Abstract
It is of considerable interest to determine how diverse subtypes of γ-aminobutyric acidergic (GABAergic) interneurons integrate into the functional network of the cerebral cortex. Using inducible in vivo genetic fate mapping approaches, we found that interneuron precursors arising from the medial ganglionic eminence (MGE) and caudal ganglionic eminence (CGE) at E12.5, respectively, populate deep and superficial cortical layers in a complementary manner in the mature cortex. These age-matched populations initiate tangential migration into the cortex simultaneously, migrate above and below the cortical plate in a similar ratio, and complete their entrance into the cortical plate by P1. Surprisingly, while these 2 interneuron populations show a comparable layer distribution at P1, they subsequently segregate into distinct cortical layers. In addition, the initiation of the radial sorting within each lineage coincided well with the upregulation of the potassium/chloride cotransporter KCC2. Moreover, layer sorting of a later born (E16.5) CGE-derived population occurred with a similar time course to the earlier born E12.5 cohorts, further suggesting that this segregation step is controlled in a subtype specific manner. We conclude that radial sorting within the early postnatal cortex is a key mechanism by which the layer-specific integration of GABAergic interneurons into the emerging cortical network is achieved.
Keywords: caudal ganglionic eminence, fate mapping, genetics, medial ganglionic eminence, migration
Introduction
In mammals, higher order information processing involved in cognition and long-term memory occurs within the 6-layered neocortex. This processing relies both on pyramidal cells that project their axons across cortical layers and areal subdivisions, and γ-aminobutyric acidergic (GABAergic) interneurons that project locally (Kawaguchi and Kubota 1997; McBain and Fisahn 2001; Markram et al. 2004; Klausberger and Somogyi 2008). As numerous diseases that compromise higher cortical function have been associated with the misplacement of both pyramidal and interneuron populations (Kitamura et al. 2002; Cobos et al. 2005; Gressens 2006), it is of considerable interest to understand how these circuits are assembled.
At least in rodents, the majority of cortical interneurons are generated within the ventral telencephalon, in the medial and caudal ganglionic eminences (MGEs and CGEs) (Corbin et al. 2001; Nery et al. 2002; Flames et al. 2007; Ghanem et al. 2007). We recently found that the MGE and CGE, respectively, contribute approximately 70% and 30% of the total cortical interneuron population (Miyoshi et al. 2010) and each gives rise to distinct interneuron subtypes (Xu et al. 2004; Butt et al. 2005; Miyoshi et al. 2007, 2010). Moreover, these populations arise in 2 distinct neurogenic waves, with the MGE-derived interneurons starting at around E9.5 and peaking at approximately E13.5 (Miyoshi et al. 2007), and the CGE counterparts initiating and peaking 3 days later (Miyoshi et al. 2010) (see Fig. 1).
Figure 1.
The MGE and CGE provide interneurons into cortical layers through a distinct temporal methodology. A schematic drawing comparing the contribution of MGE- versus CGE-derived interneurons with different cortical layers based on birthdate. While MGE-derived interneurons exhibit inside-out layering, interneurons derived from the CGE preferentially occupy superficial layers irrespective of their birthdate. Importantly, MGE- and CGE-derived interneurons generated at E12.5 (red bracket) show a complementary bias in their contribution to deep versus superficial cortical layers, respectively.
Within the cortex, the pyramidal cell layers are formed in an “inside-out” manner, with later born cells migrating past earlier born populations to occupy more superficial laminae (Angevine and Sidman 1961; Rakic 1974). Previous work including our own (Miller 1985; Fairen et al. 1986; Valcanis and Tan 2003; Miyoshi et al. 2007) has shown that interneurons derived from the MGE follow an inside-out pattern of layer integration within the cortex by approximately matching the birthdates of pyramidal cells. In contrast, cortical interneurons derived from the CGE consistently contribute ∼75% of their population to the superficial layers and ∼25% to the deep layers, regardless of their birthdates (Miyoshi et al. 2010) (see Fig. 1). Thus, for CGE-derived interneurons, the laminar positioning within the cortex is not strictly dictated by birthdate. This raises a possibility that the inside-out layering of MGE-derived interneuron populations may just be correlative. Regardless, MGE- and CGE-derived interneurons clearly use different strategies to select their appropriate cortical laminae. To better understand how MGE- versus CGE-derived interneurons differentially migrate into specific cortical layers, we utilized inducible in vivo genetic fate mapping methods to birthdate and permanently label interneuron cohorts at embryonic stages and then we assessed their migratory routes at later developmental stages.
Here, we report that MGE- and CGE-derived interneurons born at E12.5 are complementary in their final laminar distribution within the cortex, with each cohort occupying primarily deep and superficial layers, respectively. Surprisingly, however, these populations initiate tangential migration into the cortex simultaneously, take similar migratory routes, and show a similar layer distribution at P1. Only after this stage does each population sort itself out into the appropriate cortical layers. Furthermore, the critical time window for this postnatal sorting was found to occur several days earlier in the MGE-derived population than the age-matched CGE cohort. This radial sorting period coincides well with the increase in potassium/chloride cotransporter KCC2 expression within each interneuron cohort. Moreover, radial sorting of later born (E16.5) CGE-derived cohorts is found to take a similar time course as the E12.5 CGE-derived cohorts, suggesting that the final laminar location of interneurons is determined during the postnatal period in which they are sorted. We conclude that the selective sorting of interneurons within the early postnatal cortex is a crucial step for the assembly of mature neuronal networks. Moreover, the timing of their segregation into the appropriate cortical laminae is predicted by their region of origin and hence interneuron subtype rather than by their birthdate.
Materials and Methods
In Vivo Fate Mapping
For our inducible genetic fate mapping strategy, we have employed either the Olig2-CreER driver and the Z/EG reporter (for MGE-derived populations) (Miyoshi et al. 2007) or the Mash1BAC-CreER driver and the RCE:loxP reporter (Jackson Laboratories stock number 10701) (for CGE-derived populations) (Sousa et al. 2009; Miyoshi et al. 2010). Compound males carrying appropriate combinations of these alleles were crossed to Swiss Webster females. To induce CreER activity, we orally administered 4 mg of tamoxifen (Sigma) to pregnant females bearing E12.5 or E16.5 embryos between noon and 2 PM.
Histology and Cell Count
To be consistent with our former studies, we have analyzed the somatosensory barrel field to compare fate-mapped cells in the mature cortex. For embryonic and early postnatal analyses of MGE- and CGE-derived populations, we compared coronal sections from similar rostral–caudal levels. We analyzed the layering from E12.5 MGE and CGE fate-mapped populations during a number of postnatal stages: P1 (n = 3), P3 (n = 3), P5 (n = 2), P7 (n = 2), and P21 (n = 3) (Miyoshi et al. 2007, 2010). Layering of E16.5 CGE-derived interneurons were also analyzed at P1, P3, P5, P7 (n = 2), and P21 (n = 3).
Immunohistochemistry on 12-μm cryosections was performed as described previously (Miyoshi et al. 2007). Briefly, mice were anesthetized, and transcardiac perfusions were carried out with 4% formaldehyde/phosphate-buffered saline (PBS) solution. Brains were dissected and postfixed in the same fixative for 1 h on ice. Following a brief rinse in PBS, brains were kept in 25% sucrose/PBS (w/v) solution at 4 °C until equilibrated. Cryosections were rinsed in PBS, and, following 30 min of blocking, the primary antibody reaction was carried out overnight at 4 °C. All the reactions were done in the presence of 1.5% donkey or goat serum and 0.1% Triton-X containing PBS solutions. To visualize the enhanced green fluorescent protein (EGFP) expression, a 3,3′-Diaminobenzidine-coupled immunohistochemistry was carried out following the X-gal staining of forebrain sections (Butt et al. 2005; Miyoshi et al. 2007).
Antibodies were used at the following concentrations: mouse anti-Nkx2-1 (TTF-1) (1:200; PROGEN), mouse anti-Mash1 (1:1000; BD Pharmingen), rabbit anti-RORß (1:1000; Diagenode), rabbit anti-GFP (1:2000; Molecular Probes), rat anti-GFP (1:2000; Nacalai Tesque), goat anti-GFP (1:2000; Rockland), rabbit anti-Cleaved caspase 3 (1:1000; Cell Signaling Technology), and rabbit anti-KCC2 (1:2000; Upstate). Secondary antibodies conjugated with Alexa Fluor dyes 488, 594, or 680 (Molecular Probes) (1:2000 dilutions) or AMCA (Jackson Immunoresearch) (1:500 dilution) raised from the same host used for blocking serum were chosen for visualizing the signals. Fluorescent images were captured using a cooled-CCD camera (Princeton Scientific Instruments) using Metamorph software (Universal Imaging).
Results
Age-Matched MGE- and CGE-Derived Interneurons Born at E12.5 Show Complementary Layer Distributions within the Mature Cortex
In our previous studies, we took advantage of an inducible genetic fate mapping strategy (Zinyk et al. 1998; Branda and Dymecki 2004; Miyoshi and Fishell 2006) to characterize temporally distinct cohorts of cortical interneuron subtypes derived from the MGE (Olig2-CreER; Z/EG, Miyoshi et al. 2007) and the CGE (Mash1BAC-CreER; RCE:loxP, Miyoshi et al. 2010). The MGE and CGE not only give rise to distinct subtypes of cortical interneurons but they also differentially contribute to cortical layers. MGE-derived interneurons, similar to pyramidal cells, are generated in an inside-out manner (Miyoshi et al. 2007) (Fig. 1, top). In contrast, regardless of when they are generated, CGE-derived interneurons preferentially target the superficial layers of the cortex (Fig. 1, bottom) (Miyoshi et al. 2010). Here, we find that the age-matched interneuron populations born at E12.5 from the MGE and CGE show a complementary bias in their layering within the mature cortex. While E12.5 MGE-derived interneurons contribute mainly to the deep cortical layers, age-matched CGE-derived populations preferentially reside in the superficial cortex (Fig. 1, red bracket). How then do these 2 populations born at the same time come to be differentially localized within the cortex?
Tangential Migratory Pathways of E12.5 MGE- and CGE-Derived Interneurons Are Indistinguishable
In principle, the distinct layering patterns of E12.5 age-matched MGE- versus CGE-derived cortical interneurons could be achieved through a number of different mechanisms. Most simply, CGE-derived interneurons may be delayed in reaching the cortex compared with the MGE-derived population, thereby being relegated to the later developing superficial layers. If this idea is correct, one would expect to see that the CGE-derived population is delayed in its migration from the ventral telencephalon to the cortex. However, age-matched E12.5 MGE- and CGE-derived interneurons are both first observed to migrate tangentially through the cortical intermediate zone within 2 days of their birth (Fig. 2A). This indicates that there is no difference in the initiation of tangential migration within E12.5 MGE- versus CGE-derived interneuron populations.
Figure 2.
Age-matched E12.5 MGE- and CGE-derived interneurons are indistinguishable during tangential migration period. (A) The migration pathways of E12.5 MGE- (Olig2-CreER; Z/EG, Miyoshi et al. 2007) and CGE- (Mash1BAC-CreER; RCE:loxP, Miyoshi et al. 2010) derived populations were compared at E14.5. After 2 days, both interneuron populations transited from their site of origin in the ventral telencephalon to the cortex. The MGE domain is delineated by Nkx2-1 expression (left), and the dorsolateral limit of the CGE is indicated by Mash1 expression (right). Note that interneurons first appear in the intermediate zone in both cases (inset). (B) An E14.5 coronal section from the telencephalon carrying GAD1-EGFP, Nkx2-1BAC-Cre, and R26R-stop-LacZ (left). The entire GABAergic population is labeled by EGFP (brown), while the majority of MGE-derived cells are fate mapped as indicated by their beta-galactosidase enzymatic reactivity (blue) (right). A high magnification of cortex showing both MGE- (blue) and CGE- (brown without blue) derived cells migrating through the intermediate, marginal, and subventricular zones of the cortex. (C) Tangential migration of E12.5 MGE- (left) or CGE- (right) derived interneurons at E16.5. Fate-mapped cells express EGFP, and cortical histology is revealed by 4′,6-diamidino-2-phenylindole (DAPI) nuclear counter staining (pseudocolored in red). Fate-mapped cells from both the E12.5 MGE and CGE take similar tangential migration paths with migrating above (MZ, around 25%) and below (IZ and SVZ, around 60%) the cortical plate. CP, cortical plate; IZ, intermediate zone; MZ, marginal zone; SVZ, subventricular zone; VZ, ventricular zone. Scale bars: (A) 200 μm, (B, C) 100 μm.
Previous analyses have demonstrated that the marginal and the intermediate/subventricular zones are the 2 prominent routes taken by tangentially migrating interneurons as they enter the cortex (Marin and Rubenstein 2003; Kriegstein and Noctor 2004; Nakajima 2007; Stanco et al. 2009). We were able to visualize MGE- versus CGE-derived interneuron migratory streams at E14.5 (Fig. 2B) by comparing the Nkx2-1BAC-Cre; R26R-stop-LacZ labeled population (Xu et al. 2008) (MGE-derived, in blue) with those that solely expressed EGFP under the regulation of GAD1 promoter (Tamamaki et al. 2003) (CGE-derived, in brown). We found that both MGE- and CGE-derived interneurons can tangentially migrate above and below the cortical plate (Fig. 2B). Following this observation, we hypothesized that distinct interneuron lineages may facilitate their entry into specific cortical layers by utilizing the tangential migration pathway closer to their target layers. If this were the case, one would predict that E12.5 MGE-derived interneurons would preferentially migrate below the cortical plate, while CGE-derived cortical interneurons would migrate above it. However, at E16.5, we found that irrespective of whether they originated in the E12.5 MGE or the CGE, there was no obvious preference in the proportion that migrated above (MGE: 24.4%, CGE: 27.4%) or below (MGE: 61.9%, CGE: 56.3%) the cortical plate (Fig. 2C). Thus, selective routing of MGE- versus CGE-derived interneurons cannot explain their complementary laminar positioning within the mature cortex.
Age-Matched E12.5 MGE- and CGE-Derived Interneurons Show Comparable Layer Distributions at P1 and Only Subsequently Sort into Distinct Layers
After reaching the cortical area where interneurons will integrate, these cells change their mode of migration from tangential to radial in order to invade the cortical plate (Ang et al. 2003; Marin and Rubenstein 2003; Kriegstein and Noctor 2004; Yokota et al. 2007). Based on BrdU birthdating studies, interneurons born on E12.5 are known to complete their invasion into the cortical plate by P0 (Hevner et al. 2004). Consistent with this observation, both E12.5 MGE- and CGE-derived interneurons fate mapped by our inducible genetic strategies had migrated into the cortical plate by P1 (Fig. 3A,B, and D). Next, we analyzed the layer distribution of these populations at different postnatal stages and compared it with their final distribution at P21. To precisely distinguish the immature cortical layers, we combined the layer-specific pyramidal neuron marker RORß (layers IV and V) (Nakagawa and O'Leary 2003) with nuclear 4′,6-diamidino-2-phenylindole (DAPI) staining (Fig. 3A,B). Surprisingly, at P1, the layer distribution of the 2 populations did not appear to differ significantly (Fig. 3D, left and middle panels). Indeed, at this stage, the ratio of cells in either population within superficial (I–III, blue colors) or deep (IV–VI, red colors) layers was almost identical (Fig. 3D). Hence, up until P1, we were not able to observe any obvious differences between the migratory behaviors of E12.5 MGE- and CGE-derived interneurons.
Figure 3.
MGE- and CGE-derived interneurons differentially sort into distinct cortical layers after P1. (A) Representative examples of E12.5 MGE-derived interneurons in P1, P3, and P5 cortices. Triple-labeled sections for EGFP, RORß (marks layers IV and V), and 4′,6-diamidino-2-phenylindole (DAPI) nuclear counter stain (pseudocolored in red) are each shown with fate-mapped cells labeled in green. (B, C) Representative examples of E12.5 (B) and E16.5 (C) CGE-derived interneurons in P1, P3, P5, and P7 cortices. (D) The layer distribution of E12.5 MGE- and E12.5 or E16.5 CGE-derived cortical interneurons at P1, P3, P5, P7, and their final locations at P21. At P1, the superficial to deep layer ratio of interneurons derived from the E12.5 MGE and CGE are quite similar (compare blue and red color bar graphs). However, by P3, we observe a decrease in the numbers of layer I and an increase in the numbers of layer V interneurons in the E12.5 MGE-derived population, suggesting a superficial to deep layer migration of this population (dotted arrow). This superficial to deep migration continues till around P5, at which time these neurons have almost completed integrating into their target layers. By contrast, in the E12.5 CGE-derived interneuron population, the number of fate-mapped cells decreased in layers I and VI and increased in layers II/III. This suggests a net cell migration of CGE-derived cells toward layer II/III from both deeper and more superficial layers (dotted arrows). This migration seems to finalize at around P7, which is few days later than the ones derived from the age-matched MGE. Interestingly, although they are born 4 days later, E16.5 CGE-derived interneurons at P1 occupy the cortical layers in a similar proportion to the 2 lineages originated from E12.5. After this period, E16.5 CGE-derived interneurons are sorted into target layers in a very similar manner to the ones from earlier at E12.5. WM, white matter; IZ, intermediate zone. Scale bars 50 μm.
By P3, the number of MGE-derived interneurons in layer I was dramatically reduced, and the percentage of this population located in deep layers (V and VI) increased proportionately (Fig. 3D). This suggests that the MGE-derived interneurons translocated from layer I to the deeper layers during the intervening 2 days. This change in layer distribution from superficial to deep continued until P5 (Fig. 3D), at which point this population appears to have achieved its mature distribution (Fig. 3D, left panel, compare P5 with the final distribution at P21). Analysis of the age-matched CGE-derived population over this same time period showed a different pattern of layer redistribution (Fig. 3B,D) with primarily a marked increase in the percentage in layers II/III (Fig. 3D, middle panel). This suggests a net cell migration of CGE-derived interneurons toward layer II/III from deeper and more superficial layers (Fig. 3D, middle panel, dotted arrows). This trend continued, resulting in around 75% of this population being found in the superficial layers by P7 (Fig. 3D). Notably, it appears that despite sharing an identical birthdate, this radial sorting occurs several days later in CGE-derived populations than the ones derived from the MGE (Fig. 3D).
We have previously reported that CGE-derived interneurons preferentially target superficial layers irrespective of their birthdate (Miyoshi et al. 2010) (Fig. 1). This prompted us to investigate the layer distribution of E16.5 CGE-derived interneurons during the early postnatal period (Fig. 3C). At P1, the superficial versus deep layer distribution of the E16.5 CGE-derived population was found to be similar to the MGE- and CGE-derived populations fate mapped from the E12.5 time point (Fig. 3D, compare blue vs. red of P1 bar graphs). This suggests that the later born CGE-derived population also initially occupies all cortical layers at P1. Interestingly, E16.5 CGE-derived interneurons showed a similar change in their layer distribution to the E12.5 CGE-derived cohorts after P1, suggesting that they segregate to their cortical layers in a manner indistinguishable from those born from this same structure at the earlier time point (compare Fig. 3D right with the middle panel, dotted arrows). This supports the idea that the sorting of distinct interneuron lineages is differentially controlled within the cortical plate.
We considered the possibility that selective cell death might contribute to the observed changes in cell distributions within the cortex; however, analysis for cleaved caspase3 expression did not reveal any cell death in either fate-mapped population during this period (P1 MGE: 0/111, P1 CGE: 0/95, P3 MGE: 0/90, P3 CGE: 0/112) (Supplementary Figures). This data is consistent with previous work examining cell death in interneuron populations during postnatal stages (Hevner et al. 2004). This suggests that the selective layer distribution of MGE- versus CGE-derived cortical interneurons that occurs after P1 is not due to selective cell death but is the result of their directed sorting subsequent to entering the cortical plate (see the model, Fig. 5).
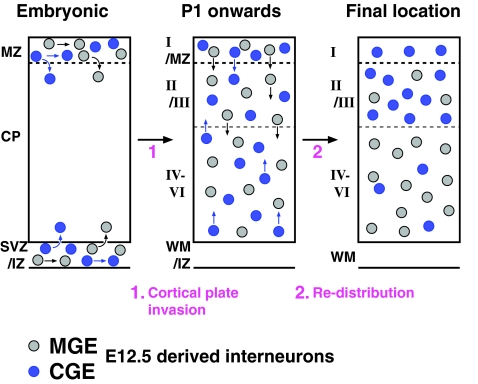
Figure 5.
Postnatal radial sorting of E12.5 MGE- and CGE-derived interneurons upon entering the cortical plate. A summary diagram illustrating that the layer-specific integration of E12.5-derived cortical interneurons only occurs after entering the cortical plate. During embryogenesis, interneurons tangentially migrate through the MZ (marginal zone), IZ (intermediate zone), and SVZ (subventricular zone). E12.5-generated interneurons switch their mode of migration from tangential to radial as they invade the cortical plate. At P1, both MGE- and CGE-derived interneurons are relatively uniformly distributed across all cortical layers. After P1, E12.5 MGE- and CGE-derived interneurons become preferentially distributed into deep and superficial cortical layers, respectively. This sorting step takes place at P1 onward until interneurons reach their final locations.
KCC2 Expression Is Upregulated within the Interneurons during the Critical Period of Radial Sorting
We have found that the radial sorting of age-matched interneurons derived from the E12.5 MGE occurs several days earlier than the CGE-derived counterpart (Fig. 3D). This selective sorting in the cortical plate may be mediated by the differential maturation timing between these age-matched cohorts. Previous work has suggested that interneuron maturation is accompanied by the expression of the potassium/chloride cotransporter KCC2 (Ben-Ari et al. 1989; Rivera et al. 1999; Bortone and Polleux 2009), which switches GABA from an excitatory to an inhibitory neurotransmitter (Ben-Ari et al. 1989; Owens and Kriegstein 2002). Since E12.5 MGE-derived interneurons reach their final positions earlier than CGE ones (Fig. 3D), we hypothesized that they also mature earlier and would hence initiate expression of KCC2 earlier. We therefore analyzed KCC2 expression in our E12.5 MGE- and CGE-derived populations (Fig. 4A) and indeed found that the former show proportionately higher numbers of cells expressing KCC2 at earlier time points (MGE: P1: 15% [15/101], P3: 16% [15/93], P5: 23% [16/71] vs. CGE: P1: 3% [3/99], P3: 5% [4/75], P5: 18% [9/50], P7: 25% [35/142]) (Fig. 4B). Although representing only a subset of each of these populations, this result is consistent with the notion that MGE-derived interneurons stop radial migration 2 days earlier than their CGE-derived counterparts.
Figure 4.
KCC2 upregulation occurs during the radial sorting of interneurons. (A) Representative examples of KCC2-positive (arrowhead) and KCC2-negative (open arrowhead) cells at P3 derived from the E12.5 MGE and CGE, respectively. (B) A graph showing the proportion of KCC2-positive interneurons fate mapped from the E12.5 MGE and CGE. An increase in KCC2 expression occurs at the time interneurons are actively sorted (E12.5 MGE-derived: around P3, E12.5 CGE-derived: around P5). Scale bar 50 μm.
Discussion
In this study, we have examined how MGE- and CGE-derived interneuron populations attain their characteristic layering within the cortex. We found that this is achieved by differential sorting of these populations within the cortical plate during the early postnatal period. Although the notion that a temporal matching exists between the pyramidal neurons and the cortical interneurons works as a general principle (Miller 1985; Fairen et al. 1986), a large percentage of CGE-derived interneurons (approximately 30%) are produced well in advance of the cortical layers they are destined to occupy (Miyoshi et al. 2010). Here, we have directly examined age-matched interneuron populations derived from E12.5 and found that despite sharing a common birthdate, migration route and timing of entry into the cortical plate, MGE- and CGE-derived interneurons possess intrinsically different propensities for the cortical layer they target. Moreover, the MGE-derived population attains its mature distribution 2 days before the one derived from the CGE by radially sorting at an earlier time window. We suggest that this radial cell sorting is a method employed by early versus late born CGE-derived interneurons in order to compensate for their difference in birthdates and to target similar cortical layers. In addition, we have found that there is a temporal correlation between the increase in KCC2 expression and the radial sorting period of the interneuron populations. Taken together, we conclude that distinct interneuron subtypes are intrinsically programmed to respond to the cortical environment in order to integrate into the target layers (see the model, Fig. 5).
Diverse Cortical Interneuron Subtypes Show Stereotypic Patterns of Tangential Migration and Integration into the Cortical Plate
Until recently, cortical interneurons were thought to arise almost exclusively from the MGE. As such, the embryonic origin of different interneuron subtypes was not considered to be a factor in determining their developmental behavior. The realization that cortical interneurons arise from at least 2 distinct embryonic territories raises 2 specific questions as to how the positioning of each interneuron subtype within precise cortical layers is achieved. First, do different cortical interneuron subtypes derived from distinct origins integrate into the cortex by taking differential migratory routes? Second, to what extent does temporal matching explain the positioning of specific interneuron subtypes to particular cortical laminae? Our recent finding that approximately 30% of all cortical interneurons do not follow an inside-out layering pattern (Miyoshi et al. 2010) bears on both of these issues. A definitive answer to the first question must await the means to track individual interneuron subtypes from their origins. However, our present analysis, coupled with the knowledge that MGE- and CGE-derived interneurons are completely complementary in terms of the subtypes they give rise to (Miyoshi et al. 2010), demonstrates that different subtypes are not obliged to follow distinct migratory paths. Still, it is possible that particular interneuron subtypes may have favored routes of migration both into and within the cortex. In contrast, the present study allowed us to address the second question by focusing on 2 populations, one that is MGE-derived and born coincident with the pyramidal cell layers they will ultimately reside in and a CGE-derived population that is not (Fig. 1).
The earliest cohort of interneurons from both the MGE and CGE seem to first reach the intermediate/subventricular zones rather than the marginal zone (Fig. 2A and data not shown) (Miyoshi et al. 2007, 2010). Since the MGE is medially located in the ventral telencephalon, interneurons derived from this structure need to avoid the striatum in order to reach the cortex (Marin and Rubenstein 2003). They take either of 2 pathways, one migrating close to the ventricular/subventricular zones of the lateral ganglionic eminence and CGE dorsally located to the striatum or the other ventrolaterally avoiding the striatum. By taking the former route, which is relatively shorter than the latter, MGE-derived interneurons seem to reach the intermediate/subventricular zones in the cortex earlier than they reach the marginal zone. Interneurons derived from the CGE are always first found tangentially migrating at the caudal levels of the telencephalon (Miyoshi et al. 2010). Although they presumably have no need to avoid the striatum and are located closer to the cortex compared with the MGE, they also first appear in the intermediate/subventricular zones in the cortex and only later at the marginal zone. In fact, although at different rostral–caudal levels of the cortical regions, the distance interneurons have traveled into the cortex from the MGE and CGE is comparable at 2 days after birth (Fig. 2A).
The Layering of MGE- and CGE-Derived Cortical Interneurons Is Intrinsically Programmed
During the postnatal stages, migration of GABAergic cells has been found in multiple forebrain regions (Marin and Rubenstein 2003). A large population can be found in the rostral migratory stream, which is a pathway originating from the subventricular zone progenitor niche and migrating toward the anteriorly located olfactory bulb (Merkle et al. 2007). In the molecular layer of rat hippocampus, a more subtle migration of GABAergic interneurons has also been demonstrated (Dupuy-Davies and Houser 1999; Morozov and Freund 2003; Morozov et al. 2006). While it is apparent that cortical interneurons continue to migrate during early postnatal stages, their behavior has not been revealed until this study due to the lack of the means to distinguish individual lineages at this period. Addressing this issue is further complicated by the fact that subtype-specific molecular markers (e.g., Parvalbumin, Vasoactive intestinal polypeptide, Reelin and Neuropeptide Y) are only expressed in interneuron lineages beginning at around 1 week after birth.
MGE-derived interneurons come to populate cortical layers in an inside-out manner by temporally matching the production of pyramidal neurons (Miyoshi et al. 2007). In contrast, CGE-derived interneurons mostly target superficial layers irrespective of their birthdates (Miyoshi et al. 2010). Hence, it is apparent that the mechanisms involved in the layer acquisition of MGE- and CGE-derived interneurons are quite distinct. This may be a reflection of differences in the presumptive somal locations of these 2 populations. As both pyramidal cells and MGE-derived interneurons are restricted to layers II–VI and excluded from layer I, temporal matching likely provides a parsimonious means for these populations to establish cortical circuitry. In contrast, approximately 20% of the CGE-derived population contributes to layer I, with the majority of the remainder being relegated to layers II/III that are born last.
CGE-derived cortical interneurons accommodate to the late generation of their target pyramidal layers by delayed neurogenesis. However, this strategy can only partially explain how they target the superficial layers appropriately, as a third of this population is born significantly prior to the generation of the superficial layers of the cortex. As such, this study provides evidence that the layering of interneurons is intrinsically determined rather than simply a product of interneurons selecting a cortical layer based on it being appropriately mature. The age-matched interneuron populations derived from the E12.5 MGE and CGE are indistinguishable in their layer distribution at P1 when they have completed their entrance into the cortical plate. Although they are similarly uniformly distributed into all cortical layers at this stage, these 2 populations soon start to differentially respond to their surrounding cortical environment. MGE-derived interneurons migrate toward deep layers immediately after P1. However, the CGE-derived populations redistribute into the target layers more gradually and only achieve their adult location within layers II/III at around P7. This clearly demonstrates that the critical time windows for postnatal sorting are distinct for age-matched E12.5 MGE- and CGE-derived interneuron populations. One possible explanation for this differential behavior is that E12.5 MGE-derived interneurons mature more quickly than age-matched cohorts derived from the CGE. This would be consistent with our finding that at P1 and P3 a significantly higher proportion of MGE-derived interneurons express KCC2 compared with cohorts derived from the CGE (Fig. 4B). Only after P3, does the CGE-derived population show an increase in the numbers of cells expressing KCC2. Although the timing of KCC2 upregulation within interneuron subtypes correlates well with the critical time window for them undergoing radial sorting, it is still unclear whether this change in KCC2 expression is functionally important for the differential positioning of MGE- versus CGE-derived interneurons. For the MGE-derived interneurons, the upregulation of KCC2 expression has been suggested to be involved in the arrest of tangential migration (Bortone and Polleux 2009). It will be interesting in the future to more directly compare the role of KCC2 in MGE- and CGE-derived interneurons during the postnatal period when radial sorting occurs. In addition, it will be informative to directly test the maturity of MGE- and CGE-derived interneuron populations at early postnatal stages by analyzing their intrinsic firing properties with electrophysiological methods.
Since the CGE-derived cortical interneurons have not been systematically studied until recently, it came as a surprise to find that they preferentially target superficial layers irrespective of their birthdates (Miyoshi et al. 2010). By comparing the layering of E12.5 versus E16.5 CGE-derived populations through the early postnatal stages, we conclude that this is achieved by the later born population entering the cortical plate much more quickly than the ones born at earlier time points. Four days after birth, approximately 85% of the E12.5-derived population is located outside the cortical plate (Fig. 2C). By contrast, the majority of E16.5 CGE-derived interneurons are able to enter the cortical plate 4 days later at P1 and hence attain a distribution indistinguishable from the much earlier born E12.5 CGE-derived population (Fig. 3D). This indicates that irrespective of their birthdate, CGE-derived interneurons synchronously enter the cortical plate at P1. We conclude that the delayed competence of E12.5 CGE-derived interneurons to undergo the radial sorting is the method used to adjust for the difference in the early versus late born CGE-derived cohorts and to ensure that they target similar cortical layers.
In vivo transplantation studies further support the idea that the layer targeting of interneurons is intrinsically determined. When donor MGE- or CGE-derived cells are heterotopically transplanted back into the host CGE or MGE, respectively, interneurons choose their target layers in accordance with their donor origin (Nery et al. 2002; Butt et al. 2005). However, homotopic, heterochronic in vivo transplantations carried out on MGE-derived cells (Pla et al. 2006) revealed that these cells can recalibrate their selection of cortical laminae, if sufficiently mature. When E15.5 MGE-derived cells were transplanted into an E12.5 host MGE, they changed their laminar preference in favor of the host environment. This result is consistent with the temporal matching hypothesis, as in this circumstance the donor E15.5 MGE-derived cells were precociously mature and thus able to populate deeper layers of cortex than they would normally occupy.
Here, we have provided evidence that distinct interneuron subtypes behave differently within the early postnatal cortex in order to reach their final laminar destinations. This argues that distinct interneuron subtypes are intrinsically programmed to undergo differential radial migration. Elucidating the genetic programs underlying this dynamic process will not only allow us to further understand the developmental process of GABAergic interneurons but also is a key for elucidating the underlying etiology of multiple neurodevelopmental disorders, whose cause is presently unrevealed.
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/
Funding
National Institute of Health grants (RO1NS039007, RO1MH071679); Simons Foundation and New York State through their New York State Stem Cell Science initiative; National Alliance for Research on Schizophrenia and Depression (to G.M.).
Acknowledgments
We thank Dr Hirohide Takebayashi for providing us the Olig2-CreER driver, Drs Chin Xu and Stewart Anderson for Nkx2-1BAC-Cre driver, and Dr Yuchio Yanagawa for GAD1-EGFP line. We thank Lihong Yin for her technical help in genotyping animals. Finally, we are greatly appreciative to all the Fishell laboratory members for their support and input on this work. We would also like to thank Allison Roberta and Joe Marlin for critically reading this manuscript. Finally, we especially thank Drs Rob Machold and Theofanis Karayannis for their intellectual input and many helpful suggestions in the design and interpretation of these experiments and in the writing of this manuscript. Conflict of Interest: None declared.
References
- Ang ES, Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Nery S, Fishell G. Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci. 2001 doi: 10.1038/nn749. 4:1177–1182. [DOI] [PubMed] [Google Scholar]
- Dupuy-Davies S, Houser CR. Evidence for changing positions of GABA neurons in the developing rat dentate gyrus. Hippocampus. 1999;9:186–199. doi: 10.1002/(SICI)1098-1063(1999)9:2<186::AID-HIPO9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Fairen A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol. 1986;251:67–83. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N, Yu M, Long J, Hatch G, Rubenstein JL, Ekker M. Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J Neurosci. 2007;27:5012–5022. doi: 10.1523/JNEUROSCI.4725-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P. Pathogenesis of migration disorders. Curr Opin Neurol. 2006;19:135–140. doi: 10.1097/01.wco.0000218228.73678.e1. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Englund C, Kohtz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–618. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Miller MW. Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Brain Res. 1985;355:187–192. doi: 10.1016/0165-3806(85)90040-9. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. Directing neuron-specific transgene expression in the mouse CNS. Curr Opin Neurobiol. 2006;16:577–584. doi: 10.1016/j.conb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov YM, Ayoub AE, Rakic P. Translocation of synaptically connected interneurons across the dentate gyrus of the early postnatal rat hippocampus. J Neurosci. 2006;26:5017–5027. doi: 10.1523/JNEUROSCI.0272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci. 2003;18:1213–1222. doi: 10.1046/j.1460-9568.2003.02852.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, O'Leary DD. Dynamic patterned expression of orphan nuclear receptor genes RORalpha and RORbeta in developing mouse forebrain. Dev Neurosci. 2003;25:234–244. doi: 10.1159/000072271. [DOI] [PubMed] [Google Scholar]
- Nakajima K. Control of tangential/non-radial migration of neurons in the developing cerebral cortex. Neurochem Int. 2007;51:121–131. doi: 10.1016/j.neuint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Pla R, Borrell V, Flames N, Marin O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci. 2006;26:6924–6934. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci U S A. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Gashghaei HT, Han C, Watson H, Campbell KJ, Anton ES. Radial glial dependent and independent dynamics of interneuronal migration in the developing cerebral cortex. PLoS One. 2007;2:e794. doi: 10.1371/journal.pone.0000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinyk DL, Mercer EH, Harris E, Anderson DJ, Joyner AL. Fate mapping of the mouse midbrain-hindbrain constriction using a site-specific recombination system. Curr Biol. 1998;8:665–668. doi: 10.1016/s0960-9822(98)70255-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.