Abstract
Background/Aims
Natural killer (NK) cells provide early defense against viral infections by killing infected cells and producing cytokines that inhibit viral replication. NK cells also interact with dendritic cells (DCs) and this reciprocal interaction regulates both innate and adaptive immunity. Genetic studies have suggested that NK cell activity is a determinant of HCV infectious outcome but a functional correlation was not established. We hypothesized that increased NK cell activity during acute HCV infection will correlate with spontaneous viral clearance.
Methods
We used multiparametric flow cytometry to monitor longitudinally the phenotype and activity of NK cells in a cohort of intravenous drug users following HCV exposure. Three groups were studied: acute HCV with chronic evolution (n=13); acute resolving HCV (n=11); and exposed un-infected individuals (n=10). We examined the expression of several NK cell activating and inhibitory receptors, IFN-γ production and CD107a degranulation upon stimulation and the kinetics of NK cell responses relative to T cell responses.
Results
We observed decreased expression of the inhibitory NKG2A receptor on NK cells following spontaneous HCV clearance. In addition, we’ve observed increased NK cell degranulation during acute HCV irrespective of infectious outcome. NK cells peak responses preceded or coincided with peak T cell responses. Furthermore, NK cell degranulation correlated with the magnitude of HCV-specific T cells.
Conclusions
Our results demonstrate that NK cells are activated during acute HCV regardless of infection outcome and may play an indirect role through induction and priming of T cell responses.
Keywords: Innate immunity, Viral hepatitis, cytotoxicity, cytokines
Introduction
The majority of individuals exposed to hepatitis C virus (HCV) develop persistent infection and chronic liver disease [1]. Acute HCV is characterized by a significant delay in the onset of adaptive T cell responses despite active viral replication. This suggests a failure of innate immunity to contain viral replication and provide the necessary signals to prime efficient adaptive immunity critical to spontaneous viral clearance [2, 3]. Natural killer (NK) cells are the most important effector population of the innate immune response. Two NK cell subsets can be distinguished based on their differential expression of CD56 and CD16: immunoregulatory CD3−CD56brightCD16− and cytolytic CD3−CD56dimCD16+ [4]. NK cells provide an early defense line against viral infections by killing infected cells and producing cytokines that can directly inhibit viral replication and trigger the adaptive immune response. NK cells use inhibitory and activation receptors as a mean of controlling their activity. NK cells interact with dendritic cells (DCs) and this reciprocal interaction results in regulation of both innate and adaptive immune responses [5, 6]. DCs can activate NK cells by binding to NKp30 on the surface of NK cells and by secreting numerous cytokines such as IL-12 [7]. In return, NK cells secrete IFN-γ and TNF-α which induce DC maturation and trigger the adaptive immune response [8]. In addition, NK cells can also kill immature DCs and inhibit their capacity to prime or tolerize adaptive T cell responses [5, 9].
Two observations highlighted the potential role of NK cells during the early phase of HCV infection. First, HCV surface glycoprotein E2 can bind CD81 on the surface of NK cells and inhibit cytotoxicity and IFN-γ production [10, 11], but Yoon et al. have recently demonstrated that exposure of NK cells from healthy donors to in vitro-produced HCV virions did not influence their function [12]. Second, genes encoding the inhibitory NK cell receptor killer-cell immunoglobulin like receptor (KIR)2DL3 and its human leukocyte antigen C group 1 (HLA-C1) ligand directly influence resolution of HCV infection in individuals homozygous for these genes [13, 14]. These observations suggest that inhibition of NK function during the early phase of HCV may contribute to viral persistence.
Several groups have studied NK cells during chronic HCV infection but the results were conflicting regarding NK cell frequency, cytotoxicity, cytokine production and receptor expression [15-20]. This probably reflects the complexity of activation and inhibitory signals that control NK cells. Only one study has compared NK cell function in chronic HCV patients with spontaneous resolvers from a single source outbreak [15]. They demonstrated that the frequency of the CD56dim NK cell subset was decreased in individuals with chronic HCV and that NK cells expressed higher frequency of the NKG2A/C/E receptors [15] but the activity of NK cells during acute HCV, where their role would be most prominent, and its correlation with infectious outcome was not studied.
In this study, we used multiparametric flow cytometry to monitor longitudinally the phenotypic and functional changes in NK cells from a unique cohort of intravenous drug users (IDUs) at high risk of HCV infection before and during acute HCV infections that progressed to spontaneous resolution or viral persistence. In addition, we monitored NK cells activity in a group of HCV-exposed but uninfected individuals. We demonstrate that NK cell degranulation is increased during acute HCV regardless of infection outcome. We also observed a decline in NKG2A expression on NK cells following spontaneous viral clearance and CD161 expression in infections progressing to chronicity. Finally, we show that NK cell response peak prior to T cell responses and that NK cell degranulation correlates with the magnitude of the HCV-specific T cell response suggesting an indirect role for NK cells in priming adaptive immune responses.
Patients and Methods
Study subjects and clinical follow-up
A total of 34 HCV exposed individuals and 10 normal donors were included in this study. HCV acutely infected subjects were recruited among high-risk IDUs participating in the Montreal Acute HepC cohort study (HEPCO) [21], the methadone treatment and the Hepatology clinics at St-Luc hospital of the Centre Hospitalier de l’Université de Montréal (CHUM). This study was approved by the institutional ethics committee (Protocols # SL05.014 and SL05.025) and conducted according to the Declaration of Helsinki. All participants signed informed consent upon enrolment. Acute HCV infection (n=24) was defined as detection of positive HCV RNA and/or HCV antibodies following a previous negative test in the past 6 months, or positive HCV RNA with concomitant negative HCV antibodies tests. The mean follow-up interval between the last aviremic and the first viremic time point was 12 weeks (range: 1-25 weeks), and the estimated time of infection was defined as the median between the last aviremic and the first viremic time point. Duration of infection was defined as the time (in weeks) post estimated time of infection. Spontaneous viral resolution (n=11) or persistent infection (n=13) was defined as the absence or presence of HCV RNA at 12 weeks post enrolment. This classification is due to recent Canadian guidelines that recommend IFN therapy to all HCV acutely infected patients if they remain HCV positive by week 12 [22]. All patients included in this study were either ineligible or refused IFN therapy. Their classification as acute or chronic did not change whether they were classified at week 12 or 24. Exposed un-infected (n=10) are IDUs who have admitted sharing a needle or injection materials with an HCV infected individual but remained HCV RNA and HCV antibody negative. In this study, three time points were analyzed for each patient representing three phases of HCV infection: Pre-infection baseline, acute HCV and follow-up. Baseline was defined as time before HCV infection or reported needle sharing for exposed un-infected (range: −1 to −22 weeks; mean −10 weeks). Baseline samples were available for 8 chronic patients, 5 spontaneous resolvers and 5 exposed un-infected. Acute HCV was defined as 12 weeks (range: 6 to 17 weeks) post estimated time of infection for HCV infected individuals and 4 weeks (range: 0 to 12 weeks) after needle sharing for exposed un-infected. The follow-up time point was defined as 52 weeks (range: 34 to 62 weeks) post estimated time of infection for HCV infected individuals or 36 weeks (range: 20 to 56 weeks) after needle sharing for exposed un-infected. All patients tested negative for human immunodeficiency virus (HIV) and hepatitis B virus (HBV).
HCV RNA testing and quantification
Qualitative HCV RNA tests were performed using an automated COBAS AmpliPrep/COBAS Amplicor HCV test, version 2.0 (sensitivity, 50 IU/ml) (Roche Molecular Systems, Inc., Branchburg, NJ). HCV genotyping was done using standard sequencing for the NS5B region, and was performed by the Laboratoire de Santé Publique du Québec (Ste-Anne-de-Bellevue, QC, Canada) as part of the clinical follow-up of patients. Additional HCV RNA quantification was performed using an in-house quantitative real-time reverse transcription-PCR assay as previously described [23].
Flow cytometry antibodies and reagents
Directly conjugated antibodies against the following molecules were used: CD3-Pacific Blue (clone UCHT1), CD16-allophycocyanin (APC)-Cy7 (clone 3G8) or CD16-APC-H7 (clone 3G8), CD56- phycoerythryin (PE)-Cy7 (clone B159), CD107a-PE-Cy5 (clone H4A3), CD158a-fluorescein isothiocyanate (FITC) (clone HP-3E4), , CD158b-FITC (clone CH-L), CD161-PE-Cy5 (clone DX12), NKB1-FITC (clone DX9), NKG2D-APC (clone 1D11), NKp30-Alexa 647 (clone P30-15), and IFN-γ-APC (clone B27) (all from BD Biosciences, San Jose, CA, USA); CD69-PE-Texas Red (ECD) (clone TP1-55-3), and NKp44-PE (clone Z231) (both from Beckman Coulter, Marseille, France); NKG2A-PE (clone #131411) (from R&D Systems, Minneapolis, MN, USA). Live cells were identified using an Aqua Live/Dead fixable dead cell Stain Kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s protocol. We’ve used four phenotypic panels and one functional panel. “Fluorescence minus one” control stains were used to determine background levels of staining. Multiparameter flow cytometry was performed using a standard BD LSR II instrument equipped with blue (488 nm), red (633 nm), and violet (405 nm) lasers (BD Biosciences,) to systematically perform seven-color staining using FACSDiva software (BD Biosciences). Compensation was performed with single fluorochromes and BD CompBeads (BD Biosciences). Data files were analyzed using FlowJo software, version 8.6.3 for Mac (Tree Star, Inc., Ashland, OR).
Multiparametric phenotypic characterization of NK cells
All flow cytometry assays were performed on cryopreserved samples. For phenotypic analysis, 1-2 × 106 peripheral blood mononuclear cells (PBMCs) were stained with surface antibodies for 30 min at 4°C and washed twice in fluorescence-activated cell sorting (FACS) buffer (1× phosphate-buffered saline [PBS], 1% fetal bovine serum [FBS], 0.02% NaN3), and fixed in FACS Fix buffer (1× PBS, 1% formaldehyde).
Intracellular cytokine staining (ICS) and CD107a degranulation assay
2 × 106 PBMCs were incubated with anti-CD107a antibody and either culture media as a negative control or K562 leukemia target cell line (ATCC, Manassas, VA, USA) at 37°C in R-10 medium (RPMI medium [Invitrogen, Carlsbad, CA, USA] supplemented with 10% FBS). Following 1 h of stimulation, 10 μg/ml of brefeldin A (Sigma-Aldrich, St-Louis, MO, USA) and 6 μg/ml of monensin sodium salt (Sigma-Aldrich) were added, and cells were incubated for a total of 6 h. Cells were washed with FACS buffer, stained for viability and cell surface antigens, then permeabilized using BD Cytofix/Cytoperm solution (BD Bioscience). Cells were then stained with anti-IFN-γ antibody for 30 min, washed twice in BD Perm/Wash buffer (BD Biosciences), and fixed in FACS fix buffer. For analysis, cells were gated on viable CD3− CD56birght CD16− and CD3−CD56dimCD16+/lo NK cells (Figure 1A). Percent specific expression is calculated as the background-adjusted function in the presence or absence of target cell line.
Figure 1. No change in frequency of CD56dimCD16+ NK cells following HCV-exposure.
A) Strategy for gating on the two NK cell subsets by flow cytometry: viable lymphocytes CD3−CD56dimCD16+ and CD3−CD56brightCD16−. Frequency of B) CD3−CD56dimCD16+ NK cells and C) CD3−CD56brightCD16− NK cells was determined ex-vivo in patients with HCV chronic evolution (•), HCV spontaneous resolution (Δ), exposed un-infected (■) and healthy donors (○). The acute phase of HCV infection is represented by the shaded area. Mean is represented by a horizontal bar. *p < 0.05; **p < 0.01; ***p < 0.001. 2-way ANOVA (repeated measures) or 1-way ANOVA (comparison with healthy donors).
IFN-γ ELISPOT
HCV-specific T cell responses were measured 3 weeks (range: 0 to 14 weeks) after the NK cell responses were measured (12 weeks; range: 6 to 17 weeks after estimated time of infection). 96-well polyvinylidene diflouride-backed microtiter plates (Millipore, Bedford, MA, USA) were pre-wet with 35% ethanol (15 μl/well) for one minute, washed with PBS and coated overnight at 4°C with 100 μl/well (3 μg/ml) of anti-IFN-γ capture mAb (BD Biosciences). Plates were washed with PBS and blocked for 2 hours at 37°C with 200 μl/well of R-10 medium. Cryo-preserved PBMCs were thawed quickly in a 37°C water bath and washed in R-10 medium. 2 × 105 PBMCs/well were stimulated in duplicate with the various peptides pools at a final concentration of 3 μg/ml of each peptide in AIM-V-HS medium (AIMV complete medium [Invitrogen], 2% Human Serum AB [Wisent, Saint-Bruno, QC, Canada]) for 36 h at 37°C, 5% CO2. Patients were stimulated with 11 peptide pools spanning the entire HCV polyprotein and corresponding to HCV genotype 1a (H77 sequence) or genotype 3a (K3a/650 sequence), according to patient’s infecting HCV genotype. Patients infected with other HCV genotypes as well as exposed un-infected were stimulated with peptides corresponding to HCV genotype 1a (H77 sequence). Peptides were obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources, Manassas, VA, USA). At the end of the incubation period, plates were washed with PBS-T (PBS, 0.05% Tween-20) then incubated with biotinylated anti-IFN-γ antibody (clone 250 4S.B3) (BD Biosciences) at 0.5 μg/ml in PBS/0.5% BSA for 2 h at room temperature. Plates were washed and incubated with Streptavidin-Alkaline Phosphatase conjugate (Bio-Rad Laboratories, Hercules, CA, USA) (1:1000) in PBS/0.5% BSA for 1 hour at room temperature. Spots were developed using Alkaline Phosphatase conjugate substrate kit (Bio-Rad Laboratories) for 5 minutes and stopped using tap water. Spots were counted using an Immunospot Analyzer Instrument (Cellular Technology Ltd [CTL], Shaker Heights, OH, USA). PBMCs incubated with media alone served as negative controls. Positive controls were: PMA (phorbol myristate acetate [Sigma-Aldrich]) 50 ng/ml – Ionomycin (Sigma-Aldrich) 1 μg/ml and a control peptide pool CEF (NIH AIDS Research and Reference Reagent Program, Germantown, MD, USA) at 0.5 μg/ml. Specific Spot forming cells (SFC) were calculated as (mean number of spots in test wells-mean number of spots in media control wells) and normalized to SFC/106 PBMCs. A response was scored positive if greater than 50 SFC/106 PBMCs.
Statistical analysis
Comparisons between patient groups with HCV chronic evolution, spontaneous resolution and exposed un-infected, during baseline, acute and follow-up phases of infection, were evaluated by 2-way ANOVA (repeated measures). Data were analyzed with SigmaStat 3.5 for Windows (Systat Software, Inc., Chicago, IL, USA). Comparisons between HCV chronic evolution, spontaneous resolution, exposed un-infected and healthy donors were evaluated by 1-way ANOVA. Correlations were evaluated by Pearson’s test if data passed normality test or by Spearman’s test if data did not pass normality. Comparisons between groups of patients bearing certain KIR and HLA genes were evaluated by the two-tailed t test for independent samples, or 1-way ANOVA if more than 2 groups. Data were analyzed with GraphPad Prism 5.02 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Acute HCV infection is associated with increased NK cell degranulation
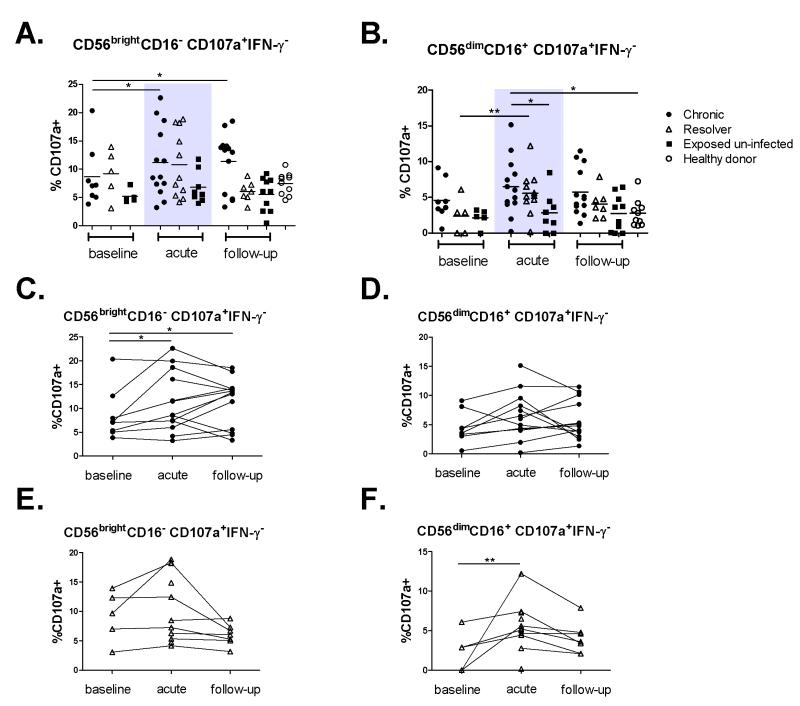
Thirty-four HCV exposed IDUs and 10 normal healthy donors were studied. As described in Materials and Methods, three patient groups were identified based on outcome following HCV exposure: a) Patients developing acute HCV with chronic evolution (n=13); b) Patients with acute resolving HCV (n=11) and c) HCV-exposed un-infected who remained HCV RNA and anti-HCV antibody negative (n=10). Patients’ demographics and characteristics are listed in Table 1. First, we monitored longitudinally the frequency of the two NK cell subsets CD56dimCD16+ and CD56brightCD16−. NK cell gating strategy by flow cytometry is shown in Figure 1A. The frequency of the CD56dimCD16+ NK cell subset was decreased in all patient groups as compared to healthy donors (Figure 1B) but it did not change significantly within each group relative to the phase of HCV infection. Similarly, no changes were observed in the frequency of CD56brightCD16− NK cell subset following HCV exposure in any of the groups studied (Figure 1C). Next, we studied the functional capacity of NK cells following HCV exposure. We monitored the cytokine production and cytotoxic potential of the two NK cell subsets by monitoring IFN-γ production by ICS and CD107a expression, a degranulation marker that is indicative of the cytotoxicity of NK cells [24] in response to co-culture with the classical NK cell target K562 cells. Representative staining and gating strategy are presented in supplementary Figure S1. The frequency of total CD107a and IFN-γ positive NK cells, as well as NK cells single positive for either CD107a or IFN-γ or double positive for both functions was evaluated. We did not observe any changes in the frequency of total degranulating (%CD107a+) NK cells between patient groups or time points (Figure S2). In contrast, we observed increased degranulation of the CD56brightCD16− subset in chronics during acute HCV and follow-up as compared to baseline (Figures 2A). Similarly, there was a trend towards increased degranulation of the CD56dimCD16+ subset in the same patients. Such increase was statistically significant when compared to exposed uninfected and healthy donors (Figure 2B). Furthermore, we observed a statistically significant increase in degranulation of CD56dimCD16+ in spontaneous resolvers during acute HCV as compared to their baseline (Figures 2B). When the results were stratified by patient, we continued to observe increased degranulation for the majority of individual patients with HCV chronic evolution (Figures 2C and 2D) and spontaneous resolution (Figures 2E and 2F). Altogether, these results demonstrate increased NK cell degranulation during acute HCV infection regardless of infection outcome.
Table 1. Demographics and Characteristics of patients and donors.
| n | Gender | Age | Race | Genotype | |
|---|---|---|---|---|---|
| (% M) | (median yr) | (Caucasian) | 1/2/3 | ||
| HCV chronic evolution | 13 | 92% | 32 | 11/13 | 2/0/8 |
| HCV spontaneous resolution | 11 | 63% | 27 | 8/11 | 1/2/4 |
| Exposed un-infected | 10 | 70% | 39 | 9/10 | N/A |
| Healthy donors | 10 | 60% | 31 | 7/10 | N/A |
Figure 2. Acute HCV infection is associated with increased NK cell degranulation regardless of infection outcome.
Degranulation was measured by CD107a surface staining in patients with HCV chronic evolution (•), HCV spontaneous resolution (Δ), exposed un-infected (■) and healthy donors (○). PBMCs were co-incubated with or without K562 target cells. Background expression of CD107a was subtracted from expression with target cells. A) Frequency of CD107a+IFN-γ− cells gated on CD56brightCD16− NK cells and B) CD56dimCD16+ NK cells. C) Longitudinal changes in frequency of CD107a+IFN-γ− cells represented for each individual patient in the HCV chronics group (•) gated on CD56brightCD16− NK cells and D) CD56dimCD16+ NK cells. For each individual patient, different infection time points are joined by a line. E) Longitudinal changes in frequency of CD107a+IFN-γ− cells in individual patients in the spontaneous resolvers group (Δ) gated on CD56brightCD16− NK cells and F) CD56dimCD16+ NK cells. For each individual patient, different infection time points are joined by a line. The acute phase of HCV infection is represented by the shaded area. Mean is represented by a horizontal bar. *p < 0.05; **p < 0.01; ***p < 0.001. 2-way ANOVA (repeated measures) or 1-way ANOVA (comparison with healthy donors).
Reduced IFN-γ production in HCV infected IDUs
We examined the frequency of total IFN-γ+ (Supplementary Figure S2), single CD107a−IFN-γ+ producing cells (Figure 3A) and CD107a+IFN-γ+ double positive cells (Figure 3B) in the different NK cell subsets following stimulation with K562 cells. We observed a general trend of decreased frequency of IFN-γ producing cells in all chronics and spontaneous resolvers at all time points as compared to healthy donors (Figures S2, 3A and 3B). The exposed uninfected group demonstrated an intermediate level of IFN-γ production. In one comparison, we observed increased CD107a−IFN-γ+ frequency in exposed uninfected subjects within the CD56dimCD16+ NK subset compared to chronics during the follow-up phase (Figures 3B). Nevertheless, the trend was always towards lower IFN-γ production as compared to healthy donors suggesting that opioid usage may influence cytokine production capacity of NK cells [25, 26]. Finally, as illustrated in Figures 3C and 3D, the frequency of NK cells positive for both functions (CD107a+IFN-γ+) was decreased in all three groups of patients compared to healthy donors during the follow-up phase. This decrease was only observed in the CD56brightCD16− NK cell subset (Figure 3C). The frequency of CD107a+IFN-γ+ remained unchanged overtime within the CD56dimCD16+ NK cell in all groups and was comparable to the frequencies observed in healthy donors (Figure 3D). These data suggest dissociation in NK cell functions where IFN-γ production does not follow the same trend as degranulation. Furthermore, they suggest that opioid usage may influence the cytokine producing capacity of NK cells.
Figure 3. HCV infection is associated with decreased IFN-γ regardless of infectious outcome.
Cytokine production was measured by intracellular IFN-γ in patients with HCV chronic evolution (•), HCV spontaneous resolution (Δ), exposed un-infected (■) and healthy donors (○). PBMCs were co-incubated with or without K562 target cells. Background expression of IFN-γ was subtracted from expression with target cells. A) Frequency of CD107a-IFN-γ+ cells gated on CD56brightCD16− NK cells and B) CD56dimCD16+ NK cells. C) Frequency of CD107a+IFN-γ+ cells gated on CD56brightCD16− NK cells and D) CD56dimCD16+ NK cells. The acute phase of HCV infection is represented by the shaded area. Mean is represented by a horizontal bar. *p < 0.05; **p < 0.01; ***p < 0.001. 2-way ANOVA (repeated measures) or 1-way ANOVA (comparison with healthy donors).
Longitudinal phenotypic analysis for the expression of activation and inhibitory receptors by NK cells during acute HCV
To determine whether increased NK cell activity during acute HCV is related to changes in expression of activating or inhibitory receptors on their surface, we monitored such longitudinal changes in expression of the activation marker (CD69), the NK cell activating receptors (NKG2D, NKp30 and NKp44) and the NK cell inhibitory receptors (CD161, NKG2A, KIR2DL1/S1, KIR2DL2/L3/S2 and KIR3DL1) on NK cells directly ex-vivo following HCV exposure. Representative staining for each marker is presented in supplementary Figures S3 and S4. As illustrated in Figure 4A and 4B, we observed decreased expression of the activation marker CD69 on both NK cell subsets in chronics and spontaneous resolvers at all time points studied as compared to healthy donors. Analysis of the frequency of NKG2A+ NK cells revealed decreased expression of this receptor by the CD56brightCD16− NK cell subset following HCV viral clearance (Figure 4C). Finally, we observed decreased frequency of CD161+ CD56dimCD16+ NK cells during baseline and follow-up phases in patients with chronic evolution compared to healthy donors (Figure 4D). We observed no significant difference in the expression of NKG2D, NKp30, NKp44, KIR2DL1/S1 and KIR2DL2/L3/S2 between the different groups of patients or between the different HCV infection phases studied (data not shown). These results reflect the complexity of the inhibitory and activating signals governing NK cell activity. They suggest that decreased expression of NKG2A might be predictive of HCV spontaneous clearance while decreased expression of CD161 might be predictive of chronic evolution.
Figure 4. Expression of activation markers, activating receptors and inhibitory receptors by NK cells.
Expression of phenotypic markers by NK cells was determined ex-vivo in patients with HCV chronic evolution (•), HCV spontaneous resolution (Δ), exposed un-infected (■) and healthy donors (○). A) Frequency of CD69+ cells gated on CD56brightCD16− NK cells and B) CD56dimCD16+ NK cells. C) Frequency of NKG2A+ cells gated on CD56brightCD16− D) Frequency of CD161+ cells gated on CD56dimCD16+ NK cells. The acute phase of HCV infection is represented by the shaded area. Mean is represented by a horizontal bar. *p < 0.05; **p < 0.01; ***p < 0.001. 2-way ANOVA (repeated measures) or 1-way ANOVA (comparison with healthy donors).
NK cell peak precedes or overlaps with peak HCV-specific T cell responses
To characterize the kinetics of induction of innate NK cell responses relative to adaptive T cell responses during acute HCV, NK cell and T cell activity was monitored longitudinally in 12 acutely infected patients (6 spontaneous resolvers and 6 chronics). NK cell activity was measured by degranulation of the CD56dimCD16+ NK cell population or IFN-γ production of the CD56brightCD16− NK cell population and T cell responses measured by IFN-γ secretion in response to overlapping HCV peptides spanning the immunodominant HCV core and NS3 proteins [27-30] using the enzyme linked immunospot (ELISPOT) assay. For four out of the six spontaneous resolvers (patients R1 to R4), the peak NK cell response preceded or overlapped the peak T cell response (Figure 5A). In patient R5, although the highest NK cell activity is at the earliest time point tested and T cell responses peak afterwards, the lack of early samples makes it difficult to ascertain that the peak NK cell response was not missed. Finally, patient R6 was the only spontaneous resolver where we observed peak NK cell response after the peak T cell response. For the patients with chronic evolution (Figure 5B), two of the six individuals (C1 and C2) had a peak NK cell response before the peak T cell response, three patients (C3 to C5) had coinciding response peaks and one patient (C6) had a peak NK cell response after the peak T cell response. In conclusion, for the majority of HCV-infected patients, the NK cell response developed before the T cell response.
Figure 5. NK cell peak precedes or coincides with peak HCV-specific T cell responses.
NK cell activity was measured by degranulation of the CD56dimCD16+CD107a+IFN-γ−NK cell population or IFN-γ production by the CD56brightCD16−CD107a−IFN-γ+ NK cell population and T cell responses measured by IFN-γ secretion in response to overlapping HCV peptides spanning the immunodominant HCV core and NS3 proteins [27-30] using the ELISPOT assay were measured longitudinally in a subset of HCV acutely infected patients with either spontaneous resolution (n=6, Panel A) or chronic evolution (n=6, Panel B). Plasma HCV viral load was measured by an in-house real time PCR assay (Sensitivity 1000 IU/ml) and represented as grey area graph. For patients R3-R6, HCV-RNA was below the sensitivity of our assay. Patients R3 and R6 were HCV RNA+ by qualitative PCR (sensitivity 50 IU/ml) at acute HCV diagnosis and negative afterwards. Patient R4 was never HCV-RNA+, acute HCV was diagnosed based on a positive anti-HCV antibody test following a previous negative test within a 16 week interval. Patient R5 was HCV RNA+ by qualitative PCR (sensitivity 50 IU/ml) at acute HCV diagnosis and the first time point tested then negative afterwards. Patient C5 tested HCV RNA+ by qualitative PCR (sensitivity 50 IU/ml) at all time points studied but samples were not available for viral load quantification.
NK cell activity does not correlate with decline in HCV viral load
As observed in Figures 5A and 5B, the peak NK cell activity, although it precedes T cell responses, did not induce any major change in viral load in particular in the patients with chronic evolution (Figure 5B). To determine if NK cell activity may contribute to decreasing HCV plasma viral load, HCV RNA viral load was quantified by real-time RT-PCR in plasma of all patients with HCV chronic evolution and spontaneous resolution during the acute phase. No correlation was established between the highest viral load during acute HCV and NK cell degranulation or IFN-γ production (data not shown). It is important to note that HCV RNA measured in the majority of spontaneously resolved patients, was below the level of quantification which may have affected the analysis. Further analysis is needed at the early stages of HCV infection to elucidate this point.
NK cell degranulation correlates positively with the magnitude of HCV-specific T cell responses
NK cells can interact with DCs and the cross-talk between these two cell types regulates both the innate and adaptive immune responses [6]. We sought to determine whether NK cell activity can affect the induction of adaptive immune responses irrespective of infectious outcome. We examined the correlation between NK cell activity measured by degranulation or cytokine production and the magnitude of the HCV-specific T cell response during acute HCV. T cell responses were measured by IFN-γ ELISPOT in response to overlapping HCV peptide pools spanning the entire HCV polyprotein. Results for patients with HCV chronic evolution and spontaneous resolution are shown in Figure 6. In the exposed un-infected group, only 1 out of the 10 patients had a detectable ELISPOT response above background levels and was not considered in this analysis. We observed a positive correlation between the frequency of CD107a+IFN-γ− CD56dimCD16+ NK cells and the magnitude of the HCV-specific T cell response (Figure 6A and 6B). A similar trend was observed for total CD107a+ but did not reach statistical significance (Figure S5). In contrast, we observed a negative correlation between the frequency of CD107a−IFN-γ+ CD56brightCD16− NK cells and the HCV-specific T cell response (Figure 6C and 6D). A similar trend was also observed for total IFN-γ+ but did not reach statistical significance (Figure S5). Finally, there was no correlation between frequency of CD107a+IFN-γ+ NK cells and ELISPOT data (Figure 6E and 6F). These correlations remained unchanged even after excluding one outlier patient (spontaneous resolver) with a T cell response of ~4000 spot forming cells (SFC)/million PBMCs (data not shown). These results demonstrate a dichotomy between the two NK cell functions and suggest that NK cell degranulation but not IFN-γ production is a more critical parameter in the cross-talk between innate and adaptive immunity at least during acute HCV.
Figure 6. NK cell degranulation correlates positively but IFN-γ correlates negatively with HCV-specific T cell adaptive immune response.
NK cell degranulation and IFN-γ production were determined in patients with HCV chronic evolution and HCV spontaneous resolution during the acute phase of HCV as shown in Figures 2 and 3. The magnitude of the HCV-specific T cell response approximately 1 month later in these same patients was determined by IFN-γ secretion in response to overlapping HCV peptides spanning the entire HCV polyprotein using the ELISPOT assay. A) Correlation between HCV-specific T cell response and frequency of CD107a+IFN-γ− cells gated on CD56brightCD16+ NK cells, B) frequency of CD107a+IFN-γ− cells gated on CD56dimCD16− NK cells, C) frequency of CD107a−IFN-γ+ cells gated on CD56brightCD16+ NK cells, D) frequency of CD107a−IFN-γ+ cells gated on CD56dimCD16− NK cells, E) frequency of CD107a+IFN-γ+ cells gated on CD56brightCD16+ NK cells, F) frequency of CD107a+IFN-γ+ cells gated on CD56dimCD16− NK cells. Correlation determined with Spearman test.
Discussion
We have analyzed the longitudinal evolution of the NK cells phenotype and function during acute HCV infections in a unique cohort of IDUs with either spontaneous resolution or chronic evolution. Furthermore, we have examined NK cell activity in a cohort of HCV-exposed but uninfected high risk IDUs. Although HCV exposure is self reported and might not be completely accurate, this group served as an important control for the influence of drug use on the activity of NK cells. A variable pattern of expression of activating and inhibitory receptors was observed reflecting the complex signals involved in activation of NK cells. We demonstrated that NKG2A expression is down modulated following spontaneous viral clearance and decreased expression of CD161 in persistent viremia suggesting that they can be predictive of spontaneous resolution or chronic evolution, respectively. We’ve demonstrated increased NK cell activity during acute HCV as demonstrated by increased degranulation. Furthermore, we’ve demonstrated that the peak NK cell activity precedes or coincides with peak T cell responses. Finally, NK cell degranulation correlated positively while IFN-γ production correlated negatively with the magnitude of HCV-specific adaptive T cell responses.
Similar to previous reports that have studied NK cell function during chronic HCV [15, 17, 19], we demonstrate that CD56dimCD16+ NK cells are significantly depleted in HCV infected patients as compared to healthy donors and irrespective of acute infection outcome. Depletion of this NK cell subset could result from persistent stimulation and their differentiation into activated CD56bright cells [31]. Although more recent studies suggest that CD56bright NK cells differentiate into CD56dim NK cells [32, 33]. Indeed, we did not observe any increase in the frequency of CD56bright NK cells or in the ratio of CD56dim/CD56bright in any of the patient groups or phases studied (data not shown). Another possibility is reduced survival of CD56dim NK cells or enhanced NK cell migration from the blood to the liver could also explain this depletion although several studies have demonstrated that total NK cell numbers in the liver are normal [34] or even decreased [35, 36] during HCV infection.
We demonstrate that NK cell degranulation is increased during acute HCV irrespective of acute HCV outcome towards chronic evolution or spontaneous resolution. However, we observe that NK cells from chronic patients degranulated more but NK cells from all HCV infected individuals produced less IFN-γ as compared to healthy donors and exposed uninfected. These results demonstrate that NK cells are activated during acute HCV infection and suggest a dichotomy or dissociation between NK cell degranulation and cytokine production functions. Indeed, recent studies have demonstrated that NK cells in chronic HCV patients are polarized towards cytotoxicity [37, 38] and that this polarization is likely due to in vivo stimulation by IFN-α induced by HCV infection.
Reduced NK cell IFN-γ production in HCV-infected patients could be explained by two mechanisms. First, HCV surface glycoprotein E2 was shown to bind to CD81 on the surface of NK cells and inhibit cytotoxicity and IFN-γ production [10, 11]. Although an interesting possibility, it would not explain the low IFN-γ production during baseline. Furthermore, another study by Yoon et al.[12] has recently demonstrated that exposure of NK cells from healthy donors to in vitro-produced HCV virions did not influence their function. Another possible explanation for low IFN-γ production by NK cells, in this particular cohort, is opioid abuse. It has been reported that heroin abuse can reduce NK cell activity [25, 26]. Specific opiate receptors are expressed on immune cells that may modulate their functions. Indeed, morphine and its derivatives interact with the mu receptor expressed on various immune cell types including lymphocytes and NK cells. Multiple in vitro and in vivo experiments have demonstrated that it can suppress production of cytokines and proliferation of lymphocytes and inhibit NK cell activity in humans, monkey and rodents [39-41]. Drug abuse in our cohort of HCV-exposed IDUs could account for low IFN-γ production during baseline. Interestingly, exposed un-infected IDUs exhibit an intermediate level of IFN-γ production that is higher than chronic HCV-infected IDUs in some instances but lower than normal donors in other instances suggesting that drugs may influence NK cell activity but are not likely to be the only factor.
Expression of the early activation marker CD69 was decreased on NK cells from HCV-infected patients with chronic evolution and spontaneous resolution but intermediate on exposed uninfected individuals. This decrease was observed during baseline, acute and follow-up phases, basically reflecting the same pattern as IFN-γ production. Indeed, CD69 expression positively correlated with CD107a−IFN-γ+ and CD107a+IFN-γ+ but negatively correlated with CD107a+IFN-γ− (data not shown). The fact that HCV E2 glycoprotein has also been shown to decrease CD25 expression [10], another activation marker, on NK cells, further supports a direct inhibitory in vivo mechanism by HCV viral particles.
We demonstrate normal expression of the inhibitory receptor NKG2A in HCV chronic patients. This is in contrast with other studies that demonstrated that NKG2A is upregulated on NK cells during chronic HCV [18, 42] and that it correlates with increased necro-inflammatory score in the liver [43] and increased IL-10 production by NK cells [44]. It is possible that this is upregulation is related to the development of chronic liver disease. Most of the patients in our cohort were followed for less than a year and had not developed any serious liver disease or inflammation as compared to more than 20 years of chronic infection in other studies [15]. Interestingly, we have also demonstrated that expression of NKG2A on NK cells is down modulated following spontaneous viral clearance. This probably reflects a shut-off mechanism to guard against liver inflammation and the development of immune suppressive IL-10 NK cells. Alternatively, it was demonstrated that HCV infected hepatocytes express enhanced levels of HLA-E and that the HLA-A2 restricted epitope HCV core AA (35-44) stabilizes HLA-E expression [18]. Since NKG2A binds to HLA-E, enhanced expression of NKG2A by NK cells from chronic HCV patients resulted in reduced cytolysis of HLA-E expressing hepatocytes [18]. Reduced expression of NKG2A by spontaneous resolvers in our study could be a mechanism for these patients to escape cytotoxic impairment induced by HLA-E expressing infected hepatocytes. We also demonstrate low frequencies of CD161 expressing NK cells in HCV chronically infected patients. CD161 is another inhibitory receptor that was also shown to be expressed at low frequencies by NK cells of HIV chronically infected patients [24]. These results suggest that low CD161 expression might be associated with chronic HCV and possibly other viral infections.
It was reported that genes encoding the inhibitory NK cell receptor KIR2DL3 and its HLA-C1 ligand directly influence resolution of HCV infection in individuals homozygous for these genes [13]. Because of the limited number of patients homozygous for KIR2DL3 and HLA-C1, it was impossible to establish a statistically significant correlation between these genes and increased NK cell activity. In addition, the heterogeneous population studied, composed of HCV-infected patients with chronic evolution or spontaneous resolution, and exposed un-infected, may have also influenced our results. Studying NK cell activity in a much larger and homogenous group of HCV infected individuals will be essential to elucidate this point in future studies.
Here we have characterized the kinetics of the changes in NK and HCV-specific T cell function during acute HCV and have demonstrated that the peak NK cell response measured by degranulation or IFN-γ production preceded the peak T cell response measured by ELISPOT for the majority of HCV chronics and spontaneous resolvers. These results suggests that NK cells could represent the dominant cytotolytic effector population during early HCV infection.
We demonstrate that NK cell degranulation, a surrogate measure of cytotoxicity [45], correlated with the magnitude of the HCV-specific T cell response measured by ELISPOT. It is possible that killing of HCV-infected hepatocytes by NK cells during acute HCV would decrease viral load and allow for the development of an efficient adaptive immune response. However, this possibility is limited since NK cell activity did not correlate with viral load. It is more likely that destruction of HCV-infected hepatocytes by NK cells facilitates uptake of apoptotic bodies by migratory DCs and enhance antigen transfer to the lymph nodes and priming of HCV-specific CD4+ and CD8+ T cells. Indeed, a recent study in mice demonstrated that NK cell-mediated killing of target cells triggers antigen-specific CD8+ and CD4+ T cell-mediated and humoral responses [46]. In addition to killing infected and cancerous cells, NK cells can also kill immature DCs [9, 47]. Killing of immature DCs presenting HCV antigens could limit the development of tolerized adaptive T cell responses and favor the development of antiviral T cells, a process known as DC editing [6].
We observed a negative correlation between NK cell IFN-γ production and the magnitude of the HCV-specific T cell response. This was a surprising result as IFN-γ is important for inducing the maturation of DCs. However, it was shown that production of TNF-α by NK cells was more potent than IFN-γ at inducing DC maturation [8]. Correlation between production of TNF-α by NK cells and magnitude of HCV-specific T cell responses will need further investigation. In addition, the effect of IFN-γ is more local in the immuno-tolerant environment of the liver and this may not enhance antigen presentation and might be neutralized by several other tolerizing signals and mediators (e.g. IL-10) [48, 49].
In conclusion, we propose that NK cells are activated during acute HCV regardless of infection outcome. This suggests that NK cell activity might not be directly implicated in HCV clearance. However, NK cells may play an indirect role through enhanced transfer of HCV antigens to lymph nodes and induction of T cell responses. Because of the well described NK-DC crosstalk, it is also tempting to speculate that NK cells might edit DCs presenting HCV antigens by inducing DC maturation or killing immature DCs with tolerogenic potential and thus favor the generation of an optimal antiviral T cell response.
Supplementary Material
Supplementary Figure S1. Representative flow cytometry staining for CD107a and IFN-γ. Degranulation was measured by CD107a surface staining and cytokine production was measured by intracellular IFN-γ. PBMCs were co-incubated with (bottom row) or without (top row) K562 target cells. CD107a+ and IFN-γ+ cells were gated on both NK cell subsets: CD56brightCD16− (left column) and CD56dimCD16+/lo (right column).
Supplementary Figure S2. Total CD107a expression and total IFN-γ production. Degranulation was measured by CD107a surface staining in patients with HCV chronic evolution (•), HCV spontaneous resolution (Δ), exposed un-infected (■) and healthy donors (○). PBMCs were co-incubated with or without K562 target cells. Background expression of CD107a was subtracted from expression with target cells. A) Frequency of CD107a+ cells gated on CD56brightCD16− NK cells and B) CD56dimCD16+ NK cells. C) Frequency of IFN-γ+ cells gated on CD56brightCD16− NK cells and D) CD56dimCD16+ NK cells. The acute phase of HCV infection is represented by the shaded area. Mean is represented by a horizontal bar. *p < 0.05; **p < 0.01; ***p < 0.001. 2-way ANOVA (repeated measures) or 1-way ANOVA (comparison with healthy donors).
Supplementary Figure S3. Representative flow cytometry staining for inhibitory receptors. Five inhibitory receptors (NKG2A, CD161, KIR2DL1/S1, KIR2DL2/L3/S2 and KIR3DL1) were examined ex-vivo in both NK cell subsets: CD56brightCD16− (top row) and CD56dimCD16+/lo (bottom row).
Supplementary Figure S4. Representative flow cytometry staining for activation markers and activating receptors. One activation marker (CD69) and three activating receptors (NKp44, NKp30 and NKG2D) were examined ex-vivo in both NK cell subsets: CD56brightCD16− (top row) and CD56dimCD16+/lo (bottom row).
Supplementary Figure S5. Total CD107a expression and total IFN-γ production do not correlate with HCV-specific T cell responses. NK cell degranulation and IFN-γ production were determined in patients with HCV chronic evolution and HCV spontaneous resolution during the acute phase of HCV as shown in Figures S2. The magnitude of the HCV-specific T cell response approximately 1 month later in these same patients was determined by IFN-γ secretion in response to overlapping HCV peptides spanning the entire HCV polyprotein using the ELISPOT assay. A) Correlation between HCV-specific T cell response and frequency of CD107a+ cells gated on CD56brightCD16+ NK cells, B) frequency of CD107a+ cells gated on CD56dimCD16− NK cells, C) frequency of IFN-γ+ cells gated on CD56brightCD16+ NK cells, D) frequency of IFN-γ+ cells gated on CD56dimCD16− NK cells. Correlation determined with Spearman test.
Acknowledgements
We thank Robert Boileau for instrumental help with the statistical analysis and Hassen Kared for critical reading of the manuscript. This study was supported by grants from the Dana Foundation, the Canadian Institutes for Health Research (CIHR) (MOP-74524) and the Fonds de la Recherche en Santé du Québec (FRSQ) AIDS and Infectious Disease Network (Réseau SIDAMI). S. Pelletier is the recipient of a Ph.D. scholarship from the National Canadian Research Training Program on Hepatitis C. J. Bruneau holds a senior clinical research award from FRSQ. N. H. Shoukry holds a joint New Investigator Award from the Canadian Foundation for Infectious Diseases and CIHR.
Abbreviations
- DC
Dendritic cells
- ELISPOT
Enzyme Linked Immunospot assay
- HBV
Hepatitis B virus
- HCV
Hepatitis C Virus
- HIV
Human immunodeficiency virus
- ICS
Intracellular Cytokine Staining
- IDUs
Intravenous Drug Users
- IFN-γ
Interferon gamma
- NK
Natural killer cells
- PBMC
Peripheral Blood Mononuclear cells
Footnotes
Competing financial interests: None
References
- 1.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002 Nov;36(5 Suppl 1):S21–29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 2.Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 3.Shoukry NH, Cawthon AG, Walker CM. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu Rev Microbiol. 2004;58:391–424. doi: 10.1146/annurev.micro.58.030603.123836. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001 Nov;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 5.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005 Feb;5(2):112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 6.Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, et al. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev. 2006 Dec;214:219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002 Feb 4;195(3):343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Arico M, Moretta L, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005 Jul 15;106(2):566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 9.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002 Feb 4;195(3):335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crotta S, Stilla A, Wack A, D’Andrea A, Nuti S, D’Oro U, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002 Jan 7;195(1):35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002 Jan 7;195(1):43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009 Jan;49(1):12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004 Aug 6;305(5685):872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 14.Romero V, Azocar J, Zuniga J, Clavijo OP, Terreros D, Gu X, et al. Interaction of NK inhibitory receptor genes with HLA-C and MHC class II alleles in Hepatitis C virus infection outcome. Mol Immunol. 2008 May;45(9):2429–2436. doi: 10.1016/j.molimm.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE, et al. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008 Aug;57(8):1121–1128. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 16.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007 Aug 15;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 17.Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006 Mar;43(3):573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 18.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006 Jun;55(6):869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005 Oct;79(19):12365–12374. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corado J, Toro F, Rivera H, Bianco NE, Deibis L, De Sanctis JB. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997 Sep;109(3):451–457. doi: 10.1046/j.1365-2249.1997.4581355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox AL, Page K, Bruneau J, Shoukry NH, Lauer GM, Kim AY, et al. Rare birds in North America: acute hepatitis C cohorts. Gastroenterology. 2009 Jan;136(1):26–31. doi: 10.1053/j.gastro.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman M, Shafran S, Burak K, Doucette K, Wong W, Girgrah N, et al. Management of chronic hepatitis C: consensus guidelines. Can J Gastroenterol. 2007 Jun;21(Suppl C):25C–34C. [PMC free article] [PubMed] [Google Scholar]
- 23.Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, et al. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008 Oct;82(20):10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter G, Malenfant JM, Delabre RM, Burgett NC, Yu XG, Lichterfeld M, et al. Increased natural killer cell activity in viremic HIV-1 infection. J Immunol. 2004 Oct 15;173(8):5305–5311. doi: 10.4049/jimmunol.173.8.5305. [DOI] [PubMed] [Google Scholar]
- 25.Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am J Ther. 2004 Sep-Oct;11(5):354–365. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 26.Novick DM, Ochshorn M, Ghali V, Croxson TS, Mercer WD, Chiorazzi N, et al. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. J Pharmacol Exp Ther. 1989 Aug;250(2):606–610. [PubMed] [Google Scholar]
- 27.Diepolder HM, Gerlach JT, Zachoval R, Hoffmann RM, Jung MC, Wierenga EA, et al. Immunodominant CD4+ T-cell epitope within nonstructural protein 3 in acute hepatitis C virus infection. J Virol. 1997;71(8):6011–6019. doi: 10.1128/jvi.71.8.6011-6019.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, et al. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004 Sep;127(3):924–936. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Lauer GM, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, et al. Full-breadth analysis of CD8+ T-cell responses in acute hepatitis C virus infection and early therapy. J Virol. 2005 Oct;79(20):12979–12988. doi: 10.1128/JVI.79.20.12979-12988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyk-Pearson S, Tester IA, Lezotte D, Sasaki AW, Lewinsohn DM, Rosen HR. Differential antigenic hierarchy associated with spontaneous recovery from hepatitis C virus infection: implications for vaccine design. J Infect Dis. 2006 Aug 15;194(4):454–463. doi: 10.1086/505714. [DOI] [PubMed] [Google Scholar]
- 31.Robertson MJ, Caligiuri MA, Manley TJ, Levine H, Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990 Nov 15;145(10):3194–3201. [PubMed] [Google Scholar]
- 32.Caligiuri MA. Human natural killer cells. Blood. 2008 Aug 1;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, et al. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007 Apr 15;178(8):4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 34.Deignan T, Curry MP, Doherty DG, Golden-Mason L, Volkov Y, Norris S, et al. Decrease in hepatic CD56(+) T cells and V alpha 24(+) natural killer T cells in chronic hepatitis C viral infection. J Hepatol. 2002 Jul;37(1):101–108. doi: 10.1016/s0168-8278(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 35.Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003 Jan;38(1):67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 36.Kawarabayashi N, Seki S, Hatsuse K, Ohkawa T, Koike Y, Aihara T, et al. Decrease of CD56(+)T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000 Nov;32(5):962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009 Sep;137(3):1151–1160. 1160, e1151–1157. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 38.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010 Jan;138(1):325–335. e321–322. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003 Apr;16(2):209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ugen KE, Nyland SB. Injecting drugs of abuse and immunity: implications for HIV vaccine testing and efficacy. Springer Semin Immunopathol. 2006 Nov;28(3):281–287. doi: 10.1007/s00281-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Barke RA, Ma J, Charboneau R, Roy S. Opiate abuse, innate immunity, and bacterial infectious diseases. Arch Immunol Ther Exp (Warsz) 2008 Sep-Oct;56(5):299–309. doi: 10.1007/s00005-008-0035-0. [DOI] [PubMed] [Google Scholar]
- 42.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007 Feb;37(2):445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 43.Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N, et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol. 2009 Sep;51(3):458–467. doi: 10.1016/j.jhep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 44.Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004 Nov 15;173(10):6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 45.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004 Nov;294(1-2):15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Krebs P, Barnes MJ, Lampe K, Whitley K, Bahjat KS, Beutler B, et al. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood. 2009 Jun 25;113(26):6593–6602. doi: 10.1182/blood-2009-01-201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005 Oct;6(10):1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 48.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006 Oct;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 49.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Representative flow cytometry staining for CD107a and IFN-γ. Degranulation was measured by CD107a surface staining and cytokine production was measured by intracellular IFN-γ. PBMCs were co-incubated with (bottom row) or without (top row) K562 target cells. CD107a+ and IFN-γ+ cells were gated on both NK cell subsets: CD56brightCD16− (left column) and CD56dimCD16+/lo (right column).
Supplementary Figure S2. Total CD107a expression and total IFN-γ production. Degranulation was measured by CD107a surface staining in patients with HCV chronic evolution (•), HCV spontaneous resolution (Δ), exposed un-infected (■) and healthy donors (○). PBMCs were co-incubated with or without K562 target cells. Background expression of CD107a was subtracted from expression with target cells. A) Frequency of CD107a+ cells gated on CD56brightCD16− NK cells and B) CD56dimCD16+ NK cells. C) Frequency of IFN-γ+ cells gated on CD56brightCD16− NK cells and D) CD56dimCD16+ NK cells. The acute phase of HCV infection is represented by the shaded area. Mean is represented by a horizontal bar. *p < 0.05; **p < 0.01; ***p < 0.001. 2-way ANOVA (repeated measures) or 1-way ANOVA (comparison with healthy donors).
Supplementary Figure S3. Representative flow cytometry staining for inhibitory receptors. Five inhibitory receptors (NKG2A, CD161, KIR2DL1/S1, KIR2DL2/L3/S2 and KIR3DL1) were examined ex-vivo in both NK cell subsets: CD56brightCD16− (top row) and CD56dimCD16+/lo (bottom row).
Supplementary Figure S4. Representative flow cytometry staining for activation markers and activating receptors. One activation marker (CD69) and three activating receptors (NKp44, NKp30 and NKG2D) were examined ex-vivo in both NK cell subsets: CD56brightCD16− (top row) and CD56dimCD16+/lo (bottom row).
Supplementary Figure S5. Total CD107a expression and total IFN-γ production do not correlate with HCV-specific T cell responses. NK cell degranulation and IFN-γ production were determined in patients with HCV chronic evolution and HCV spontaneous resolution during the acute phase of HCV as shown in Figures S2. The magnitude of the HCV-specific T cell response approximately 1 month later in these same patients was determined by IFN-γ secretion in response to overlapping HCV peptides spanning the entire HCV polyprotein using the ELISPOT assay. A) Correlation between HCV-specific T cell response and frequency of CD107a+ cells gated on CD56brightCD16+ NK cells, B) frequency of CD107a+ cells gated on CD56dimCD16− NK cells, C) frequency of IFN-γ+ cells gated on CD56brightCD16+ NK cells, D) frequency of IFN-γ+ cells gated on CD56dimCD16− NK cells. Correlation determined with Spearman test.