Abstract
An assessment of the total protein composition of filovirus (ebolavirus and marburgvirus) virions is currently lacking. In this study, liquid chromatography-linked tandem mass spectrometry of purified ebola and marburg virions was performed to identify associated cellular proteins. Host proteins involved in cell adhesion, cytoskeleton, cell signaling, intracellular trafficking, membrane organization, and chaperones were identified. Significant overlap exists between this data set and proteomic studies of disparate viruses, including HIV-1 and influenza A, generated in multiple cell types. However, the great majority of proteins identified here have not been previously described to be incorporated within filovirus particles. Host proteins identified by liquid chromatography-linked tandem mass spectrometry could lack biological relevance because they represent protein contaminants in the virus preparation, or because they are incorporated within virions by chance. These issues were addressed using siRNA library-mediated gene knockdown (targeting each identified virion-associated host protein), followed by filovirus infection. Knockdown of several host proteins (e.g. HSPA5 and RPL18) significantly interfered with ebolavirus and marburgvirus infection, suggesting specific and relevant virion incorporation. Notably, select siRNAs inhibited ebolavirus, but enhanced marburgvirus infection, suggesting important differences between the two viruses. The proteomic analysis presented here contributes to a greater understanding of filovirus biology and potentially identifies host factors that can be targeted for antiviral drug development.
An important advance in virology in recent years has been the realization that multiple host proteins are incorporated into viral particles. This observation is supported by studies with multiple viruses (e.g. HIV-1 and influenza A), produced in multiple cell types (1–3). Interestingly, there is substantial overlap between host proteins identified in these diverse studies, suggesting a significant role for these gene products in viral biology (4). An assessment of the total protein composition of viral particles can be provided by proteomic techniques, including liquid chromatography tandem mass spectroscopy (LC-MS/MS)1. For example, Shaw et al., performed LC-MS/MS analysis on protein lysates from highly purified influenza A viral particles (3). Along with nine virus-encoded proteins, 36 host proteins were identified, including highly abundant and cytoplasm-localized proteins (β-actin, tubulin, and GAPDH), as well as membrane-bound proteins (CD9 and CD81). Chertova et al., identified a larger cohort of 253 host proteins incorporated into HIV-1 virions (1). The functional significance of most of these virion-localized host proteins remains to be elucidated. However, studies of this type promise to teach us something about viral biology and important host-pathogen interactions. For example, examination of host proteins incorporated into the viral envelope of HIV-1 can provide information about the particular type of host cell producing the virus, both in vitro and in vivo (5).
Filoviruses (ebolaviruses and marburgvirus) cause severe hemorrhagic fever in humans (and nonhuman primates) with exceptionally high case fatality rates. Confirmed human marburgvirus infections, occurring between 1967 and 2007, yielded a case fatality rate of 81.9% (6). During this same time period, the combined case fatality rate for Zaire and Sudan ebolavirus infections was 69.8%. Fortunately, outbreaks in human populations are rare, and can be contained with strict patient isolation and barrier nursing practices. Although there are currently no licensed vaccines or therapeutics to prevent or treat filovirus infections, progress is being made in this area (7, 8). In a recent report, antisense-based treatments protected nonhuman primates against lethal Zaire ebolavirus and marburgvirus infections, and have received approval from U.S. Food and Drug Administration to proceed with human clinical trials (7). A threat to public health and safety continues to exist because of sporadic filovirus outbreaks in endemic areas, and the potential for use of these agents as bioweapons (9).
Filovirions are filamentous particles with near uniform diameter and variable elongated shape and length (10). The filovirus genome codes for seven proteins; the nucleoprotein NP, the RNA-dependent RNA polymerase L, glycoprotein GP1,2, and the viral proteins (VP) VP35, VP24, VP30, and VP40 (11). Ebolaviruses, but not marburgvirus, additionally express two secreted forms of GP1,2, sGP and ssGP (12, 13). NP, L, VP35, and VP30 make up the nucleocapsid surrounding the linear 19 kb, single-stranded, negative-sense RNA genome. VP40 is the major matrix protein and functions in viral assembly and budding (14). VP24 is a minor matrix protein that can interact with the viral membrane and has a role in nucleocapsid formation (15–18). GP is found embedded within, and extending from the viral membrane. Filovirus entry, transcription, trafficking, assembly, and budding have been studied in some detail (14, 19–24). Importantly, several investigations have described specific host-pathogen interactions, implicating the involvement of host proteins in the filovirus lifecycle (25). For example, interactions between VP40 and host proteins TSG101 and Nedd4 may be critical for viral assembly and budding (14). Interactions with host kinases (IKKε and TBK-1) have been described for VP35 (26). Additionally, filovirus NP and VP30 proteins are highly phosphorylated, suggesting a direct interaction with a host kinase (20).
As with other viruses, there is evidence that specific host proteins are incorporated into filovirus particles. Kolesnikova et al., detected actin, Lamp-1, and Rab11 in purified marburgvirus virions by Western blot analysis (27). Actin has also been detected in marburgvirus particles by immunoelectron microscopy (28). However, an assessment of the total protein composition of filovirions has not yet been reported. To further characterize the host protein content of filovirions, we performed LC-MS/MS on protein lysates from sucrose-band-purified ebola (Zaire ebolavirus, Kikwit isolate) and marburg (Lake Victoria marburgvirus, Musoke isolate) virions. This analysis successfully identified each ebola- and marburgvirus-encoded protein. Additionally, 66 virion-associated host proteins were identified. To examine biological relevance, we hypothesized that host proteins specifically incorporated into virions would be essential for productive viral infection. To address this idea, a custom siRNA library, targeting each of the putative virion-associated proteins, was used to identify host proteins that play a role during ebola or marburgvirus infection. Eleven proteins influencing viral infection or production were identified in this manner. Interestingly, knockdown of select host genes produced opposite effects on ebolavirus versus marburgvirus infection, suggesting potentially important differences between the two viruses. These data provide a better understanding of host pathways and proteins utilized during the filovirus life cycle and may provide novel targets for antivirus drug development.
EXPERIMENTAL PROCEDURES
Viral Sample Preparation and LC-MS/MS
Experiments with infectious viruses were carried out in biosafety level 4 maximum-containment laboratories (USAMRIID, Fort Detrick, MD). High-dose ionizing radiation-inactivated ebolavirions (Zaire ebolavirus, Kikwit isolate), or marburgvirions (Lake Victoria marburgvirus, Musoke isolate) were combined in a 1:1 ratio with SDS-PAGE loading buffer and heated to 95 °C for 15 min. Samples were then cooled and loaded onto a 10% Tris-HCl SDS-PAGE gel (BioRad) and subjected to electrophoresis at 150 V for 60 min. Proteins in the gel were stained with Coomassie blue overnight and then destained in water. Gel slices (20 per lane) were macerated in 200 μl of 50% acetonitrile (ACN) in 50% ammonium bicarbonate (50 mm, pH 8.0) and incubated for 15 min. The gel mixture was centrifuged at 12,000 × g with the wash being discarded. The process was repeated three times following which 100 μl of ACN was added. The gel mixture was dried in a speed vacuum concentrator, resuspended in enough trypsin digest solution (Promega) to cover the gel material, and then incubated overnight at 37 °C. The amount of trypsin digest solution was recorded and on the following day an equal volume of 50% ACN was added. The mixture was then incubated for 15 min at room temperature, centrifuged, and the supernatant collected. After repeating the process, the supernatants were pooled and dried in a speed vacuum concentrator following which 100 μl of ACN was added. The digest was again dried, repeating the process three times. Samples were desalted using a C-18 PepClean column (Pierce Biotechnology, Inc, Rockford, IL) according to manufacturer's instructions. Samples were then dried and stored at 0 °C.
LC-MS/MS analyses were performed using an Agilent 1100 nanopump coupled to a 2000 QTRAP with nanospray source (Applied Biosystems, Foster City, CA) controlled by Analyst software (Applied Biosystems, version 1.4). Each sample was resuspended in 10 μl of 5% ACN and 0.1% formic acid in water and 8 μl was loaded onto the LC-MS/MS system. An LC gradient of 5%–60% was generated with 95% ACN and 0.1% formic acid in water over 35 min. MS/MS peak lists were generated by the Analyst software (version 1.4). All MS/MS data were searched against the theoretical spectra of the NCBI nr database (release 2.2.12 of 9–7-2005) using taxonomies Homo Sapiens (255,836 sequences searched), Zaire ebolavirus (9 sequences searched), or Lake Victoria marburgvirus (7 sequences searched) using Mascot software (Matrix Science, version NT2.0, Torrance, CA). The search parameters included a maximum of one missed cleavage by trypsin, variable modification of oxidized methionine, charge state of + 2 to + 3, an MS tolerance of ±1.2 Da, and an MS/MS tolerance of ±0.8 Da. The resulting data were then filtered for low scoring hits by rejecting single peptides with a score lower than 42 for hits observed after searching the H. sapiens database.
Over-Representation Analysis
The PANTHER classification system (version 6.1, release date January 6, 2009) and analysis package was used to identify significantly over-represented gene functional groups in our proteomics dataset (http://www.pantherdb.org/) (29, 30). Sixty-five of 66 virion-associated host genes were present in the PANTHER ontologies. Annotation enrichment was measured using the right-tailed hypergeometric distribution (Fisher's exact test), with the background equal to the entire set of Homo-sapiens annotated genes. p Values were corrected for multiple testing using a Bonferonni procedure, described on the PANTHER website, where the number of tests is dependent on the level of the biological process or molecular function annotation. A p value of ≤ 0.05 was considered significant.
siRNA Transfection and Virus Infection
A custom siRNA library, targeting the transcript of each putative filovirion-associated host protein was designed and obtained from Dharmacon (Lafayette, CO). For each gene, a pool of four individual siRNAs was provided lyophilized in v-bottom 96-well plates. siRNA pools were resuspended in 1× siRNA buffer (Dharmacon) at a concentration of 1 pmol/μl. 293T cells were transfected with 75 nm (final concentration) siRNA and 0.5 μl Lipofectamine 2000 (Invitrogen) per well of a 96-well plate. Dharmacon nontargeting siRNA pool was added to the library plate and transfected with the same parameters as a control.
Twenty-four hours following siRNA transfection, cells were moved to a BIOSAFETY LEVEL 4 maximum-containment laboratory and infected with ebolavirus at a multiplicity of infection of three, or marburgvirus (multiplicity of infection of one). Three days following infection, cells were fixed by the addition of 10% neutral buffered formalin, and incubation at 4 °C for 3 days. For hit-confirmation experiments, 100 μl of cell culture supernatant was collected, before cell fixation, and added to 300 μl Trizol LS (Invitrogen).
Quantitative Real-Time PCR
To measure the viral titer in cell culture supernatants, RNA was isolated from Trizol samples using the MegaMax 96 RNA extraction kit (Ambion, Austin, TX). Viral RNA was detected using custom virus-specific TaqMan primer/probe sets (Applied Biosystems), 5 μl input RNA per 20 μl reaction, RNA UltraSense One-Step Quantitative RT-PCR system (Invitrogen), and a 7900HT Fast real-time PCR instrument (Applied Biosystems). Serial dilutions of RNA from viral samples of known titer (102–107 genomic copies per reaction) were used as standards. PCR reactions were set up using the parameters and cycling conditions provided by the RNA UltraSense kit (Invitrogen).
To check for knockdown of target transcripts, RNA was harvested 48 h following transfection of siRNAs using the Qiagen RNeasy mini RNA isolation kit. Transcript level was measured as described above, but using inventoried TaqMan gene expression assays (Applied Biosystems) and 50 ng of input RNA per reaction.
Automated Fluorescence-Microscopy Image Capture and Data Analysis
Following fixation for 72 h in 10% formalin, cells were washed three times in PBS, and then blocked for 1 h at room temperature in PBS with 3% bovine serum albumin (Sigma Aldrich). Cells were then incubated with mouse monoclonal antibodies detecting ebola (antibody 6D8) or marburg (antibody 5E2) GP protein at 1:1000 and 1:250, respectively, for 2 h at room temperature. Cells were washed in PBS and incubated with goat anti-mouse Alexa-488-conjugated secondary antibody (Invitrogen) (1:1000, 1 h, room temperature). Cells were counter stained with Hoechst 33342 (to visualize nuclei), and CellMask Deep Red cytoplasmic stain (Invitrogen). Acquisition and analysis of fluorescence microscopy images were carried out on an Opera confocal high-content screening instrument (model 3842-Quadrapole Excitation High Sensitivity, Perkin Elmer) running Acapella image analysis software (Perkin Elmer). Additionally, a Discovery-1 automated fluorescence microscope (Molecular Devices, Sunnyvale, CA) using MetaExpress software was utilized. In each case, a 10× air objective was used for the collection of six to nine image fields per well. Approximately 15,000 total cells are analyzed per well. Data from one instrument are consistent with the other, and a particular instrument was chosen for use on the basis of availability. For each well, the total number of cells, and the number of GP1,2-positive cells were recorded. Threshold levels for detection are set by examining control cells without virus infection, or cells stained with isotype control antibodies.
RESULTS
Identification of Host Proteins Associated with Purified ebola and marburg Virions
A proteomic analysis of purified ebola and marburg virions was carried out using liquid chromatography-linked tandem mass spectroscopy (LC-MS/MS), with the aim of identifying host proteins that are specifically incorporated into filovirus particles. Ebola or marburg viral particles produced in African green monkey kidney epithelial cells (VeroE6) were collected and band-purified on a sucrose gradient. The use of VeroE6 cells allows viral replication to high titer, and because of extensive genomic and protein sequence homology enables successful protein identification by comparison to the human protein database. Purified viral particles were dissolved in SDS-PAGE loading buffer and size fractionated by gel electrophoresis. Following staining to visualize the proteins, 20 gel slices were excised from each lane and subjected to trypsin digestion. Resulting tryptic peptides were analyzed by LC-MS/MS and identified using Mascot software. LC-MS/MS data were compared against the Zaire ebolavirus, Lake Victoria marburgvirus, and the human genome databases to identify proteins present in the viral particles.
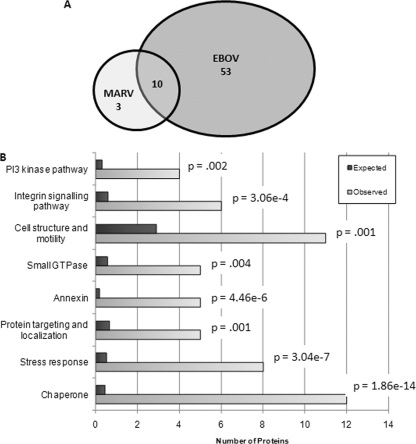
As expected, each ebola- and marburg-encoded protein was detected by the LC-MS/MS analysis (not shown). Additionally, peptides from the viral protein lysates mapped to 70 human protein accession numbers. We were able to map these protein sequences to 66 unique human proteins (Table I). If a protein was identified by more than one peptide, the highest Mascot sore is given. For proteins identified with one peptide, the peptide sequence and additional information is provided (Table II). Of the 66 host proteins, most were identified only in ebola virions. Far fewer host proteins were identified in marburg virions (Fig. 1A). The results of a gene-function over-representation analysis suggest a nonrandom incorporation of host proteins into filovirions (Fig. 1B). The proteins listed in Table I were mapped to various molecular functions, biological processes, and biological pathways using the PANTHER classification system (http://www.pantherdb.org) (29). The expected number of genes in each category is calculated by examining 66 proteins chosen at random from the human genome. These expected frequencies differ from the observed functional group distributions for the 66 proteins identified in our examination of filovirus particles. Statistical analysis reveals that several distinct gene functional classifications are significantly over-represented in our dataset (Fig. 1B) (30).
Table I. Host proteins identified in ebola and/or marburg virions by LC-MS/MS. Proteins listed in this table were identified in ebola virions only, unless otherwise noted.
| Protein Class | Gene Symbol | Gene ID | Protein Name | Accession | High# Score | Number of Unique Peptides | % Coverage |
|---|---|---|---|---|---|---|---|
| Chaperone | DNAJB2 | 3300 | DnaJ (Hsp40) homolog, subfamily B, member 2 | gi 250082 | 52 | 1 | 3 |
| HSPA1A | 3303 | Heat shock 70kDa protein 1A | gi 386785 | 212 | 3 | 5 | |
| HSPA1L | 3305 | Heat shock 70kDa protein 1-like | gi 188492 | 78 | 1 | 1 | |
| HSPA5 | 3309 | Heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa), BiP, GRP78 | gi 1143492 | 126 | 1 | 3 | |
| HSPA8 | 3312 | Heat shock 70kDa protein 8 | gi 5729877 | 375 | 5 | 10 | |
| HSPB1 | 3315 | Heat shock 27kDa protein 1 | gi 662841 | 48 | 2 | 10 | |
| HSP90AA1 | 3320 | Heat shock protein 90kDa α(cytosolic), class A member 1 | gi 61656605 | 279 | 1 | 5 | |
| HSP90AB1 | 3326 | Heat shock protein 90kDa α(cytosolic), class B member 1 | gi 306891 | 350 | 4 | 7 | |
| VCP | 7415 | Valosin-containing protein | gi 6005942 | 56 | 1 | 1 | |
| YWHAG | 7532 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, γ polypeptide. 14–3-3γ | gi 5726310 | 78 | 1 | 4 | |
| YWHAZ | 7534 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, ζ polypeptide. 14–3-3ζ | gi 21735625, gi 4262000 | 137 | 2 | 9 | |
| TRAP1 | 10131 | TNF receptor-associated protein 1 | gi 1082886 | 88 | 1 | 2 | |
| YWHAQ | 10971 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, θ polypeptide | gi 5803227 | 123 | 2 | 9 | |
| Cytoskeleton, Actin, Actin-related, and Actin-binding | ACTB* | 60 | Actin, β | gi 178024, gi 28336 | 183 | 2 | 6 |
| ACTG1* | 71 | Actin, γ1 | gi 178045 | 214 | 2 | 15 | |
| TUBA1A§ | 7846 | Tubulin, α1a | gi 37492 | 51 | 1 | 2 | |
| ACTN4 | 81 | Actinin, α4 | gi 2804273, gi 3157976 | 111 | 1 | 3 | |
| ACTN1 | 87 | Actinin, α1 | gi 28334 | 116 | 2 | 2 | |
| CFL1* | 1072 | Cofilin 1 (non-muscle) | gi 5031635 | 86 | 1 | 12 | |
| PFN1 | 5216 | Profilin 1 | gi 1943532 | 55 | 1 | 8 | |
| VIM | 7431 | Vimentin | gi 37852 | 257 | 4 | 10 | |
| TAGLN2 | 8407 | Transgelin 2 | gi 4507357 | 48 | 1 | 5 | |
| Cell Adhesion | CLDN3* | 1365 | Claudin 3 | gi 4502875 | 67 | 1 | 7 |
| GPC4 | 2239 | Glypican 4 | gi 3420277 | 85 | 1 | 2 | |
| ITGA3 | 3675 | Integrin, α3 (antigen CD49C, α3 subunit of VLA-3 receptor) | gi 220141 | 67 | 1 | <1 | |
| VTN | 7448 | Vitronectin | gi 36573 | 69 | 1 | 3 | |
| CLDN1* | 9076 | Claudin 1 | gi 4559278 | 52 | 1 | 7 | |
| G Protein, small GTPase, and related | ARF1 | 375 | ADP-ribosylation factor 1 | gi 1065362 | 258 | 2 | 27 |
| ARF6 | 382 | ADP-ribosylation factor 6 | gi 7767049 | 75 | 1 | 6 | |
| GNB2 | 2783 | Guanine nucleotide binding protein (G protein), beta polypeptide 2 | gi 306785 | 69 | 1 | 2 | |
| RAB5C | 5878 | RAB5C, member RAS oncogene family | gi 642532 | 117 | 2 | 10 | |
| RAN | 5901 | RAN, member RAS oncogene family | gi 4092054 | 56 | 1 | 5 | |
| RAB11B | 9230 | RAB11B, member RAS oncogene family | gi 4758986 | 57 | 1 | 5 | |
| GNB2L1 | 10399 | Guanine nucleotide binding protein (G protein), beta polypeptide 2-like 1 | gi 62896535 | 64 | 1 | 3 | |
| Annexins | ANXA1* | 301 | Annexin A1 | gi 442631 | 68 | 1 | 3 |
| ANXA2* | 302 | Annexin A2 | gi 16306978, gi 18645167 | 347 | 5 | 19 | |
| ANXA4* | 307 | Annexin A4 | gi 189617 | 135 | 2 | 6 | |
| ANXA5 | 308 | Annexin A5 | gi 809190 | 186 | 2 | 12 | |
| Ribosomal Proteins | RPL3 | 6122 | Ribosomal protein L3 | gi 337580 | 61 | 1 | 3 |
| RPL5 | 6125 | Ribosomal protein L5 | gi 1173054 | 46 | 1 | 4 | |
| RPL18 | 6141 | Ribosomal protein L18 | gi 4506607 | 96 | 1 | 6 | |
| RPS6 | 6194 | Ribosomal protein S6 | gi 337514 | 75 | 1 | 4 | |
| Other Enzymes | ATP1A1§ | 476 | ATPase, Na+/K+ transporting, α1 polypeptide | gi 179212 | 48 | 1 | 1 |
| ATP5A1 | 498 | ATP synthase, H+ transporting, mitochondrial F1 complex, α subunit 1, cardiac muscle | gi 15030240 | 155 | 2 | 5 | |
| LDHA§ | 3939 | Lactate dehydrogenase A | gi 5031857 | 46 | 1 | 4 | |
| GGT1 | 2678 | γ-glutamyltransferase 1 | gi 306767 | 177 | 2 | 4 | |
| GGT3 | 2679 | γ-glutamyltransferase 3 | gi 183136 | 93 | 1 | 6 | |
| GLUD2 | 2747 | Glutamate dehydrogenase 2 | gi 31815 | 46 | 1 | 2 | |
| NTRK1 | 4914 | Neurotrophic tyrosine kinase, receptor, type 1 | gi 37403 | 223 | 3 | 5 | |
| PKM2 | 5315 | Pyruvate kinase, muscle | gi 35505 | 99 | 2 | 3 | |
| PLG | 5340 | Plasminogen | gi 38051823 | 47 | 1 | 1 | |
| UBE2N | 7334 | Ubiquitin-conjugating enzyme E2N (UBC13 homolog, yeast) | gi 18017605 | 84 | 1 | 7 | |
| PDIA4 | 9601 | Protein disulfide isomerase family A, member 4 | gi 4758304 | 63 | 1 | 1 | |
| Vesicular Trafficking | TSG101 | 7251 | Tumor susceptibility gene 101 | gi 5454140 | 42 | 1 | 2 |
| VPS28 | 51160 | Vacuolar protein sorting 28 homolog (S. cerevisiae) | gi 7705885 | 54 | 1 | 4 | |
| FAM125A | 93343 | Family with sequence similarity 125, member A | gi 39644809 | 108 | 1 | 5 | |
| Miscellaneous | A2M* | 2 | α-2-macroglobulin | gi 177872 | 64 | 1 | 1 |
| GRB2 | 2885 | Growth factor receptor-bound protein 2 | gi 4504111 | 43 | 1 | 4 | |
| HLA-C | 3107 | Major histocompatibility complex, class I, C | gi 28357 | 53 | 1 | 2 | |
| PLTP | 5360 | Phospholipid transfer protein | gi 5453914 | 48 | 1 | 2 | |
| UBC | 7316 | Ubiquitin C | gi 2627129 | 69 | 1 | 2 | |
| MLF2 | 8079 | Myeloid leukemia factor 2 | gi 4885487 | 74 | 1 | 4 | |
| HIST1H2BO | 8348 | Histone cluster 1, H2bo | gi 31979 | 67 | 1 | 11 | |
| HIST2H4A | 8370 | Histone cluster 2, H4a | gi 32097 | 72 | 1 | 11 | |
| KIAA1529 | 57653 | KIAA1529 | gi 7959325 | 50 | 1 | 1 | |
| DCD* | 117159 | Dermcidin | gi 16751921 | 69 | 1 | 10 |
* Proteins identified in both ebola and marburg virions.
§ Proteins identified in marburg only.
# A Mascot score above 54 is considered highly significant. Scores below 54 are statistically significant, but of lower confidence.
Table II. Proteins identified by LC-MS/MS with one peptide.
| Gene Symbol | Peptide ID Sequence | Precursor m/z | Precursor charge | Expect Value |
|---|---|---|---|---|
| DNAJB2 | R.VEVEEDGQLK.S | 573.2 | 2 | 0.0067 |
| HSPA1L | K.VEIIANDQGNR.T | 614.72 | 2 | 1.30E-05 |
| VCP | R.EVDIGIPDATGR.L | 621.7718 | 2 | 0..26 |
| YWHAG | R.YLAEVATGEK.R | 541.17 | 2 | 1.30E-05 |
| TRAP1 | GVVDSEDIPLNLSR | 757.26 | 2 | 1.20E-06 |
| TUBA1A | DVNAAIATIK | 508.22 | 2 | 0.01 |
| CFL1 | EILVGDVGQTVDDPYATFVK | 1083.42 | 2 | 1.30E-06 |
| PFN1 | DSPSVWAAVPGK | 607.23 | 2 | 0.0028 |
| TAGLN2 | NFSDNQLQEGK | 640.14 | 2 | 0.012 |
| CLDN3 | VYDSLLALPQDLQAAR | 886.87 | 2 | 0.00014 |
| GPC4 | TFAQGLAVAGDVVSK | 731.76 | 2 | 2.50E-06 |
| ITGA3 | K.TVEDVGSPLK.Y | 523.1049 | 2 | 0.0004 |
| VTN | DVWGIEGPIDAAFTR | 823.8 | 2 | 9.60E-05 |
| CLDN1 | VFDSLLNLSSTLQATR | 883.32 | 2 | 0.0043 |
| ARF6 | FNVWDVGGQDK | 632.74 | 2 | 3.30E-05 |
| GNB2 | LLVSASQDGK | 505.19 | 2 | 1.50E-04 |
| RAN | LVLVGDGGTGK | 508.25 | 2 | 0.0034 |
| RAB11B | VVLIGDSGVGK | 522.35 | 2 | 0.0025 |
| GNB2L1 | DETNYGIPQR | 596.74 | 2 | 0.0005 |
| ANXA1 | TPAQFDADELR | 632.18 | 2 | 0.00012 |
| RPL3 | K.NNASTDYDLSDK.S | 671.66 | 2 | 0.00056 |
| RPL5 | R.FPGYDSESK.E | 515.7 | 2 | 0.028 |
| RPL18 | K.ILTFDQLALDSPK.G | 730.8 | 2 | 2.4E-07 |
| RPS6 | K.DIPGLTDTTVPR.R | 642.7 | 2 | 2.30E-05 |
| ATP1A1 | SPDFTNENPLETR | 760.24 | 2 | 0.012 |
| LDHA | DLADELALVDVIEDK | 829.32 | 2 | 0.019 |
| GGT3 | NIDQAVTAALETR | 701.28 | 2 | 5.2E-07 |
| GLUD2 | DDGWEVIEGYR | 713.22 | 2 | 0.019 |
| PLG | LSSPADITDK | 523.68 | 2 | 0.027 |
| UBE2N | TNEAQAIETAR | 602.23 | 2 | 4.10E-06 |
| PDIA4 | VDATAETDLAK | 567.21 | 2 | 0.00059 |
| TSG101 | DGTISEDTIR | 553.69 | 2 | 0.067 |
| VPS28 | AMDEIQPDLR | 594.21 | 2 | 0.0042 |
| FAM125A | DMQGLSLDAASQPSK + OX (M) | 782.23 | 2 | 9.1E-09 |
| A2M | LPPNVVEESAR | 605.76 | 2 | 0.0004 |
| GRB2 | ATADDELSFK | 548.74 | 2 | 0.052 |
| HLA-C | DYIALNEDLR | 611.27 | 2 | 0.0065 |
| PLTP | AVEPQLQEEER | 664.21 | 2 | 0.012 |
| UBC | TITIEVEPSDTIENVK | 894.34 | 2 | 1.40E-05 |
| MLF2 | LAIQGPEDSPSR | 635.25 | 2 | 3.60E-05 |
| HIST1H2BO | AMGIMNSFVNDIFER + 2 OX (M) | 888.81 | 2 | 0.00012 |
| HIST2H4A | TVTAMDVVYALK + OX (M) | 663.79 | 2 | 3.30E-05 |
| KIAA1529 | MDESKEGSIQGLEEMQVER + 2 OX (M) | 742.85 | 3 | 0.005 |
| DCD | DAVEDLESVGK | 581.21 | 2 | 0.00013 |
Fig. 1.
Virus distribution and gene ontology over-representation analysis for virion-associated host proteins. A, A Venn diagram of virus distribution for the identified virion-associated host proteins is shown. Many more ebola-associated host proteins (EBOV) were identified compared with marburg-associated proteins (MARV). B, Over-representation analysis. For the genes on Table I, observed frequencies of proteins mapping to a particular molecular function, biological process, or biological pathway, were compared with expected frequencies. Several of these gene classifications (on the y axis) were significantly over-represented in Table I, compared with the human genome as a whole. For each group, the expected number of proteins is plotted along with the observed number of proteins with this classification. The Bonferonni-corrected p value is given for each.
Identification of Virion-associated Host Proteins Essential for Infection
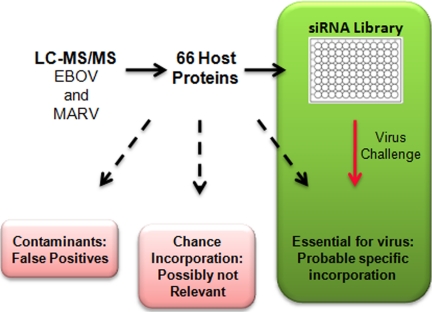
It is possible that some cellular proteins identified by LC-MS/MS represent contaminants in the purified virus sample, and are not associated with the virions at all. Other proteins may be incorporated into virions by chance, because of their high abundance in the cell, presence in the host cell membrane, or interactions with other host proteins directly involved in the viral lifecycle. With this in mind, we utilized a custom siRNA library, targeting the transcripts for each virion-associated cellular protein, to identify host proteins that are required for productive virus infection (Fig. 2). Protein contaminants, or proteins incorporated by chance, are likely not essential for viral infection or production, and would thus not likely score as hits in this assay. Rather, hits from the screen should represent true-positive and biologically relevant virion-associated proteins identified by LC-MS/MS (Fig. 2).
Fig. 2.
Strategy to identify true virion-associated host proteins that are essential for viral infection/production. We combined LC-MS/MS proteomic analysis with a siRNA library screen. In addition to host proteins with specific virus incorporation, some proteins detected by LC-MS/MS may be contaminants of the virus preparation, or may be incorporated by chance. The siRNA screen identifies host proteins that are essential for productive virus infection. Ideally, using these techniques in series allows us to priority rank proteins that are likely not contaminants or proteins incorporated by chance.
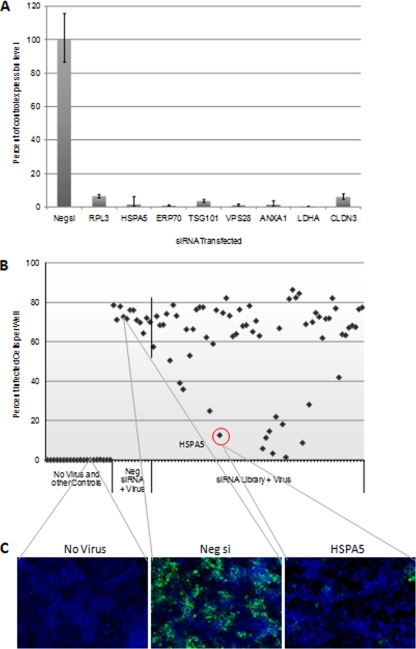
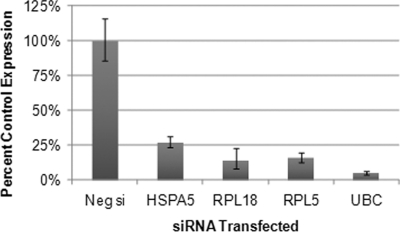
We first evaluated the performance of the siRNA library. 293T cells were transfected with the library, in 96-well plate format. Forty-eight hours later, RNA was harvested from eight wells distributed around the library plate. qRT-PCR was performed to determine whether knockdown of the target transcripts was successful. Each gene tested displayed reduced transcript level following siRNA library transfection (Fig. 3A). This success rate suggests high knockdown efficiency for the siRNA library as a whole.
Fig. 3.
siRNA library screen to identify essential virion-associated host proteins. A, Spot check of siRNA library-mediated transcript knockdown to assess library performance. Following transfection, RNA was isolated from eight wells of the siRNA library. qRT-PCR was performed to measure transcript level. The gene symbol for each gene tested is shown. For each gene, expression level was plotted as a percentage of transcript level for that gene in negative control siRNA (Neg si) treated wells. For simplicity, the Neg si bar for only one gene is shown here. B, Example data from one plate of the siRNA library screen against ebolavirus. Following library transfection and ebola infection, immunostaining was performed to detect cells expressing ebola GP protein. The percentage of infected cells (cells expressing GP) was determined in each well of the library and plotted versus treatment and well position. The data point for HSPA5 siRNA is circled. The vertical line in the graph separates the negative control siRNA wells from the experimental wells. C, Example images from the automated immunofluorescence analysis are shown. Infected cells were detected with a mouse monoclonal antibody to ebola GP, and a green fluorescent secondary antibody. Cell nuclei, stained with Hoechst 33342, are blue.
To identify virion-associated host proteins important for infection, cells were transfected with the siRNA library and then infected with ebolavirus or marburgvirus. Following infection, fluorescent immunostaining was used to detect cells expressing viral antigen (GP1,2 protein), as a measure of the number of virus-infected cells in each well. Fluorescence microscopy, image capture, and data analysis were carried out on an automated high-content imaging system. From each well on the siRNA library plate, fluorescence microscopy images were collected from multiple fields. From each image, the total number of cells, and the number of virus-infected cells were determined, yielding the percentage of infected cells in each well. Fig. 3B shows data from one representative ebola-infected siRNA library plate. The percentage of infected cells observed in each well of the library was plotted versus well position. For this plate, wells transfected with nontargeting, negative control siRNA, exhibited an average percent infection of ∼72% (Fig. 3B). Preliminary experiments confirmed that this particular control siRNA did not influence infection (data not shown). Most siRNAs in the library allowed a level of infection similar to control siRNA. However, several siRNAs clearly and dramatically reduced the number of infected cells observed following ebolavirus challenge (Fig. 3B). Example images from this experiment are shown in Fig. 3C.
For each virus, data from repeat experiments and replicate plates were normalized and averaged together. As a first filter, siRNAs were excluded from further analysis if they reduced total cell number (indicating possible toxicity) by more than twofold compared with negative control siRNA. Next, siRNAs were considered hits if they reduced, or increased, the percentage of infected cells by greater than 50%, compared with negative control siRNA wells (Fig. 4). We identified siRNAs that were effective inhibitors of both ebolavirus and marburgvirus. Interestingly, several siRNAs scored as enhancing marburgvirus infection. Further, select siRNAs inhibited ebolavirus infection, whereas moderately enhancing marburgvirus infection. A screening experiment was performed with ebolavirus utilizing a lower virus dose, and lower baseline level of infection (fewer infected cells in control siRNA wells). Even with these infection parameters, infection-enhancing siRNAs were not identified for ebolavirus (data not shown). On the basis of the siRNA library screening results, we expect the 11 proteins scoring as hits in this assay to be true virion-incorporated host proteins that play a direct role in filovirus biology.
Fig. 4.
siRNA library screen hits. The siRNA library screen was performed with ebolavirus (EBOV), and marburgvirus (MARV) infection. For each virus, data from replicate plates and repeat experiments were averaged. siRNAs targeting the genes listed here inhibited or enhanced EBOV and/or MARV infection. The numbers shown indicate percent of control infection (nontargeting siRNA transfected wells). Hits shaded green inhibited the infection to ≤50% of control wells. Hits shaded red/dark orange enhanced infection to ≥150% of controls. siRNAs listed here met these criteria for at least one of the viruses.
Confirmation of siRNA Library Screen Data
Experiments were performed to confirm data obtained with the siRNA library screen. The three siRNAs, which inhibited both ebolavirus and marburgvirus infections, were chosen for further study (Fig. 4). Additionally siRNA targeting RPL5 was investigated further based on the apparent opposite effect on infection between ebola and marburg viruses. siRNAs were obtained for these select hits and transfected into 293T cells. Following transfection, the transcript level of each target gene was significantly reduced, as determined by quantitative, reverse-transcription PCR (qRT-PCR) analysis (Fig. 5). Cells were also infected with ebolavirus or marburgvirus following transfection with siRNA. Following virus infection, viral genomic RNA was isolated from cell culture supernatants from each well. Using these samples, viral titer was measured by qRT-PCR. Compared with controls, transfection of each siRNA significantly reduced the amount of newly produced ebolavirus released from cells into the cell culture medium (Fig. 6A). These results are consistent with the immunofluorescence detection of ebola-infected cells from the same plate (Fig. 6B). Knockdown of HSPA5 also significantly inhibited marburgvirus infection and production, as measured by qRT-PCR (Fig. 6D), or immunofluorescence detection of infected cells (Fig. 6E). In both of these assays, siRNA for RPL5 significantly enhanced marburgvirus infection (Fig. 6D and 6E), in agreement with the siRNA library screen data. siRNAs for RPL18 and UBC did not significantly affect marburgvirus titers in the qRT-PCR assay (Fig. 6D), but did inhibit the infection according to the immunofluorescence assay (Fig. 6E). This discrepancy is under investigation, but unresolved at this time.
Fig. 5.
qRT-PCR analysis of target transcripts following siRNA transfection in 293T cells. Four hits from the siRNA screen were chosen for validation. The siRNAs were transfected into 293T cells and RNA was harvested 48 h later. qRT-PCR was performed to examine transcript level for each gene. For each gene, the expression level is plotted as a percentage of transcript level seen in control nontargeting siRNA (Neg si) transfected samples. For simplicity, the Neg si bar for only one gene is shown here. Each siRNA significantly reduced the expression of its target gene at the transcript level.
Fig. 6.
Confirmation of hits from the siRNA library screen. 293T cells were transfected with the indicated siRNAs. Cells were then infected with ebolavirus (A–C), or marburgvirus (D–F). Three days following infection, viral genomic RNA was detected in cell culture supernatants by qRT-PCR (A and D). The number of viral genomic copies per PCR reaction was plotted for each siRNA transfected. On the same plates, the cells were fixed and then processed for immunofluorescence detection of virus infected cells. The average percent infected value (compared with neg si wells) is shown for each siRNA (B and E). From the immunofluorescence analysis, the average number of cells analyzed in each well is also shown (C and F). For the marburgvirus experiment, a lower multiplicity of infection was used in an attempt to clearly observe enhancement of infection. *p < .01.
Transfection of these siRNAs did not result in gross cytotoxicity, as indicated by the number of cells in each well, or by visual inspection. Following siRNA transfection and viral infection, the total number of cells per image field was recorded during the automated immunofluorescence imaging procedure (Fig. 6C and 6F). Transfection of each siRNA resulted in only a modest decrease in cell number per well, compared with negative control siRNA transfection. On the basis of these data, we do not believe the effect of each siRNA on viral infection was because of a change in cell number or cytotoxicity.
DISCUSSION
We report here LC-MS/MS analysis of protein lysates from highly purified ebolavirus and marburgvirus virions. These experiments identified 66 host proteins presumably associated with ebola and/or marburg viral particles. Proteomic studies, similar to our own, have been performed for several other viruses (1–3). The overlap between data sets from these investigations, and our own, supports the veracity of the observations. Additionally, these data identify common host proteins that likely play a role in the lifecycle of divergent viruses. We also recognize the inherent difficulties with proteomic studies of this type, and have addressed some of these issues here. One important concern is the purity of the starting material for LC-MS/MS analysis. Membrane-bound vesicles of several types (e.g. exosomes), with a size and density similar to viral particles, can be released from cells into the culture medium and copurified with virions during sample preparation (31, 32). Copurification of exosomes (known to harbor multiple cellular proteins), or other vesicle types, with viral particles could produce a background protein signature in subsequent proteomics analysis.
A second, and independent, question is that of biological relevance. It is difficult to draw conclusions from the simple identification of host proteins incorporated into viral particles. Although the over-representation gene ontology analysis suggests a nonrandom incorporation of host proteins into virions, this virion-association could still be largely irrelevant for virus biology. For example, many proteins identified in our study are associated with the plasma membrane, and lipid rafts in particular. During the budding process, probably from lipid raft microdomains, filovirions acquire a lipid bilayer membrane of host cell origin (33, 34). Therefore, it is possible that select lipid raft-associated host proteins are incorporated into the filovirion membrane by chance.
To partly address the above data interpretation issues, we employed a siRNA library screen, which targeted for knockdown the transcript of each putative virus-associated protein identified in our proteomic analysis. Knockdown of several genes significantly impacted (inhibited or enhanced) ebolavirus and/or marburgvirus infections. This screen served as an empirical filter for biological relevance of the putative virion-associated host proteins. Additionally, the proteins scoring as hits in this assay are likely not contaminants of the virus purification procedure. Instead, our confidence is increased that the hits represent true virion-associated host proteins. For proteins not scoring as a hit in this assay, one cannot conclude that they definitively represent nonvirion-associated contaminants, or that they are irrelevant for viral biology. Rather, the screen provided positive evidence of relevance for select genes and allowed us to priority rank proteins for future, more focused study. Still, only a fraction of the host proteins identified by LC-MS/MS scored as hits in the siRNA screen. One possible explanation is the choice of different cell lines for the virus generation (Vero), and siRNA experiments (293T). With this arrangement, we may fail to identify particular genes as relevant. Host proteins that are required for virus production in Vero cells, may not be present or required in 293T cells. This can also be said when comparing 293T cells with a more physiologically relevant human cell type (e.g. primary human macrophages or dendritic cells). The use of primary human cells in future studies should mitigate unnecessary complications in our host target and drug discovery efforts.
The siRNA library screens, and hit confirmation experiments, identify virion-associated host proteins that play a significant role at some point in the virus lifecycle. Ongoing and future experiments will attempt to answer whether or not these host proteins are required to be incorporated into the virion for successful productive infection. We do not know whether these host proteins play any role within the virion, or whether virion-incorporation is required for subsequent infection of new target cells. Utilizing virus isolated from cells deficient in select host proteins of interest (siRNA-transfected cells, or mouse knockout cells) could help elucidate answers to these questions.
Strikingly, many more host proteins were identified in ebola virions than in marburg. Whether this represents a true difference in biology between the two is currently under investigation in our laboratory. Congruent with the smaller number of marburg-associated proteins, a smaller number of host proteins were identified as critical for marburg infection by the siRNA library screen compared with ebola. However, the siRNA library screen did identify proteins that impacted both ebola and marburg infections, but were identified by LC-MS/MS as present only in ebola virions (e.g. HSPA5). Therefore, the marburg proteomic data set could contain false negatives. Alternatively, HSPA5, and other hits, are involved in the marburgvirus lifecycle, but not stably incorporated into marburg virions.
To date, only a small number of cellular proteins have been identified within filovirus virions or virus-like particles (VLPs). VLPs can be produced by transfection of the ebola or marburg matrix protein VP40. Upon expression, VP40 assembles at the cell membrane and directs the budding of VP40-containing VLPs, with morphology similar to ebola or marburg viral particles (33, 35, 36). Host proteins actin, Tsg101, and Nedd4 have been detected in ebola VP40-induced VLPs (37–39). However, Kolesnikova et al., recently failed to detect Tsg101 or actin (or tubulin), in marburg VP40-dependent VLPs (40). Even so, the same research group previously detected actin, Lamp-1, and Rab11 in purified marburg virions by Western blot (27). HSPA5, tubulin, and annexin A2 were examined but not detected in marburg viral particles (27). In contrast, our proteomic analysis detected HSPA5, Tsg101, actin, tubulin, and annexins A1, A2, A4, and A5 in ebola and/or marburg virions by LC-MS/MS.
Examination of the entire cohort of virion-associated proteins reinforces previous observations concerning filovirus biology, assembly, and budding. There is evidence that lipid rafts are important for filovirus assembly and budding. For example, upon transfection, ebola VP40 and GP were detected in lipid rafts purified by sucrose gradient centrifugation (33, 34). VP40 mutants with diminished capacity to associate with lipid rafts were partially defective in VLP production (34). Additionally, the lipid raft marker phospholipid GM1 colocalizes with VP40 and GP, and can be detected in the membrane of purified ebola virus particles (33). Consistent with these observations, approximately half of the host proteins identified by LC-MS/MS have a literature-documented association with lipid rafts (Fig. 7).
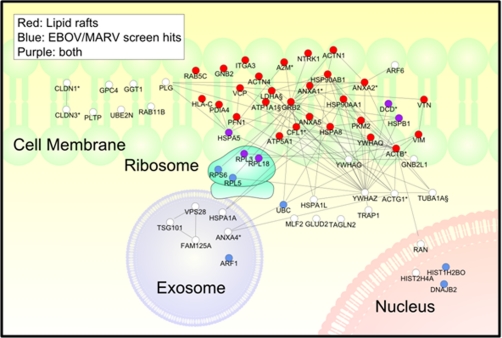
Fig. 7.
Protein interaction and cellular localization map of filovirion-associated host proteins. For the proteins listed in Table I, cell localization and protein interaction information was examined using Ingenuity Pathways Analysis software. Any protein with an annotation for plasma membrane or lipid raft was placed at this position in the diagram. Approximately half the identified virion-associated proteins are integral membrane, or membrane-associated proteins, and located within or at lipid raft microdomains (red circles). Hits in the siRNA library screen are colored purple (also associated with lipid rafts), or blue (no known lipid raft association). White circles indicate proteins that have no known association with lipid rafts, and also did not score as hits in the siRNA library screen. Literature-described possible protein interactions are indicated by connecting lines.
There is also evidence that filoviruses utilize components of the vacuolar protein sorting pathway, including proteins making up ESCRT-1 (host cell endosomal sorting complex required for transport-1), to carry out assembly and budding (19). Previous work demonstrated that ebola VP40 can partially redirect the ESCRT-1 proteins Tsg101, VPS4, VPS28, and VPS37B to the cell surface (41, 42). Importantly, deletion mutants or point mutants of Tsg101 or VPS4 can act in a dominant-negative manner and partially inhibit VP40-dependent VLP release (38, 40, 42). Work utilizing a mouse model suggests that targeting the vacuolar protein sorting pathway may be an effective therapeutic strategy against filovirus infections. Silvestri et al., designed phosphorodiamidite morpholino antisense oligos spanning the start codon of mouse VPS4 (42). These PMOs effectively inhibited expression of VPS4 and significantly protected mice from lethal ebolavirus challenge (42). Consistent with a role for ESCRT-1 proteins in filovirus biology, our study detected Tsg101 and VPS28 as incorporated into ebola virions. However, according to the siRNA library screen, they are not absolutely necessary for productive ebolavirus or marburgvirus infection. One report suggests that Tsg101 siRNA effectively inhibits VLP formation when ebola VP40 is transfected alone (43). However, this effect is much less dramatic when VP40 is cotransfected with GP and NP. Although the authors detect Tsg101 incorporated within VLPs, VLP formation does take place efficiently in the absence of Tsg101 incorporation (43). Our work indicates that Tsg101 siRNA is not an effective inhibitor in the context of infection with authentic ebolavirus.
One might presume that the presence of a host protein in the viral particle is predicated on a direct interaction between one or more viral protein and the incorporated host protein. Ebola and/or marburg VP40, and other viral proteins, have been shown to interact with several host proteins. VP40-interacting proteins include, among others, HSPA5, HSPA8, Tsg101, Nedd4, tubulin, NEDL1, AIP1, AIP4, AIP5, and Sec24C (34, 36, 40, 43–46). Interactions with host proteins have also been described for VP35. VP35 is a potent inhibitor of the host antiviral interferon (IFN) response (47, 48). A recent report describes a direct interaction between VP35 and the host kinases IKKepsilon and TBK-1 (26). These interactions prevent phosphorylation of IRF-3 and subsequent induction of IFN-β. Additional virus/host protein interactions include ebola GP and BST2, and VP24 and karyopherin-α 1 (49–51). Finally, filoviral NP and VP30 proteins are highly phosphorylated, suggesting a direct interaction with a host kinase (20).
Four ribosomal proteins were identified as incorporated into ebola virions. Besides their role in translation, it is now appreciated that ribosomal proteins have several functions away from the ribosome, including protein chaperone activity and regulation of transcription (52–54). Our proteomic analysis identified RPL18, RPL5, RPL3, and RPS6 as present in ebola virions. Although each of these was not detected in marburg virions, each may play an important role in ebola and marburg biology. According to the siRNA library screen data, reduced expression of each of these ribosomal proteins effectively inhibited ebola infection in 293T cells. Knockdown of RPL18 and RPL3 also inhibited marburgvirus, whereas siRNAs directed against RPS6 and RPL5 moderately enhanced marburg infection in this assay. It is notable that all four ebola-associated ribosomal proteins have a known link to viral biology. RPL3 regulates ribosomal frame shifting required for maintenance of yeast M1 killer virus (55). RPL5 is an rRNA nuclear export protein, and also regulates HIV-1 Rev-dependent nuclear export of viral RNAs, a process required for HIV-1 replication (56). RPS6 binds to the nsp2 protein of several alphaviruses and regulates viral gene expression (57). These authors demonstrated that siRNA-mediated reduction of RPS6 inhibits gene expression from Venezuelan equine encephalitis replicon particles (57). Importantly, although the interaction between RPS6 and nsp2 may occur in the context of intact ribosomes, RPS6 siRNA had little effect on global host translation. Finally, RPL18 of Arabidopsis thaliana interacts with P6 of cauliflower mosaic virus (58).
Heat-shock proteins (HSPs) are known to play an important role in the life cycle of diverse viruses. Several viral proteins have been shown to interact with HSPs, and in some cases require HSPs to be fully active (59). Additionally, HSPs have been identified in purified virions of HIV-1 (1, 60), HHV-4 (61), HHV-5 (62), HHV-1 (2), and vaccinia virus (63). The hsp70 family member, HSPA5 (GRP78, BiP) is a resident of the endoplasmic reticulum and has a known role in virus biology. In particular, HSPA5 acts as a chaperone for the envelope proteins of hepatitis B virus, Sendai virus, HIV-1, and sindbis virus (64–67). HSPA5 can also be detected at the cell surface, and has been described as a receptor or co-receptor for dengue virus serotype 2 and coxsackievirus A9 (68–71). In our proteomic analysis, this protein was detected as associated with ebola virions but not marburg. This is consistent with work by Kolesnikova et al., who failed to detect HSPA5 in purified marburg virions by Western blot (27). However, we observed that knockdown of HSPA5 transcripts with siRNA dramatically and consistently reduced the number of cells infected with ebola or marburg viruses. Likewise, reduction of HSPA5 inhibited the release of nascent ebola or marburg virions into the media of infected cell cultures, as measured by qRT-PCR.
Preclinical investigations suggest that inhibition of Hsp90 or HSPA5 could develop into effective antiviral therapeutic strategies. For example, Connor et al. demonstrated that pharmacologic or siRNA-mediated inhibition of Hsp90 can inhibit replication of several negative-strand RNA viruses, including vesicular stomatitis virus, multiple paramyxoviruses (SV5, HPIV-2, HPIV-3, and SV41), and the La Crosse bunyavirus (72). Similarly, cleavage and depletion of HSPA5 using the SubAB subtilase cytotoxin inhibits hCMV virus production in human fibroblasts (73). Knockdown of HSPA5 also inhibits dengue virus serotype 2 production (74). However, apparently contradictory reports exist regarding HSPA5 knockdown and hepatitis B virus (HBV). Huang et al., report that HSPA5 siRNA decreases the amount of HBV DNA detected in the supernatants of infected cell cultures (75). Alternatively, Ma et al., describe HSPA5 as a potent antiviral protein in the context of HBV infection (76). In that report, siRNA-mediated reduction of HSPA5 increases the production of HBV virions in HepG2 cells.
Localization in filovirus particles has not previously been reported for the great majority of the proteins we identified with LC-MS/MS analysis. Additionally, we demonstrated that several of these virion-incorporated host proteins are critical for filovirus infection/production. This new information opens up novel avenues of investigation into filovirus biology. It is our hope that several of these host proteins, and associated host/pathogen interactions, will become targets for antiviral therapeutic intervention.
Acknowledgments
We thank Jens Kuhn for a critical review of this manuscript. We thank Lian Dong for performing the qRT-PCR viral titer measurements.
Footnotes
* A portion of the research described herein was sponsored by the Defense Threat Reduction Agency (JSTO-CBD project number 44.10022-08-RD-B and TMTI project number 0048-09-RD-T). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army. During a portion of this research, KBS was appointed to the Postgraduate Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and USAMRMC.
1 The abbreviations used are:
- LC-MS/MS
- liquid chromatography-linked tandem mass spectrometry
- ACN
- acetonitrile
- qRT-PCR
- quantitative reverse transcription PCR
- VP
- viral protein
- VLP
- virus-like particle.
REFERENCES
- 1. Chertova E., Chertov O., Coren L. V., Roser J. D., Trubey C. M., Bess J. W., Jr., Sowder R. C., 2nd, Barsov E., Hood B. L., Fisher R. J., Nagashima K., Conrads T. P., Veenstra T. D., Lifson J. D., Ott D. E. (2006) Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J. Virol. 80, 9039–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loret S., Guay G., Lippé R. (2008) Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 82, 8605–8618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw M. L., Stone K. L., Colangelo C. M., Gulcicek E. E., Palese P. (2008) Cellular proteins in influenza virus particles. PLoS Pathog 4, e1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maxwell K. L., Frappier L. (2007) Viral proteomics. Microbiol. Mol. Biol. Rev. 71, 398–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawn S. D., Roberts B. D., Griffin G. E., Folks T. M., Butera S. T. (2000) Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection. J. Virol. 74, 139–145 [PMC free article] [PubMed] [Google Scholar]
- 6. Kuhn J. H., Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. 1st ed, Calisher C. H. (ed.) 2008, SpringerWien, NewYork, 413. [PubMed] [Google Scholar]
- 7. Warren T. K., Warfield K. L., Wells J., Swenson D. L., Donner K. S., Van Tongeren S. A., Garza N. L., Dong L., Mourich D. V., Crumley S., Nichols D. K., Iversen P. L., Bavari S. (2010) Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat. Med. 16, 991–994 Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 8. Burnett J. C., Henchal E. A., Schmaljohn A. L., Bavari S. (2005) The evolving field of biodefence: therapeutic developments and diagnostics. Nat. Rev. Drug Discov. 4, 281–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bray M. (2003) Defense against filoviruses used as biological weapons. Antiviral Res. 57, 53–60 [DOI] [PubMed] [Google Scholar]
- 10. Geisbert T. W., Jahrling P. B. (1995) Differentiation of filoviruses by electron microscopy. Virus Res. 39, 129–150 [DOI] [PubMed] [Google Scholar]
- 11. Hoenen T., Groseth A., Falzarano D., Feldmann H. (2006) Ebola virus: unravelling pathogenesis to combat a deadly disease. Trends Mol. Med. 12, 206–215 [DOI] [PubMed] [Google Scholar]
- 12. Volchkov V. E., Feldmann H., Volchkova V. A., Klenk H. D. (1998) Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. U.S.A. 95, 5762–5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Volchkova V. A., Feldmann H., Klenk H. D., Volchkov V. E. (1998) The nonstructural small glycoprotein sGP of Ebola virus is secreted as an antiparallel-orientated homodimer. Virology 250, 408–414 [DOI] [PubMed] [Google Scholar]
- 14. Hartlieb B., Weissenhor n W. (2006) Filovirus assembly and budding. Virology 344, 64–70 [DOI] [PubMed] [Google Scholar]
- 15. Noda T., Halfmann P., Sagara H., Kawaoka Y. (2007) Regions in Ebola virus VP24 that are important for nucleocapsid formation. J. Infect. Dis. 196 Suppl 2, S247–250 [DOI] [PubMed] [Google Scholar]
- 16. Hoenen T., Groseth A., Kolesnikova L., Theriault S., Ebihara H., Hartlieb B., Bamberg S., Feldmann H., Ströher U., Becker S. (2006) Infection of naive target cells with virus-like particles: implications for the function of ebola virus VP24. J. Virol. 80, 7260–7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Y., Xu L., Sun Y., Nabel G. J. (2002) The assembly of Ebola virus nucleocapsid requires virion-associated proteins 35 and 24 and posttranslational modification of nucleoprotein. Mol. Cell. 10, 307–316 [DOI] [PubMed] [Google Scholar]
- 18. Feldmann H., Klenk H. D., Sanchez A. (1993) Molecular biology and evolution of filoviruses. Arch. Virol. Suppl. 7, 81–100 [DOI] [PubMed] [Google Scholar]
- 19. Harty R. N. (2009) No exit: targeting the budding process to inhibit filovirus replication. Antiviral Res. 81, 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Becker S., Mühlberger E. (1999) Co- and posttranslational modifications and functions of Marburg virus proteins. Curr. Top Microbiol. Immunol. 235, 23–34 [DOI] [PubMed] [Google Scholar]
- 21. Sanchez A. (2007) Analysis of filovirus entry into vero e6 cells, using inhibitors of endocytosis, endosomal acidification, structural integrity, and cathepsin (B and L) activity. J. Infect. Dis. 196, S251–258 [DOI] [PubMed] [Google Scholar]
- 22. Martínez M. J., Biedenkopf N., Volchkova V., Hartlieb B., Alazard-Dany N., Reynard O., Becker S., Volchkov V. (2008) Role of Ebola virus VP30 in transcription reinitiation. J. Virol. 82, 12569–12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weik M., Enterlein S., Schlenz K., Mühlberger E. (2005) The Ebola virus genomic replication promoter is bipartite and follows the rule of six. J. Virol. 79, 10660–10671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aman M. J., Bosio C. M., Panchal R. G., Burnett J. C., Schmaljohn A., Bavari S. (2003) Molecular mechanisms of filovirus cellular trafficking. Microbes Infect. 5, 639–649 [DOI] [PubMed] [Google Scholar]
- 25. Dolnik O., Kolesnikova L., Becker S. (2008) Filoviruses: Interactions with the host cell. Cell Mol. Life Sci. 65, 756–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prins K. C., Cárdenas W. B., Basler C. F. (2009) Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J. Virol. 83, 3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kolesnikova L., Bugany H., Klenk H. D., Becker S. (2002) VP40, the matrix protein of Marburg virus, is associated with membranes of the late endosomal compartment. J. Virol. 76, 1825–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kolesnikova L., Bohil A. B., Cheney R. E., Becker S. (2007) Budding of Marburgvirus is associated with filopodia. Cell Microbiol. 9, 939–951 [DOI] [PubMed] [Google Scholar]
- 29. Thomas P. D., Campbell M. J., Kejariwal A., Mi H., Karlak B., Daverman R., Diemer K., Muruganujan A., Narechania A. (2003) PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 13, 2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas P. D., Kejariwal A., Guo N., Mi H., Campbell M. J., Muruganujan A., Lazareva-Ulitsky B. (2006) Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 34, W645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Théry C., Ostrowski M., Segura E. (2009) Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 [DOI] [PubMed] [Google Scholar]
- 32. Théry C., Zitvogel L., Amigorena S. (2002) Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 33. Bavari S., Bosio C. M., Wiegand E., Ruthel G., Will A. B., Geisbert T. W., Hevey M., Schmaljohn C., Schmaljohn A., Aman M. J. (2002) Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195, 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panchal R. G., Ruthel G., Kenny T. A., Kallstrom G. H., Lane D., Badie S. S., Li L., Bavari S., Aman M. J. (2003) In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc. Natl. Acad. Sci. U.S.A. 100, 15936–15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jasenosky L. D., Neumann G., Lukashevich I., Kawaoka Y. (2001) Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 75, 5205–5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harty R. N., Brown M. E., Wang G., Huibregtse J., Hayes F. P. (2000) A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. U.S.A. 97, 13871–13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Licata J. M., Simpson-Holley M., Wright N. T., Han Z., Paragas J., Harty R. N. (2003) Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J. Virol. 77, 1812–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yasuda J., Nakao M., Kawaoka Y., Shida H. (2003) Nedd4 regulates egress of Ebola virus-like particles from host cells. J. Virol. 77, 9987–9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han Z., Harty R. N. (2005) Packaging of actin into Ebola virus VLPs. Virol J. 2, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kolesnikova L., Strecker T., Morita E., Zielecki F., Mittler E., Crump C., Becker S. (2009) Vacuolar protein sorting pathway contributes to the release of Marburg virus. J. Virol. 83, 2327–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin-Serrano J., Eastman S. W., Chung W., Bieniasz P. D. (2005) HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J. Cell Biol. 168, 89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silvestri L. S., Ruthel G., Kallstrom G., Warfield K. L., Swenson D. L., Nelle T., Iversen P. L., Bavari S., Aman M. J. (2007) Involvement of vacuolar protein sorting pathway in Ebola virus release independent of TSG101 interaction. J. Infect. Dis. 196, S264–270 [DOI] [PubMed] [Google Scholar]
- 43. Urata S., Noda T., Kawaoka Y., Morikawa S., Yokosawa H., Yasuda J. (2007) Interaction of Tsg101 with Marburg virus VP40 depends on the PPPY motif, but not the PT/SAP motif as in the case of Ebola virus, and Tsg101 plays a critical role in the budding of Marburg virus-like particles induced by VP40, NP, and GP. J. Virol. 81, 4895–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamayoshi S., Noda T., Ebihara H., Goto H., Morikawa Y., Lukashevich I. S., Neumann G., Feldmann H., Kawaoka Y. (2008) Ebola virus matrix protein VP40 uses the COPII transport system for its intracellular transport. Cell Host Microbe. 3, 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruthel G., Demmin G. L., Kallstrom G., Javid M. P., Badie S. S., Will A. B., Nelle T., Schokman R., Nguyen T. L., Carra J. H., Bavari S., Aman M. J. (2005) Association of ebola virus matrix protein VP40 with microtubules. J. Virol. 79, 4709–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin-Serrano J., Zang T., Bieniasz P. D. (2001) HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7, 1313–1319 [DOI] [PubMed] [Google Scholar]
- 47. Basler C. F., Mikulasova A., Martinez-Sobrido L., Paragas J., Mühlberger E., Bray M., Klenk H. D., Palese P., Garcia-Sastre A. (2003) The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77, 7945–7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feng Z., Cerveny M., Yan Z., He B. (2007) The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J. Virol. 81, 182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reid S. P., Leung L. W., Hartman A. L., Martinez O., Shaw M. L., Carbonnelle C., Volchkov V. E., Nichol S. T., Basler C. F. (2006) Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J. Virol. 80, 5156–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reid S. P., Valmas C., Martinez O., Sanchez F. M., Basler C. F. (2007) Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J. Virol. 81, 13469–13477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaletsky R. L., Francica J. R., Agrawal-Gamse C., Bates P. (2009) Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 106, 2886–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lindström M. S. (2009) Emerging functions of ribosomal proteins in gene-specific transcription and translation. Biochem. Biophys. Res. Commun. 379, 167–170 [DOI] [PubMed] [Google Scholar]
- 53. Kovacs D., Rakacs M., Agoston B., Lenkey K., Semrad K., Schroeder R., Tompa P. (2009) Janus chaperones: assistance of both RNA- and protein-folding by ribosomal proteins. FEBS Lett. 583, 88–92 [DOI] [PubMed] [Google Scholar]
- 54. Wool I. G. (1996) Extraribosomal functions of ribosomal proteins. Trends Biochem. Sci. 21, 164–165 [PubMed] [Google Scholar]
- 55. Peltz S. W., Hammell A. B., Cui Y., Yasenchak J., Puljanowski L., Dinman J. D. (1999) Ribosomal protein L3 mutants alter translational fidelity and promote rapid loss of the yeast killer virus. Mol. Cell. Biol. 19, 384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schatz O., Oft M., Dascher C., Schebesta M., Rosorius O., Jaksche H., Dobrovnik M., Bevec D., Hauber J. (1998) Interaction of the HIV-1 rev cofactor eukaryotic initiation factor 5A with ribosomal protein L5. Proc. Natl. Acad. Sci. U.S.A. 95, 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Montgomery S. A., Berglund P., Beard C. W., Johnston R. E. (2006) Ribosomal protein S6 associates with alphavirus nonstructural protein 2 and mediates expression from alphavirus messages. J. Virol. 80, 7729–7739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leh V., Yot P., Keller M. (2000) The cauliflower mosaic virus translational transactivator interacts with the 60S ribosomal subunit protein L18 of Arabidopsis thaliana. Virology. 266, 1–7 [DOI] [PubMed] [Google Scholar]
- 59. Mayer M. P. (2005) Recruitment of Hsp70 chaperones: a crucial part of viral survival strategies. Rev. Physiol. Biochem. Pharmacol. 153, 1–46 [DOI] [PubMed] [Google Scholar]
- 60. Kolegraff K., Bostik P., Ansari A. A. (2006) Characterization and role of lentivirus-associated host proteins. Exp. Biol. Med. (Maywood) 231(3), 252–263 [DOI] [PubMed] [Google Scholar]
- 61. Johannsen E., Luftig M., Chase M. R., Weicksel S., Cahir-McFarland E., Illanes D., Sarracino D., Kieff E. (2004) Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. U.S.A. 101, 16286–16291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Varnum S. M., Streblow D. N., Monroe M. E., Smith P., Auberry K. J., Pasa-Tolic L., Wang D., Camp D. G., 2nd, Rodland K., Wiley S., Britt W., Shenk T., Smith R. D., Nelson J. A. (2004) Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78, 10960–10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Resch W., Hixson K. K., Moore R. J., Lipton M. S., Moss B. (2007) Protein composition of the vaccinia virus mature virion. Virology 358, 233–247 [DOI] [PubMed] [Google Scholar]
- 64. Awe K., Lambert C., Prange R. (2008) Mammalian BiP controls posttranslational ER translocation of the hepatitis B virus large envelope protein. FEBS Lett. 582, 3179–3184 [DOI] [PubMed] [Google Scholar]
- 65. Tomita Y., Yamashita T., Sato H., Taira H. (1999) Kinetics of interactions of sendai virus envelope glycoproteins, F and HN, with endoplasmic reticulum-resident molecular chaperones, BiP, calnexin, and calreticulin. J. Biochem. 126, 1090–1100 [DOI] [PubMed] [Google Scholar]
- 66. Earl P. L., Moss B., Doms R. W. (1991) Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J. Virol. 65, 2047–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mulvey M., Brown D. T. (1995) Involvement of the molecular chaperone BiP in maturation of Sindbis virus envelope glycoproteins. J. Virol. 69, 1621–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jindadamrongwech S., Thepparit C., Smith D. R. (2004) Identification of GRP 78 (BiP) as a liver cell expressed receptor element for dengue virus serotype 2. Arch Virol. 149, 915–927 [DOI] [PubMed] [Google Scholar]
- 69. Cabrera-Hernandez A., Thepparit C., Suksanpaisan L., Smith D. R. (2007) Dengue virus entry into liver (HepG2) cells is independent of hsp90 and hsp70. J. Med. Virol. 79, 386–392 [DOI] [PubMed] [Google Scholar]
- 70. Triantafilou K., Fradelizi D., Wilson K., Triantafilou M. (2002) GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J. Virol. 76, 633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Upanan S., Kuadkitkan A., Smith D. R. (2008) Identification of dengue virus binding proteins using affinity chromatography. J. Virol. Methods. 151, 325–328 [DOI] [PubMed] [Google Scholar]
- 72. Connor J. H., McKenzie M. O., Parks G. D., Lyles D. S. (2007) Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology 362, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Buchkovich N. J., Maguire T. G., Yu Y., Paton A. W., Paton J. C., Alwine J. C. (2008) Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly. J. Virol. 82, 31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Limjindaporn T., Wongwiwat W., Noisakran S., Srisawat C., Netsawang J., Puttikhunt C., Kasinrerk W., Avirutnan P., Thiemmeca S., Sriburi R., Sittisombut N., Malasit P., Yenchitsomanus P. T. (2009) Interaction of dengue virus envelope protein with endoplasmic reticulum-resident chaperones facilitates dengue virus production. Biochem. Biophys. Res. Commun. 379, 196–200 [DOI] [PubMed] [Google Scholar]
- 75. Huang K. L., Lai Y. K., Lin C. C., Chang J. M. (2009) Involvement of GRP78 in inhibition of HBV secretion by Boehmeria nivea extract in human HepG2 2.2.15 cells. J. Viral Hepat. 16, 367–375 [DOI] [PubMed] [Google Scholar]
- 76. Ma Y., Yu J., Chan H. L., Chen Y. C., Wang H., Chen Y., Chan C. Y., Go M. Y., Tsai S. N., Ngai S. M., To K. F., Tong J. H., He Q. Y., Sung J. J., Kung H. F., Cheng C. H., He M. L. (2009) Glucose-regulated protein 78 is an intracellular antiviral factor against hepatitis B virus. Mol. Cell. Proteomics 8, 2582–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]