Abstract
Semantic judgments involve both representations of meaning plus executive mechanisms that guide knowledge retrieval in a task-appropriate way. These 2 components of semantic cognition—representation and control—are commonly linked to left temporal and prefrontal cortex, respectively. This simple proposal, however, remains contentious because in most functional neuroimaging studies to date, the number of concepts being activated and the involvement of executive processes during retrieval are confounded. Using functional magnetic resonance imaging, we examined a task in which semantic representation and control demands were dissociable. Words with multiple meanings like “bank” served as targets in a double-prime paradigm, in which multiple meaning activation and maximal executive demands loaded onto different priming conditions. Anterior inferior temporal gyrus (ITG) was sensitive to the number of meanings that were retrieved, suggesting a role for this region in semantic representation, while posterior middle temporal gyrus (pMTG) and inferior frontal cortex showed greater activation in conditions that maximized executive demands. These results support a functional dissociation between left ITG and pMTG, consistent with a revised neural organization in which left prefrontal and posterior temporal areas work together to underpin aspects of semantic control.
Keywords: ambiguity, inferior frontal gyrus, semantic memory, semantic priming, temporal cortex
Introduction
Understanding the meaning of words requires 2 neural processes to interact: first, there must be activation of previously acquired conceptual knowledge of the word, and secondly, in many situations, executive mechanisms are required to direct retrieval of such information in a task-appropriate way. Research that supports this dissociation between semantic control and representation stresses the importance of a distributed network in left temporal and prefrontal cortex. Left temporal cortex is thought to be a key substrate for storing semantic knowledge (Hickok and Poeppel 2004; Indefrey and Levelt 2004; Vigneau et al. 2006; Binder et al. 2009), while left inferior frontal gyrus (LIFG) has been linked to semantic control processes during meaning retrieval (Thompson-Schill et al. 1997; Wagner et al. 2001; Bookheimer 2002; Badre 2008). Accordingly, patients with damage to posterior temporal cortex show poor language comprehension, while those with damage to LIFG show semantic retrieval problems but relatively intact knowledge of word meaning in tasks that minimize executive control demands (Hart and Gordon 1990; Chertkow et al. 1997; Thompson-Schill et al. 1998; Robinson et al. 2005; Novick et al. 2009).
In line with this view, the functional neuroimaging literature emphasizes the role of LIFG in tasks that involve significant executive control over semantic selection or retrieval. Studies have shown that brain activation increases in LIFG when words appear in weak as opposed to strong semantic environments, since contextual cues that guide the retrieval of target concepts are lacking (e.g., Roskies et al. 2001; Wagner et al. 2001; Zempleni et al. 2007; Kuperberg et al. 2008; Ruff et al. 2008; Chou et al. 2009). The same activation pattern is observed when more than one response option might be appropriate and competing information needs to be inhibited (e.g., during verb generation with many appropriate associated responses) (Thompson-Schill et al. 1997; Badre et al. 2005; Snyder et al. 2007; Bedny et al. 2008; Nagel et al. 2008; Snijders et al. 2009). However, close inspection of the findings of these studies suggests that the neural substrate of semantic control is complex, involving additional brain regions beyond LIFG. Imaging studies often reveal left posterior middle temporal gyrus (pMTG) coactivation—together with prefrontal cortex—during manipulations of semantic control (e.g.,Thompson-Schill et al. 1997; Wagner et al. 2001; Noppeney et al. 2004; Badre et al. 2005; Gold et al. 2006; Kuperberg et al. 2008). Similarly, patients with semantic aphasia (SA), who have deficits in the executive regulation of meaning retrieval, can show posterior temporal as well as prefrontal lesions (Jefferies and Lambon Ralph 2006; Jefferies et al. 2008; Noonan et al. 2009; Corbett et al. 2009a). It is therefore likely that distinct regions of temporal lobe are important for semantic control and representation.
Strong support for a functional dissociation within the left temporal lobe is provided by direct comparisons between SA patients with temporoparietal infarcts and individuals with semantic dementia (SD). SD patients show atrophy focused on the anterior and inferior aspects of temporal cortex (although as the disease progresses, atrophy can extend into posterior temporal regions or rostrally into frontal cortex) (Mummery et al. 2000; Hodges and Patterson 2007). SD produces a gradual deterioration of semantic knowledge, starting with fine-grained knowledge of specific concepts (e.g., that a camel has a hump) and progressing to more basic semantic information (e.g., that a camel is an animal) (Rogers et al. 2004). Moreover, patients with SD show highly consistent performance when the same concepts are tested in verbal and visual tasks, suggesting that anterior inferior temporal cortex provides a key repository of amodal semantic knowledge (Bozeat et al. 2000). In contrast, SA patients with either left prefrontal or posterior temporal infarcts show inconsistent performance across different tests that tap the same concepts. Their ability to retrieve conceptual information is related to the executive demands of tasks (Jefferies and Lambon Ralph 2006; Noonan et al. 2009; Corbett et al. 2009b)—for example, they have difficulty selecting appropriate concepts in the face of potent distractors. Picture naming can be substantially improved in SA but not SD by the provision of phonological cues (e.g., /t/ for “tiger”). SA patients also show poorer picture naming performance when the initial phoneme of an inappropriate, semantically related response is provided (e.g., /l/ from lion, when “tiger” is the correct answer), increasing demands on semantic selection and inhibition processes (Jefferies et al. 2008; Noonan et al. 2009; Soni et al. 2009). SA patients with prefrontal and posterior temporal lesions show strikingly similar semantic deficits characterized by poor semantic control (Berthier 2001; Jefferies and Lambon Ralph 2006; Noonan et al. 2009). Therefore, qualitatively different patterns of semantic deficits arise from anterior and posterior temporal lesions (in SD and SA respectively), suggesting a dissociable role for these brain areas in semantic representation and control respectively.
The present study seeks convergent evidence for this hypothesis using functional magnetic resonance imaging (fMRI). Inconsistencies in the conclusions of previous neuroimaging studies might follow from the fact that in most behavioral tasks, semantic representation and control demands are confounded. Typically, as the level of semantic control during meaning retrieval increases, the demands on semantic representations are also high. We offer a paradigm that pulls these 2 components of semantic cognition apart by studying the comprehension of words with multiple meanings like “bank” (i.e., homonyms).
Homonym processing is thought to increase both control and representational demands: more than one concept may be activated for the same lexical item (e.g., bank), and there may be competition between the alternative meanings of one word (e.g., between the 2 meanings of bank referring to the financial institution vs. riverside). According to most models of ambiguity resolution, meaning frequency and contextual constraints determine the degree to which multiple meanings of homonyms are activated and hence the amount of semantic retrieval that is expected (Duffy et al. 1988; Rayner et al. 1994; Simpson 1994; Giora 1999; Sereno et al. 2003; Peleg and Eviatar 2008; Noonan et al. 2009): single meaning retrieval is likely when the more frequent, dominant concept is favored, whereas the recovery of multiple meanings occurs when the context allows for both interpretations of the homonym (“Jane had a bad day when she damaged her heel”).
Consistent with the traditional view that temporal cortex is crucial for storing word meaning, brain activation in left pMTG (Brodmann area [BA] 21) and mid portions of inferior temporal gyrus (mid-ITG) has been shown to increase during long epochs of equibiasing contexts in which both interpretations of an ambiguous word are equally likely, presumably because conceptual representations corresponding to both interpretations are activated (Snijders et al. 2009). Most ambiguity studies, however, have not modeled periods of sustained multiple meaning activation but have increased retrieval demands more indirectly, for example, by using subordinately biasing contexts (“The bank was steep”). Here, the reader is misguided and needs to replace an initial interpretation with its alternative subordinate meaning, leading to the effortful suppression of the dominant concept (Rayner et al. 1994; Simpson 1994). Studies using this method again observed activation in left temporal lobe although this was limited to posterior aspects of middle and ventrolateral temporal cortex (BA 21/37) (Gennari et al. 2007; Bedny et al. 2008; but cf. Rodd et al. 2005; Zempleni et al. 2007; Hoenig and Scheef 2009). In contrast, homonyms that were resolved toward their dominant meaning or that required less task-induced semantic analysis (e.g., lexical decision) did not yield additional activation in these areas (Copland et al. 2003, 2007; Zempleni et al. 2007; Grindrod et al. 2008). This work supports the essential role of pMTG in semantic processing; however, the co-occurrence of high semantic control demands with multiple meaning activation in these conditions makes it impossible to determine whether this posterior temporal activation reflects additional conceptual retrieval during homonym processing or, according to an opposing viewpoint, aspects of executive control.
Isolating these 2 components is nontrivial. Bedny et al. (2008) addressed this issue by using a conjunction approach—contrasting several conditions of high with low semantic competition—while Hoenig and Scheef (2009) entered behavioral measures of semantic interference into their analysis of fMRI data. Both studies demonstrated that blood oxygen level–dependent (BOLD) activity in dorsolateral or inferior frontal cortex was influenced by executive aspects of ambiguity resolution unlike temporal cortex. However, neither investigation experimentally separated semantic representational requirements from control demands.
Our aim was to investigate the possibility of a double dissociation between semantic representation (in anterior ITG) and semantic control (in posterior MTG). To achieve this, we used a novel paradigm to create, for the first time, experimental conditions that differentially loaded onto the amount of information being retrieved as opposed to executive demands. Conditions that triggered multiple meaning retrieval of homonyms did not require maximal semantic control. Homonyms were preceded by 2 primes that related to their different meanings (e.g., game–dance–ball). Participants had to decide whether the final target word was related to either of the preceding primes. Unlike past studies, meaning suppression was not obligatory for successful task performance, as both primes (game, dance) were related to the target (ball). In this special situation, homonym retrieval is “facilitated” by the activation of both concepts. Multiple priming studies have shown that—despite an interference effect elicited by the activation of 2 competing meanings—reaction times (RT) are faster when both concepts of a homonym are addressed, compared with priming of a single meaning (Balota and Paul 1996; Chwilla and Kolk 2003; Milberg et al. 2003; Kandhadai and Federmeier 2007). A conjunction analysis was therefore performed on contrasts of multiple versus single meaning priming to uncover specific and robust brain activation linked to the computation of multiple meanings in the absence of strong competition/inhibition demands (Conjunction 1). We also included an “unambiguous” control condition, where both primes converged onto a single meaning (lion–stripe–tiger), to exclude cognitive systems involved in multiple priming of a single concept (Whitney et al. 2009).
To differentiate this region from brain structures supporting executive aspects of ambiguity resolution, we compared retrieval of subordinate and dominant meanings. Retrieval of the subordinate interpretation (e.g., dance–ball) requires maximal executive resources because the dominant meaning must be inhibited in order to establish a semantic link between the prime and target. This process was not obligatory when both meanings of a homonym were primed, suggesting that semantic control demands were substantially reduced (although not eliminated) in this condition. The lowest executive resources were required in trials that addressed the dominant interpretation of ambiguous words. Conjunction 2 therefore identified brain areas that responded during subordinately biasing contexts and also when these trials were compared with conditions of lowest levels of semantic control (i.e., dominant contexts).
Materials and Methods
Participants
Imaging and behavioral data from 15 male, right-handed, native German speakers were analyzed (mean age = 28.93 years, standard deviation [SD] = 7.11; mean years of education = 12.60 years, SD = 1.92). Further details are described in Whitney et al. (2009). Subjects were healthy and showed average- or above-average–estimated verbal IQ as assessed by the German multiple choice vocabulary test (MWT-B; Lehrl et al. 1995) (mean estimated verbal IQ = 111.93, SD = 14.55). Written consent was obtained from each subject before testing. The study was approved by the local ethics committee.
Task
Participants performed a relatedness judgment task on the last word shown in a triplet (lion–stripe–tiger). Upon appearance of the final word (target), subjects had to decide whether the target was related to any of the preceding items (primes) by pressing a button with their left index (“yes”) or middle (“no”) finger. Primes were presented consecutively for 200 ms each before the target appeared on the screen for 1000 ms.
Stimuli
Word triplets consisted of 2 primes followed by 1 target word, which was either a homonym or unambiguous. Prime–target relationships were systematically manipulated to yield 4 basic conditions: 1) In an ambiguous double-related condition, the 2 related primes diverged onto different concepts of a homonym (RRa) (e.g., game–dance–ball). 2) In an unambiguous double-related control condition, both primes addressed the same meaning of an unambiguous target (RR) (e.g., lion–stripe–tiger). 3) In single-related trials, only one prime was related to the homonym (the other was unrelated). In one condition, the dominant meaning was primed (Rd) (e.g., game–pillow–ball). 4) In the other condition, the subordinate meaning was primed (Rs) (e.g., dance–clock–ball). To eliminate position effects, each prime appeared once in the first and second positions in the triplet, yielding a total of 8 conditions for the current fMRI analysis. Table 1 displays all 8 conditions plus 8 additional manipulations, which were used to calculate the behavioral priming effects (PEs) (see Behavioral Analysis below). These included 2 unambiguous single-related trials (R1: lion–bread–tiger; R2: stripe–rest–tiger), analogue to the ambiguous conditions Rd and Rs, and 2 novel unrelated conditions, in which none of the primes were related to either an ambiguous (UUa; pillow–clock–ball) or unambiguous target (UU; bread–rest–tiger). Again, prime position was altered. These conditions were irrelevant for the fMRI study since we were interested in the simultaneous activation of multiple versus single meanings of the same ambiguous word. Details on stimulus construction for these conditions can be obtained from Whitney et al. (2009).
Table 1.
Examples of word triplets in the multiple prime paradigm
| Ambiguous |
Unambiguous |
||||||||
| Prime sequence | Prime1 | Prime2 | Target | Prime sequence | Prime1 | Prime2 | Target | ||
| RRa | rev− | Game | Dance | Ball | RR | rev− | Lion | Stripe | Tiger |
| rev+ | Dance | Game | Ball | rev+ | Stripe | Lion | Tiger | ||
| Rd | rev− | Game | Pillow | Ball | R1 | rev− | Lion | Bread | Tiger |
| rev+ | Pillow | Game | Ball | rev+ | Bread | Lion | Tiger | ||
| Rs | rev− | Dance | Clock | Ball | R2 | rev− | Stripe | Rest | Tiger |
| rev+ | Clock | Dance | Ball | rev+ | Rest | Stripe | Tiger | ||
| UUa | rev− | Pillow | Clock | Ball | UU | rev− | Bread | Rest | Tiger |
| rev+ | Clock | Pillow | Ball | rev+ | Rest | Bread | Tiger | ||
Note: Conditions that are used for the fMRI analysis are displayed in bold. Prime sequence denotes whether primes appear in canonical (rev−) or reversed (rev+) order. RRa = ambiguous double-related trials, Rd/Rs = single-related trials that address the dominant/subordinate meaning of a homonym, UUa = ambiguous unrelated trials, RR = double-related trials, R1/R2 = single-related trials, UU = unrelated trials.
For the 8 conditions relevant for fMRI, 25 homonyms and their corresponding dominant and subordinate meanings were selected from the association norms of German homonyms (Moritz et al. 2001). Each homonym had a preferred meaning, which was at least 20% more frequent than its alternative, subordinate interpretation. Dominant and subordinate meanings (i.e., target concepts) did not differ with respect to familiarity (dominant: M = 5.49, SD = 1.01; subordinate: M = 5.01, SD = 1.29), concreteness (dominant: M = 5.09, SD = 1.22; subordinate: M = 5.36, SD = 1.51), or imageability (dominant: M = 5.46, SD = 1.56; subordinate: M = 4.97, SD = 1.07) as rated by a sample of 15 naive subjects on a 7-point scale (P > 0.16). The distribution of concepts denoting natural kinds (10/7), man-made artifacts (11/13)—including tools (3/1)—and abstract concepts (4/5) was also similar across dominant/subordinate interpretations (chi-square = 1.79, P = 0.41).
To form single-related trials, one of the related primes in the triplet was substituted by an unrelated prime and matched to the (removed) related prime in length in syllables (≤4) and letters (≤9) and frequency as assessed by the CELEX database (Baayen et al. 1993). Stimuli for the unambiguous double-related condition were taken from the indirectly related word triplets in Spitzer et al. (1993) and matched to the ambiguous items along the same parameters. Stimuli characteristics are summarized in Table 2.
Table 2.
Mean values of orthographic and lexical characteristics of words that were used to create the 8 conditions for the fMRI experiment (standard deviation in parentheses)
| Target |
Related primes |
Unrelated primes |
||||||
| Amb | Unamb | Amb |
Unamb |
Amb |
||||
| Rd | Rs | R1 | R2 | U1 | U2 | |||
| Ball | Tiger | Game | Dance | Lion | Stripe | Pillow | Clock | |
| Syll | 1.68 (0.62) | 1.48 (0.48) | 1.72 (0.43) | 1.56 (0.49) | 1.72 (0.48) | 1.56 (0.48) | 1.76 (0.66) | 1.60 (0.50) |
| Let | 5.36 (1.55) | 4.84 (1.35) | 5.36 (0.99) | 4.96 (1.38) | 5.08 (1.59) | 5.24 (1.14) | 5.08 (1.41) | 5.12 (1.48) |
| Freq | 46.48 (24.44) | 86.08 (241.31) | 93.36 (61.30) | 59.52 (64.82) | 46.88 (104.75) | 71.76 (45.99) | 30.6 (44.73) | 51.84 (146.84) |
Note: Amb/Unamb = ambiguous/unambiguous condition, Syll = number of syllables, Let = number of letters, and Freq = total frequency (spoken and written) per million.
Finally, each prime–target and prime–prime relationship in the triplet was rated by another 12 participants on a scale from 1 (=not related) to 7 (=related). We made sure that 1) the ambiguous and unambiguous targets in the double-related conditions were primed to a similar extent by the preceding related words (average relatedness for ambiguous prime–target pair: M = 6.34, SD = 0.68; unambiguous prime–target pair: M = 6.45, SD = 0.45; P = 0.32); 2) that the 2 related primes in these triplets were unrelated to each other to ensure independent PEs (ambiguous prime–prime pair: M = 2.28, SD = 1.42; unambiguous prime–prime pair: M = 2.89, SD = 1.25; P = 0.15); and 3) that unrelated primes in the single-related conditions were indeed unrelated to the target (M = 1.32, SD = 0.39) (for a more detailed analysis, see Whitney et al. 2009). The association strength between prime–target pairs being biased toward the subordinate versus dominant meaning was uniformly high (>6) but differed significantly (dominant prime–target pair: M = 6.62, SD = 0.38; subordinate prime-target pair: M = 6.08, SD = 0.81; P < 0.01).
The 8 conditions were distributed evenly across 4 lists (along with the remaining 8 manipulations from Table 1). A list contained 100 trials, so that each condition was represented by 6–7 individual trials. These trials were presented 1) once or twice in isolation, 2) once in a sequence of 2, and 3) once in a sequence of 3 consecutive trials of the same condition (“mini blocks”) to enhance BOLD signal strength during event-related designs (Amaro and Barker 2006; Kircher et al. 2009; Sass et al. 2009a, 2009b). The intertrial interval (ITI) was mostly short between uniform sequences (1–2 s) and longer (2.5–5 s) between different trial types. The order of conditions was pseudorandomized, and the sequence of lists was counter-balanced across subjects. In total, participants saw 400 word triplets, of which 200 (8 conditions × 25 triplets) were relevant to the current fMRI investigation (for more details, see Whitney et al. 2009).
fMRI Procedure
The conditions were presented in a rapid event-related design in 4 separate scanning sessions. Each session contained 100 trials and lasted 7 min 48 s. At the beginning of each trial, a fixation cross appeared for 500 ms in the centre of the screen. Visually presented primes and targets followed and were shown individually at the same position with an interstimulus interval of 0 ms. Primes were presented for 200 ms each and the target for 1000 ms. The appearance of the hash symbol indicated the end of the trial and was shown for the entire ITI duration. The ITI was jittered and lasted between 1 and 5 s. Subjects were equipped with MRI-compatible goggles (VisuaStim XGA, Resonance Technology, Inc., http://www.mrivideo.com/) and a 2-button response device, which was used to record relatedness judgment decisions at target presentation. Presentation of stimuli was controlled by a computer using the Presentation 10.1 software package (Neurobehavioral Systems, http://www.neurobs.com/) and synchronized with the beginning of the sixth scan.
Data Acquisition
For each subject, 4 series of -weighted axial echo-planar imaging (EPI) scans were acquired at 1.5-T (Gyroscan Intera, Philips Medical Sytems), which were aligned parallel to an imaginary line that connects the anterior and posterior commissure. A circularly polarized phase array head coil and standard gradients were used: number of slices, 31; slice thickness, 3.5 mm; interslice gap, 0.35 mm; matrix size, 64 × 64; field of view, 240 × 240 mm; echo time, 30 ms; and repetition time, 2.8 s. Each series consisted of 167 functional volumes. To minimize head movement, cushions were placed between the participant's head and the coil, and participants were instructed to keep as still as possible during scanning (e.g., avoid swallowing).
fMRI Data Analysis
Preprocessing and first-level statistical analyses of MR data are identical to the procedure described in Whitney et al. (2009) and were performed using Statistical Parametric Mapping software (SPM5) implemented in MATLAB 7.0 (Mathworks Inc.). Images were realigned to the first image and unwarped to correct for the interaction of movement and susceptibility artifacts during image acquisition. Overall, movement was minimal as the maximum change in translation and rotation for each participant was less than one voxel size (i.e., 3.5 mm) and less than 1°, respectively. Each slice was then shifted relative to the acquisition time of the middle slice using a sinc interpolation. Volumes were normalized into standard stereotaxic anatomical MNI space by using the transformation matrix calculated from the first EPI scan of each subject and the EPI template. Afterward, the normalized data with a resliced voxel size of 4 × 4 × 4 mm were smoothed with a 10-mm full-width at half-maximum isotropic Gaussian kernel to accommodate intersubject variation in brain anatomy. The time series data were high-pass filtered with a high-pass cutoff of 1/128 Hz. The autocorrelation of the data was estimated and corrected for.
All conditions (including those that were not analyzed in this experiment) were modeled to accurately resolve variance in the data. First-level statistics were performed on the full set of 8 basic relatedness manipulations instead of 16 conditions (see Table 1) because behavioral analysis revealed no effect of prime position, allowing data to be pooled (see Table 3). The expected hemodynamic response at target onset was modeled for each of the 8 event types with the canonical hemodynamic response function (HRF) (Friston et al. 1998) and its temporal derivative. The functions were convolved with the event train of stimulus onsets to create covariates in a general linear model. The volume of interest was restricted to gray matter voxels by the use of an inclusive mask created from the segmentation of the standard brain template. Subsequently, parameter estimates of the HRF regressor for each of the different conditions were calculated from the least-mean-squares fit of the model to the time series.
Table 3.
Reaction time (RT), error rate, and PEs (pooled over prime position) recorded during fMRI (standard deviations are shown in parentheses)
| Ambiguous |
Unambiguous |
||||||
| RT | Error rate | PE (pooled) | RT | Error rate | PE (pooled) | ||
| RRa rev− | 909.26 (315.37) | 3.73 (5.34) | 443.52 (282.75) | RR rev− | 788.93 (298.52) | 0.56 (1.47) | 557.99 (281.35) |
| RRa rev+ | 917.63 (320.75) | 3.88 (4.52) | RR rev+ | 794.72 (290.22) | 1.40 (2.59) | ||
| Rd rev− | 955.37 (314.50) | 11.85 (7.16) | 363.22 (238.11) | R1 rev− | 997.39 (319.04) | 13.74 (11.03) | 344.43 (218.73) |
| Rd rev+ | 1032.14 (323.63) | 18.48 (14.06) | R1 rev+ | 1013.78 (304.05) | 13.98 (8.69) | ||
| Rs rev− | 1137.00 (391.07) | 32.74 (15.43) | 297.68 (237.33) | R2 rev− | 1007.63 (334.85) | 10.47 (7.85) | 338.84 (219.56) |
| Rs rev+ | 1081.59 (314.24) | 35.38 (15.59) | R2 rev+ | 1014.30 (252.84) | 20.41 (12.85) | ||
| UUa rev− | 1380.14 (499.18) | 12.42 (12.49) | UU rev− | 1340.44 (432.16) | 17.21 (7.96) | ||
| UUa rev+ | 1333.80 (439.12) | 8.82 (7.36) | UU rev+ | 1359.18 (441.59) | 9.35 (7.67) | ||
Note: RRa = ambiguous double-related trials, Rd/Rs = single-related trials that address the dominant/subordinate meaning of a homonym, UUa = ambiguous unrelated trials, RR = double-related trials, R1/R2 = single-related trials, UU = unrelated trials. rev−/rev+ denotes whether primes appeared in their canonical order or were reversed.
Second-level statistics were calculated in several steps. First, a random-effects group analysis was performed by entering parameter estimates of all first-level contrasts into a flexible factorial analysis of variance (ANOVA). Second, conjunction analyses were performed on contrasts from the group-level. Conjunction analyses have the advantage to reveal brain areas that are robustly activated across different sets of contrasts and hence strengthen the reliability of our results given the relatively small sample size (Thirion et al. 2007). Conjunction 1 examined activation of multiple versus single meanings of ambiguous words (RRa ∩ RRa > Rd ∩ RRa > Rs), and thus highlighted brain regions involved in semantic representation. An exclusive mask of the unambiguous double-related condition (RR) was used (P < 0.001, uncorrected) to cancel out activation related to multiple priming in the absence of multiple concept activation. In contrast, Conjunction 2 (Rs ∩ Rs > Rd) examined the semantic control network. This analysis examined activation related to meaning competition and suppression during the retrieval of subordinate (Rs) as opposed to dominant meanings (Rd). Differential contrasts that were entered into the conjunction were masked inclusively by the minuend at the same threshold (P < 0.001).
All analyses were corrected on a voxel-wise threshold of P < 0.001. A Monte Carlo simulation of the brain volume of the current study was conducted to establish an appropriate voxel contiguity threshold (Slotnick et al. 2003). Assuming an individual voxel type I error of P < 0.001, a cluster extent of 12 contiguous resampled voxels was indicated as necessary to correct for multiple voxel comparisons at P < 0.05. Anatomical labels for activated brain areas were computed using the SPM anatomy toolbox (Eickhoff et al. 2005). Detailed anatomical maps were available for inferior (Caspers et al. 2008) and superior parietal cortex (Scheperjans et al. 2008), visual cortex (BA 17, 18) (Amunts et al. 2000), motor (Geyer et al. 1996) and premotor cortex (Geyer 2004), inferior frontal gyrus (BA 44, 45) (Amunts et al. 1999), and amygdala and hippocampus (Amunts et al. 2005). Activation clusters that did not match any probability map (e.g., lateral temporal cortex) or that overlapped only marginally with any existing map (< 30%) were labeled using the wfu_pickatlas tool implemented in SPM (Maldjian et al. 2003).
Comparison of fMRI Findings with Earlier Studies
We used DataViewer3D (Gouws et al. 2009) to superimpose brain activation from previous ambiguity studies onto our own findings within a single template in MNI space (Colin27_T1_seg_MNI.nii). We plotted all activation peaks in left temporal lobe that referred to the comprehension of visually-presented ambiguous versus unambiguous material (Gennari et al. 2007; Bedny et al. 2008; Hoenig and Scheef 2009; Snijders et al. 2009) or, where calculated, contrasts between subordinate and dominant contexts (Zempleni et al. 2007). Studies that used ambiguous words but did not require strong semantic analysis (e.g., lexical decision) were excluded (Copland et al. 2003, 2007).
In a second analysis, we examined the extent to which studies employing ambiguous words and other manipulations of semantic control overlapped. Studies that manipulated semantic control demands using visually-presented unambiguous material during comprehension tasks were compared with the peaks identified above. All 12 studies either employed a relatedness judgment task or semantic categorization (Ochsner et al. 2009; Race et al. 2009; Thompson-Schill et al. 1997; Roskies et al. 2001; Wagner et al. 2001; Noppeney and Price 2004; Noppeney et al. 2004; Zhang et al. 2004; Badre et al. 2005; Snyder et al. 2007; Chou et al. 2009; Liu et al. 2009). Activation peaks within left temporal cortex and LIFG (including peaks that were detected in neighboring regions but that reflected LIFG activation) were plotted. We plotted the most significant peak in each specific substructure of LIFG (e.g., BA 44, 44/6, 45, 47, 47/10) for each experiment when more than one was reported.
Results
Behavioral Analysis
RT data recorded during fMRI were screened for errors and outliers (±2 SD). Following Balota and Paul (1996) and Chwilla and Kolk (2003), PEs were calculated for ambiguous and unambiguous conditions separately by subtracting each of the related conditions from the unrelated condition. The PE data were entered into a 2 × 3 × 2 repeated-measures ANOVA with ambiguity (ambiguous, unambiguous), relation (RR, RU, UR), and position (first, second) as within-subject factors. For the interpretation of our fMRI data, it was important to demonstrate that the ambiguous double-related condition (game–dance–ball) was characterized by the retrieval of multiple concepts. Therefore, RT data needed to show 1) facilitation from both primes at homonym retrieval, that is, larger PEs in the ambiguous double-related than the single-related conditions (RRa > Rd, RRa > Rs); 2) an interference effect when both meanings were addressed, that is, lower PEs in the ambiguous double-related condition than in equivalent unambiguous trials (RRa < RR); and 3) evidence that this interference effect could not be attributed to PE differences between ambiguous and unambiguous single-related trials, that is, similar PEs for each of the single-related conditions across ambiguity levels (Rd = R1, Rs = R2).
The behavioral data, displayed in Table 3, were consistent with multiple meaning retrieval during the ambiguous double-related condition (game–dance–ball). There was a main effect of ambiguity (F1,14 = 5.70, P = 0.03) and condition (F2,28 = 55.37, P < 0.001) and an ambiguity by condition interaction (F2,28 = 12.75, P < 0.001). PEs were generally larger for unambiguous than ambiguous trials (P = 0.03). As expected, PEs were largest for the double-related condition (P < 0.001) and similar across both single-related conditions (P = 0.18).
Since no effect of position was observed (F1,14 < 1), data were pooled accordingly. The reduced 2 × 3 ANOVA replicated the main effect of ambiguity and condition and the ambiguity by condition interaction. Individual planned comparisons (paired t-tests) revealed significant differences between ambiguity levels only for the double-related condition, with larger PEs for the unambiguous than ambiguous condition (t14 = 5.15, P < 0.001). A separate ANOVA for ambiguous trials confirmed that PEs for the double-related condition were larger than PEs for each of the single-related trials (P < 0.005). The same was true for unambiguous items (P < 0.001). However, while the 2 single-related trials were similar among unambiguous conditions (P = 1.0), PEs for single-related trials that addressed the subordinate meaning of a homonym were smaller than those for dominant meanings (P = 0.04).
fMRI Analysis
Activation during Multiple Meaning Priming (RRa)
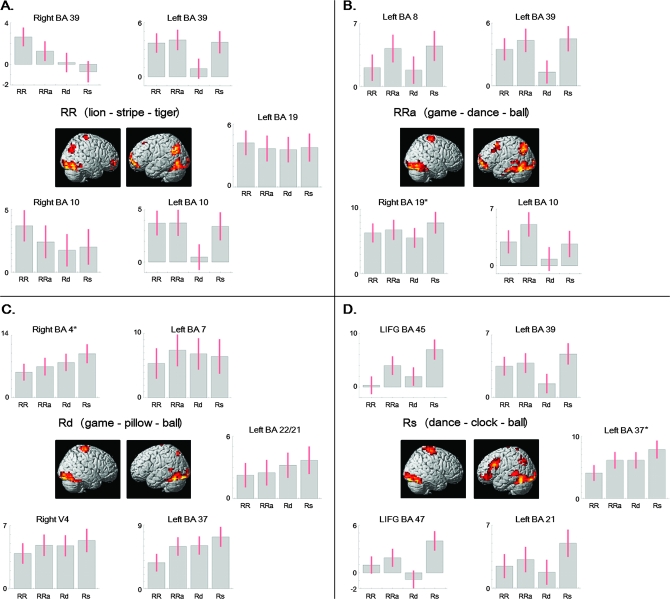
Brain activation during ambiguous double-related trials (game–dance–ball) comprised neural structures that are associated with word reading, motor responses, and multiple-related priming (Fig. 1B, Table 4A). Beside strongest BOLD signal changes in bilateral occipitotemporal cortices (V3, V4, BA 17, 18, 19, 37)—including left mid-ITG (BA 20) and left pMTG (BA 21)—cerebellum, and right motor cortex (BA 4, 6), distributed activation emerged in left angular gyrus and adjacent BA 7, ventral parts of left frontal cortex (MFG, SFG, IFG), and more dorsal left superior and middle frontal gyri (BA 8). Medial temporal activation spanned the left and right hippocampal area and adjoining fusiform gyrus, right caudate, left rectal gyrus, and left putamen.
Figure 1.
Main effects. Brain activation during (A) double priming of unambiguous targets (RR) and priming of ambiguous words in which either (B) both meanings of the homonym are primed (RRa) or (C) the dominant (Rd) or (D) the subordinate interpretation (Rs) is addressed individually. Parameter estimates (with 90% confidence interval) are extracted from coordinates at peak activation for various brain regions using SPM (for MNI coordinates, see Table 4). * = the same brain region (i.e., matched in MNI coordinates) was activated in more than one contrast: right BA 4 was activated in all conditions; right BA 19 was activated in RR, RRa, and Rs; and left BA 37 was activated in RRa and Rs. Activation is corrected for multiple comparisons (P < 0.05, cluster extent = 12 voxels).
Table 4.
Brain activation during (A) double priming of unambiguous (RR; lion-stripe-tiger) and ambiguous targets (RRa; game-dance-ball) and (B) single meaning priming of the dominant (Rd; game-pillow-ball) and subordinate (Rs; dance-clock-ball) interpretation of homonyms
| (A) | RR |
RRa |
||||||||||||
| Brain region | Activation peaks | BA | x | y | z | Z | Voxels | Activation peaks | BA | x | y | z | Z | Voxels |
| Left occipitotemporal (plus cerebellum) | IOG | 19 | –48 | –72 | –8 | 5.52 | 159 | ITG | 37 | –48 | –60 | –24 | 6.81 | 761 |
| IOG | V4 | –44 | –76 | –16 | 5.15 | ITG | V4 | –40 | –76 | –20 | 5.45 | |||
| ITG | 37 | –48 | –60 | –24 | 5.02 | ITG | 20 | –60 | –24 | –24 | 4.64 | |||
| Cerebellum | –44 | -52 | -28 | 4.25 | Cerebellum | –44 | –52 | –28 | 6.39 | |||||
| LG | 18 | –28 | –96 | –16 | 4.13 | IOG | V3 | –32 | –88 | –20 | 5.27 | |||
| IOG | 18 | –20 | –100 | –12 | 3.43 | IOG | 37 | –48 | –68 | –12 | 4.93 | |||
| MOG | 18 | –20 | –100 | 4 | 4.00 | 14 | FFG | EC | –28 | –16 | –32 | 4.06 | ||
| MOG | 18 | –20 | –100 | 0 | 3.92 | |||||||||
| IOG | 17 | –12 | –100 | -8 | 3.70 | |||||||||
| Right occipitotemporal (plus cerebellum) | ITG | 19 | 48 | –68 | –20 | 6.36 | 214 | ITG | 19 | 48 | –68 | –20 | 6.47 | |
| IOG | 19 | 48 | –72 | –12 | 6.03 | ITG | V4 | 40 | –84 | –16 | 6.18 | |||
| IOG | V4 | 36 | –88 | –12 | 5.79 | ITG | 20 | 48 | –36 | –24 | 4.79 | |||
| MTG | 21 | 64 | –40 | –12 | 3.67 | MOG | 18 | 28 | –100 | 4 | 3.57 | |||
| Cerebellum | 40 | –40 | –28 | 3.38 | ||||||||||
| Left posterior temporal | MTG | 21 | –64 | –52 | 4 | 4.23 | ||||||||
| Left temporoparietooccipital | AG | 39/PGa | –40 | –64 | 36 | 5.34 | 158 | AG | 39 | –36 | –68 | 36 | 5.92 | 175 |
| AG | 39/PGp | –40 | –68 | 28 | 4.74 | SPL | 7a | –32 | –72 | 56 | 4.98 | |||
| SPL | 7a | –28 | –72 | 56 | 3.63 | AG | 39/PGp | –40 | –68 | 28 | 4.61 | |||
| Precuneus | 7b | –8 | –80 | 56 | 3.81 | |||||||||
| Right temporoparietooccipital | MOG | 39/PGp | 48 | –68 | 28 | 4.58 | 59 | |||||||
| AG | 40 | –64 | 44 | 4.40 | ||||||||||
| Right motor areas | PrecG | 4 | 36 | –28 | 72 | 4.73 | 28 | PrecG | 4 | 36 | –28 | 72 | 5.40 | 63 |
| PrecG | 6 | 28 | –28 | 72 | 4.50 | PrecG | 6 | 28 | –28 | 72 | 4.89 | |||
| Left superior frontal | SFG | 8 | –16 | 24 | 56 | 4.22 | 12 | MFG | 8 | –52 | 8 | 44 | 4.31 | 82 |
| SFG | 8 | –20 | 20 | 52 | 3.67 | |||||||||
| Left ventral frontal | OrbG | 10 | –28 | 56 | –8 | 4.91 | 99 | MFG | 10 | –28 | 60 | 4 | 5.28 | 109 |
| IFGorb | 11/47 | –44 | 44 | –16 | 4.60 | IFGorb | 11/47 | –44 | 44 | –16 | 4.79 | |||
| SFG | 10 | –20 | 64 | 8 | 3.75 | SFG | 10 | –16 | 64 | 24 | 3.78 | |||
| Right ventral frontal | OrbG | 10 | 48 | 52 | –4 | 4.67 | 30 | |||||||
| SFG | 10 | 32 | 64 | 0 | 4.65 | |||||||||
| RectG | 11 | 4 | 56 | –16 | 4.60 | 13 | ||||||||
| Left hippocampus | Hippocampus | –16 | –12 | –20 | 4.00 | 25 | Hippocampus | –24 | –20 | –12 | 4.45 | 21 | ||
| Hippocampus | SUB | –24 | –20 | –16 | 4.93 | 37 | ||||||||
| Amygdala | SF | –16 | –8 | –20 | 4.10 | |||||||||
| Right hippocampus | Thalamus | 16 | –32 | 8 | 4.13 | 74 | FFG | EC | 32 | –8 | –36 | 5.01 | 32 | |
| Hippocampus | CA | 32 | –36 | –4 | 4.09 | FFG | 36 | –16 | –32 | 3.91 | ||||
| PHG | SUB | 20 | –24 | –16 | 3.59 | |||||||||
| PHG | CA | 36 | –24 | –20 | 3.55 | |||||||||
| Right amygdala | FFG | LB | 32 | –4 | –36 | 4.53 | 23 | |||||||
| PHG | EC | 24 | –8 | –32 | 4.10 | |||||||||
| Amydala | SF | 28 | 0 | –12 | 3.89 | 28 | ||||||||
| Putamen | 28 | –4 | 8 | 3.88 | ||||||||||
| Sublobar | ACC | 25 | 0 | 12 | –4 | 5.16 | 67 | RectG | 11/47 | –16 | 20 | –12 | 4.79 | 119 |
| Caudate | 8 | 20 | –4 | 4.84 | Caudate | 8 | 20 | 0 | 4.68 | |||||
| Caudate | –20 | –8 | 24 | 4.71 | 24 | Putamen | 16 | 4 | 4 | 3.94 | ||||
| Thalamus | 0 | –16 | 12 | 4.51 | 17 | Putamen | –28 | 0 | –4 | 3.96 | 13 | |||
| (B) | Rd |
Rs |
||||||||||||
| Brain region | Activation peaks | BA | x | y | z | Z | Voxels | Activation peaks | BA | x | y | z | Z | Voxels |
| Left occipitotemporal | ITG | 37 | –44 | –60 | –24 | 6.94 | 251 | ITG | 37 | –48 | –60 | –24 | 7.79 | 342 |
| MOG | V3 | –32 | –88 | –20 | 5.95 | LG | V3 | –36 | –88 | –16 | 5.82 | |||
| IOG | V4 | –40 | –76 | –20 | 5.45 | |||||||||
| IOG | 19 | –48 | –72 | –8 | 4.61 | |||||||||
| IOG | 17 | –12 | –100 | –8 | 3.98 | |||||||||
| ITG | 37 | –56 | –64 | –12 | 3.63 | |||||||||
| MOG | V3 | –28 | –100 | 8 | 3.59 | |||||||||
| Left posterior temporal | STG/MTG | 22/21 | –64 | –48 | 8 | 4.18 | 14 | MTG | 21 | –64 | –36 | 0 | 5.09 | |
| Right occipitotemporal | IOG | V4 | 40 | -84 | –12 | 6.11 | 197 | ITG | 19 | 48 | –68 | –20 | 6.89 | 217 |
| ITG | 19 | 52 | –72 | –8 | 5.81 | LG | V3 | 28 | –92 | –16 | 6.83 | |||
| IOG | 17 | 12 | –96 | –12 | 4.16 | LG | 18 | 20 | –92 | –16 | 6.64 | |||
| LG | 18 | 16 | –92 | –16 | 3.96 | IOG | V4 | 44 | –80 | –12 | 6.39 | |||
| Left temporo-parieto-occipital | SPL | 7a | –28 | –72 | 56 | 4.42 | 24 | AG | 39/PGa | –36 | –64 | 36 | 6.17 | 57 |
| IPL | 40/hIP1 | –36 | –56 | 40 | 5.11 | |||||||||
| Precuneus | 5 | –4 | –48 | 72 | 4.32 | 15 | ||||||||
| Precuneus | 5 | –4 | –48 | 72 | 4.32 | 15 | ||||||||
| IFG | IFGop | 45 | –52 | 24 | 32 | 5.67 | 212 | |||||||
| IFGorb | 47 | –48 | 32 | –4 | 5.06 | |||||||||
| OrbG | 11 | –44 | 48 | –8 | 4.20 | |||||||||
| IFGtri | 45 | –48 | 32 | 12 | 3.96 | |||||||||
| Right motor areas | PrecG | 4 | 36 | –28 | 72 | 6.10 | 85 | PrecG | 4 | 36 | –28 | 72 | 6.81 | 136 |
| PrecG | 6 | 16 | –24 | 76 | 4.35 | PrecG | 6 | 16 | –16 | 76 | 4.13 | |||
| ParacL | 3b | 8 | –40 | 76 | 3.89 | |||||||||
| Left ventral frontal | RectG | 11 | –4 | 56 | –16 | 4.07 | 24 | |||||||
| OrbG | 10 | –28 | 56 | –8 | 4.03 | |||||||||
| OrbG | 11 | 0 | 60 | –12 | 3.97 | |||||||||
| Left hippocampus | Hippocampus | FD | –28 | –20 | –16 | 4.69 | 33 | |||||||
| Hippocampus | SUB | –16 | –12 | –20 | 3.41 | |||||||||
| Sublobar | Corpus Callosum | –20 | 28 | 0 | 4.11 | 16 | Corpus Callosum | –12 | 24 | –4 | 4.47 | 146 | ||
| Caudate | –12 | 16 | –4 | 3.71 | Caudate | 12 | 8 | 8 | 3.74 | |||||
| Putamen | 32 | 0 | –4 | 3.52 | ||||||||||
| Caudate | –20 | –4 | 24 | 4.34 | 17 | Midbrain | –12 | –16 | –20 | 4.72 | 43 | |||
| Thalamus | –16 | –20 | 16 | 4.31 | ||||||||||
| Putamen | 24 | 8 | –8 | 4.29 | 24 | Putamen | –28 | 4 | –4 | 4.09 | 17 | |||
| Putamen | 24 | –4 | 12 | 4.29 | 16 | |||||||||
| Cerebellum | –4 | –72 | –24 | 4.10 | 48 | Cerebellum | –12 | -56 | –16 | 4.05 | 15 | |||
Note: “Activation peaks” lists brain regions that correspond to global and local maxima. Local maxima are reported for large clusters and are listed beneath the global maximum, for which the overall cluster size is provided. If 2 or more local maxima could be assigned to the same macroanatomical structure, only the maximum with the highest Z value is listed. ACC = anterior cingulate cortex, AG = angular gyrus, FFG = fusiform gyrus, IFGop/orb/tri = inferior frontal gyrus pars opercularis/orbitalis/triangularis, IOG = inferior occipital gyrus, IPL = inferior parietal lobule, ITG = inferior temporal gyrus, LG = lingual gyrus, MFG = middle frontal gyrus, MOG = middle occipital gyrus, MTG = middle temporal gyrus, OrbG = orbital gyrus, ParacL = paracentral lobule, PHG = parahippocampal gyrus, PrecG = precentral gyrus, RectG = rectal gyrus, SFG = superior frontal gyrus, OrbG = orbital gyrus, SPL = superior parietal lobule, STG = superior temporal gyrus; hIP1 = ventral intraparietal area in the intraparietal sulcus, PGa/p = anterior/posterior aspect of the caudal inferior parietal cortex, CA = cornu ammonis, EC = entorhinal cortex, FD = fascia dentata, SUB = subiculum, and LB/SF = laterobasal/superficial group of the amygdala.
Activation during Single Meaning Priming (RR, Rd, Rs)
A similar pattern emerged for the unambiguous double-related condition, which served as a control condition and addressed the same concept (lion–stripe–tiger). Activation increased in bilateral occipitotemporal (V4, BA 18, 19, 37, 21) and left parietal cortex (BA 7, 39), left ventral frontal lobe (BA 10, 11/47), right motor areas (BA 4, 6), and cerebellum. However, activation spread neither into left pMTG nor into more anterior parts of ITG. Additional clusters were observed in right angular gyrus and most ventral aspects of right frontal cortex (BA 10, 11). A smaller cluster was observed in left superior frontal gyrus (BA 8). Medial activation was observed in left and right amygdala and hippocampus, which extended into adjacent right thalamus, right parahippocampal and fusiform gyri, and putamen. Caudate, thalamus, and anterior cingulate cortex were also activated (Fig. 1A, Table 4A).
Priming of the dominant meaning during ambiguous single-related trials (game–pillow–ball) relied, again, on bilateral occipitotemporal areas (V3, V4, BA 17, 18, 19, 37) including the cerebellum, right motor cortex (BA 4, 6), and a separate cluster in left pMTG/STG (BA 21/22). Activation in left superior parietal lobule (BA 7) was reduced. Medially, left hippocampus, caudate, thalamus, corpus callosum, and right putamen showed BOLD response increases. No ventral frontal activation emerged (Fig. 1C, Table 4B).
Finally, when the subordinate meaning of the homonym was addressed in ambiguous single-related trials (dance–clock–ball), pMTG (BA 21) was recruited alongside bilateral inferior occipital and temporal cortices (V3, V4, BA 18, 19, 37), cerebellum, left angular gyrus and adjacent BA 40 and 5, left ventral frontal areas (orbital and rectal gyri), right motor areas (BA 4, 6), and neighboring regions in BA 3. For the first time, activity was seen in LIFG covering pars opercularis, triangularis (BA 45) and orbitalis (BA 47), and orbital gyrus (BA 11). Further, right caudate, left and right putamen, left midbrain, and corpus callosum were recruited (Fig. 1D, Table 4B).
Contrasts of Multiple versus Single Meaning Priming
RRa > RR.
In the most stringent contrast, priming of 2 different interpretations of a homonym (game–dance–ball) as opposed to double priming of a single concept of an unambiguous word (lion–stripe–tiger) yielded peak activation in left mid-ITG (BA 20) and left cerebellum.
RRa > Rd.
BOLD responses were stronger during ambiguous double-related trials than for single-related conditions that addressed the dominant meaning of a homonym (game–pillow–ball) in left mid-ITG (BA 20), left angular gyrus (BA 39), and ventral (BA 10, 11) and dorsal parts (MFG; BA 6) of the left frontal lobe.
RRa > Rs.
When contrasted with single-related trials that primed the subordinate meaning (dance–pillow–ball), the ambiguous double-related condition showed stronger responses in left mid-ITG (BA 20), left inferior parietal cortex (BA 19/39), and right hippocampus. Results are listed in Table 5A.
Table 5.
Brain activation during contrasts of (A) multiple versus single meaning retrieval and (B) contrasts of high versus lower semantic control demands
| Coordinates |
|||||||
| Activation peak | BA | x | y | z | Z | Cl | |
| (A) Multiple versus single meaning retrieval | |||||||
| RRa > RR | ITG | 20 | –48 | –24 | –20 | 4.13 | 13 |
| Cerebellum | –24 | –48 | –24 | 3.78 | 12 | ||
| RRa > Rd | ITG | 20 | –60 | –24 | –24 | 5.43 | 44 |
| AG | 39 | –44 | –64 | 32 | 5.12 | 63 | |
| MFG | 10 | –28 | 60 | 8 | 5.08 | 88 | |
| SFG | 10 | –20 | 64 | 20 | |||
| OrbG | 10 | –28 | 56 | –4 | |||
| OrbG | 11 | –44 | 48 | –12 | |||
| MFG | 6 | –32 | 12 | 60 | 4.75 | 46 | |
| RRa > Rs | IPC | 19/39 | –44 | –76 | 40 | 4.09 | 14 |
| Hippocampus | 28 | –4 | –36 | 3.97 | 17 | ||
| ITG | 20 | –60 | –24 | –24 | 3.69 | 15 | |
| Conjunction 1: RRa ∩ RRa > Rd ∩ RRa > Rs (exclusively masked by RR) | ITG* | 20 | –60 | –24 | –24 | 3.69 | 15 |
| (B) High versus low semantic control demands | |||||||
| Rs > Rd | IFGorb | 47 | –48 | 32 | –4 | 5.84 | 34 |
| OrbG | 10 | –4 | 60 | –8 | 4.56 | 16 | |
| OrbG | 11 | –16 | 56 | –12 | |||
| IFGtri | 44 | –48 | 16 | 28 | 4.30 | 105 | |
| IFGtri | 45 | –48 | 28 | 16 | |||
| AG | 39/PGa | –36 | –64 | 36 | 4.28 | 15 | |
| Midbrain | 4 | –8 | –16 | 4.01 | 17 | ||
| MTG | 21 | –60 | –24 | –4 | 3.95 | 12 | |
| Conjunction 2: Rs ∩ Rs > Rd | IFGorb* | 47 | –48 | 32 | –4 | 5.06 | 34 |
| IFGtri* | 44 | –48 | 16 | 28 | 4.30 | 105 | |
| IFGtri* | 45 | –48 | 28 | 16 | |||
| AG | 39/PGa | –36 | –64 | 36 | 4.28 | 15 | |
| OrbG | 11 | 0 | 60 | –12 | 3.97 | 16 | |
| MTG* | 21 | –68 | –36 | –4 | 3.94 | 12 | |
| Midbrain | –8 | –20 | –20 | 3.59 | 17 | ||
Note: Cortical areas that are activated across contrasts and, thus identified by the conjunction analyses, are displayed in bold. Brain activation is corrected for multiple comparisons (P < 0.05, cluster extent [Cl] = 12 voxels). Each differential contrast is inclusively masked by its minuend at P < 0.001 (uncorrected). Areas marked by an asterisk (*) are displayed in Figure 3. AG = angular gyrus, IFGorb/tri = inferior frontal gyrus pars orbitalis/triangularis, IPC = inferior parietal cortex, ITG = inferior temporal gyrus, MFG = middle frontal gyrus, MTG = middle temporal gyrus, OrbG = Orbital gyrus, PGa = anterior aspect of the caudal IPC, and SFG = superior frontal gyrus.
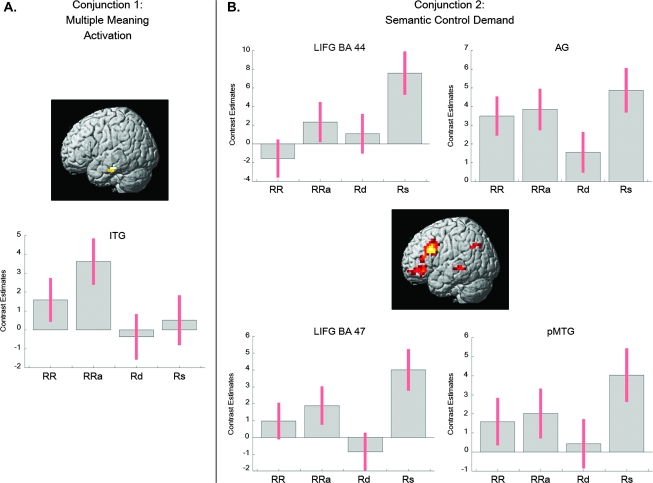
Conjunction 1: analysis of multiple versus single meaning contrasts—RRa ∩ RRa > Rd ∩ RRa > Rs (exclusively masked by RR).
Left mid-ITG (BA 20) was the only brain region that yielded significantly stronger BOLD activity during multiple meaning retrieval of homonyms as opposed to each of the single meaning retrieval conditions (Fig. 2A; Table 5A).
Figure 2.
Results of the conjunction analyses. Brain activation refers to contrasts of (A) multiple versus single meaning activation of homonyms (RRa ∩ RRa > Rd ∩ RRa > Rs; exclusively masked by RR) and (B) highest versus lowest forms of semantic control (Rs ∩ Rs > Rd). Contrast estimates and 90% confidence interval are plotted for peak activation in left ITG (BA 20), LIFG (BA 44, 47), left pMTG (BA 21), and left angular gyrus (AG; BA 39) using SPM (for MNI coordinates, see Table 5). Activation is corrected for multiple comparisons (P < 0.05, cluster extent = 12 voxels).
Contrasts of High versus Low Semantic Control Demands
Rs > Rd.
Targets that were primed toward their subordinate meaning in ambiguous single-related trials (dance–pillow–ball) were expected to elicit maximal semantic control demands and engaged LIFG (BA 44, 45, 47), left orbital gyrus (BA 10, 11), left angular gyrus, left pMTG, and midbrain compared with homonyms that were primed toward their dominant interpretation (game–pillow–ball) (Table 5B).
Conjunction 2: analysis of high versus lowest semantic control demands—Rs ∩ Rs > Rd.
The conjunction revealed activation in the same set of distributed brain areas as the contrast Rs > Rd (Fig. 2B; Table 5B).
Comparison of fMRI Findings with Earlier Studies
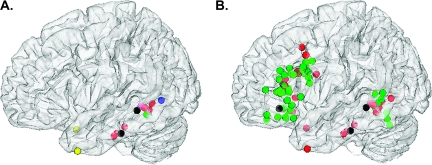
Figure 3A shows temporal peak activations from the current study, associated with semantic representation (Conjunction 1) and semantic control (Conjunction 2) respectively, alongside temporal lobe peaks from previous studies of ambiguity resolution (i.e., ambiguous > unambiguous material; retrieval of subordinate > dominant concepts of homonyms). The mid-ITG and pMTG sites identified by our analyses (shown in black) fall within 2 spatially distinct clusters of temporal activations seen across previous studies. Left pMTG was consistently activated during situations that required the suppression of alternative interpretations (Gennari et al. 2007; Zempleni et al. 2007; Bedny et al. 2008; Snijders et al. 2009), consistent with our proposal that this region plays a role in semantic control. In contrast, mid-ITG was activated by studies that required the 2 alternative meanings of ambiguous words to be maintained over time before meaning selection eventually took place (Snijders et al. 2009) in line with the notion that this region is sensitive to the representational demands of semantic tasks. One study revealed activation outside both target areas (Hoenig and Scheef 2009), despite using a standard sentence verification paradigm—that is, participants had to judge whether a target word, shown afterwards, was consistent with the content of a previously presented sentence related to the subordinate meaning of a homonym (e.g., the teacher played the organ–music?).
Figure 3.
(A) Left temporal lobe activation during ambiguity resolution (i.e., ambiguous > unambiguous material, retrieval of subordinate > dominant concepts of homonyms). (B) Left temporal and inferior frontal activation during tasks of high versus low semantic control demands for ambiguous (red) and unambiguous (green) stimuli. Brain activation is superimposed onto a semi-transparent MNI template using DataViewer3D. Black dots refer to the result of the conjunction analyses, reflecting multiple versus single meaning retrieval in mid-ITG and highest versus lowest semantic control demand in pMTG and LIFG (see Table 5). Color codes in Figure 3A: red = Snijders et al. (2009), green = Zempleni et al. (2007), blue = Gennari et al. (2007), pink = Bedny et al. (2008), and yellow = Hoenig et al. (2009).
Figure 3B shows the overlap in temporal and inferior frontal cortex between studies of semantic control that employed ambiguous words (in red) and unambiguous stimuli (in green). It illustrates that 1) activation in pMTG is not restricted to ambiguity resolution but also occurs when semantic control demands are manipulated using unambiguous material and 2) pMTG often coactivates with LIFG, irrespective of ambiguity. This overlap between ambiguous and unambiguous stimuli was restricted to LIFG and left pMTG—it did not extend into more anterior and inferior regions of temporal cortex, consistent with our hypothesis that these regions are not part of the extended semantic control network.
Discussion
Our aim was to clarify whether subregions within left temporal cortex (pMTG, ITG) support different aspects of semantic cognition, and in particular, whether pMTG activation relates to control processes during meaning retrieval as opposed to semantic storage per se. We were able to dissociate semantic control and representation by investigating ambiguous words since these words project onto several semantic concepts—thus engaging increased meaning representation—and also evoke enhanced semantic control processes under certain situations. Unlike earlier studies, the current investigation separated these 2 components of semantic cognition within a single experiment because maximal semantic control demands and multiple meaning activation loaded onto different experimental conditions.
When 2 meanings of a homonym were activated in the absence of strong semantic competition/suppression during ambiguous double-related trials (game–dance–ball), brain responses were observed in left mid-ITG (BA 20) and pMTG (BA 21). A conjunction approach on contrast images, however, revealed that BA 20 was the only site that was significantly more engaged during multiple meaning retrieval of homonyms compared with any of the ambiguous single-related conditions, indicating that mid-ITG plays a key role in nonexecutive aspects of meaning representation. Further, activation in BA 20 was independent of general processes of multiple-related priming: the activation remained even when the unambiguous double-related condition (lion–stripe–tiger) was used as a mask. Since this control condition involved a single concept, it was not expected to be linked to brain areas contributing to semantic representation (i.e., mid-ITG).
In contrast, maximal semantic control processes were elicited during ambiguous single-related trials that primed the subordinate meaning (dance–clock–ball). These mapped onto activation increases in LIFG (BA 44, 45, 47) and pMTG. In these trials, the highly favored dominant meaning needed to be suppressed for successful task performance. In contrast, meaning competition/suppression was not expected during single-related dominant contexts (game–clock–ball). A subsequent conjunction analysis revealed that the left prefrontal and posterior temporal activations observed during subordinately biasing contexts were also present when these trials were compared with the least executively demanding condition (i.e., single-related dominant trials).
These findings advance our knowledge of the neural organization of semantic cognition. Distinct regions in left temporal lobe (pMTG, mid-ITG) were found to react differently to increases in semantic control demands and the number of meanings being retrieved. Increases in the number of meanings being retrieved did not influence activation in pMTG, unlike mid-ITG. In contrast, enhanced semantic executive demands altered neural responses in pMTG but not mid-ITG. These results suggest that pMTG works in conjunction with LIFG as part of a distributed frontotemporal control network.
Role of Mid-ITG in Semantic Representation
Previous studies employing ambiguous words with multiple meanings have observed activation in either pMTG or more anterior inferior temporal structures (Rodd et al. 2005; Gennari et al. 2007; Zempleni et al. 2007; Bedny et al. 2008; Hoenig and Scheef 2009; Snijders et al. 2009) (see Fig. 3A). However, since multiple meaning activation was confounded with strong semantic executive demands in these studies, the role of posterior temporal areas in ambiguity resolution was unresolved. The possibility of a more heterogeneous function of temporal lobe was raised by Snijders et al. (2009), who reported substantial activation in left mid-ITG (BA 20) in a task that encouraged multiple interpretations of a homonym to be maintained for long periods before meaning selection—for example, during long epochs of equibiasing contexts (see Fig. 3A). In line with this research, we propose that activation of multiple, context-appropriate concepts of homonyms results specifically in mid-ITG activation, while the degree of strategic requirements during semantic retrieval (e.g., meaning suppression) can be linked to pMTG activity instead.
This purported functional specialization of posterior and mid-inferior temporal regions, derived from ambiguity research, is supported by neuropsychological studies. Patients with SD show degradation of semantic knowledge following core atrophy of anterior and inferior aspects of temporal lobe (Hodges et al. 1992; Mummery et al. 2000; Jefferies and Lambon Ralph 2006; Hodges and Patterson 2007). These regions of atrophy overlap with the mid-ITG activation observed in the current study. In contrast, multimodal semantic impairment in the context of stroke aphasia (SA) is associated with deregulated semantic cognition but not a loss of semantic knowledge per se. SA patients have lesions in LIFG and temporoparietal regions, including pMTG, providing convergent evidence that this region contributes to executive control over semantic activation (Jefferies and Lambon Ralph 2006).
Our activation peak in mid-ITG coincides with an anterior inferior temporal site identified recently as critical for semantic processing (Binney et al. 2010). Using a multidisciplinary approach, Binney and colleagues found that left lateral anterior temporal cortex and adjacent anterior fusiform gyrus are activated when healthy volunteers perform a synonym judgment task; moreover, these sites are associated with impaired performance by patients with SD on the same task. A recent meta-analysis of neuroimaging studies of verbal semantic tasks in healthy participants revealed that the same brain areas, spanning mid-ITG, form an integral part of the neural network that stores conceptual knowledge (Binder et al. 2009; see also Martin 2007; Patterson et al. 2007). These areas constitute the anterior extension of the ventral-visual pathway for word reading that runs, in posterior-to-anterior direction, along the inferior occipital and temporal lobes, mediating progressively complex presemantic processes (Dehaene et al. 2005; Dien 2009).
In contrast, the function ascribed to posterior sections of left temporal cortex, which is the region that activates during high degrees of meaning suppression in our study (i.e., during single-related subordinate trials), is more diverse. Although pMTG has often been implicated in meaning representation (see Binder et al. 2009), it is not the focus of atrophy in SD (Mummery et al. 1999; Garrard and Hodges 2000) and, as discussed below, activation of this region is sensitive—like LIFG—to manipulations of semantic control.
The Role of Left pMTG in Semantic Control
Strong evidence for an extended semantic control network, comprising left pMTG as well as LIFG, is provided by the ambiguity literature itself, which has frequently observed activation increases in both of these regions during situations of strong meaning suppression (Rodd et al. 2005; Gennari et al. 2007; Zempleni et al. 2007; Bedny et al. 2008; Snijders et al. 2009) (Fig. 3B). Similarly, close inspection of fMRI studies using unambiguous stimuli implicate both inferior frontal and posterior temporal structures in aspects of semantic control and selection (Thompson-Schill et al. 1997; Wagner et al. 2001; Noppeney et al. 2004; Badre et al. 2005; Snyder et al. 2007; Chou et al. 2009). This is clearly demonstrated by Figure 3B, which presents activation peaks (high > low control) in left temporal and inferior frontal cortex. Although there are clearly more peaks in LIFG across studies, pMTG is also frequently implicated, and, importantly, the distribution of peaks from studies of ambiguity resolution overlap with those manipulating control demands in unambiguous words. These findings imply that similar control mechanisms (e.g., competition, selection, or inhibition of irrelevant semantic knowledge) operate in both contexts. Most intriguingly, this overlap between ambiguous and unambiguous stimuli was restricted to LIFG and pMTG—it did not extend into more anterior and inferior regions of temporal cortex, consistent with our hypothesis that these regions are the key substrate for meaning representation and not part of the extended semantic control network.
As mentioned above, these imaging findings are in line with neuropsychological data from patients with multimodal semantic problems resulting from stroke (SA). SA patients show fluctuating semantic performance that is highly sensitive to the executive requirements of semantic tasks (Jefferies and Lambon Ralph 2006; Jefferies et al. 2007, 2008; Noonan et al. 2009; Corbett et al. 2009a). SA patients have infarcts affecting either left prefrontal or temporoparietal regions, and these groups show highly similar neuropsychological profiles, characterized by poor executive control over semantic activation (Berthier 2001; Jefferies and Lambon Ralph 2006). This provides strong support for the view that left posterior temporal and prefrontal areas form a distributed semantic executive system (see also Noppeney et al. 2004; Gold et al. 2006; Kuperberg et al. 2008; Noonan et al. 2009).
The proposed functional specialization of regions within temporal cortex is also supported by connectivity analyses, which revealed strong anatomical and functional links between pMTG and anterior aspects of LIFG (for a review, see Friederici 2009). Direct pathways also exist between pMTG and structures within temporal cortex that have been implicated in storing semantic knowledge (i.e., fusiform gyrus) (Saur et al. 2010). Further, recordings of resting state activity revealed that left pMTG correlated with LIFG and parietal lobule (see below), while left ITG was part of another, functionally distinct neural circuit (Wig et al. 2009). These findings support the view that pMTG and LIFG (BA 45/47) act in concert to retrieve and manipulate semantic knowledge, which is stored in “representation areas” in anterior and inferior temporal cortex. Damage to pMTG thus evokes problems in executive aspects of meaning retrieval and not loss of semantic knowledge per se (as in SA patients).
The Large-Scale Semantic Control Network
Apart from left prefrontal and posterior temporal structures, brain regions in left inferior parietal lobule (IPL) were also engaged during high levels of semantic control (see Fig. 2). This brain region—like LIFG and pMTG—is often damaged in SA patients with deregulated semantic control, supporting the view that IPL is vital for executive–semantic functions (Jefferies et al. 2007; Noonan et al. 2009; Corbett et al. 2009b). Imaging data have further shown that IPL interacts with medial and lateral prefrontal regions (e.g., supplementary motor area, anterior cingulate cortex, dorsolateral prefrontal cortex) as part of a “multiple-demand” system, which is believed to support all cognitively challenging tasks independent of stimulus modality or domain (Owen et al. 2000; Duncan 2006, 2010; Dosenbach et al. 2008). Semantic tasks that require additional executive resources might therefore recruit brain regions that are sensitive to semantic-specific control functions (e.g., IFG, pMTG) and cognitive control more widely (e.g., IPL).
Closer inspection of our imaging data revealed that, although IPL, pMTG and LIFG were all more strongly involved in tasks that required high versus lowest semantic control demand (i.e., Rs > Rd), the activation profile differed across these 3 regions for the other conditions. Differences were most pronounced between parietal and frontal/temporal structures (see Figs 1 and 2): left parietal lobule participated in unambiguous and ambiguous double-related conditions (RR, RRa), while frontal and temporal brain areas showed no response. IPL might therefore denote a functionally distinct component in the semantic control network. In line with this view, Nagel et al. (2008) reported that left IPL and dorsolateral prefrontal cortex were both engaged during high semantic and nonsemantic selection processes, while LIFG showed a specific response to semantic manipulations. Similarly, a meta-analysis revealed that LIFG and pMTG formed a core part of the semantic control network, whereas left inferior and superior parietal lobule and dorsolateral prefrontal cortex were engaged during different forms of executive functioning (KA Noonan, E Jefferies, F Corbett, MA Lambon Ralph, in preparation).
In sum, this study identifies a double dissociation between processes related to semantic control and representation in posterior and inferior aspects of temporal cortex within a single fMRI paradigm for the first time. When several semantic representations were activated in the presence of weak meaning competition/suppression, mid-ITG (BA 20) responded consistently, strengthening the established role for left inferior temporal cortex in the storage of meaning. In contrast, more posterior structures in middle temporal gyrus (BA 21) worked in concert with LIFG and IPL to support aspects of semantic control during meaning retrieval. Although our proposal that the semantic control network extends into left posterior temporal lobe regions is controversial, it receives support from 1) other neuroimaging studies that have manipulated semantic control demands using ambiguous and unambiguous words and 2) studies of semantically impaired stroke aphasic patients, who show sensitivity to manipulations of semantic control following posterior temporal as well as inferior frontal lesions.
Funding
German Research Foundation (IRTG 1328); Welcome Project (078734/Z05/Z to E.J.).
Acknowledgments
Conflict of Interest: None declared.
References
- Amaro E, Jr, Barker GJ. Study design in fMRI: basic principles. Brain Cogn. 2006;60:220–232. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space—where and how variable? Neuroimage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX Lexical Database. Philadelphia (PA): Lingustic Data Consortium, University of Pennsylvania; 1993. [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Balota DA, Paul ST. Summation of activation: evidence from multiple primes that converge and diverge within semantic memory. J Exp Psychol Learn Mem Cogn. 1996;22:827–845. doi: 10.1037//0278-7393.22.4.827. [DOI] [PubMed] [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL. Semantic adaptation and competition during word comprehension. Cereb Cortex. 2008;18:2574–2585. doi: 10.1093/cercor/bhn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier ML. Unexpected brain-language relationships in aphasia: evidence from transcortical sensory aphasia associated with frontal lobe lesions. Aphasiology. 2001;15:99–130. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJ, Lambon Ralph MA. The Ventral and Inferolateral Aspects of the Anterior Temporal Lobe Are Crucial in Semantic Memory: Evidence from a Novel Direct Comparison of Distortion-Corrected fMRI, rTMS, and Semantic Dementia. Cereb Cortex Advance Access published February 26, 2010 doi: 10.1093/cercor/bhq019. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D, Deaudon C, Whitehead V. On the status of object concepts in aphasia. Brain Lang. 1997;58:203–232. doi: 10.1006/brln.1997.1771. [DOI] [PubMed] [Google Scholar]
- Chou TL, Chen CW, Wu MY, Booth JR. The role of inferior frontal gyrus and inferior parietal lobule in semantic processing of Chinese characters. Exp Brain Res. 2009;198:465–475. doi: 10.1007/s00221-009-1942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwilla DJ, Kolk HH. Event-related potential and reaction time evidence for inhibition between alternative meanings of ambiguous words. Brain Lang. 2003;86:167–192. doi: 10.1016/s0093-934x(02)00527-8. [DOI] [PubMed] [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Eastburn M. Neural correlates of semantic priming for ambiguous words: an event-related fMRI study. Brain Res. 2007;1131:163–172. doi: 10.1016/j.brainres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenery HJ. Brain activity during automatic semantic priming revealed by event-related functional magnetic resonance imaging. Neuroimage. 2003;20:302–310. doi: 10.1016/s1053-8119(03)00279-9. [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Ehsan S, Lambon Ralph MA. Different impairments of semantic cognition in semantic dementia and semantic aphasia: evidence from the non-verbal domain. Brain. 2009a;132:2593–2608. doi: 10.1093/brain/awp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Lambon Ralph MA. Exploring multimodal semantic control impairments in semantic aphasia: evidence from naturalistic object use. Neuropsychologia. 2009b;47:2721–2731. doi: 10.1016/j.neuropsychologia.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cogn Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dien J. The neurocognitive basis of reading single words as seen through early latency ERPs: a model of converging pathways. Biol Psychol. 2009;80:10–22. doi: 10.1016/j.biopsycho.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SA, Morris RK, Rayner K. Lexical ambiguity and fixation times in reading. J Mem Lang. 1988;27:429–446. [Google Scholar]
- Duncan J. EPS Mid-Career Award 2004: brain mechanisms of attention. Q J Exp Psychol (Colchester) 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Garrard P, Hodges JR. Semantic dementia: clinical, radiological and pathological perspectives. J Neurol. 2000;247:409–422. doi: 10.1007/s004150070169. [DOI] [PubMed] [Google Scholar]
- Gennari SP, MacDonald MC, Postle BR, Seidenberg MS. Context-dependent interpretation of words: evidence for interactive neural processes. Neuroimage. 2007;35:1278–1286. doi: 10.1016/j.neuroimage.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol. 2004;174:I, 1–VIII. doi: 10.1007/978-3-642-18910-4. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Giora R. On the priority of salient meanings: studies of literal and figurative language. J Pragmat. 1999;31:919–929. [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouws A, Woods W, Millman R, Morland A, Green G. DataViewer3D: An Open-Source, Cross-Platform Multi-Modal Neuroimaging Data Visualization Tool. Front Neuroinformatics. 2009;3:9. doi: 10.3389/neuro.11.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindrod CM, Bilenko NY, Myers EB, Blumstein SE. The role of the left inferior frontal gyrus in implicit semantic competition and selection: an event-related fMRI study. Brain Res. 2008 Sep;1229:167–178. doi: 10.1016/j.brainres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Scheef L. Neural correlates of semantic ambiguity processing during context verification. Neuroimage. 2009;45:1009–1019. doi: 10.1016/j.neuroimage.2008.12.044. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Baker SS, Doran M, Lambon Ralph MA. Refractory effects in stroke aphasia: a consequence of poor semantic control. Neuropsychologia. 2007;45:1065–1079. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Ralph MA. Deficits of knowledge versus executive control in semantic cognition: insights from cued naming. Neuropsychologia. 2008;46:649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandhadai P, Federmeier KD. Multiple priming of lexically ambiguous and unambiguous targets in the cerebral hemispheres: the coarse coding hypothesis revisited. Brain Res. 2007;1153:144–157. doi: 10.1016/j.brainres.2007.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T, Sass K, Sachs O, Krach S. Priming words with pictures: neural correlates of semantic associations in a cross-modal priming task using fMRI. Hum Brain Mapp. 2009;30:4116–4128. doi: 10.1002/hbm.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Lakshmanan BM. Neuroanatomical distinctions within the semantic system during sentence comprehension: evidence from functional magnetic resonance imaging. Neuroimage. 2008;40:367–388. doi: 10.1016/j.neuroimage.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S, Triebig G, Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand. 1995;91:335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Deng X, Peng D, Cao F, Ding G, Jin Z, Zeng Y, Li K, Zhu L, Fan N. Modality- and task-specific brain regions involved in Chinese lexical processing. J Cogn Neurosci. 2009;21:1473–1487. doi: 10.1162/jocn.2009.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Milberg W, Blumstein S, Giovanello KS, Misiurski C. Summation priming in aphasia: evidence for alterations in semantic integration and activation. Brain Cogn. 2003;51:31–47. doi: 10.1016/s0278-2626(02)00500-6. [DOI] [PubMed] [Google Scholar]
- Moritz S, Mersmann K, Quast C, Andresen B. [Association norms for 68 German homonyms] Z Exp Psychol. 2001;48:226–238. [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJS, Vandenbergh R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122:61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Schumacher EH, Goebel R, D'Esposito M. Functional MRI investigation of verbal selection mechanisms in lateral prefrontal cortex. Neuroimage. 2008;43:801–807. doi: 10.1016/j.neuroimage.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Corbett F, Lambon Ralph MA. Elucidating the nature of deregulated semantic cognition in semantic aphasia: evidence for the roles of prefrontal and temporo-parietal cortices. J Cogn Neurosci. 2009;22:1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Phillips J, Price C. The neural areas that control the retrieval and selection of semantics. Neuropsychologia. 2004;42:1269–1280. doi: 10.1016/j.neuropsychologia.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of abstract semantics. Neuroimage. 2004;22:164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Novick J, Kan IP, Trueswell J, Thompson-Schill SL. A case for conflict across multiple domains: memory and language impairments following damage to ventrolateral prefrontal cortex. Cogn Neuropsychol. 2009;26:527–567. doi: 10.1080/02643290903519367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JD. Neural systems supporting the control of affective and cognitive conflicts. J Cogn Neurosci. 2009;21:1842–1855. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Schneider WX, Duncan J. Executive control and the frontal lobe: current issues. Exp Brain Res. 2000;133:1–2. [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peleg O, Eviatar Z. Hemispheric sensitivities to lexical and contextual information: evidence from lexical ambiguity resolution. Brain Lang. 2008;105:71–82. doi: 10.1016/j.bandl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Race EA, Shanker S, Wagner AD. Neural priming in human frontal cortex: multiple forms of learning reduce demands on the prefrontal executive system. J Cogn Neurosci. 2009;21:1766–1781. doi: 10.1162/jocn.2009.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K, Pacht JM, Duffy SA. Effects of prior encounter and global discourse bias on the processing of lexically ambiguous words: evidence from eye fixations. J Mem Lang. 1994;33:527–544. [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. A failure of high level verbal response selection in progressive dynamic aphasia. Cogn Neuropsychol. 2005;22:661–694. doi: 10.1080/02643290442000239. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. J Cogn Neurosci. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Ruff I, Blumstein SE, Myers EB, Hutchison E. Recruitment of anterior and posterior structures in lexical-semantic processing: an fMRI study comparing implicit and explicit tasks. Brain Lang. 2008;105:41–49. doi: 10.1016/j.bandl.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass K, Krach S, Sachs O, Kircher T. Lion - tiger - stripes: neural correlates of indirect semantic priming across processing modalities. Neuroimage. 2009a;45:224–236. doi: 10.1016/j.neuroimage.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Sass K, Sachs O, Krach S, Kircher T. Taxonomic and thematic categories: neural correlates of categorization in an auditory-to-visual priming task using fMRI. Brain Res. 2009b;1270:78–87. doi: 10.1016/j.brainres.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Saur D, Schelter B, Schnell S, Kratochvil D, Kupper H, Kellmeyer P, Kummerer D, Kloppel S, Glauche V, Lange R, et al. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage. 2010;49:3187–3197. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Sereno SC, Brewer CC, O'Donnell PJ. Context effects in word recognition: evidence for early interactive processing. Psychol Sci. 2003;14:328–333. doi: 10.1111/1467-9280.14471. [DOI] [PubMed] [Google Scholar]
- Simpson GB. Context and the processing of ambiguous words. In: Gernsbacher MA, editor. Handbook of psycholinguistics. San Diego (CA): Academic Press; 1994. pp. 359–374. [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Snijders TM, Vosse T, Kempen G, Van Berkum JJ, Petersson KM, Hagoort P. Retrieval and unification of syntactic structure in sentence comprehension: an FMRI study using word-category ambiguity. Cereb Cortex. 2009;19:1493–1503. doi: 10.1093/cercor/bhn187. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Feigenson K, Thompson-Schill SL. Prefrontal cortical response to conflict during semantic and phonological tasks. J Cogn Neurosci. 2007;19:761–775. doi: 10.1162/jocn.2007.19.5.761. [DOI] [PubMed] [Google Scholar]
- Soni M, Lambon Ralph MA, Noonan K, Ehsan S, Hodgson C, Woollams AM. “L” is for tiger: effects of phonological (mis)cueing on picture naming in semantic aphasia. J Neurolinguistics. 2009;22:538–547. [Google Scholar]
- Spitzer M, Braun U, Hermle L, Maier S. Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol Psychiatry. 1993;34:864–877. doi: 10.1016/0006-3223(93)90054-h. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Whitney C, Grossman M, Kircher TT. The influence of multiple primes on bottom-up and top-down regulation during meaning retrieval: evidence for 2 distinct neural networks. Cereb Cortex. 2009;19:2548–2560. doi: 10.1093/cercor/bhp007. [DOI] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. J Neurophysiol. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempleni MZ, Renken R, Hoeks JC, Hoogduin JM, Stowe LA. Semantic ambiguity processing in sentence context: evidence from event-related fMRI. Neuroimage. 2007;34:1270–1279. doi: 10.1016/j.neuroimage.2006.09.048. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Zhuang J, Ma L, Yu W, Peng D, Ding G, Zhang Z, Weng X. Semantic processing of Chinese in left inferior prefrontal cortex studied with reversible words. Neuroimage. 2004;23:975–982. doi: 10.1016/j.neuroimage.2004.07.008. [DOI] [PubMed] [Google Scholar]