Abstract
In the last decade there has been increasing awareness of the virulence and changing epidemiology of Clostridium difficile (C. difficile). While the vast majority of clinical cases of C. difficile are associated with antimicrobial or nosocomial exposure, this syndrome has been well described in the absence of antibiotic use. We present an unusual case of fatal, non-antibiotic associated C. difficile colitis following Salmonella serotype Saintpaul gastroenteritis in a previously healthy young person. We review the typical risk factors for C. difficile colitis and fulminant disease. We also review the epidemiology of community-acquired C. difficile-associated disease (CA-CDAD) and highlight Salmonella infection as a potential risk factor for development of CA-CDAD.
KEY WORDS: Clostridium difficile, Salmonella, community-acquired infections
INTRODUCTION
In the last decade there has been increasing awareness of the virulence and changing epidemiology of C. difficile- associated disease (CDAD)1–3. While the vast majority of clinical cases of CDAD are associated with antimicrobial or nosocomial exposure, this syndrome has been well described in the absence of antibiotic use. A number of other risk factors have now been identified for C. difficile infection; however, antecedent Salmonella infection has only rarely been reported4. We present an unusual case of fulminant, non-antibiotic associated C. difficile colitis following Salmonella Saintpaul gastroenteritis.
CASE REPORT
A previously healthy 20-year-old woman had developed an acute gastroenteritis 3 weeks prior to admission. Her illness was characterized by fever, abdominal pain and up to ten watery, non-bloody bowel movements per day. She was evaluated by her physician, but received no antibiotics. Her stool cultures grew Salmonella Saintpaul. She reported a reduction in stool volume, fever and abdominal pain over the next 2 weeks, but continued to have loose stools.
Three days prior to admission, her symptoms recurred. She had progressive nausea and vomiting, increasing stool frequency, and severe abdominal pain. Initially, she was admitted to an outlying hospital with a temperature of 102°F, a non-surgical abdomen, and a leukocytosis of 30,000 cells/ml. Twelve hours after admission, she developed a rigid abdomen requiring surgical exploration. Laparotomy revealed 1 l of clear peritoneal fluid and marked bowel wall thickening without evidence of perforation. Sigmoidoscopy demonstrated a friable, erythematous rectal mucosa with a seropurulent exudate. She developed septic shock, requiring vasopressor support, and was transferred to our institution with a diagnosis of inflammatory bowel disease 37 h following admission to the outlying hospital. She had received two doses of ampicillin-sulbactam and one dose of gentamicin prior to transfer.
On arrival to our facility, the patient was alert and oriented. Her temperature was 38.1°C, blood pressure 90/60 mmHg, pulse 120 beats per minute, respiratory rate twenty four breaths per minute, and oxygen saturation 96% on room air. Her physical exam was remarkable for a mildly tender, distended abdomen with hypoactive bowel sounds. She was also noted to have anasarca. Admission studies revealed leukocytosis (24,500 cells/ml), hypoalbuminemia (1.8 mg/dl), and the presence of fecal leukocytes. Her hematocrit and renal function were normal. An HIV test was negative. Abdominal radiographs noted extensive bowel wall edema and “thumbprinting” (Fig. 1). Antibiotics were empirically broadened to include imipenem-cilastatin and intravenous vancomycin on arrival. A flexible sigmoidoscopy was performed within several hours of admission and demonstrated erythematous colonic mucosa with extensive pseudomembranes consistent with pseudomembranous colitis. Intravenous metronidazole and rectal vancomycin were added. Oral vancomycin could not be administered due to the severity of her ileus.
Figure 1.
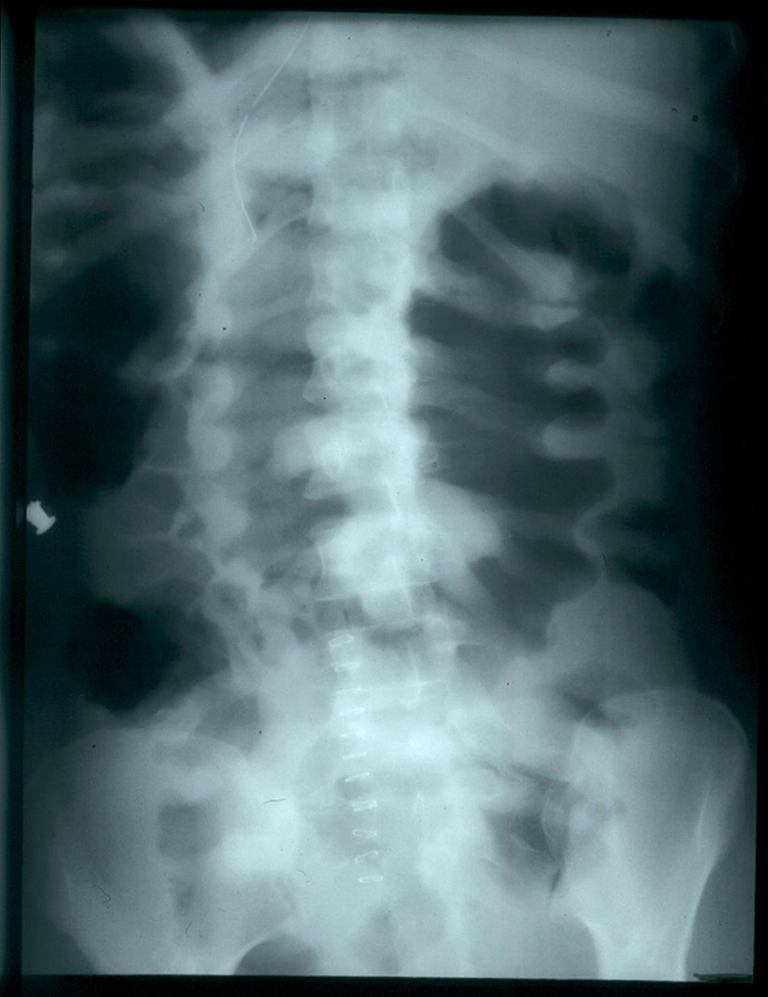
Radiographic “thumbprinting” (wide transverse bands associated with haustral fold thickening).
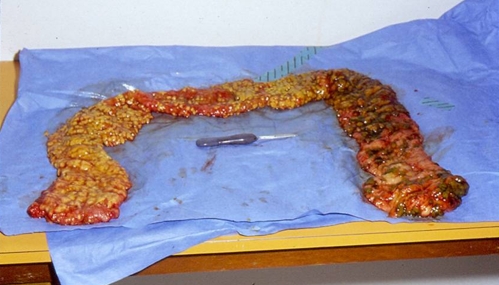
On hospital day 3 the C. difficile cell culture cytotoxin assay returned positive, and Salmonella Saintpaul was isolated from her admission stool specimen. She developed worsening sepsis and respiratory failure, requiring a total colectomy on hospital day 5 (Fig. 2). Pathology revealed extensive pseudomembranes and mucosal friability throughout the entire colon, without evidence of perforation. Despite the colectomy, she continued to clinically deteriorate and died because of complications related to acute respiratory distress syndrome and sepsis on hospital day 14. Tissue gram stain of the involved colon revealed numerous gram-positive bacilli consistent with C. difficile.
Figure 2.
Gross specimen showing extensive pseudomembranes on the colonic mucosa.
DISCUSSION
This case illustrates an unusual case of fulminant, non-antibiotic associated C. difficile infection following Salmonella gastroenteritis in an otherwise healthy young person. Typically, CDAD presents with mild to moderate, non-bloody diarrhea that may be accompanied by lower abdominal cramping3. Systemic symptoms, such as fever, abdominal pain and distention, and watery diarrhea, are hallmarks of more severe disease. CDAD has been thought to result from antibiotic exposure with alteration of the normal colonic flora, followed by colonization and subsequent proliferation of this organism and expression of its toxin(s). The toxins are essential for the development of diarrhea and colitis, though it is not known why some people acquire asymptomatic colonization and others develop frank C. difficile colitis. Both host factors and virulence factors have been implicated3,5.
Non-antibiotic associated C. difficile has been well described in the literature6–8. In one study 26% of C. difficile cases were non-antibiotic associated7. Other known risk factors for the development of C. difficile infection include advanced age and hospitalization, gastrointestinal procedures and surgery, and exposure to certain non-antibiotic medications1,3. For example, use of proton pump inhibitors has been associated with an increased risk of CDAD in both inpatients and outpatients9–11; however, the mechanism has not been elucidated, and not all studies have observed this association12.
C. difficile is considered primarily a nosocomial infection; however, the ambulatory incidence is considerable, approximately 7.6–29 cases per 100,000 population depending on the population studied and definition used10,12,13. Recent reports have demonstrated that community-acquired C. difficile-associated disease (CA-CDAD) may be on the rise8,10. Possible reasons for this increase include increasing exposure to antibiotic and non-antibiotic medications, exposure to more virulent strains, increasing health-care exposures, and more vigilant testing and reporting among providers1,7,10,13, In addition, The United States Centers for Disease Control and Prevention (CDC) has reported an increase in severe CA-CDAD infection in populations previously considered to be at low risk13. Wilcox and colleagues report that approximately one-third of CA-CDAD cases had neither recent antibiotic exposure nor hospitalization in the preceding 6 months12.
Fulminant C. difficile colitis, defined as colitis accompanied by systemic toxic effects and shock and resulting in need for colectomy or death, occurs in approximately 3% of hospitalized patients with CDAD14,15. In a retrospective review by Sailhamer and colleagues, the mortality rate associated with fulminant C. difficile colitis was 34.7%. Independent risk factors for mortality include age >70 years, severe leukocytosis or leukopenia (white blood cell count ≥35,000 cells/ml or <4,000 cells/ml) or bandemia (neutrophil bands ≥10%), and cardiorespiratory failure. Patients admitted to a surgical service were more likely to undergo early colectomy and experienced lower mortality rates (12.8% vs. 39.3%) than those admitted to nonsurgical departments16.
Treatment of CDAD depends on disease severity. First-line therapy for an initial episode of mild CDAD is oral metronidazole17,18. Oral vancomycin therapy is now the preferred first-line agent for severe or fulminant CDAD, and for patients with multiple relapses17–19. There is not a standard definition of “severe” CDAD. A severity score was developed by Zar and colleagues in which one point is given for age >60 years, temperature >38.3°C, an albumin level of <2.5 mg/dl or white blood cell count of >15,000 cells/mm3, and two points for presence of pseudomembranous colitis or hospitalization in the intensive care unit19. Severe CDAD is diagnosed in those patients with a severity score of ≥2 points. The Infectious Disease Society of America (IDSA) uses the following criteria to define severe CDAD: leukocytosis of >15,000 cells/mm or a serum creatinine level ≥1.5 times the premorbid level18. A multi-pronged approach of oral and rectal vancomycin, as well as intravenous metronidazole can also be used for severe and fulminant disease. The optimal medical treatment for these patients is unclear, and early surgical, gastroenterology and infectious disease consultation is recommended16,17.
The most distinctive feature of this case is that C. difficile colitis was preceded by Salmonella gastroenteritis. Non-typhoidal Salmonella infections are usually self-limited illnesses, and typically not treated with antibiotics. Co-infection with both C. difficile and Salmonella has been reported previously in two patients; however, unlike our patient, both experienced a relatively benign clinical course4. A small proportion of patients with non-typhoidal Salmonella infections may develop more severe syndromes, including one case report of pseudomembranous colitis20.
Though Salmonella is not known to precipitate CDAD, it has been shown to result in intestinal inflammation and alter the intestinal flora5. It is by this latter mechanism that antibiotics are known to result in CDAD; thus, our hypothesis is that the patient’s prior infection with Salmonella served to alter her normal colonic flora enough to promote the growth of C. difficile. A C. difficile toxin was not obtained at the patient’s incident visit, so it is unknown whether she was co-infected at that time. Her continued positive stool culture for Salmonella was attributed to prolonged stool shedding of bacteria.
To our knowledge, this is the first case of CA-CDAD in which the preceding risk factor appears to have been Salmonella infection, and which then progressed to fulminant colitis and death. Why our patient, an otherwise healthy young person, experienced such a severe course remains unknown. Existing guidelines do not currently recommend empiric C. difficile treatment for all hospitalized patients with colitis. If there is clinical suspicion of C difficile, treatment should be started while the test is pending17,18. In retrospect, perhaps oral vancomycin or metronidazole should have been started earlier in our patient; however, even with appropriate diagnosis and management, fulminant C. difficile colitis remains a highly lethal disease16. Further research is needed.
This case also has implications for the management of community-acquired diarrhea in patients who lack usual risk factors for CDAD. The CDC recommends that clinicians consider the diagnosis of CDAD in all patients with severe diarrhea even if they lack traditional risk factors such as recent hospitalization or antimicrobial use13. The recently updated IDSA Guidelines for C. difficile infection recommend that when severe or complicated infection is suspected, clinicians should initiate empirical treatment as soon as the diagnosis is suspected18.
Acknowledgements
The manuscript has not been simultaneously submitted elsewhere. The manuscript was prepared solely by the authors listed, and the authors listed on the manuscript have contributed sufficiently to the article to be included as authors.
Conflict of Interest None disclosed.
References
- 1.Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758–64. doi: 10.7326/0003-4819-145-10-200611210-00008. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. Clostridium difficile - More difficult than ever. N Engl J Med. 2008;359(18):1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Sunenshine RH, McDonald LC. Clostridium difficile-associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2):187–97. doi: 10.3949/ccjm.73.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Grinblat JWA, Grosman B, Dicker D, Beloosesky Y. Diarrhea in elderly patients due to Clostridium difficile associated with Salmonella and Shigella infection. Arch Gerontol Geriatr. 2004;39:277–82. doi: 10.1016/j.archger.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 5.Santos RL, Raffatellu M, Bevins CL, et al. Life in the inflamed intestine, Salmonella style. Trends Microbiol. 2009;17(11):498–506. doi: 10.1016/j.tim.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dial SMDM, Kezouh AP, Dascal AMD, Barkun AMDM, Suissa SP. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ. 2008;179(8):767–72. doi: 10.1503/cmaj.071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbut F, Decre D, Lalande V, et al. Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP-ribosyltransferase)-producing strains. J Med Microbiol. 2005;54(2):181–5. doi: 10.1099/jmm.0.45804-0. [DOI] [PubMed] [Google Scholar]
- 8.Surveillance for community-associated Clostridium-difficile--Connecticut, 2006. MMWR. 2008;57(13):340–43. [PubMed]
- 9.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33–8. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dial S, Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989–95. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 11.Dial S, Delaney JAC, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ. 2006;175(7):745–8. doi: 10.1503/cmaj.060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox MH, Mooney L, Bendall R, Settle CD, Fawley WN. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother. 2008;62(2):388–96. doi: 10.1093/jac/dkn163. [DOI] [PubMed] [Google Scholar]
- 13.Severe Clostridium difficile—associated disease in populations previously at low risk. MMWR 2005;54(47):1201–5. [PubMed]
- 14.Rubin M, Bodenstein L, Kent K. Severe Clostridium difficile colitis. Dis Colon Rectum. 1995;38:350–4. doi: 10.1007/BF02054220. [DOI] [PubMed] [Google Scholar]
- 15.Dallal R, Harbrecht B, Boujoukas A, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363–72. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433–9. doi: 10.1001/archsurg.2009.51. [DOI] [PubMed] [Google Scholar]
- 17.Gerding DN, Muto CA, Owens JRC. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46(s1):S32–42. doi: 10.1086/521860. [DOI] [PubMed] [Google Scholar]
- 18.Cohen Stuart H, Gerding Dale N, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Contr Hosp Epidemiol 31(5):431-55. [DOI] [PubMed]
- 19.Zar FA, Bakkanagari SR, Moorthi KMLST, Davis MA. A Comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-Associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 20.Mönkemüeller KPI, Walther F, Peitz U, Fry LC, Malfertheiner P. Pseudomembranous colitis due to Salmonella enterica serotype infantis. Endoscopy. 2006;38(05):546. doi: 10.1055/s-2006-925343. [DOI] [PubMed] [Google Scholar]