daily for 5 days. |
|
| Outcomes | Clinical evaluation of eradication of lesions over 5 day study period using WHO criteria (0 to 4 scale). | |
| Notes | No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Randomised double‐blind placebo controlled study. Patients were randomly assigned to receive in a double blind manner vitamin E or placebo". |
| Allocation concealment? | Unclear risk | No details given. |
| Blinding of participants/carers? | Low risk | Double blind. |
| Blinding of outcome assessors? | Low risk | Ddouble blind. |
| Incomplete outcome data addressed? | Unclear risk | Unclear information on withdrawals which were either before or after randomisation. 0% or 25% lost to follow‐up. |
| Free of selective reporting? | Low risk | Planned outcome described and reported. |
| Free of other bias? | Low risk | Yes. |
| Methods | Location: Germany. Number of centres: One. Funding source: Ute Huneke‐Stiftung & Leukamie Liga e.V. Recruitment period: August 1994 to February 1996. |
|
| Participants | Inclusions: Adults with a haematological neoplasia or malignant lymphoma scheduled for bone marrow transplantation. Eligible patients had mucositis pain. 20 patients were randomised. Exclusion: those who refused consent, failed to understand the protocol for pain therapy and measurement, who had prior chronic or recurrent opioid medication, those with a history of alcohol or drug abuse. |
|
| Interventions | Gr A (n = 10) Staff controlled pethidine infusion (100 mg pethidine continuously on first day, 200 mg on second and 300 mg on third. From day 4 this was increased up to 400 mg. Supplemental bolus doses of 25 mg pethidine were administered through BMT staff "when needed". Gr B (n = 10) Patient controlled pethidine (PCA device delivering 150 mg pethidine/day continuously and 25 mg per demand dose. The lockout time was 45 min and speed of injection of a single bolus dose to 600 mg/h). |
|
| Outcomes | Patient VAS pain scale. 6 times per day. Pethidine consumption was also monitored (mg/day shown in figure). Patient mean pain scores were also recorded. | |
| Notes | All patients also received ondansetron via continuous infusion. No power calculation reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Randomised assignment". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants/carers? | High risk | PCA versus staff controlled analgesia. |
| Blinding of outcome assessors? | High risk | Pain assessed by patient. |
| Incomplete outcome data addressed? | Low risk | One patient from group B excluded from analysis. No reason given but unlikely to affect results. Drop out 5%. |
| Free of selective reporting? | Low risk | Pplanned outcomes described and reported. |
| Free of other bias? | Low risk | Yes. |
BMT = bone marrow transplantation TBI = total body irradiation VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agüeros 2004 | Abstract, insufficient information (I). |
| Allison 1995 | Not RCT (sucralfate plus fluconazole). |
| Atkins 2004 | Abstract, insufficient information. |
| Awada 2004 | Not RCT and mucositis not primary outcome. |
| Barker 1991 | No useable data, only medians given (sucralfate versus diphenhydramine syrup/kaolin‐pectin). |
| Beltran 1990 | Abstract, insufficient information (bencidamide mouthwash versus placebo). |
| Biswal 2002 | Abstract, insufficient information (PCA versus CI). |
| Biswal 2003 | Prevention not treatment of mucositis (honey). |
| Bondi 1997 | No useable data, outcome looks at progression of mucositis (tobramycin polymyxin E amphotericin versus diphenhydramine). |
| Carnel 1990 | No useable data, unclear how many patients in each group (lidocaine 1% cocaine versus dyclonine hydrochloride versus diphenhydramine kaolin‐pectin versus placebo). |
| Cerchietti 2002 | No relevant outcomes (morphine mouthwash versus 'magic' mouthwash). |
| Cerchietti 2003 | Problems with design and no mucositis outcomes in form which can be used in the review. |
| Chambers 2006 | Abstract, insufficient information (RK‐0102 oral rinse). |
| Chapman 1997 | No useable data, severity scores given (morphine versus hydromorphone). |
| Cheng 2004 | Prevention of mucositis not treatment. |
| Collins 1996 | No relevant outcomes (morphine versus hydromorphone). |
| Collova 2004 | Abstract, insufficient information. |
| Connor 1996 | Abstract, insufficient information (PCA versus CI). |
| Crawford 1994 | Abstract, insufficient information. |
| Domenge 1999 | Abstract, insufficient information (fluconazole versus amphotericin B). |
| Ehrnrooth 1999 | Abstract, insufficient information (morphine versus tricyclic antidepressant). |
| Eren 2007 | Study published in turkish language with English abstract. Unit of randomisation is chemotherapy cycles not patients (rGM ‐ CSF versus standard care comprising chlorhexidine + sodium bicarbonate + Vitamin E + mycostatin + ranitidine). |
| Evans 1998 | Abstract, insufficient information (GM‐CSF versus placebo). |
| Evensen 2001 | Prevention not treatment of mucositis (Na‐sucrose octasulfate versus placebo). |
| Ferretti 1987 | Abstract, insufficient information (chlorhexidine versus placebo). |
| Foote 1996 | Abstract, insufficient information (antibiotic lozenge‐tobramycin, polymyxin, amphotericin B versus placebo). |
| Giles 2002 | Abstract, insufficient information. |
| Giles 2004 | Prevention of mucositis not treatment. |
| Girdler 1995 | No useable data, number of ulcers and area covered, means but no SDs (epidermal growth factor versus placebo). |
| Gobetti 1999 | Abstract, insufficient information. |
| Haritha 2009 | Abstract, insufficient information (oral morphine). |
| Hejna 2000 | Abstract, insufficient information (GM‐CSF mouthwashes). |
| Kin‐Fong Cheng 2006 | Not eligible as excluded patients with severe (> 2) oral mucositis. |
| Kostler 2005 | Not RCT. |
| Labbate 2003 | Not RCT (contacted authors to check but no reply to e‐mail 10/8/06). |
| Lever 1987 | Study halted early ‐ protocol violation (benzydamine versus HSC mouthwashes). |
| Lilleby 2006 | Prevention of mucositis not treatment. |
| Lockhart 2005 | Prevention of mucositis not treatment. |
| Mantovani 2003 | Not RCT (GM‐CFS). |
| Marinoni 1996 | Abstract, insufficient information (many interventions). |
| Meredith 1997 | Design only treats when mucositis present but the results include patients not treated (sucralphate versus placebo). |
| Mitrokhin 2003 | Translated from Russian. Not RCT. |
| Naidu 2005 | Data presented within groups (comparing change from baseline) only no comparison between groups (Oral polyherb). |
| Oguchi 1998 | Not RCT (polymer film). |
| Oshitani 1990 | Not RCT (sodium alginate versus no treatment control). |
| Papila 1999 | Unclear if RCT ‐ no reply to letter or e‐mail (sucralfate versus placebo). |
| Pession 1997 | Abstract, insufficient information. |
| Pouli 1999 | Abstract, insufficient information. |
| Rades 2004 | Prevention of mucositis not treatment. |
| Radmard 2002 | Abstract, insufficient information. |
| Rothwell 1990 | No useable data, results presented as means (rinse‐hydrocortisone, nystatin, tetracyclin, diphenhydramine versus placebo). |
| Schmid 2006 | No oral outcome and wrong intervention for mucositis. |
| Schwerkkoske 1999 | Abstract, insufficient information. |
| Shaiova 2004 | No appropriate outcomes and unsure of design. |
| She 2000 | Unclear if RCT and insufficient information. |
| Shen 2004 | Unclear if RCT and insufficient information. |
| Sprinzl 2001 | Trial designed so that allocated patients received intervention on onset of mucositis, however outcomes measured at time from beginning of study. Patients had been on intervention for different times. We felt we could not use the data (GM‐CSF versus hydrocortisone mouthwash). |
| Stokman 2004 | Not RCT. |
| Su 2004 | Abstract, insufficient information. |
| Svanberg 2004 | Abstract, insufficient information. |
| Teshima 1986 | Insufficient information on trial to include and no response to letter (sent in Japanese). |
| Valcarcel 2002 | Abstract, insufficient information. |
| Vayne‐Bossert 2010 | Cross‐over study with carry‐over effect from first to second period. Only 9 patient in cross‐over, too few to undertake first period analysis. |
| Vela‐Ojeda 1996 | Abstract, insufficient information. |
CI = continuous infusion GM‐CSF = granulocyte macrophage‐colony stimulating factor PCA = patient controlled analgesia RCT = randomised controlled trial SD = standard deviation
Contributions of authors
Jan Clarkson (JC) and Helen Worthington (HW) wrote the protocol and review. HW co‐ordinated the review and wrote the letters to authors. HW, JC and Susan Furness independently and in duplicate assessed the eligibility of trials, extracted data and assessed the risk of bias of the trials. HW conducted the statistical analysis. Tasneem Khalid provided advice on the interventions and Stefan Meyer and Martin McCabe provided input on the cancer treatments and the assessment of mucositis, along with methodological input and checking of data.
Sources of support
Internal sources
The University of Manchester, UK.
Scottish Executive Health Department, UK.
University of Dundee, UK.
NHS Education for Scotland, UK.
Manchester Biomedical Research Centre, University of Manchester, UK.
Cancer Research UK, UK.
Teenage Cancer Trust, UK.
External sources
NIDCR grant ref 1 DE01 6950‐01, USA.
Declarations of interest
None known.
Notes
Changes from protocol. The title has changed. The hypotheses have changed. The original first hypothesis has been expanded into two hypotheses one for eradication and one for improvement of mucositis. The second original hypothesis has been changed from "there is no difference in the proportion of patients with relief of pain" to "there is no difference in the mean pain scores". This change was made as all studies reported pain as mean and standard deviations of VAS scores.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
- Baharvand M, Sarrafi M, Alavi K, Jalali Moghaddam E. Efficacy of topical phenytoin on chemotherapy‐induced oral mucositis; a pilot study. Daru2010; Vol. 18, issue 1:46‐50. [PMC free article] [PubMed]
- Barber C, Powell R, Ellis A, Hewett J. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: a preliminary, double centre, randomised controlled trial on patients with radiotherapy‐induced oral mucositis. Supportive Care in Cancer 2007;15(4):427‐40. [DOI] [PubMed] [Google Scholar]
- Chiara S, Nobile MT, Vincenti M, Gozza A, Pastrone I, Rosso M, et al. Sucralfate in the treatment of chemotherapy‐induced stomatitis: a double‐blind, placebo‐controlled pilot study. Anticancer Research 2001;21(5):3707‐10. [PubMed] [Google Scholar]
- Coda BA, O'Sullivan B, Donaldson G, Bohl S, Chapman CR, Shen DD. Comparative efficacy of patient‐controlled administration of morphine, hydromorphone, or sufentanil for the treatment of oral mucositis pain following bone marrow transplantation. Pain 1997;72(3):333‐46. [DOI] [PubMed] [Google Scholar]
- Cubucku CE, Sevenir B. Debridement could be a solution to promote healing of established mucositis in children. European Archives of Paediatric Dentistry 2007;8(2):105‐12. [DOI] [PubMed] [Google Scholar]
- Dodd MJ, Dibble SL, Miaskowski C, MacPhail L, Greenspan D, Paul SM, et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy‐induced mucositis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2000;90(1):39‐47. [DOI] [PubMed] [Google Scholar]
- Dodd MJ, Miaskowski C, Greenspan D, MacPhail L, Shih AS, Shiba G. Radiation‐induced mucositis: a randomized clinical trial of micronized sucralfate versus salt & soda mouthwashes. Cancer Investigation 2003;21(1):21‐33. [DOI] [PubMed] [Google Scholar]
- Ehrnrooth E, Grau C, Zachariae R, Andersen J. Randomized trial of opioids versus tricyclic antidepressants for radiation‐induced mucositis pain in head and neck cancer. Acta Oncologica 2001;40(6):745‐50. [DOI] [PubMed] [Google Scholar]
- El‐Housseiny AA, Saleh SM, El‐Masry AA, Allam AA. The effectiveness of vitamin 'E' in the treatment of oral mucositis in children receiving chemotherapy. The Journal of Pediatric Dentistry 2007;31(3):167‐70. [PubMed] [Google Scholar]
- Genot‐Klastersky MT, Klastersky J, Awada F, Awada A, Crombez P, Martinez MD, et al. The use of low‐energy laser (LEL) for the prevention of chemotherapy‐ and/or radiotherapy‐induced oral mucositis in cancer patients: results from two prospective studies. Supportive Care in Cancer 2008;16(12):1381‐7. [DOI] [PubMed] [Google Scholar]
- Hejna M, Kostler WJ, Raderer M, Steger GG, Brodowicz T, Scheithauer W, et al. Decrease of duration and symptoms in chemotherapy‐induced oral mucositis by topical GM‐CSF: results of a prospective randomised trial. European Journal of Cancer 2001;37(16):1994‐2002. [DOI] [PubMed] [Google Scholar]
- Hill HF, Chapman CR, Kornell JA, Sullivan KM, Saeger LC, Benedetti C. Self‐administration of morphine in bone marrow transplant patients reduces drug requirement. Pain 1990;40(2):121‐9. [DOI] [PubMed] [Google Scholar]
- Hill HF, Mackie AM, Coda BA, Iverson K, Chapman CR. Patient‐controlled analgesic administration. A comparison of steady‐state morphine infusions with bolus doses. Cancer 1991;67(4):873‐82. [DOI] [PubMed] [Google Scholar]
- Hill HF, Coda BA, Mackie AM, Iverson K. Patient‐controlled analgesic infusions: alfentanil versus morphine. Pain 1992;49(3):301‐10. [DOI] [PubMed] [Google Scholar]
- Kaushal V, Verma K, Manocha S, Hooda HS, Das BP. Clinical evaluation of human placental extract (placentrex) in radiation‐induced oral mucositis. International Journal of Tissue Reaction 2001;23(3):105‐10. [PubMed] [Google Scholar]
- Kim JH, Chu F, Houde R. A clinical study of benzydamine for the treatment of radiotherapy‐induced mucositis of the oropharynx. International Journal of Tissue Reaction 1985;VII(3):215‐8. [PubMed] [Google Scholar]; Kim JH, Chu FC, Lakshmi V, Houde R. Benzydamine HCl, a new agent for the treatment of radiation mucositis of the oropharynx. American Journal of Clinical Oncology 1986;9(2):132‐4. [DOI] [PubMed] [Google Scholar]
- Kostrica R, Rottenberg J, Kvech J, Betka J, Jablonicky P. Randomised, double‐blind comparison of efficacy and tolerability of diclofenac mouthwash versus placebo in mucositis of oral cavity by radiotherapy. Journal of Drug Assessment 2002;5(2):99‐113. [Google Scholar]
- Kuhn A, Porto FA, Miraglia P, Brunetto AL. Low‐level infrared laser therapy in chemotherapy‐induced oral mucositis: a randomized placebo‐controlled trial in children. Journal of Paediatric Hematological Oncology 2009;31(1):33‐7. [DOI] [PubMed] [Google Scholar]
- Ghosh C, Loprinzi CL, Camoriano J, Sloan J, Steen P, Michalak J, et al. Evaluation of sucralfate for alleviating 5FU induced stomatitis. Proceedings of Annual Meeting American Society for Clinical Oncology. 1996:Abs A1729. [Google Scholar]; Loprinzi CL, Ghosh C, Camoriano J, Sloan J, Steen PD, Michalak JC, et al. Phase III controlled evaluation of sucralfate to alleviate stomatitis in patients receiving fluorouracil‐based chemotherapy. Journal of Clinical Oncology 1997;15(3):1235‐8. [DOI] [PubMed] [Google Scholar]
- Mackie AM, Coda BC, Hill HF. Adolescents use patient‐controlled analgesia effectively for relief from prolonged oropharyngeal mucositis pain. Pain 1991;46(3):265‐9. [DOI] [PubMed] [Google Scholar]
- Malik IA, Moid I, Haq S, Sabih M. A double‐blind, controlled, randomized trial to evaluate the role of tetrachlorodecaoxide in the management of chemotherapy‐induced oral mucositis. Journal of Pain and Symptom Management 1997;14(2):82‐7. [DOI] [PubMed] [Google Scholar]
- Masucci G, Broman P, Kelly C, Lindahl S, Malmberg L, Reizenstein J, et al. Therapeutic efficacy by recombinant human granulocyte/monocyte‐colony stimulating factor on mucositis occurring in patients with oral and oropharynx tumors treated with curative radiotherapy: a multicenter open randomized phase III study. Medical Oncology 2005;22(3):247‐56. [DOI] [PubMed] [Google Scholar]
- Papila C, Papila I, Yanardag H, Cansiz H, Cagatay P. The use of granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) as a mouthwash in patients with chemotherapy‐induced oral mucositis. International Review of Allergology and Clinical Immunology 2003;9:1. [Google Scholar]
- Pillitteri LC, Clark RE. Comparison of a patient controlled analgesia system with continuous infusion for administration of parenteral diamorphine for mucositis. Bone Marrow Transplantation 1996;17(Suppl 1):S169. [DOI] [PubMed] [Google Scholar]; Pillitteri LC, Clark RE. Comparison of a patient‐controlled analgesia system with continuous infusion for administration of diamorphine for mucositis. Bone Marrow Transplantation 1998;22(5):495‐8. [DOI] [PubMed] [Google Scholar]; Pillitteri LC, Clark RE. Comparison of patient‐controlled analgesia with continuous diamorphine infusion for mucositis. Bone Marrow Transplantation 1998;21(1):S264. [DOI] [PubMed] [Google Scholar]
- Porta C, Moroni M, Nastasi G. Allopurinol mouthwashes in the treatment of 5‐fluorouracil‐induced stomatitis. American Journal of Clinical Oncology 1994;17(3):246‐7. [DOI] [PubMed] [Google Scholar]; Porta C, Moroni M, Nastasi G, Montecucco CM, Caporali R, Notario A. Allopurinol mouthwashes can reduce oral mucositis in chemotherapy‐treated patients. Annals of Oncology 1994;5(Suppl 8):208. [Google Scholar]
- Schedler MGJ, Bost P, Federspil P, Pautler M, Schatzle W. Treatment of radiogenic mucositis in patients with head and neck tumors with polyvalent intramuscular immunoglobulin [Die behandlung der strahleninduzierten mukositis bei kopf‐/halstumoren mit intramuskular verabreichtem polyvalentem immunoglobin]. Tumordiagnostik und Therapie 1994;15:184‐91. [Google Scholar]
- Schubert MM, Newton RE. The use of benzydamine HCI for the management of cancer therapy‐induced mucositis: preliminary report of a multicentre study. International Journal of Tissue Reaction 1987;IX(2):99‐103. [PubMed] [Google Scholar]
- Syrjala KL, Cummings C, Donaldson GW. Hypnosis or cognitive behavioral training for the reduction of pain and nausea during cancer treatment: A controlled clinical trial. Pain 1992;48(2):137‐46. [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive‐behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain 1995;63(2):189‐98. [DOI] [PubMed] [Google Scholar]
- Valcarcel D, Sanz MA Jr, Sureda A, Sala M, Munoz L, Subira M, et al. Mouth‐washings with recombinant human granulocyte‐macrophage colony stimulating factor (rhGM‐CSF) do not improve grade III‐IV oropharyngeal mucositis (OM) in patients with hematological malignancies undergoing stem cell transplantation. Results of a randomized double‐blind placebo‐controlled study. Bone Marrow Transplantation 2000;29(9):783‐7. [DOI] [PubMed] [Google Scholar]
- Wadleigh RG, Redman RS, Graham ML, Krasnow SH, Anderson A, Cohen MH. Vitamin E in the treatment of chemotherapy‐induced mucositis. The American Journal of Medicine 1992;92(5):481‐4. [DOI] [PubMed] [Google Scholar]
- Zucker TP, Flesche CW, Germing U, Schroter S, Willers R, Wolf HH, et al. Patient‐controlled versus staff‐controlled analgesia with pethidine after allogeneic bone marrow transplantation. Pain 1998;75(2‐3):305‐12. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Agüeros P, Bárcena M, Campo M, Lucero P, Bermudez A, Yanez L. Efficacy of vitamin E during acute mucositis in bone marrow transplantation. Bone Marrow Transplantation 2004;33(Suppl 1):S291. [Google Scholar]
- Allison RR, Vongtama V, Vaughan J, Shin KH. Symptomatic acute mucositis can be minimized or prophylaxed by the combination of sucralfate and fluconazole. Cancer Investigations 1995;13(1):16‐22. [DOI] [PubMed] [Google Scholar]
- Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, et al. Randomized phase II study of multiple dose levels of CCI‐779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. Journal of Clinical Oncology 2004;22(5):909‐18. [DOI] [PubMed] [Google Scholar]
- Awada A, Gil T, Sales F, Dubuisson M, Vereecken P, Klastersky J, et al. Prolonged schedule of temozolomide (Temodal) plus liposomal doxorubicin (Caelyx) in advanced solid cancers. Anticancer Drugs 2004;15(5):499‐502. [DOI] [PubMed] [Google Scholar]
- Barker G, Loftus L, Cuddy P, Barker B. The effects of sucralfate suspension and diphenhydramine syrup plus kaolin‐pectin on radiotherapy‐induced mucositis. Oral Surgery, Oral Medicine, Oral Pathology 1991;71(3):288‐93. [DOI] [PubMed] [Google Scholar]
- Beltran A, Ochoa Carrillo FJ, Ibieta BR, Fuentes EP, Zeichner I. Double blind controlled study with placebo to demonstrate the efficacy and safety of bencidamide mouth washes for radiation mucositis. Journal of Cancer Research and Clinical Oncology 1990;116 Suppl:639(Abs A5.280.12). 2254383 [Google Scholar]
- Biswal BM, Zakaria A, Nik Min A. Topical application of honey in the management of radiation mucositis: A randomised study. International Journal of Cancer 2002;13:480(Abs P1109). [Google Scholar]
- Biswal BM, Zakaria A, Ahmad NM. Topical application of honey in the management of radiation mucositis: a preliminary study. Supportive Care in Cancer 2003;11(4):242‐8. [DOI] [PubMed] [Google Scholar]
- Bondi E, Baroni C, Prete A, Gatti M, Carrassi A, Lodi G, et al. Local antimicrobial therapy of oral mucositis in paediatric patients undergoing bone marrow transplantation. Oral Oncology 1997;33(5):322‐6. [DOI] [PubMed] [Google Scholar]; Pession A, Prete A, Bondi E, Baroni C, Melchionda F, Riolo U, Paolucci G. Treatment of oral mucositis in children undergoing bone marrow transplantation: comparison between two different regimes. Bone Marrow Transplantation 1997;19(Suppl):S123(Abs P491). [Google Scholar]
- Carnel SB, Blakeslee DB, Oswald SG, Barnes M. Treatment of radiation‐ and chemotherapy‐induced stomatitis. Otolaryngology ‐ Head & Neck Surgery 1990;102(4):326‐30. [DOI] [PubMed] [Google Scholar]
- Cerchietti LC, Navigante AH, Bonomi MR, Zaderajko MA, Menendez PR, Pogany CE, et al. Effect of topical morphine for mucositis‐associated pain following concomitant chemoradiotherapy for head and neck carcinoma. Cancer 2002;95(10):2230‐6. [DOI] [PubMed] [Google Scholar]
- Cerchietti LC, Navigante AH, Bonomi MR, Zaderajko MA, Menendez PR, Pogany CE, et al. Effect of topical morphine for mucositis‐associated pain following concomitant chemoradiotherapy for head and neck carcinoma. Cancer 2002;95(10):2230‐6. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Welsh DV, Scrimger RA, Zehn W, Epstein JB, Troha J, et al. RK‐0202 for radiation‐induced oral mucositis. Journal of Clinical Oncology 2006;24:18S. [Google Scholar]
- Chapman CR, Donaldson GW, Jacobson RC, Hautman B. Differences among patients in opioid self‐administration during bone marrow transplantation. Pain 1997;71(3):213‐23. [DOI] [PubMed] [Google Scholar]
- Cheng KK. Children's acceptance and tolerance of chlorhexidine and benzydamine oral rinses in the treatment of chemotherapy‐induced oropharyngeal mucositis. European Journal of Oncology Nursing 2004;8(4):341‐9. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Geake J, Grier HE, Houck CS, Thaler HT, Weinstein HJ, et al. Patient‐controlled analgesia for mucositis pain in children: a three‐period crossover study comparing morphine and hydromorphone. Journal of Pediatrics 1996;129(5):722‐8. [DOI] [PubMed] [Google Scholar]
- Collova E, Castagna L, Nozza A, Perfetti V, Patrone F, Danova M, et al. New approaches for reduction of oral mucositis in patients undergoing high‐dose chemotherapy with hematopoietic stem cell support. Bone Marrow Transplantation 2004;33(Suppl 1):S323‐S4. [Google Scholar]
- Connor CA, Aston S, Fegan CD, Milligan DW. A comparison of patient controlled analgesia (PCA) versus conventional pain control for mucositis after bone marrow transplantation. Bone Marrow Transplantation 1996;17(Suppl):S161. [Google Scholar]
- Crawford J, Glaspy J, Vincent M, Tomita D, Mazanet R. Effect of filgrastim (r‐metHug‐CSF) on oral mucositis in patients with small cell lung cancer (SCLC) receiving chemotherapy (cyclophosphamide, doxorubicin and etoposide, CAE). Proceedings of the Annual Meeing of American Society for Clinical Oncology. 1994; Vol. 13:Abs A1523. [Google Scholar]
- Domenge C, Lefebvre JL, Esnault Y. Randomized study of fluconozole (FCA) oral suspension (OS) versus amphotericin b (ab) oral suspension in the treatment of oropharingeal mucositis in head and neck cancer patients (HNCP). European Journal of Cancer 1999;35 Suppl(4):161(Abs 603). 10211105 [Google Scholar]
- Ehrnrooth E, Grau C, Zachariae I, Andersen JW. A randomised trial of opioid versus tricyclic antidepressants for radiation induced mucositis pain in patients with head and neck cancer (Abstract). European Journal of Cancer 1999;35 Suppl(4):175(Abs 666). [DOI] [PubMed] [Google Scholar]
- Eren M, Akyuz C, Yalcin B, Varan A, Kutluk T, Buyukpamukcu M. Effectiveness of rhGM ‐ CSF mouthwashes on chemotherapy induced mucositis in childhood solid tumours [Cocukluk cagi kanserlerinde kemoterapi ile iliskili mukozit tedavisinde granulosit makrofaj koloni stimulan faktorum agiz bakimindaki yeri]. Interantional Journal of Hematology and Oncology 2007;17(2):70‐8. [Google Scholar]
- Evans G, Mahendra P, Brightwell M, Jestice K, Marcus RE. A double blind randomised trial of oral GM‐CSF mouthwashes versus placebo in the treatment of mucositis following PBSC / bone marrow transplantation. Bone Marrow Transplantation 1998;21 Suppl(1):S88. [Google Scholar]
- Evensen JF, Bjordal K, Jacobsen AB, Lokkevik E, Tausjo JE. Effects of Na‐sucrose octasulfate on skin and mucosa reactions during radiotherapy of head and neck cancers‐‐a randomized prospective study. Acta Oncologica 2001;40(6):751‐5. [DOI] [PubMed] [Google Scholar]
- Ferretti G, Lillich T, Raybould T, Ash R, Henslee J, MacDonald J, et al. Effect of chlorhexidine on the oral complications of patients receiving chemotherapy. Proceedings of Annual Meeting of American Society for Cinical Oncology. 1987; Vol. 6:268(Abs 1053). [Google Scholar]
- Foote RL, Loprinzi CL, Gulavita S, Sloan J, Earle J, Burk M, et al. Randomised trial of a nonabsorbable antibiotic lozenge for alleviation of radiation‐induced mucositis. Proceedings of Annual Meeting American Society of Clinical Oncology. 1996:(Abs A1728). [Google Scholar]
- Giles FJ, Hurd DD, Rodriguez R, Weisdorf DJ, Martin PJ, Wingard JR, et al. A phase III, randomized, placebo‐controlled, double‐blind, multicenter study (RPCDBM) of iseganan HCL oral solution (Iseganan) in reducing severity of oral mucositis (OM) in patients receiving stomatotoxic chemotherapy (CT). Blood 2002;100(11):(Pt 2). [Google Scholar]
- Giles FJ, Rodriguez R, Weisdorf DJ, Wingard JR, Martin PJ, Fleming TR, et al. A phase III, randomized, double‐blind, placebo‐controlled, study of iseganan for the reduction of stomatitis in patients receiving stomatotoxic chemotherapy. Leukemia Research 2004;28(6):559‐65. [DOI] [PubMed] [Google Scholar]
- Girdler NM, McGurk M, Aqual S, Prince M. The effect of epidermal growth factor mouthwash on cytotoxic‐induced oral ulceration. A phase I clinical trial. American Journal of Clinical Oncology 1995;18(5):403‐6. [DOI] [PubMed] [Google Scholar]
- Gobetti JP. Comparison of two commercial products in relieving radiation mucositis discomfort (IADR Abstract 1999). Journal of Dental Research 1999;March Special Issue:518(Abs 3301). [Google Scholar]
- Haritha C, Shankar V, Patel MS. Oral morphine gargles: a cost effective approach for pain relief in patients with chemoradiation induced acute oral mucositis in head and neck cancers. Journal of Clinical Oncology 2009;27(15S):Abstract number e 20504. [Google Scholar]
- Hejna M, Kostler W, Raderer M, Steger G, Brodowicz T, Scheithauer W, et al. A prospective randomized trial on the efficacy in GM‐CSF mouthwashes for the treatment of chemotherapy‐ induced oral mucositis. Proceedings of the Annual Meeting of American Society of Clinical Oncology. 2000; Vol. 19:(Abs 2407). [Google Scholar]
- Kin‐Fong Cheng K, Ka Tsui Yuen J. A pilot study of chlorhexidine and benzydamine oral rinses for the prevention and treatment of irradiation mucositis in patients with head and neck cancer. Cancer Nursing 2006;29(5):423‐30. [DOI] [PubMed] [Google Scholar]
- Kostler WJ, Hejna M, Zielinski CC. Palifermin and chemotherapy‐induced oral mucositis. New England Journal of Medicine 2005;352(12):1264‐5. [PubMed] [Google Scholar]
- Labbate R, Lehn CN, Denardin OVP. Effects of chlorhexidine mouthwash on radiation induced mucosistis in head and neck cancer. Revista Brasileira de Otorrinolaringologia 2003;69(3):349‐54. [Google Scholar]
- Lever SA, Dupuis LL, Chan HS. Comparative evaluation of benzydamine oral rinse in children with antineoplastic‐induced stomatitis. Drug Intelligence for Clinical Pharmacology 1987;21(4):359‐61. [DOI] [PubMed] [Google Scholar]
- Lilleby K, Garcia P, Gooley T, McDonnnell P, Taber R, Holmberg L, et al. A prospective, randomized study of cryotherapy during administration of high‐dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplantation 2006;37(11):1031‐5. [DOI] [PubMed] [Google Scholar]
- Lockhart PB, Brennan MT, Kent ML, Packman CH, Norton HJ, Fox PC, et al. Randomized controlled trial of pilocarpine hydrochloride for the moderation of oral mucositis during autologous blood stem cell transplantation. Bone Marrow Transplantation 2005;35(7):713‐20. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Massa E, Astara G, Murgia V, Gramignano G, Lusso MR, et al. Phase II clinical trial of local use of GM‐CSF for prevention and treatment of chemotherapy‐ and concomitant chemoradiotherapy‐induced severe oral mucositis in advanced head and neck cancer patients: an evaluation of effectiveness, safety and costs. Oncology Reports 2003;10(1):197‐206. [PubMed] [Google Scholar]
- Marinoni L, Galletti C, Caruana S, Saccomando A, Castelli N, et al. Treatment of mucositis‐related pain in patients undergoing CPC transplantation. Bone Marrow Transplantation 1996;17(Suppl):S168. [Google Scholar]
- Meredith R, Salter M, Kim R, Spencer S, Weppelmann B, Rodu B, et al. Sucralfate for radiation mucositis: results of a double‐blind randomized trial. International Journal of Radiation Oncology, Biology, Physics 1995;32(S1):285. [DOI] [PubMed] [Google Scholar]; Meredith R, Salter M, Kim R, Spencer S, Weppelmann B, Rodu B, et al. Sucralfate for radiation mucositis: results of a double‐blind randomized trial. International Journal of Radiation Oncology, Biology, Physics 1997;37(2):275‐9. [DOI] [PubMed] [Google Scholar]
- Mitrokhin SD. [Itraconazole (Orungal) in the treatment of mycotic infections in oncological patients]. Antibiotiki i Khimioterapiia 2003;48(2):16‐21. [PubMed] [Google Scholar]
- Naidu MU, Ramana GV, Ratnam SV, Sudhavani T, Naidu KJ, Roy P, et al. A randomised, double‐blind, parallel, placebo‐controlled study to evaluate the efficacy of MF 5232 (Mucotrol), a concentrated oral gel wafer, in the treatment of oral mucositis. Drugs in R&D 2005;6(5):291‐8. [DOI] [PubMed] [Google Scholar]
- Oguchi M, Shikama N, Sasaki S, Gomi K, Katsuyama Y, Ohta S, et al. Mucosa‐adhesive water‐soluble polymer film for treatment of acute radiation‐induced oral mucositis. International Journal of Radiation Oncology, Biology, Physics 1998;40(5):1033‐7. [DOI] [PubMed] [Google Scholar]
- Oshitani T, Okada K, Kushima T, Suematsu T, Obayashi K, Hirata Y, et al. [Clinical evaluation of sodium alginate on oral mucositis associated with radiotherapy]. Nippon Gan Chiryo Gakkai Shi 1990;25(6):1129‐37. [PubMed] [Google Scholar]
- Papila C, Paplia I, Resuli A, Koksal S. Efficacy of sucralfate in oral mucositis induced by systemic chemotherapy with 5‐fluorouracil. Journal of BUON 1999;4:321‐3. [Google Scholar]
- Pession A, Prete A. Treatment of oral mucositis in children undergoing bone marrow transplantation: comparison between two different regimen. Bone Marrow Transplantation 1997;19 Suppl:S123(Abs P489). [Google Scholar]
- Pouli A, Delistrati V, Giannakaki A, Kostelidou D, Pagoni M, Karakasis D, et al. A prospective randomized trial of GM‐CSF mouthwash versus sodium bicarbonate mouthwash in the treatment of stomatitis following allogeneic bone marrow transplantation. Bone Marrow Transplantation 1999;23(Suppl 1):S166. [Google Scholar]
- Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiotherapy & Oncology 2004;70(3):261‐4. [DOI] [PubMed] [Google Scholar]
- Radmard A, Niewohner‐Desbordes U, Wagner W. [rhGM‐CSF in the treatment of radiogenic mucositis: prospective randomised phase‐II study on the effectivity and tolerance of rhGM‐CSF in the treatment of oral mucositis under radiotherapy of head and neck tumors]. Strahlentherapie und Onkologie 2002;178(Suppl 1):24. [Google Scholar]
- Rothwell BR, Spektor WS. Palliation of radiation‐related mucositis. Special Care in Dentistry 1990;10(1):21‐5. [DOI] [PubMed] [Google Scholar]
- Schmid I, Schmitt M, Streiter M, Meilbeck R, Albert MH, Reinhardt D, et al. Parenteral nutrition is not superior to replacement fluid therapy for the supportive treatment of chemotherapy induced oral mucositis in children. European Journal of Cancer 2006;42(2):205‐11. [DOI] [PubMed] [Google Scholar]
- Schwerkkoske J, Schwartzberg L, Weaver CH, Schwertschlag US, Goodfellow J, Berdosian CL. A phase I double‐masked, placebo‐controlled study to evaluate tolerability of Neumega (rhIL‐11; Oprelvekin) to reduce mucositis in patients with solid tumors or lymphoma receiving high‐dose chemotherapy (CT) with autologous peripheral blood stem cell reinfusion (PBSCT). Proceedings of the American Society of Clinical Oncology. 1999; Vol. 18:584a. [Google Scholar]
- Shaiova L, Lapin J, Manco LS, Shasha D, Hu K, Harrison L, et al. Tolerability and effects of two formulations of oral transmucosal fentanyl citrate (OTFC; ACTIQ) in patients with radiation‐induced oral mucositis. Supportive Care in Cancer 2004;12(4):268‐73. [DOI] [PubMed] [Google Scholar]
- She H, Ding Z, Guo QM, Cheng BQ. Prevention and treatment of oral mucositis caused by radiotherapy in patients with nasopharyngeal carcinoma. Journal of Ningxia Medical College 2000;22(2):99‐100. [Google Scholar]
- Shen YF, Ma BL, Huang H. Effect of vitamin B12 and dexasone on acute radiation stomatitis. Chinese New Medicine 2004;5(6):509‐10. [Google Scholar]
- Sprinzl GM, Galvan O, Vries A, Ulmer H, Gunkel AR, Lukas P, et al. Local application of granulocyte‐macrophage colony stimulating factor (GM‐CSF) for the treatment of oral mucositis. European Journal of Cancer 2001;37(16):2003‐9. [DOI] [PubMed] [Google Scholar]
- Stokman MA, Wachters FM, Koopmans P, Burgerhof JG, Groen HJ, Spijkervet FK, et al. Outcome of local application of amifostine (WR‐1065) on epirubicin‐induced oral mucositis. A phase II study. Anticancer Research 2004;24(5B):3263‐7. [PubMed] [Google Scholar]
- Su YB, Vickers A, Zelefsky MJ, Kraus DH, Shaha AR, Shah JP, et al. Double‐blind, randomized trial of granulocyte‐colony stimulating factor (GCSF) versus (v.) placebo during postoperative radiation (RT) for advanced resectable squamous cell head and neck cancer (SCCHN): impact on mucositis. Journal of Clinical Oncology 2004;22(Suppl):14S. [Google Scholar]
- Svanberg A, Öhrn K. Cryotherapy during chemotherapy ‐ could it delay or alleviate the development of mucositis?. Bone Marrow Transplantation 2004;33(Suppl 1):S292. [Google Scholar]
- Teshima T. [Clinical evaluation of kenalog ointment for radiation mucositis of patients with head and neck cancer]. Yakuri to Chiryo (Japanese Pharmacology and Therapeutics) 1986;14(11):7163‐6. [Google Scholar]
- Valcarcel D, Sanz M, Sureda A, Sala M, Munoz L, Subira M, et al. Topically applied recombinant human granulocyte‐macrophage colony stimulating factor (rhGM‐CSF) for oropharyngeal mucositis (OM) in stem cell transplantation recipients: a randomized double‐blind placebo‐controlled study. Bone Marrow Transplantation 2002;(Suppl 2):S20. [DOI] [PubMed] [Google Scholar]
- Vayne‐Bossert P, Escher M, Vautibault CG, Dulguerov P, Allal A, Desmeules J, et al. Effect of topical morphine (mouthwash) on oral pain due to chemotherapy‐ and/or radiotherapy‐induced mucositis: a randomized double‐blinded study. Journal of Palliative Medicine 2010;13(2):125‐8. [DOI] [PubMed] [Google Scholar]
- Vela‐Ojeda J, Tripp‐Villaneuva F, Sanchez‐Cortes E, Gonzales‐Llaven J. Topical oral granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) for the treatment of mucositis after bone marrow transplantation. Blood 1996;88(10):253b. [Google Scholar]
Additional references
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
- Bellm LA, Cunningham G, Durnell L, Eilers J, Epstein JB, Fleming T. Defining clinically meaningful outcomes in the evaluation of new treatments for oral mucositis: oral mucositis patient provider advisory board. Cancer Investigations 2002;20(5‐6):793‐800. [DOI] [PubMed] [Google Scholar]
- Clarkson JE, Worthington HV, Eden OB. Interventions for preventing oral candidiasis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2007, Issue 1. [Art. No.: CD003807. DOI: 10.1002/14651858.CD003807.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauw BE. Practical modalities for prevention of fungal infections in cancer patients. European Journal of Clinical Microbiology & Infectious Diseases 1997;16(1):32‐41. [DOI] [PubMed] [Google Scholar]
- Denning DW, Donnelly JP, Hellreigel KP, Ito J, Martino P, van't Wout JW. Antifungal prophylaxis during neutropenia or allogeneic bone marrow transplantation: what is the state of the art? Ad HOC Working Group. Chemotherapy 1992;38 Suppl(1):43‐9. [DOI] [PubMed] [Google Scholar]
- Donnelly JP, Bellm LA, Epstein JB, Sonis ST, Symonds RP. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infectious Diseases 2003;3(7):405‐12. [DOI] [PubMed] [Google Scholar]
- Fung SM, Ferrill MJ. Granulocyte macrophage‐colony stimulating factor and oral mucositis. Annals of Pharmacotherapy 2002;36(3):517‐20. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
- Knox JJ, Puodziunas AL, Feld R. Chemotherapy‐induced oral mucositis. Prevention and management. Drugs Aging 2000;17(4):257‐67. [DOI] [PubMed] [Google Scholar]
- Kowanko I, Long L, Hodgkinson B, Evans D. The effectiveness of strategies for preventing and treating chemotherapy and radiation induced oral mucositis in patients with cancer. 1‐84. Adelaide, S. Australia, Australia: Joanna Briggs Institute for Evidence Based Nursing and Midwifery, 1998. [Google Scholar]
- Lortholary O, Dupont B. Antifungal prophylaxis during neutropenia and immunodeficiency. Clinical Microbiology Reviews 1997;10(3):477‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Annals of Internal Medicine 2001;134(8):657‐62. [DOI] [PubMed] [Google Scholar]
- Plevova P. Prevention and treatment of chemotherapy‐ and radiotherapy‐induced oral mucositis: a review. Oral Oncology 1999;35(5):453‐70. [DOI] [PubMed] [Google Scholar]
- Shaw MJ, Kumar ND, Duggal M, Fiske J, Lewis DA, Kinsella T. Oral management of patients following oncology treatment: literature review. British Journal of Oral & Maxillofacial Surgery 2000;38(5):519‐24. [DOI] [PubMed] [Google Scholar]
- Shih A, Miaskowski C, Dodd MJ, Stotts NA, MacPhail L. A research review of the current treatments for radiation‐induced oral mucositis in patients with head and neck cancer. Oncology Nursing Forum 2002;29(7):1063‐80. [DOI] [PubMed] [Google Scholar]
- Stevens DA. Therapy for opportunistic fungal infections: past, present and future. Indian Journal of Cancer 1995;32(1):1‐9. [PubMed] [Google Scholar]
- Sutherland SE, Browman GP. Prophylaxis of oral mucositis in irradiated head‐and‐neck cancer patients: a proposed classification scheme of interventions and meta‐analysis of randomized controlled trials. International Journal of Radiation Oncology, Biology, Physics 2001;49(4):917‐30. [DOI] [PubMed] [Google Scholar]
- Symonds RP. Treatment‐induced mucositis: an old problem with new remedies. British Journal of Cancer 1998;77(10):1689‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiotherapy Oncology 2003;66(3):253‐62. [DOI] [PubMed] [Google Scholar]
- Verdi CJ. Cancer therapy and oral mucositis. Drug Safety 1993;9(3):185‐95. [DOI] [PubMed] [Google Scholar]
- White MH. Antifungal prophylaxis. Current Opinion in Infectious Diseases 1993;6(6):737‐43. [Google Scholar]
- Worthington HV, Clarkson JE, Khalid T, Meyer S, McCabe M. Interventions for treating oral candidiasis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2010, Issue 7. [Art. No.: CD001972. DOI: 10.1002/14651858.CD001972.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington HV, Clarkson JE, Eden OB. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2007, Issue 2. [Art. No.: CD000978. DOI: 10.1002/14651858.CD000978.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Worthington HV, Clarkson JE, Eden OB. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2004, Issue 2. [Art. No.: CD001973. DOI: 10.1002/14651858.CD001973.pub2] [DOI] [PubMed] [Google Scholar]


