Abstract
Superoxide released from mitochondria forms reactive oxygen species that can cause severe oxidative damage and have been associated with aging- and disuse-induced muscle dysfunction. Superoxide is released to both the exterior and the matrix of mitochondria, where oxidative damage is not necessarily the same. This complicates determining the role of mitochondrial superoxide in eliciting oxidative stress in skeletal muscle. A newly developed capillary electrophoretic method analyzes hydroxytriphenylphosphonium ethidium, a superoxide-specific product of triphenylphosphonium hydroethidine, released to outside the mitochondria (supernatant) and retained in the matrix (pellet). In this study, we investigated the mitochondrial superoxide production of soleus (type I) and semimembranosus (type II) muscles of Fischer 344 rats affected by aging (13 vs. 26 mo) and disuse (hindlimb unloading). In agreement with previous studies, overall superoxide production increased with aging and disuse. On the other hand, the new experimental method revealed that superoxide production outside the mitochondria of the soleus does not show a significant age-related increase. Another observation was that the superoxide production increase in the matrix occurs earlier (7 days of disuse) compared with the outside mitochondria (14 days of disuse) in both muscle types. These findings indicate that superoxide release is complex as it occurs asymmetrically at both sides of the mitochondrial inner membrane, and that such release has muscle type and temporal specificity. These findings are important to refine current concepts on oxidative stress associated with muscle aging and disuse.
Keywords: triphenylphosphonium, hindlimb unloading, capillary electrophoresis, superoxide, aging, muscle, soleus, semimembranosus
mitochondria are the major sources of superoxide production in skeletal muscles (34), where superoxide is released asymmetrically to both sides of the mitochondrial inner membrane, forming two separate superoxide pools (in the matrix and outside of the mitochondria) due to the impermeability of the inner membrane to superoxide anion (24, 32). The produced superoxide in the respective compartments may ultimately be transformed into highly reactive radicals such as hydroxyl radical and peroxynitrite, which cause the oxidative damage and lead to the oxidative stress (4, 26).
The production of mitochondrial superoxide has been associated with increased oxidative stress during muscle aging and disuse, characterized by accumulation of damaged DNA, oxidized proteins, peroxidized lipids, enhanced production of oxidants, and adaptation of antioxidant systems (2–3, 6, 8–11, 14, 17–18, 20–22, 25, 31, 33). Several studies have been conducted to determine the production of reactive species with muscle aging and disuse (2, 8, 17, 19–20, 25), where either total reactive oxygen species (ROS) or superoxide in both pools (2, 19–20) or the superoxide released outside of the mitochondria (17) was measured. Although these studies show increased ROS and superoxide production with muscle aging and disuse, it is unknown whether the production of superoxide in each pool (in the matrix or outside the mitochondria) is different. We hypothesize that both hindlimb unloading (HU) (a form of disuse) and aging induce the production of mitochondrial superoxide and the effect of HU is dependent on the age of animals. Furthermore, such effects will be different at both sides of the mitochondrial inner membrane. To test such hypothesis, methods with the ability to determine simultaneously the superoxide production at both sides of the mitochondrial inner membrane are desired.
Recently, we developed a novel approach for the indirect determination of the superoxide production in the matrix and outside of the mitochondria (36). After incubation of isolated mitochondria with triphenylphosphonium hydroethidine (TPP-HE), differential centrifugation separates the supernatant and the pellet containing the superoxide-specific product hydroxytriphenylphosphonium ethidium (OH-TPP-E+) released outside and in the mitochondrial matrix, respectively. Micellar electrokinetic chromatography with laser-induced fluorescence detection (MEKC-LIF) is then used to determine the OH-TPP-E+ contents in the supernatant and the pellet (28). Other advantages of this approach are low limits of detection (∼10−18 mol), short separation times (∼3 min), and small sample consumption (∼10−9 liter) (36).
In this report we used the new method to detect superoxide production inside and outside of the mitochondria of rat soleus (type I) and semimembranosus (type II) muscles in response to muscle aging and disuse. Importantly, we discovered that superoxide outside mitochondria does not increase with aging in the soleus and that the onset of increased superoxide production with inactivity occurs earlier in the matrix than outside the mitochondria of both type I and type II muscles.
METHODS
Animals and HU.
Nineteen male Fischer 344 rats ages 13 mo (100% strain survival; n = 10) and 26 mo (25% strain survival; n = 9) were purchased from the Minneapolis Veterans Affairs Aged Rodent Colony (University of Minnesota). These rats were randomized into three groups: normal weight bearing (control), HU for 7 days, and HU for 14 days. The HU intervention was achieved as described previously (6–7). Briefly, the tail of the rat was attached to a swivel mounted at the top of the cage. The height of the suspension was adjusted to prevent the hindlimbs from contacting the floor, which permitted animals to move with their forelimbs while the hindlimbs were unloaded. All the animals were housed in a research animal facility and were checked daily for any abnormal response to suspension. The protocol of this study was approved by the University of Minnesota Institutional Animal Care and Use Committee.
Mitochondrial preparation.
Mitochondria were isolated from soleus and semimembranosus skeletal muscle following previously reported procedures (23, 36). Briefly, the muscles were minced into small pieces with scissors and washed three times with ice-cold isolation buffer (50 mM Tris·HCl, 100 mM sucrose, 100 mM KCl, 1 mM KH2PO4, 0.1 mM EGTA, and 0.2% wt/vol BSA, pH 7.4). The minced muscles were resuspended in ice-cold isolation buffer (10 ml/g tissue) supplemented with 0.2 mg/l Nargarse and incubated for 1 min. Then the minced tissues were homogenized using a Potter-Elvehjem homogenizer and the homogenate was centrifuged at 700 g for 10 min at 4°C. The supernatant was then centrifuged at 10,000 g for 10 min at 4°C. The obtained mitochondrial pellet was resuspended in ice-cold isolation buffer and washed two more times. The protein concentration of the isolated mitochondria was determined by the bicinchoninic acid (BCA) assay. Respiratory control ratio (RCR) was used to assess the quality of the mitochondrial preparation. Oxygen consumption of isolated mitochondria was measured using a FOXY-R Oxygen Sensor (Ocean Optics Dunedin, FL) and RCR value calculated as the ratio of oxygen consumption rate at state 3/state 2. The RCR values of mitochondria isolated from skeletal muscles were ∼5, indicating that the freshly isolated mitochondria were actively respiring. The membrane potential of isolated mitochondria was measured using a TPP+-selective electrode (World Precision Instruments, Sarasota, FL).
Sample preparation and MEKC-LIF analysis of OH-TPP-E+.
Isolated mitochondria were incubated with 5 μM TPP-HE (Mito-SOX red, Invitrogen, Carlsbad, CA) in the respiration buffer (10 mM HEPES, 125 mM KCl, 5 mM MgCl2, and 2 mM K2HPO4, pH 7.4) supplemented with 5 mM pyruvate/malate for 30 min at 37°C. After incubation, mitochondria were pelleted down by centrifugation at 10,000 g for 10 min. The supernatant was used to determine the amount of OH-TPP-E+ outside the mitochondrial inner membrane by MEKC-LIF (Fig. 1). Separately, the mitochondrial pellet was dissolved in mitochondrial lysis buffer (40 mM Tris·HCl, 6 mM MgCl2, and 0.5% wt/vol Triton X-100, pH 9.4) aided by sonication for 5 min (Branson ultrasonic cleaner, model 1510), then treated with 2 mg/ml proteinase K for 45 min and subsequently 400 U/ml DNase 1 for 15 min at 37°C to digest the mitochondrial DNA. The resultant dissolved mitochondrial pellet was analyzed by MEKC-LIF to determine the amount of OH-TPP-E+ in the mitochondrial matrix.
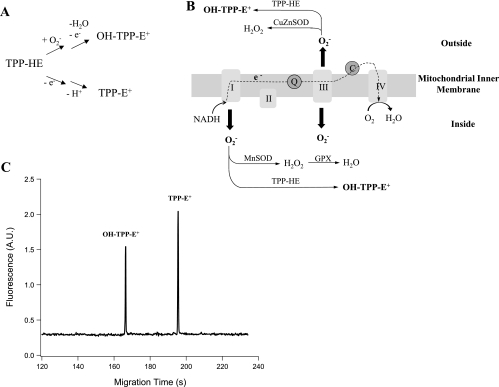
Fig. 1.
Reaction of triphenylphosphonium hydroethidine (TPP-HE) with superoxide in the mitochondria and the subsequent measurement of the oxidation products. A: oxidation of TPP-HE to form hydroxytriphenylphosphonium ethidium (OH-TPP-E+) and TPP-E+. B: formation of OH-TPP-E+ from the reaction between TPP-HE and superoxide in the mitochondrial matrix and the outside of the mitochondrial inner membrane. C: electrophoretic separation of OH-TPP-E+ and TPP-E+ by micellar electrokinetic chromatography with laser-induced fluorescence detection (MEKC-LIF).
The MEKC-LIF instrument used in this study has been described previously (36). The 488-nm line of an argon-ion laser (Melles Griot, Irvine, CA) was used for excitation and the fluorescence was selected in the 635 ± 27.5 nm range using a band-pass filter (635DF55, Omega Optical, Brattleboro, VT). Separations were carried out using 50-μm ID, 150-μm OD fused silica capillaries (Polymicro Technologies, Phoenix, AZ) at −400 V/cm in MEKC running buffer (10 mM borate and 1 mM CTAB, pH 9.4). Samples were injected by a 1-s hydrodynamic injection at 10.7 kPa, which introduced 3.8 nl of the sample into the capillary. A 5-min wash with running buffer was performed between runs.
Data analysis.
Data were presented as mean ± standard error of the mean (SE). The MEKC data were analyzed using Igor Pro software (Wavemetrics, Lake Oswego, OR) and the peak areas of OH-TPP-E+ were normalized to mitochondrial protein concentration (1 mg/ml). Standards of OH-TPP-E+ and TPP-E+ were synthesized and purified following a previously described procedure (40). MEKC-LIF analysis of these standards provided the peak areas needed to build a calibration curve and the calculation of the concentrations of OH-TPP-E+ and TPP-E+ in the samples that were analyzed. Two-way ANOVA was used to determine the effect of aging and hindlimb unloading on the net mitochondrial superoxide production in both mitochondrial matrix and outside of the mitochondrial inner membrane of soleus and semimembranosus muscles. Tukey's honest significant difference test was used as a post hoc test when the main effect of aging or HU was significant (P < 0.05).
RESULTS
Superoxide released into the mitochondrial matrix of the soleus muscle.
TPP-HE permeates the mitochondrial inner membrane and reacts with superoxide in the matrix to form the superoxide product, OH-TPP-E+ (Fig. 1, A and B). This product is then detected in the mitochondrial pellet by MEKC-LIF (Fig. 1C). The accumulation of this product in the matrix indicates that the superoxide production in the matrix changed with both HU (2-way ANOVA, P < 0.001) and aging (2-way ANOVA, P = 0.003); the effect of HU was independent of age (Fig. 2).
Fig. 2.
Superoxide production (OH-TPP-E+ amount) in the mitochondrial matrix of the soleus muscles from rats with normal weight bearing (control), 7 days of hindlimb unloading (7d HU), and 14 days of HU (14d HU). Values are means ± SE. Effect of HU was independent of age.
In adult rats, the amount of OH-TPP-E+ in the soleus muscle with 14 days of HU was 1.53-fold of the amount in the muscle with 7 days of HU, which was 3.1-fold of the value seen in the muscle with normal weight bearing (control group). In old rats, the amount of OH-TPP-E+ in the muscle with 14 days of HU was 1.58-fold of the amount in the muscle with 7 days of HU, which was 3.3-fold of the value in the muscle with normal weight bearing.
Aging also changed the superoxide production in the matrix. The soleus muscle from old rats had 30% greater amount of superoxide product than did the muscle from adult rats.
Superoxide released outside of the mitochondrial inner membrane of the soleus muscle.
TPP-HE that remains in the outside of the mitochondrial inner membrane reacts with superoxide there to form OH-TPP-E+ (Fig. 1, A and B). This product is then detected in the supernatant by MEKC-LIF (Fig. 1C). As expected the accumulation of this product outside the mitochondria indicates that superoxide production changed with HU (2-way ANOVA, P < 0.001) but did not statistically change with aging (Fig. 3).
Fig. 3.
Superoxide production (OH-TPP-E+ amount) in the outside of the mitochondrial inner membrane of the soleus muscles from rats with normal weight bearing (control), 7 days of HU, and 14 days of HU. Values are means ± SE. Effect of HU was independent of age.
In adult rats, the amount of superoxide OH-TPP-E+ in the muscle with 14 days of HU was 3.5 and 1.6 times the values seen in the muscle with normal weight bearing and 7 days of HU, respectively. In old rats, the amount of superoxide product in muscle with 14 days of HU was also greater than the values seen in the muscle with normal weight bearing and 7 days of HU.
Superoxide released into the mitochondrial matrix of the semimembranosus muscle.
Following the procedures described above for the soleus muscles, we investigated superoxide production in the matrix of the semimembranosus muscle. In the matrix of this muscle the superoxide production changed with hindlimb unloading (2-way ANOVA, P < 0.001) and aging (2-way ANOVA, P = 0.008); the change with HU was not age specific (Fig. 4).
Fig. 4.
Superoxide production (OH-TPP-E+ amount) in the mitochondrial matrix of the semimembranosus muscles from rats with normal weight bearing (control), 7 days of HU, and 14 days of HU. Values are means ± SE. Effect of HU was independent of age.
In both adult and old rats, the amount of OH-TPP-E+ in the muscle with 14 days of HU was greater than the values seen in the muscle with 7 days of HU (1.46 times the 7-day value in the adult and 1.33 times the 7-day value in the old), which were also greater than those in the muscle with normal weight bearing (1.6 times in the adult and 2.2 times in the old, respectively).
The superoxide production in the matrix of the semimembranosus also changed with aging. The semimembranosus muscle from old rats had 40% greater amount of OH-TPP-E+ than the value seen in the muscle of adult rats.
Superoxide released outside of the mitochondrial inner membrane of the semimembranosus muscle.
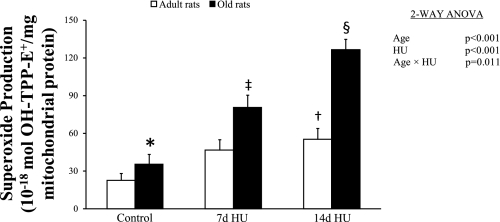
Next we investigated superoxide production outside the mitochondria of the semimembranosus muscle. The superoxide production here changed with HU, and the change was age dependent (age × HU, P = 0.011) (Fig. 5).
Fig. 5.
Superoxide production (OH-TPP-E+ amount) in the outside of the mitochondrial inner membrane of the semimembranosus muscles from rats with normal weight bearing (control), 7 days of HU, and 14 days of HU. Values are means ± SE. Effect of HU was dependent on age. *Significantly different from adult control rats. †Significantly different from adult control rats. ‡Significantly different from old control rats and rats with 14 days of HU. §Significantly different from old control rats and rats with 7 days of HU.
In adult rats, the amount of superoxide product in the muscle with 14 days of HU was 2.4-fold of the value seen in the muscle with normal weight bearing. In old rats, the amount of superoxide product in muscle progressively increased. The increased value was 2.3-fold with 7 days of HU and 3.6-fold with 14 days of HU compared with the normal weight bearing value.
In addition to disuse, the superoxide production was also affected by age outside the mitochondria of the semimembranosus. The amount of OH-TPP-E+ in the muscle was 90% higher in old rats compared with the amount in adult rats.
DISCUSSION
Overview of main findings.
We investigated the effect of muscle aging and disuse (hindlimb unloading) on the net mitochondrial superoxide production at both sides of the mitochondrial inner membrane. The mitochondria were isolated from two muscles, the soleus and semimembranosus, which are composed predominantly of type I and type II fibers, respectively. The method for detecting superoxide is based on the reaction of TPP-HE with superoxide, and the separation and detection of the formed superoxide product OH-TPP-E+ by MEKC-LIF.
In mitochondria, superoxide is mainly released by complexes I and III in the mitochondrial electron transport chain (ETC) (Fig. 1) (26). Complex I releases superoxide exclusively into the mitochondrial matrix while complex III releases superoxide toward both sides of the mitochondrial inner membrane. The impermeability of the inner membrane to superoxide defines two separate superoxide pools, inside and outside of the mitochondria, each with different potential cellular effects (24, 32). The superoxide released in the matrix and the intermembrane space could cause damage to iron-sulfur proteins by direct oxidation in the respective compartments (26). The superoxide in these two environments can be further transformed into hydrogen peroxide by Mn-superoxide dismutase (SOD) and Cu,Zn-SOD, respectively, which then subsequently reacts to form the highly reactive hydroxyl radical (4). In the presence of nitric oxide, the superoxide forms a highly reactive nitrogen species, peroxynitrite (26). All the superoxide and its derivative radicals can cause severe damage to proteins, nucleic acids, and phospholipids (4, 26), thereby leading to oxidative stress, and such damage is possibly linked to accumulation of dysfunctional mitochondria in muscle.
The production of reactive species with muscle aging and disuse has been measured in several studies, including the detection of total ROS and superoxide production (2, 8, 17, 19–20, 25). The measurement of total ROS production is generally based on the oxidation of dichlorofluorescein (2, 8, 20), which is a nonspecific probe possibly oxidized by species other than ROS such as metal centers and oxidized thiols within the cells (1). The measurement of superoxide production is based on either the reaction of cytochrome c with superoxide released outside the mitochondria (19), or an indirect assay that measures the levels of hydrogen peroxide converted from total superoxide within both pools by SOD (17). Although these methods are commonly used, they are not able to reveal simultaneously the separate changes in the production of superoxide at both sides of the mitochondrial inner membrane.
In this study we used a recently developed approach for the indirect determination of mitochondrial superoxide production, both inside and outside the mitochondria, which has advantages of short separation times, low limits of detection, and small sample capability (36). The method is not biased by OH-TPP-E+ binding and/or crossing of the mitochondrial inner membrane (36), or affected by radical formation due to sonication of the mitochondrial pellet as previously observed by others (39) because the sonication conditions used here are milder (see Supplementary Material, Part 1, available with the online version of this article). A salient feature of this qualitative method is that the TPP-HE probe accumulates within mitochondria due to the mitochondrial membrane potential, which results in a favorable reaction with superoxide vs. superoxide dismutation by SOD.
The membrane potential of isolated mitochondria from both adult and old groups was found to be ∼ 119 mV (n = 6), which based on the Nernst equation increases 100-fold the TPP-HE concentration in the matrix relative to the medium (30). Since the volume of mitochondria in a typical mitochondrial preparation (e.g., 0.1 mg/ml protein) is only ∼0.01% of the medium volume (36), the TPP-HE concentration in the medium would only decrease by ∼1%. On the other hand, a variation in membrane potential between the mitochondrial samples can cause different uptakes of TPP-HE in the mitochondria and therefore affect the production of OH-TPP-E+ (37). In this study, no significant difference in mitochondrial membrane potential was found between two age groups (121 ± 3 mV in the adult vs. 117 ± 4 mV in the old, n = 3), indicating that age may not affect the mitochondrial membrane potential. Similar results were found among groups in the presence and absence of disuse (data not shown). More importantly, a 3- to 4-mV variation in membrane potential would only result in an uncertainty of ∼11–15% in TPP-HE accumulation, which is smaller than the changes in OH-TPP-E+ (i.e., ∼50%–200%) observed under the various experimental conditions of this study. Therefore, although variations in probe accumulation due to variations in the mitochondrial membrane potential result in variations in OH-TPP-E+ levels, the measurement of membrane potential indicates that this factor is not responsible for the trends in superoxide production observed in this study.
The superoxide levels in the matrix and outside mitochondria were monitored separately by observing their respective OH-TPP-E+ levels (e.g., 25 ± 14 and 16 ± 9 amol/mg mitochondrial protein in the adult soleus muscle, respectively; Figs. 2 and 3). Given that TPP-HE is expected to be at greater concentrations in the matrix than outside the mitochondria, at first review it is surprising that the OH-TPP-E+ levels at both sides of the mitochondrial inner membrane are comparable. However, it is important to consider that superoxide levels and consequently OH-TPP-E+ levels are also modulated by SODs present in these two distinct locations (Fig. 1). Mn-SOD is more abundant in the matrix (i.e., 30 μM) whereas Cu,Zn-SOD, located outside the mitochondria, is less abundant (i.e., 6 μM) (5, 27). Moreover, some studies report that there is no detectable activity of the Cu,Zn-SOD in isolated mitochondria (15, 16). Hence, Mn-SOD is likely more effective at reducing superoxide levels in the matrix compared with Cu,Zn-SOD outside the mitochondria. Collectively, the combined effects of membrane potential and relative SODs activities prohibit a direct comparison between the OH-TPP-E+ levels in the matrix and outside the mitochondria, while the values observed here are coincidentally similar.
Besides the reaction with superoxide to form OH-TPP-E+, TPP-HE is known to react with other intracellular oxidative species (e.g., cytochromes) to form TPP-E+ and dimeric products of TPP-HE and TPP-E+ (37–38). These reactions proceed via the formation of a common intermediate radical and have the potential of depleting TPP-HE, thereby affecting the competitiveness of this probe for superoxide. Given the experimentally observed levels of TPP-E+ and the TPP-HE concentrations outside the mitochondria and in the matrix (i.e., predicted to increase ∼100-fold relative to the outside) the TPP-E+/TPP-HE ratios are ∼4 × 10−7 and ∼1 × 10−7 for the matrix and outside the mitochondria, respectively. Treatment of TPP-HE-loaded mitochondrial samples with chloranil (38) further confirmed these estimates (see Supplementary Material, Part 2, available with the online version of this article). Thus the formation of TPP-E+ would not dramatically change the initial concentration of TPP-HE during the 30 min of incubation. Dimerization is even less likely to contribute to reagent depletion because it is a second-order reaction. Furthermore, the measured TPP-E+ levels, either in the matrix or outside the mitochondria, are the same for controls and their respective samples under conditions of aging and disuse (see Supplementary Material, Part 2).
Based on kinetic arguments (see Supplementary Material), the OH-TPP-E+ formed from the reaction of TPP-HE with superoxide released into the mitochondrial matrix or outside the mitochondria is a relatively small fraction of H2O2 formed by dismutation of superoxide by SODs. This fortuitous condition suggests that superoxide measurements with the TPP-HE probe do not drastically quench the production of H2O2 by SODs, thereby making such measurements highly compatible with studies in which H2O2 plays a key role. This feature would be highly beneficial to investigate the superoxide changes simultaneously with H2O2 production and oxidative damage (e.g., carbonylation), which would further enhance our understanding of the role of compartmentalization of mitochondrial superoxide release in skeletal muscle under age-related and disuse-related conditions.
Effect of aging on the mitochondrial superoxide production in skeletal muscle.
Aging has been associated with increased oxidative stress in skeletal muscle, characterized by accumulated oxidized proteins, damaged DNA, lipid peroxidation, and enhanced production of oxidants (3, 9–10, 25). In this study, we investigated the superoxide production inside and outside of the mitochondria of soleus and semimembranosus muscles. In most cases, superoxide production increased at both sides of the mitochondrial inner membrane with age. These results suggest that the oxidative stress is greater in muscles of old rats than adult rats, which is consistent with the reported increased oxidative stress with aging (3, 9–10, 19, 25).
Interestingly, the superoxide production outside the mitochondria of soleus muscle was not different between adult and old rats. This observation is surprising because the total superoxide production in the soleus is expected to parallel age-related increase in oxidative stress in this muscle type. Absence of change in the superoxide production outside mitochondria may also be indicative of an increase of Cu,Zn-SOD activity (i.e., located outside the mitochondrial inner membrane). However, it has been reported that Cu,Zn-SOD activity does not change in type I muscle with aging (6, 11). Thus the changes in superoxide production inside the mitochondria appear to play a critical role in age-related oxidative stress in this muscle type. This observation could not have been made in previous studies that are based on total ROS/superoxide measurements (19, 25).
Effect of HU on the mitochondrial superoxide production in skeletal muscle.
There is strong evidence of increased oxidative stress with muscle disuse, characterized by increased protein oxidation as well as lipid peroxidation, and adaptation of antioxidants (14, 21, 22, 31). Muscle disuse is also reported to induce higher production of reactive species including nitric oxide, hydrogen peroxide, and total ROS in muscles such as diaphragm, soleus, and gastrocnemius (2, 8, 17, 18, 20, 33). Consistent with these findings, the results in this study show that muscle disuse (HU) is a stressful condition for both muscles in both age groups. Regardless of age, the superoxide production inside the mitochondria of both muscle types progressively increased with 7 and 14 days of HU.
In both muscle types, increased superoxide production with HU occurred earlier in the mitochondrial matrix compared with outside of mitochondria. Specifically, 7 days of HU was sufficient to detect significant increases in the matrix superoxide, while it took 14 days of HU to detect a significant change in superoxide outside of the mitochondrial matrix. These findings suggest that the early targets of HU-related oxidative stress are inside the mitochondria.
It has been reported that prolonged mechanical ventilation (an extreme form of muscle disuse) caused depressed activities of ETC complexes as well as increased production of oxidants in diaphragm muscle (17), suggesting that muscle disuse may affect the ETC complex subunits associated with enzymatic activity and electron transfer. As aforementioned, complexes I and III in the mitochondrial ETC are the two main sites of superoxide production (26). In complex I, several sites, including iron-sulfur clusters, flavine mononucleotide-containing flavoprotein, and semiquinone-binding sites, have been proposed as the superoxide production sites of this complex (4). In complex III, superoxide production is believed to occur at the “o” center of this complex (24). Inhibition of these complexes will induce superoxide production due to the block of the electron transfer through these sites (4, 24). Thus it is possible that the affected subunits by muscle disuse (HU and other disuse conditions) are located downstream from the superoxide generation sites of complexes I and III, which will affect the electron transfer through these superoxide generation sites, thereby causing the accumulation of free radicals in these sites and consequentially the induction of superoxide production from ETC (4, 26).
The increased superoxide production may even cause more damage to the proteins of these complexes as a vicious cycle (9), induce greater oxidative stress, and possibly bring about the accumulation of damaged mitochondria with defective function. Several studies have reported a decline in mitochondrial biogenesis with prolonged muscle disuse (e.g., limb immobilization and denervation), characterized by disrupted mitochondrial genomes expression, and reduced mitochondrial content and function (12, 13, 35). Mitochondrial biogenesis involves the cellular processes both for mitochondrial synthesis and degradation (12). So the decrease in mitochondrial biogenesis with muscle disuse suggests that damaged mitochondria are not able to be degraded and replenished effectively in disused muscles, which may cause an increased proportion of defective mitochondria and contribute to the elevated superoxide production in disused muscles.
A unique experimental observation that resulted from the use of the new methodology was that the increase in superoxide production outside of the mitochondria in the semimembranosus (type II muscle fibers) is age dependent. Superoxide production in relation to the controls is significant with 7 days of HU in old animals but does not show significant change until 14 days of HU in adult animals. This finding suggests that the components outside the mitochondria of semimembranosus muscle from old animals are more susceptible to the oxidative stress induced by HU than the adult muscle. The susceptibility of type II muscle to HU may be related to the greater age-related antioxidant system deterioration of this muscle type (29).
In summary, we monitored the mitochondrial superoxide production inside and outside of the mitochondrial inner membrane with a new method based on MEKC-LIF. This method is able to reveal the unique response of each superoxide pool (inside or outside the mitochondria) to muscle aging and HU, which was not feasible in previous studies based on total ROS or superoxide measurements. The superoxide production in the mitochondrial matrix of both the soleus and semimembranosus of old rats was found greater than that of adult rats, whereas the production outside the mitochondria was unchanged between the two age groups in the soleus muscle. The superoxide production at either side of the mitochondrial inner membrane also increased with prolonged HU in both muscle types, while the increase occurred earlier inside the mitochondria (i.e., 7 days) compared with outside the mitochondria (i.e., 14 days) in soleus muscle (regardless of age) and semimembranosus muscle (adult rats). The results suggest that both aging and disuse are stressful conditions in inducing the production of mitochondrial superoxide in two muscle types, whereas the induction of superoxide production is different at either side of the mitochondrial inner membrane.
GRANTS
This project was partially funded by Grants AG-21626 (L. V. Thompson), AG-017768 (L. V. Thompson), and AG-20866 (E. A. Arriaga) from the National Institutes of Health. Chiao-nan Chen was funded by the Doctoral Dissertation Fellowship from the Graduate School at the University of Minnesota.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Janice Shoeman for technical assistance.
REFERENCES
- 1. Arbogast S, Reid MB. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol 287: R698–R705, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Arbogast S, Smith J, Matuszczak Y, Hardin BJ, Moylan JS, Smith JD, Ware J, Kennedy AR, Reid MB. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol 102: 956–964, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 87: 465–470, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37: 755–767, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chang LY, Slot JW, Geuze HJ, Crapo JD. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol 107: 2169–2179, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen CN, Brown-Borg HM, Rakoczy SG, Thompson LV. Muscle disuse: adaptation of antioxidant systems is age dependent. J Gerontol A Biol Sci Med Sci 63: 461–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen CN, Ferrington DA, Thompson LV. Carbonic anhydrase III and four-and-a-half LIM protein 1 are preferentially oxidized with muscle unloading. J Appl Physiol 105: 1554–1561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falk DJ, Deruisseau KC, Van Gammeren DL, Deering MA, Kavazis AN, Powers SK. Mechanical ventilation promotes redox status alterations in the diaphragm. J Appl Physiol 101: 1017–1024, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Gianni P, Jan KJ, Douglas MJ, Stuart PM, Tarnopolsky MA. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol 39: 1391–1400, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Gunduz F, Senturk UK, Kuru O, Aktekin B, Aktekin MR. The effect of one year's swimming exercise on oxidant stress and antioxidant capacity in aged rats. Physiol Res 53: 171–176, 2004 [PubMed] [Google Scholar]
- 11. Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech Ageing Dev 116: 33–45, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Hood DA. Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90: 1137–1157, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab 34: 465–472, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Ikemoto M, Okamura Y, Kano M, Hirasaka K, Tanaka R, Yamamoto T, Sasa T, Ogawa T, Sairyo K, Kishi K, Nikawa T. A relative high dose of vitamin E does not attenuate unweighting-induced oxidative stress and ubiquitination in rat skeletal muscle. J Physiol Anthropol Appl Human Sci 21: 257–263, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Inarrea P, Moini H, Han D, Rettori D, Aguilo I, Alava MA, Iturralde M, Cadenas E. Mitochondrial respiratory chain and thioredoxin reductase regulate intermembrane Cu,Zn-superoxide dismutase activity: implications for mitochondrial energy metabolism and apoptosis. Biochem J 405: 173–179, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inarrea P, Moini H, Rettori D, Han D, Martinez J, Garcia L, Fernandez-Vizarra E, Iturralde M, Cadenas E. Redox activation of mitochondrial intermembrane space Cu,Zn-superoxide dismutase. Biochem J 387: 203–209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med 46: 842–850, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kondo H, Nakagaki I, Sasaki S, Hori S, Itokawa Y. Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol Endocrinol Metab 265: E839–E844, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic Biol Med 25: 1089–1097, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med 35: 9–16, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Lawler JM, Song W, Kwak HB. Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve 33: 200–207, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Liu MJ, Li JX, Lee KM, Qin L, Chan KM. Oxidative stress after muscle damage from immobilization and remobilization occurs locally and systemically. Clin Orthop Relat Res 246–250, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Madsen K, Ertbjerg P, Djurhuus MS, Pedersen PK. Calcium content and respiratory control index of skeletal muscle mitochondria during exercise and recovery. Am J Physiol Endocrinol Metab 271: E1044–E1050, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279: 49064–49073, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol 293: R1159–R1168, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47: 143–183, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Quijano C, Hernandez-Saavedra D, Castro L, McCord JM, Freeman BA, Radi R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J Biol Chem 276: 11631–11638, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA 103: 15038–15043, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rogers MA, Evans WJ. Changes in skeletal muscle with aging: effects of exercise training. Exerc Sport Sci Rev 21: 65–102, 1993 [PubMed] [Google Scholar]
- 30. Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A, Da Ros T, Hurd TR, Smith RAJ, Murphy MP. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 70: 222–230, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol 289: R134–R139, 2005 [DOI] [PubMed] [Google Scholar]
- 32. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki N, Motohashi N, Uezumi A, Fukada S, Yoshimura T, Itoyama Y, Aoki M, Miyagoe-Suzuki Y, Takeda S. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest 117: 2468–2476, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Poel C, Edwards JN, Macdonald WA, Stephenson DG. Mitochondrial superoxide production in skeletal muscle fibers of the rat and decreased fiber excitability. Am J Physiol Cell Physiol 292: C1353–C1360, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Wicks KL, Hood DA. Mitochondrial adaptations in denervated muscle: relationship to muscle performance. Am J Physiol Cell Physiol 260: C841–C850, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Xu X, Arriaga EA. Qualitative determination of superoxide release at both sides of the mitochondrial inner membrane by capillary electrophoretic analysis of the oxidation products of triphenylphosphonium hydroethidine. Free Radic Biol Med 46: 905–913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med 48: 983–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: Identification of homo- and heterodimers. Free Radic Biol Med 44: 835–846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zielonka J, Vasquez-Vivar J, Kalyanaraman B. The confounding effects of light, sonication, and Mn(III)TBAP on quantitation of superoxide using hydroethidine. Free Radic Biol Med 41: 1050–1057, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3: 8–21, 2008 [DOI] [PubMed] [Google Scholar]