Abstract
Resting natural killer (NK) cells in nonobese diabetic (NOD) mice have impaired immune functions compared with NK cells from other mouse strains. Here we investigated how NOD NK cells respond after mouse cytomegalovirus (MCMV) infection, using NOD mice congenic for the protective NK gene complex from C57BL/6 mice. Compared with C57BL/6 mice congenic for the H2 gene complex from NOD mice (B6.g7), NOD.NK1.1 mice fail to control early infection with MCMV. After MCMV infection, however, NOD.NK1.1 NK cells demonstrate increased cytolytic function, associated with higher expression of granzyme B, and undergo robust expansion. One week after infection, NOD.NK1.1 NK cells control MCMV replication as effectively as B6.g7 NK cells, even in the absence of T cells and B cells. Thus, the impaired cytotoxic function of NK cells in NOD mice is alleviated by viral infection, which enables NOD NK cells to efficiently control MCMV infection.
Keywords: nonobese diabetic mice, NK cells, NOD mice, MCMV, Ly49H
Natural killer (NK) cells play an important role in the immune response to viral and bacterial infections and to transformed cells (1). NK cells directly kill infected or transformed cells by release of lytic granules containing granzymes and perforin. Upon stimulation through their activating receptors, NK cells also produce proinflammatory cytokines, including IFN-γ and TNF-α, which help control infection and shape the adaptive immune response. NK cells can also limit autoimmunity by secretion of cytokines and killing activated autoreactive T cells (2, 3). Unlike T cells and B cells, each with a single unique T cell receptor or B cell receptor, NK cells express a wide array of activating receptors, including NKG2D, CD16, NKp46, NK1.1 (NKR-P1C), Ly49D, and Ly49H (4). The Ly49H receptor, expressed by C57BL/6 (B6) NK cells but not BALB/c or 129 NK cells, directly binds the m157 viral gene product that is expressed on the surface of cells infected with mouse cytomegalovirus (MCMV) (5, 6). Ly49H engagement of m157 results in NK cell–mediated cytotoxicity against the infected cell, secretion of cytokines and chemokines, and the proliferation of the Ly49H+ NK cells (7). Transgenic expression of Ly49H is sufficient to render normally MCMV-susceptible BALB/c mice resistant to MCMV infection (8). Recognition of MCMV via Ly49H is also sufficient to restore responsiveness to anergized or disarmed NK cells that do not express inhibitory receptors for self-MHC class I (9).
NK cells from nonobese diabetic (NOD) mice are impaired in their ability to reject xenograft transplants, making NOD/SCID mice, which lack B cells and T cells, a useful recipient for transplanted normal human hematopoietic cells and for human tumors (10). NK cells in NOD mice are poorly cytotoxic against a variety of targets, including YAC-1 lymphoma cells that activate the NKG2D receptor on NK cells, CHO cells that activate the Ly49D receptor on NK cells, and antibody-coated target cells, which are recognized by CD16, the intermediate affinity receptor for IgG, on NK cells (11–13). Compared with B6 NK cells, NOD NK cells are impaired in their ability to reject syngeneic MHC class I–deficient bone marrow or splenocytes in vivo. At least two defects may account for the poor cytolytic function of NOD NK cells. Upon activation with IL-2 NOD NK cells, but not B6 NK cells, up-regulate expression of the Rae-1 family of NKG2D ligands, resulting in down-modulation of NKG2D on the activated NK cells (14). This may partially account for the defective killing of YAC-1 target cells by activated NOD NK cells. IL-15, a cytokine critical for the proper development and activation of NK cells, is expressed at reduced levels in NOD mice compared with B6 mice (15), which might also contribute to the functional defects in resting NOD NK cells. Injection of IL-15 complexed with IL-15 receptor into NOD and B6 mice enhances NK cell–mediated cytotoxicity (15). Activation of NOD NK cells by treatment with IL-12 and IL-18, IFN-β, complete Freund's adjuvant (CFA), or inducers of type I IFN, such as polyI:C or tilorone, also enhances NK cell–mediated cytotoxicity, although usually not to the level of activated B6 NK cells (10, 12, 16).
Many NK cell receptors, including NK1.1, NKG2D, and the Ly49 family, are encoded within the NK receptor complex (NKC) on mouse chromosome 6 (17, 18). NOD mice have a unique NKC encoding many more activating and inhibitory Ly49 receptors than B6 mice (19). Similar to NOD mice, NOD mice congenic for the NKC from B6 mice (designated NOD.NK1.1 because they express the activating NKR-P1C receptor from B6 mice) are defective in their response to a number of stimuli, including the killing of YAC-1 tumor cells and MHC class I–deficient hematopoietic cells (11, 20). These findings indicate that the NOD NK defect is not intrinsic to the NOD NKC. Unlike B6 mice, NOD mice are susceptible to MCMV, despite possessing an allele of the gene encoding Ly49H (Klra8) (19). To determine whether the generalized defect in NK cells in NOD mice prevents control of MCMV, we have compared NK cell responses to MCMV infection between C57BL/6.g7 mice that are congenic for the unique NOD H2 locus (B6.g7) and NOD.NK1.1 mice that are congenic for the NKC from C57BL/6 mice, including Ly49HB6 (20).
Results
NOD Allele of Ly49H Does Not Bind MCMV m157.
NOD mice fail to control MCMV, despite expressing an allele of the Ly49H (Klra8) gene (19). This could be due either to hyporesponsiveness of NOD NK cells or to Ly49HNOD failing to bind MCMV m157. To address the latter possibility, we stained NK cells from B6.g7, NOD, and NOD.NK1.1 mice with an m157 Ig fusion protein, followed by antibody staining for Ly49H. The B6.g7 and NOD.NK1.1 Ly49H+ NK cells bound the m157 fusion protein, confirming that the B6 allele of Ly49H expressed by NOD.NK1.1 NK cells binds m157 (Fig. 1A) (6). Conversely, neither Ly49H+ nor Ly49H– NK cells from NOD mice bound m157 (Fig. 1A). This failure to bind MCMV m157 thus accounts for the failure of NOD mice to resist MCMV infection.
Fig. 1.
Ly49H+NOD NK cells do not bind MCMV m157. (A) NK cells from NOD, B6.g7, and NOD.NK1.1 mice were stained with an m157 fusion protein (top row) or an irrelevant IgG control (bottom row) followed by an antibody against Ly49H. (B) NK cells from B6.g7, NOD and NOD.NK1.1 mice were stained for the expression of the indicated NK cell receptors. Data are representative of (A) three or (B) two experiments.
To compare the expression of the receptors encoded by the NKC in NOD, NOD.NK1.1, and B6.g7 mice, we stained NK cells with a panel of antibodies specific for receptors or alleles of receptors found in the B6, but not the NOD, NKC. The YE1/48 antibody is specific for a polymorphic epitope on the Ly49A receptor encoded by the Klra1 gene at the telomeric end of the Ly49 gene family. YE1/48 stained B6 and NOD.NK1.1 NK cells, but not NOD NK cells (Fig. 1B), indicating that YE1/48 does not recognize the allele of Ly49A present in NOD mice (19). The NKR-P1C receptor detected by the PK136 anti-NK1.1 antibody is encoded by an allele of the Klrb1c gene located at the centromeric end of the NKC that is present in NOD.NK.1 and B6 mice but not NOD mice (Fig. 1B) (17). Additionally, NK cells from NOD.NK1.1 mice, but not NOD mice, expressed the Ly49C and Ly49I inhibitory receptors found in B6 mice (Fig. 1B). The 16a11 antibody binds to the NKG2A receptor encoded by the allele of the Klrc1 gene in B6 mice, but not other alleles of NKG2A, whereas the 20d5 antibody cross-reacts with NKG2A, NKG2C, and NKG2E encoded by all alleles of these genes (21). Accordingly, B6 and NOD.NK1.1, but not NOD, NK cells bound 16a11 mAb (NKG2AB6), whereas NK cells from all three strains bound 20d5 mAb (NKG2A/C/E) (Fig. 1B). On the basis of this staining pattern, we conclude that NOD.NK1.1 mice express the entire B6 NKC.
Resting NOD.NK1.1 NK Cells Are Hypofunctional.
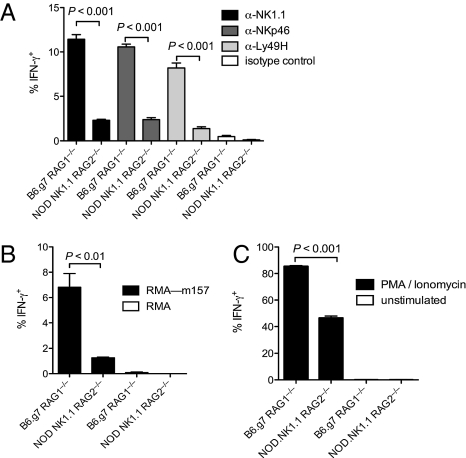
To determine whether the defects in NOD NK cell functions are due to genetic loci that differ between NOD and B6.g7 mice, we compared the responsiveness of NK cells from B6.g7 and NOD.NK1.1 mice on a Rag-deficient background. Using Rag-deficient mice eliminated the autoimmune environment in NOD mice that is dependent on T cells (22) and may compromise NK cell functions (23). Compared with B6.g7 NK cells, very few NOD.NK1.1 NK cells produced IFN-γ when the activating receptors NK1.1, Ly49H, or NKp46 were cross-linked with antibodies, despite comparable expression of NK1.1, Ly49H, and NKp46 on both populations (Figs. 1B and 2A). Ly49H+ NK cells from NOD.NK1.1 mice also failed to produce IFN-γ when incubated with target cells expressing MCMV m157, the cognate ligand for Ly49H (Fig. 2B). After stimulation with phorbol myristate acetate (PMA) and ionomycin, NOD.NK1.1 NK cells produced IFN-γ, albeit not at the same frequency as B6.g7 NK cells, thus suggesting a partial impairment in IFN-γ production by NOD.NK1.1 NK cells (Fig. 2C).
Fig. 2.
Impaired IFN-γ production by NOD.NK1.1 NK cells (A–C) Intracellular IFN-γ production by naïve B6.g7 Rag1−/− and NOD.NK1.1 Rag2−/− NK cells stimulated (A) by crosslinking NK1.1, NKp46, or Ly49H with plate-bound antibodies, an isotype-matched control antibody, (B) by co-culture with parental RMA or RMA targets transduced with MCMV m157 (RMA-m157), or (C) with PMA and ionomycin. Data are representative of five experiments with three or four mice per group per experiment.
We also analyzed the frequency of NK cells undergoing degranulation after stimulation, using surface expression of CD107a (LAMP-1) as a surrogate for lytic capacity. Unlike IFN-γ production, an equivalent frequency of NOD.NK1.1 NK cells and B6.g7 NK cells expressed CD107a when stimulated by cross-linking the NK1.1, NKp46, or Ly49H activating receptors or when Ly49H+ NK cells were incubated with m157-bearing target cells (Fig. 3 A and B). Although Ly49H+ NOD.NK1.1 NK cells degranulated normally, they were unable to kill m157-bearing target cells, suggesting a defect in their lytic machinery (Fig. 3C). NOD.NK1.1 NK cells expressed ≈2.5-fold less granzyme B than B6.g7 NK cells on a per-cell level, possibly accounting for the poor cytolytic capacity of NOD.NK1.1 NK cells (Fig. 3D). NOD.NK1.1 and B6.g7 NK cells expressed similar amounts of perforin.
Fig. 3.
Impaired cytotoxicity by NOD.NK1.1 NK cells. (A, B) Degranulation as measured by surface expression of CD107a by naïve B6.g7 Rag1−/− and NOD.NK1.1 Rag2−/− NK cells stimulated (A) by crosslinking NK1.1, NKp46, or Ly49H with plate-bound antibodies, an isotype-matched control antibody, or (B) by co-culture with parental RMA or RMA targets transduced with MCMV m157 (RMA-m157). (C) Cytotoxicity of naïve B6.g7 and NOD.NK1.1 NK cells against Ba/F3 target cells or Ba/F3 cells transduced with MCMV m157 (Ba/F3-m157). (D) Ex vivo expression of granzyme B by naïve B6.g7 and NOD.NK1.1 NK cells. Graphs indicate the average ± SEM. Data are representative of (A, B) five, (C) two, or (D) four experiments with three or four mice per group per experiment.
Ly49H+ NOD.NK1.1 NK Cells Fail to Control MCMV Early After Infection.
Ly49H+ NK cells control MCMV infection by secreting IFN-γ and direct cytolysis of MCMV infected cells via granzyme B and perforin (24). To determine whether defects in NOD.NK1.1 NK cells, which carry the protective B6 allele of Ly49H that recognizes m157 expressed by the Smith strain of MCMV (Fig. 1A), prevent control of MCMV, we measured viral titers in the spleens and livers of NOD.NK1.1 and B6.g7 mice 3 d after infection. Unlike B6.g7 NK cells, NOD.NK1.1 NK cells were completely unable to control MCMV viral replication (Fig. 4 A and B). Depletion of NK cells in B6.g7 increased the viral burden in both organs, but NK cell depletion did not alter the viral titers in NOD.NK1.1 mice, indicating that NK cells in these mice were unable to control MCMV at this early time (Fig. 4 A and B). The failure to control MCMV was not due to pathogenic T cells or B cells because NOD.NK1.1 Rag2−/− mice also failed to control MCMV infection when compared with B6.g7 Rag1−/− mice (Fig. 4 C and D).
Fig. 4.
NOD.NK1.1 NK cells fail to control MCMV early after infection. MCMV titers three days after infection with 5 × 104 pfu of MCMV in the (A) spleen and (B) liver of B6.g7 and NOD.NK1.1 mice either untreated or depleted of NK cells. MCMV titers 3 d after infection with 5 × 103 pfu of MCMV in the (C) spleen and (D) liver of B6.g7 Rag1−/− and NOD.NK1.1 Rag2−/− mice. The lower viral dose was used to avoid lethality in Rag-deficient animals. Graphs indicate the average ± SEM. Data are representative of three experiments with five mice per group per experiment.
MCMV Infection Restores Lytic Capacity of NOD.NK1.1 NK Cells.
We previously found that MCMV infection increases the expression of granzyme B in B6 NK cells (9). By 5 d after infection expression of granzyme B in NOD.NK1.1 Ly49H+ NK cells increased to levels similar to those found in Ly49H+ NK cells from infected B6.g7 mice (Fig. 5A). Unlike Ly49H+ NK cells from uninfected NOD.NK1.1 mice (Fig. 3C), NK cells from MCMV-infected NOD.NK1.1 mice were able to kill m157-bearing targets as efficiently as NK cells from MCMV-infected B6.g7 mice (Fig. 5B). Unlike lytic capacity, the capacity of NOD.NK1.1 NK cells to make IFN-γ in response to antibody cross-linking of NK1.1, NKp46, or Ly49H or coincubation with m157-bearing target cells was not significantly restored by MCMV infection (Fig. 5 C and D), suggesting that these two functional defects may arise from distinct causes.
Fig. 5.
MCMV infection restores cytolytic functions in NOD.NK1.1 NK cells. (A) Ex vivo expression of granzyme B by NK cells from MCMV-infected B6.g7 and NOD.NK1.1 mice. (B) Cytotoxicity of B6.g7 and NOD.NK1.1 NK cells against Ba/F3 cells or Ba/F3 transduced with MCMV m157 (Ba/F3-m157) 5 d after infection with MCMV. (C, D) Intracellular IFN-γ production by B6.g7 and NOD.NK1.1 NK cells stimulated (C) by crosslinking NK1.1, NKp46, or Ly49H with plate-bound antibodies, an isotype-matched control antibody, or (D) by co-culture with RMA cells or RMA transduced with MCMV m157 (RMA–m157). Graphs indicate the average ± SEM. (A–D) Data are representative of four experiments with three or four mice per group per experiment.
NOD.NK1.1 NK Cells Control MCMV Late During Infection.
Ly49H+ NK cells undergo a specific and robust expansion during MCMV infection in response to engagement with MCMV m157 (7). The frequency of Ly49H+ NK cells in both B6.g7 and NOD.NK1.1 mice increased from ≈50% to ≈80% of all NK cells by 5 d after infection, indicating that NOD.NK1.1 NK cells are not impaired in their proliferative capacity (Fig. 6A). The larger amounts of MCMV m157 present in the infected NOD.NK1.1 mice due to increased viral burden (Fig. 4) might enhance the proliferation of the Ly49H+ NK cells, thus masking an intrinsic proliferation defect in NOD.NK1.1 NK cells. This was addressed by infection of mixed bone marrow chimeric mice generated by reconstitution of NOD Rag2−/− recipients with B6.g7 Rag1−/− and NOD.NK1.1 Rag2−/− bone marrow. In these mixed chimeras, B6.g7 NK cells expanded at a higher rate for the first 15 d after MCMV infection, as evinced by a skewing of the NK population toward B6.g7 (Fig. 6B). Thus, relative to B6.g7 NK cells, NOD NK cells are intrinsically impaired in their proliferative capacity in response to infection, but this impairment might be offset by the increased amount of m157 present in the infected NOD.NK1.1 mice.
Fig. 6.
Ly49H+ NOD.NK1.1 NK cells expand normally during MCMV infection. (A) Frequency of splenic NK cells expressing Ly49H from naïve and MCMV-infected B6.g7 and NOD.NK1.1 mice prior to infection or 5 d after MCMV infection. (B) Ratio of peripheral blood B6.g7 to NOD.NK1.1 Ly49H+ NK cells in MCMV infected mixed-bone marrow chimeric mice normalized to the ratio prior to infection. Graphs indicate the average ± SEM. Data are pooled from two experiments with three or four mice per experiment.
The early control of MCMV by NK cells occurs before the extensive proliferative burst of Ly49H+ NK cells and is defective in NOD.NK1.1 mice. To determine whether NOD.NK1.1 NK cells are able to control MCMV, independent of T and B cells, after undergoing several rounds of proliferation (Fig. 6A) and up-regulating granzyme B expression (Fig. 5A), we measured MCMV titers in B6.g7 Rag1−/− and NOD.NK1.1 Rag2−/− mice. By 1 wk after infection, NOD.NK1.1 NK cells controlled MCMV replication in the spleen, liver, and salivary glands as effectively as B6.g7 NK cells (Fig. 7 A–C). That NOD.NK1.1 NK cells were able to control MCMV titers, despite their defective capacity to produce IFN-γ in vitro (Fig. 5 C and D), suggests that late viral control may be primarily mediated by cytolysis of infected cells. Additionally, because early control of MCMV is defective in NOD.NK1.1 mice, NOD.NK1.1 NK cells may be more efficient at controlling MCMV at later stages because viral titers were similar at day 7.
Fig. 7.
NOD.NK1.1 NK cells control MCMV infection as efficiently as B6.g7 NK cells after 7 d of infection. MCMV titers 7 d after infection with 5 × 103 pfu of MCMV in the (A) spleen, (B) liver, and (C) salivary glands of B6.g7 Rag1−/− and NOD.NK1.1 Rag2−/− mice either untreated or depleted of NK cells. Data are representative of three experiments with five mice per group per experiment. (D) Survival of NOD Rag2−/− mice that received 1 × 105 Ly49H+ NK cells from either B6.g7 Rag1−/− or NOD.NK1.1 Rag2−/− or PBS and infection with 2.5 × 104 pfu of MCMV. Data are pooled from three experiments with similar results. Graphs indicate the average ± SEM. The difference in survival between recipients of either B6.g7 Rag1−/− or NOD.NK1.1 Rag2−/− Ly49H+ NK cells is statistically significant (P < 0.001).
We and others have shown that adoptive transfer of adult Ly49H+ NK cells is sufficient to protect neonatal mice from MCMV infection (9, 25, 26). To determine whether NOD.NK1.1 NK cells are protective in vivo, we adoptively transferred equal numbers of B6.g7 Rag1−/− or NOD.NK1.1 Rag2−/− Ly49H+ NK cells into MCMV-susceptible NOD Rag2−/− recipients and then infected them with MCMV. We transferred a suboptimal dose of NK cells to compare the protection conferred by NOD.NK1.1 vs. B6.g7 NK cells. We found that NOD.NK1.1 Rag2−/− Ly49H+ NK cells protected 4 of the 15 recipients, whereas at this suboptimal cell dose the B6.g7 NK cells failed to protect the NOD Rag2−/− recipients (Fig. 7D). The difference in survival between recipients of either B6.g7 Rag1−/− or NOD.NK1.1 Rag2−/− Ly49H+ NK cells is statistically significant (P < 0.001).
Discussion
In this study, we examined the capacity for NK cells from NOD.NK1.1 mice to control MCMV infection. NOD mice express a unique NKC that contains an allele of Ly49H that is unable to bind to m157 from the MCMV Smith strain, and accordingly, NOD mice do not control early MCMV infection (19). Additionally, it has recently been appreciated that the repertoire of MHC class I inhibitory receptors affects the responsive potential of NK cells. Naïve NK cells that do not express inhibitory receptors for self-MHC class I are hyporesponsive to stimulation through activating receptors (27–29); however, during MCMV infection expression of self-MHC I inhibitory receptors limits NK cell control of infection (9). To directly address the functional responsiveness of NK cells in MCMV-susceptible NOD mice and MCMV-resistant B6 mice, we compared B6.g7 with NOD.NK1.1 mice, both of which express the unique H-2g7 NOD H2 locus and the B6 NKC receptors. In doing so, we have eliminated the differential contribution of NK cell licensing, as well as expression of the Ly49H receptor able to recognize m157, between the two strains.
Upon engagement with m157-bearing targets or cross-linking of the Ly49H, NK1.1, or NKp46 activating receptors, NOD.NK1.1 NK cells degranulated as efficiently as B6.g7 NK cells. Surprisingly, despite normal degranulation, naïve NOD.NK1.1 NK cells were unable to kill m157-bearing target cells. Although degranulation, as measured by CD107a expression on the surface, has often been considered equivalent to lytic capacity, these findings demonstrate that the ability to induce target cell death is not necessarily reflected by effector cell degranulation. This discrepancy between normal degranulation and defective target cell killing is likely due to low expression of granzyme B in naïve NOD.NK1.1 NK cells compared with B6.g7 NK cells. Effectively, NOD.NK1.1 NK cells are secreting granules normally upon stimulation, but they lack the effector molecules necessary to kill target cells. This may explain why NOD.NK1.1 NK cells are impaired in their ability to kill a wide array of targets that are normally susceptible to NK cell killing, such as RMA-S, CHO, YAC-1, and B2m−/− splenocytes (11–13). It may also explain why NOD/SCID and NOD Rag1−/− mice are inefficient at rejecting xenografts (10, 22). Naïve NOD.NK1.1 NK cells also failed to produce IFN-γ upon exposure to m157-bearing target cells or cross-linking of several activating receptors. These defects in cytotoxicity and possibly IFN-γ secretion prevented NOD.NK1.1 NK cells from controlling MCMV early after infection, despite expressing the Ly49HB6 receptor. This defect in viral control was not due to ongoing autoimmunity because NOD.NK1.1 Rag2−/− mice, which do not develop disease, also failed to control MCMV early after infection.
Although NOD.NK1.1 NK cells failed to control MCMV early after infection, intracellular granzyme B stores were increased after infection. This coincided with a restored ability of NOD.NK1.1 NK cells to kill m157-bearing targets. NOD mice express reduced levels of IL-15 compared with B6.g7 mice, possibly accounting for the low level of granzyme B expression in naïve mice (15). Stimulation of human or mouse NK cells with IL-15 or IL-12 is sufficient to increase expression of granzyme B (30, 31). In vivo activation with IL-15 and IL-15R complexes or CFA or in vitro stimulation with IL-12 and IL-18, IFN-β, or tilorone restores NOD NK cell cytotoxicity (3, 12, 15, 16). Upon MCMV infection, IL-15 levels likely increase, leading to an increased amounts of granzyme B; however, we have previously found that after MCMV infection NK cells from Il15−/− mice are able to kill m157-bearing targets (32), thus other stimuli such as IL-12 and/or IFN-β induced during MCMV infection may be sufficient to increase expression of granzyme B. Transgenic expression of IFN-β in the pancreas of NOD mice also enhances NK cell functions (33). Thus, it is likely the proinflammatory cytokine environment associated with viral infection, rather than direct Ly49H triggering, is responsible for the increased levels of granzyme B expression. In contrast to cytotoxicity, MCMV infection was not sufficient to restore the ability of NOD NK1.1 NK cells to produce IFN-γ upon activating receptor stimulation. This suggests that granzyme B and IFN-γ expression are differentially regulated in NOD.NK1.1 NK cells.
Despite the defects in effector functions, Ly49H+ NOD.NK1.1 NK cells expanded during infection, indicating that NOD.NK1.1 NK cells are not globally impaired. We have previously found that efficient Ly49H+ NK cell expansion during MCMV infection is necessary to efficiently control MCMV replication at later time points (34). Even in the absence of T cells and B cells, NK cells from NOD.NK1.1 Rag2−/− mice controlled late infection in the spleen, liver, and salivary glands as efficiently as NK cells from B6.g7 Rag1−/− mice. NOD.NK1.1 NK cells controlled the infection even though these cells were still impaired in their ability to produce IFN-γ, suggesting that late viral control is primarily due to direct killing of infected cells, as has been previously suggested (35). The outcome that transferred NOD.NK1.1 NK cells protect NOD.Rag2−/− from MCMV infection better than B6.g7 NK cells suggest that NOD.NK1.1 NK cells may be more efficient at killing infected cells in vivo and/or survive better under chronic inflammation. Thus, although unable to control the initial wave of MCMV replication owing to defective effector function, NOD.NK1.1 NK cells are able to up-regulate granzyme B, proliferate, and ultimately control MCMV. These findings imply that NK cells in NOD mice are not as impaired as previously considered and are able to contribute to host defense against viral immunity.
Methods
Mice.
Mark Anderson (University of California San Francisco, San Francisco, CA) provided B6.g7 mice. B6.g7 Rag1−/− mice were generated by breeding B6.g7 mice and B6 Rag1−/− mice purchased from Jackson Laboratory. Albert Bendelac (University of Chicago, Chicago, IL) provided NOD.B6-(D6Mit254-D6Mit14)/CarJ (NOD.NK1.1) mice (20). NOD.NK1.1 Rag2−/− mice were generated by breeding NOD.NK1.1 and NOD Rag2−/− mice provided by Jeffrey Bluestone (University of California San Francisco, San Francisco, CA). All mice were maintained in the specific pathogen-free animal facility of the University of California, San Francisco. The Institutional Animal Care and Use Committee of the University of California, San Francisco approved animal protocols.
NK Cell-Surface Staining.
The MCMV m157 fusion protein was described previously (6). NK cells were stained with m157 Ig fusion protein, anti-NKp46 (29A1.4), anti-Ly49H (3D10), anti-Ly49A (YE1/48), anti-Ly49C/I (5E6), anti-NKG2AB6 (16a11), anti-NKG2A/C/E (20d5), and anti-NK1.1 (PK136). Anti-NKp46, anti-Ly49H, anti-NKG2AB6, anti-NKG2A/C/E mAb were purchased from eBioscience. Anti-NK1.1 and anti-Ly49A were purchased from BioLegend. Anti-Ly49C/I was purchased from BD Biosciences.
NK Cell Stimulation.
Target cells were generated by retroviral transduction of MCMV m157 into RMA cells. Splenic NK cells from naïve or MCMV-infected mice were incubated for 6 h at a ratio of 1:1 with target cells in the presence of brefeldin A and anti-CD107 AMAb (1D4B) (BD Biosciences). Alternatively, 5 × 105 splenic NK cells were incubated on plates coated with 10 μg/mL anti-Ly49H (3D10), anti-NK1.1 (PK136), anti-NKp46 (29A1.4), or an isotype-matched control mAb or with 10 ng/mL PMA and 200 ng/mL ionomycin in the presence of brefeldin A. Cells were surface stained with mAbs to NK1.1, T cell receptor β (TCR-β) (H57-597) (BioLegend), and Ly49H, and then stained intracellularly with anti-IFN-γ mAb (XMG1.2) by using the Intracellular Staining Kit (BD Biosciences).
NK Cell-Mediated Cytotoxicity.
Naïve or 5 d after MCMV infection, splenic NK cells were enriched by labeling splenocytes with rat anti-mouse IgG mAbs (produced in our laboratories) against CD4, CD5, CD8, CD19, TER119, and Gr-1 and magnetically depleting labeled cells with anti-rat IgG-coated beads (Qiagen). Enriched NK cells were incubated in triplicate with 51Cr-labeled Ba/F3 cells or Ba/F3 cells stably expressing MCMV m157. Six hours later, supernatants were harvested and assayed for 51Cr release. Spontaneous lysis was determined by incubating target cells without effectors. Maximum lysis was determined by freeze–thaw lysis of target cells. Percentage-specific lysis = (lysis − spontaneous lysis)/(maximum lysis − spontaneous lysis) × 100.
MCMV Infection.
Mice 6–10 wk of age were infected i.p. with 0.5–5 × 104 plaque-forming units of MCMV (Smith strain). Before infection or 5 d after infection, splenic NK cells were stained for Ly49H, NK1.1, and TCR-β, and intracellularly for granzyme B (GB11) (BD Biosciences). For measurement of viral titers, spleens, salivary glands, and livers were collected, homogenized, plated on M2-10B4 cells (American Type Culture Collection) in RPMI-1640 medium without FCS and incubated for 2 h at 37 °C. RPMI-1640 medium with 10% (vol/vol) FCS and 0.75% (wt/vol) carboxymethyl cellulose was added, and samples were incubated for 7–10 d. Plaques were visualized by staining with crystal violet dye.
Bone Marrow Chimeras.
Bone marrow was harvested from the femurs and tibias of donor NOD.NK1.1 Rag2−/− and B6.g7 Rag1−/− mice. Bone marrow cells were counted and mixed at a 70:30 ratio of B6.g7 Rag1−/−/NOD.NK1.1 Rag2−/−. Lethally irradiated recipient NOD Rag2−/− mice were conditioned with 200 μg of anti-NK1.1 (PK136) at the time of transplantation to eliminate mature donor and host NK cells. Recipients were used at 10–12 wk after transplantation to allow for full reconstitution and return of hematopoietic homeostasis. Chimerism was monitored by flow cytometry of peripheral blood before infection of the mice with 3 × 104 pfu of MCMV. NK cell ratios were monitored by flow cytometric analysis of Ly49H+ NK cells and distinguished by expression of CD45.1 (NOD.NK1.1 Rag2−/−) or CD45.2 (B6.g7 Rag1−/−). Anti-CD45.1 (A20) and anti-CD45.2 (104) were purchased from BioLegend.
Transfer of Protection.
NK cells from NOD.NK1.1 Rag2−/− and B6.g7 Rag1−/− spleens were enriched as described above. Ly49H+ NK cells (1 × 105) were transferred into NOD Rag2−/− mice via i.v. injection the day before infection with 2.5 × 104 pfu of MCMV.
Statistical Analysis.
The statistical significance of differences in the percentage of IFN-γ+ and CD107a+ was determined by the unpaired, two-tailed Student's t test. The statistical significance of differences in viral titers was determined by the unpaired, two-tailed Mann–Whitney test. The statistical significance of differences in survival of recipients receiving NK cells was determined by the Mantel–Cox test. Statistics were determined with Prism software (GraphPad Software).
Acknowledgments
We thank H. Consengco for assistance with retroviral transduction. This work was supported by National Institutes of Health Grant AI066897. M.T.O. is supported by the Cancer Research Institute. J.N.B. was supported by the Juvenile Diabetes Research Foundation. L.L.L. is an American Cancer Society Professor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Lu L, et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee IF, Qin H, Priatel JJ, Tan R. Critical role for IFN-gamma in natural killer cell-mediated protection from diabetes. Eur J Immunol. 2008;38:82–89. doi: 10.1002/eji.200737189. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith HR, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 7.Dokun AO, et al. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, et al. Transgenic expression of the activating natural killer receptor Ly49H confers resistance to cytomegalovirus in genetically susceptible mice. J Exp Med. 2003;197:515–526. doi: 10.1084/jem.20021713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11:321–327. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shultz LD, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 11.Poulton LD, et al. Cytometric and functional analyses of NK and NKT cell deficiencies in NOD mice. Int Immunol. 2001;13:887–896. doi: 10.1093/intimm/13.7.887. [DOI] [PubMed] [Google Scholar]
- 12.Johansson SE, Hall H, Björklund J, Höglund P. Broadly impaired NK cell function in non-obese diabetic mice is partially restored by NK cell activation in vivo and by IL-12/IL-18 in vitro. Int Immunol. 2004;16:1–11. doi: 10.1093/intimm/dxh002. [DOI] [PubMed] [Google Scholar]
- 13.Kataoka S, et al. Immunologic aspects of the nonobese diabetic (NOD) mouse. Abnormalities of cellular immunity. Diabetes. 1983;32:247–253. doi: 10.2337/diab.32.3.247. [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara K, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 15.Suwanai H, Wilcox MA, Mathis D, Benoist C. A defective Il15 allele underlies the deficiency in natural killer cell activity in nonobese diabetic mice. Proc Natl Acad Sci USA. 2010;107:9305–9310. doi: 10.1073/pnas.1004492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee IF, Qin H, Trudeau J, Dutz J, Tan R. Regulation of autoimmune diabetes by complete Freund's adjuvant is mediated by NK cells. J Immunol. 2004;172:937–942. doi: 10.4049/jimmunol.172.2.937. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–316. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama WM, et al. cDNA cloning of mouse NKR-P1 and genetic linkage with LY-49. Identification of a natural killer cell gene complex on mouse chromosome 6. J Immunol. 1991;147:3229–3236. [PubMed] [Google Scholar]
- 19.Belanger S, Tai LH, Anderson SK, Makrigiannis AP. Ly49 cluster sequence analysis in a mouse model of diabetes: An expanded repertoire of activating receptors in the NOD genome. Genes Immun. 2008;9:509–521. doi: 10.1038/gene.2008.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carnaud C, Gombert J, Donnars O, Garchon H, Herbelin A. Protection against diabetes and improved NK/NKT cell performance in NOD.NK1.1 mice congenic at the NK complex. J Immunol. 2001;166:2404–2411. doi: 10.4049/jimmunol.166.4.2404. [DOI] [PubMed] [Google Scholar]
- 21.Vance RE, Jamieson AM, Cado D, Raulet DH. Implications of CD94 deficiency and monoallelic NKG2A expression for natural killer cell development and repertoire formation. Proc Natl Acad Sci USA. 2002;99:868–873. doi: 10.1073/pnas.022500599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shultz LD, et al. NOD/LtSz-Rag1null mice: An immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J Immunol. 2000;164:2496–2507. doi: 10.4049/jimmunol.164.5.2496. [DOI] [PubMed] [Google Scholar]
- 23.Liu R, et al. Autoreactive T cells mediate NK cell degeneration in autoimmune disease. J Immunol. 2006;176:5247–5254. doi: 10.4049/jimmunol.176.9.5247. [DOI] [PubMed] [Google Scholar]
- 24.Loh J, Chu DT, O'Guin AK, Yokoyama WM, Virgin HW., 4th Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol. 2005;79:661–667. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bukowski JF, Warner JF, Dennert G, Welsh RM. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985;161:40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. doi: 10.1016/j.cell.2010.08.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye W, Young JD, Liu CC. Interleukin-15 induces the expression of mRNAs of cytolytic mediators and augments cytotoxic activities in primary murine lymphocytes. Cell Immunol. 1996;174:54–62. doi: 10.1006/cimm.1996.0293. [DOI] [PubMed] [Google Scholar]
- 31.Salvucci O, et al. Differential regulation of interleukin-12- and interleukin-15-induced natural killer cell activation by interleukin-4. Eur J Immunol. 1996;26:2736–2741. doi: 10.1002/eji.1830261128. [DOI] [PubMed] [Google Scholar]
- 32.Sun JC, Ma A, Lanier LL. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J Immunol. 2009;183:2911–2914. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alba A, et al. Natural killer cells are required for accelerated type 1 diabetes driven by interferon-beta. Clin Exp Immunol. 2008;151:467–475. doi: 10.1111/j.1365-2249.2007.03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orr MT, et al. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J Exp Med. 2009;206:807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumaria N, et al. The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol Cell Biol. 2009;87:559–566. doi: 10.1038/icb.2009.41. [DOI] [PubMed] [Google Scholar]