Abstract
Estrogens are important in the development, maintenance and physiology of the CNS. Several studies have shown their effects on the processing of hearing in both males and females, and these effects, in part, are thought to result from regulation of the transcription of genes via their classical estrogen receptor (ER) pathway. In order to understand the spatiotemporal changes that occur with age, we have studied the expression of ERs in the central auditory pathway in prepubertal and aged CBA mice with immunohistochemistry. In prepubertal mice a clear dichotomy was noted between the expression of ERα and ERβ. ERβ-positive neurons were found in the metencephalon whereas the majority of ERα was found in mesencephalon, diencephalon or the telencephalon. In the aged animals a different pattern of ER expression was found in terms of location and overall intensity. These age-induced changes in the expression pattern were generally not uniform, suggesting that region-specific mechanisms regulate the ERs’ age-related expression. Neither the prepubertal nor the aged animals showed sex differences in any auditory structure. Our results demonstrate different age-dependent spatial and temporal changes in the pattern of expression of ERα and ERβ, suggesting that each ER type may be involved in distinct roles across the central auditory pathway in different periods of maturation.
Keywords: hearing, brain, sex dimorphism, western blot, auditory brainstem response, hearing loss
Estrogens regulate many physiological functions in the brain beyond the classic tasks of sexual development and reproductive behavior. Functions such as learning and memory, mood or pain sensitivity, neurodevelopment and neurodegeneration are greatly affected by estrogens (McCarthy, 2008; Craft et al., 2004; Gillies and McArthur, 2010). Growing evidence documents that estrogens play significant roles in auditory processing in young and middle aged females. When estradiol levels are modified, alterations in auditory perception, auditory brainstem latencies, auditory thresholds and sound localization are perturbed (Haggard and Gaston, 1978; Caruso et al., 2003a,b; Jerger and Johnson, 1988; Wharton and Church, 1990; Coleman et al., 1994). Women suffering from Ménière’s (hearing loss, vertigo, and perhaps tinnitus) exhibit enhanced symptoms during the premenstrual phase (low estrogen levels) (Morse and House, 2001). These clinical studies suggest that the auditory system of females is protected and made more sensitive by estradiol. The effects of estradiol on hearing are also well documented in experimental animals. By antagonizing estrogen actions, auditory feedback mechanisms are altered in mice (Thompson et al., 2006). When non-reproductive female midshipman fish are treated with estrogen agonists an increase in their eighth nerve temporal encoding to male vocalizations occurs in a manner that is similar to cycling females (Sisneros et al., 2004). Estradiol can rapidly modulate the physiological response of neurons during auditory processing in the zebra finch auditory association cortex by suppressing inhibitory transmission (Tremere et al., 2009). Thus, estrogens have profound effects on hearing in different species and different experimental paradigms and, in contrast to the classic view, they very often have similar effects on male and female brains.
Estrogens’ actions are mediated by estrogen receptors alpha and beta (ERα and ERβ), which belong to the nuclear receptor superfamily and act as transcription factors. The distinct roles of these two receptor types in auditory processing are not fully elucidated (McCullar and Oesterle, 2009; Charitidi et al., 2009). It is known however, that ERβ has a protective role in the hearing of mice when they are exposed to acoustic trauma (Meltser et al., 2008). In a previous study, estrogen receptors were located throughout the central auditory system of young CBA mice indicating a potential influence of estrogen in central auditory processing (Charitidi and Canlon, 2009). In the present study we examine the expression pattern of ERα and ERβ in the central auditory system before and after major hormonal changes found in prepubertal and aged mice. Characterizing the organization of ERs in the central auditory system in different ages is an essential step for comprehending the role of steroid hormones during maturation and degeneration of the hearing system, as well as understanding the effects of hormone replacement therapy.
EXPERIMENTAL PROCEDURES
Animals
18 CBA/Ca mice of both sexes were used. 6 mice were sacrificed in 4 weeks (prepubertal; 3 males and 3 females) and 6 in 26–28 months (aged; 3 males and 3 females). In addition six mice were sacrificed in order to verify the specificity of the antibodies against ERα and ERβ with western blot (3 males and 3 females, 11-weeks old). The animals were housed on an artificial 12-hour light/12-hour dark cycle with free access to food and water. The young mice were supplied by Scanbur, Sweden. The handling and housing of the young animals as well as the use of these animals was approved by the Stockholm Animal Research Ethical Committee. The aged mice were raised from birth within the University of Rochester Vivarium with ambient noise levels<50 dB sound pressure level (SPL). One of the old male mice started labored breathing when anesthetized for the auditory brainstem responses (ABRs), so ABRs were not collected on that subject, and that mouse received the perfusion directly. All procedures described below for the aged mice were approved by University of Rochester’s Animal Care and Use Committee and comply with all federal and state regulations of the USA. All efforts were made in order to keep consistency in the processing of the prepubertal and aged brains. Transcardiac perfusion of the animals and fixation of the brains were done separately using the same protocols in each facility and then the brains of the aged animals were shipped to Stockholm in 0.5% paraformaldehyde (PFA). Thereafter all brains were processed in parallel.
In mice, different strains show variability of the onset time of sexual maturation, but for CBA female mice maturation occurs at 5–6 weeks and for male mice at 8 weeks (Van Zutphen et al., 2001). The vaginal opening and first estrus or the onsets of cyclicity are the events that are most commonly used as measures of puberty, and these differ in the time that they appear in different strains of mice (Nelson et al., 1990). In this experiment the presence or absence of the vaginal opening was assessed in female prepubertal mice with a simple visual examination of the vulva of the mice (Caligioni, 2009). In female mice, menopause occurs as a gradual decrease in the levels of sex hormones leading to irregular and progressively rare estrous cycles. In males there is also a substantial decrease in the levels of testosterone and estradiol with aging (Wu et al., 2009).
Auditory brainstem responses
ABR thresholds were recorded in the free-field, similar to our previous reports (Jacobson et al., 2003; Zhu et al., 2007; Zettel et al., 2007). Mice were anesthetized with a mixture of keta mine/xylazine (120 and 10 mg/kg body weight, respectively, i.p. injection). Supplementary doses (1/3 of the initial dose) were administered as needed. Prior to recordings, the ear canals and ear drums were inspected for signs of obstruction or infection, and only those animals with clear outer and middle ears were used. While under anesthesia, body temperature was maintained at 38 °C with a servo heating pad. Recording sessions were completed in a soundproof acoustic chamber (IAC) lined with Sonex. S.c. platinum needle electrodes were placed at the vertex (non-inverting input), right mastoid prominence (inverted input) and on the back (indifferent site). Electroencephalographic (EEG) activity was differentially amplified (50 or 100×) (Grass model P511 EEG amplifier), input to an analog to digital (A/D) converter (ad1, Tucker-Davis [TDT]), and then digitized at 50 kHz. Each averaged response was based on 300–500 stimulus repetitions recorded over 10 ms epochs. Rejection of data epochs in which the single trace EEG peak-to-peak amplitude exceeded 50 µV prevented contamination by muscle and cardiac activities. ABR thresholds were recorded for frequencies of 3, 6, 12, 24, 32 and 48 kHz with thresholds defined as the minimum SPL for which any ABR wave could be reliably observed. The sound intensity was varied in 5 dB increments. For mice of the present study, near threshold response amplitudes generally increased above the noise floor background voltage level for Wave IV/V.
Tissue preparation for immunocytochemistry
Mice were anaesthetized with an i.p. injection of ketamine (50 mg/kg) (Ketalar) and xylazine (10 mg/kg) (Rompun). Transcardiac perfusion with phosphate buffer saline (PBS) at room temperature (RT) containing 0.001% v/v heparin 5000-iu/ml (LEO Pharma) followed by freshly prepared 4% PFA in PBS was performed. Brains were dissected and maintained in 4% PFA for 2 h at RT followed by 0.5% PFA in PBS at 4 °C. After fixation they were placed in 10% sucrose in PBS for 24 h at 4 °C followed by 20% sucrose in PBS for 24 h at 4 °C. Specimens were then frozen with isopentane and dry ice and stored at −80 °C. Serial coronal brain sections (40 µm thickness) on a cryostat (HM 500 M, Zeiss, Germany) at −24 °C were cut and stored in tubes with cryoprotection solution (40% phosphate buffer [0.1 M pH 7.4], 30% glycerol, 30% ethylene glycol) to be used for free-floating staining.
Immunohistochemistry
In brief, brain sections were rinsed from the cryoprotection solution in PBS 3× 10 min before permeabilization with 0.3% Triton-X in PBS for 30 min accordingly to a previous report (Charitidi and Canlon, 2009). To block endogenous peroxidase and non-specific staining sections were treated with H2O2 (3%) in PBS for 30 min and 1.5% normal goat serum (Vector S-1000) in PBS for 1 h at RT. The primary antibody was applied and the sections were incubated for 24–72 h at 4 °C on an orbital shaker. Polyclonal rabbit anti-ERα antibody (MC-20 sc-542; Santa Cruz Biotechnology) raised against a peptide mapping at the C-terminus of ERα of mouse origin and polyclonal rabbit anti-ERβ antibody (PA1-310B; Affinity Bioreagents) against residues C(467) to C(485) in the ligand-binding domain of rat ERβ were used at concentrations 0.0005 and 0.0025 mg/ml respectively. These concentrations were found to be optimal after several pilot experiments in which different concentrations and incubation times were tested. Excess primary antibody was rinsed with 0.15% Triton in PBS and secondary antibody, anti-rabbit IgG (Vector BA-1000) was added for 30 min followed by an immunoperoxidase reaction (VectaStain ABC kit PK-6100 and DAB substrate kit SK-4100; Vector Laboratories). The sections were rinsed and mounted on slides (Super-frost Slides Plus, Menzel GmbH, Germany), left to dry and further dehydrated in a graded series of ethanol dilutions (70%, 95%, 99%) and xylene before placing coverslips. The validity of the antibodies used for this study was verified by substitution of the primary antibodies by blocking solution, as well as pre-incubation of anti-ERα and anti-ERβ antibodies with an excess (1:10) of ERα-blocking peptide (sc-542 P; Santa Cruz Biotechnology) or ERβ-blocking peptide (PEP-007; Affinity Bioreagents). Additional control experiments were included in our previous study (Charitidi and Canlon, 2009).
Evaluation
The auditory pathway was assessed for ERα and ERβ immunoreactivity including the cochlear nucleus (CN) with its three main subdivisions (dorsal [DCN], anterior part of the ventral [VCA]and posterior part of the ventral cochlear nucleus [VCP]as well as the large neurons embedded in between the fibers of the 8th nerve forming the auditory nerve nucleus [AuN]), (superior olivary complex (SOC) including the nucleus of the trapezoid body (Tz), lateral superior olive (LSO), medial superior olive (MSO), superior paraolivary nucleus (SPO), medioventral (MVPO) and lateroventral (LVPO) periolivary nucleus); inferior colliculus (IC, dorsal cortex [DCIC], external cortex [ECIC], central nucleus [CIC], commissure and brachium [BIC]), lateral lemniscus (intermediate, ILL; ventral, VLL; and dorsal nucleus, DLL), medial geniculate nucleus (MGN) and auditory cortex (AuC). Coronal diagrams of the different brain levels containing the areas of interest from the stereotaxic map of the mouse brain (Franklin and Paxinos, 2008) were used as a reference in order to define with accuracy the different structures throughout the central auditory pathway.
A total of six animals per age group were assessed. Between 2 and 4 randomly chosen sections per region of interest, including the left and right hemispheres, were analyzed from each animal. Thus, from each animal at least four regions of interest were analyzed, resulting in a minimum of 24 regions of interest per age group. In accordance with previous studies of ERs distribution in the CNS (Mitra et al., 2003), an estimate of the distribution and relative density of immunoreactive cells (++++ very abundant; +++ abundant, ++ present, + few, − not present), as well as of the intensity of signal obtained (+, ++, +++, ++++ representing low, medium, high and intense immunoreactivity) was made in different levels of the brain. The assessments were not performed in a blind fashion. The intensity of staining was determined in a relative manner where the auditory region of interest was compared to that of brain regions where immunoreactivity of ERs is well established (thus characterized as very intense or ++++). The staining of the auditory regions was then approximated in relation to these established brain regions. In order to control for the influence of methodological variation in intensity we made similar comparisons to these established regions. These regions are: (a) the periaqueductal gray matter in the midbrain for ERα (only a few ERβ-positive neurons), (b) the Purkinje cells of the cerebellar cortex in the hindbrain for ERβ (negative for ERα), and (c) the medial amygdala in the forebrain for both ERα and ERβ. An Axioskop microscope (Carl Zeiss, Germany) was used; 20× and 40× objective lenses with a numerical aperture of 0.5 and 1.30 (with the use of oil) respectively were used to take the photographs.
Tissue collection and protein isolation for western blot
Mice were deeply anaesthetized with an i.p. injection of ketamine (50 mg/kg) (Ketalar) and xylazine (10 mg/kg) (Rompun). Cervical dislocation and decapitation were performed. IC, CN and ovaries were dissected and preserved at −80 °C. Tissues were placed in lysis buffer containing (in mM): 20Tris (pH 7), 1 EDTA, 1 EGTA, 150 NaCl, 2.5 Sodium Pyrophosphate, 1 Sodium Glycerophosphate, 1% v/v Triton, 1 Na3VO4, 1 DTT and complete protease inhibitor (Roche Diagnostics). The tissue was homogenized, left for 45 min on ice and centrifuged at 12,000 g for 7 min at 4 °C, and supernatant was collected as the whole cell extract. Protein concentration in lysates was assessed according to Bradford method, using BSA Protein Assay Kit (catalog no. 23227; Pierce Biotechnology). Lysates were frozen and stored at −80 °C until further processing.
Western blot
Whole cell protein fractions containing 20 µg total protein per sample were electrophoresed under reducing conditions in a 10% Bis-Tris Gel (Invitrogen). MagicMark protein standards (Invitrogen) were used to estimate the molecular weights of the proteins on the blots. Proteins were transferred to a PVDF membrane (IPFL 00010; Immobilon FL, Millipore) by semi-dry electroblotting. The membrane was washed (5× 5 min) in TBST (TBS, 20 mM Tris, 150 mM NaCl, pH 7.6, containing 0.1% Tween 20) and incubated for 1 h at RT in the blocking buffer [Odyssey Blocking Buffer (927–40,000; LI-COR Biosciences) diluted 1:1 in TBST]prior to overnight incubation with the primary antibodies at 4 °C. The primary antibodies used were polyclonal rabbit anti-ERα antibody (MC-20 sc-542; Santa Cruz Biotechnology; dilution 1/1000) or polyclonal rabbit anti-ERβ antibody (PA1-310B; Affinity Bioreagents; 1/1000). In the same time mouse monoclonal anti-GAPDH IgG (ab9484; Abcam) was used as a loading control. All primary antibodies were diluted in the blocking buffer. The membrane was washed (3× 10 min) in TBST, incubated for 1 h with a mixture of IRDye800-conjugated goat anti-rabbit IgG (926–32211; LI-COR Biosciences) and IRDye680-conjugated donkey anti-mouse IgG (926–32222; LI-COR Biosciences) diluted in the blocking buffer, then washed again (3× 10 min) in TBST and (2× 1 min) in TBS. The membrane was scanned on an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA) at 700 and 800 nm channels.
RESULTS
Lack of sex-related differences
No sex differences were found in the expression pattern of the two receptors neither in the prepubertal nor in the aged mice. Therefore, in reporting the results we do not distinguish between male and female mice.
Auditory physiology
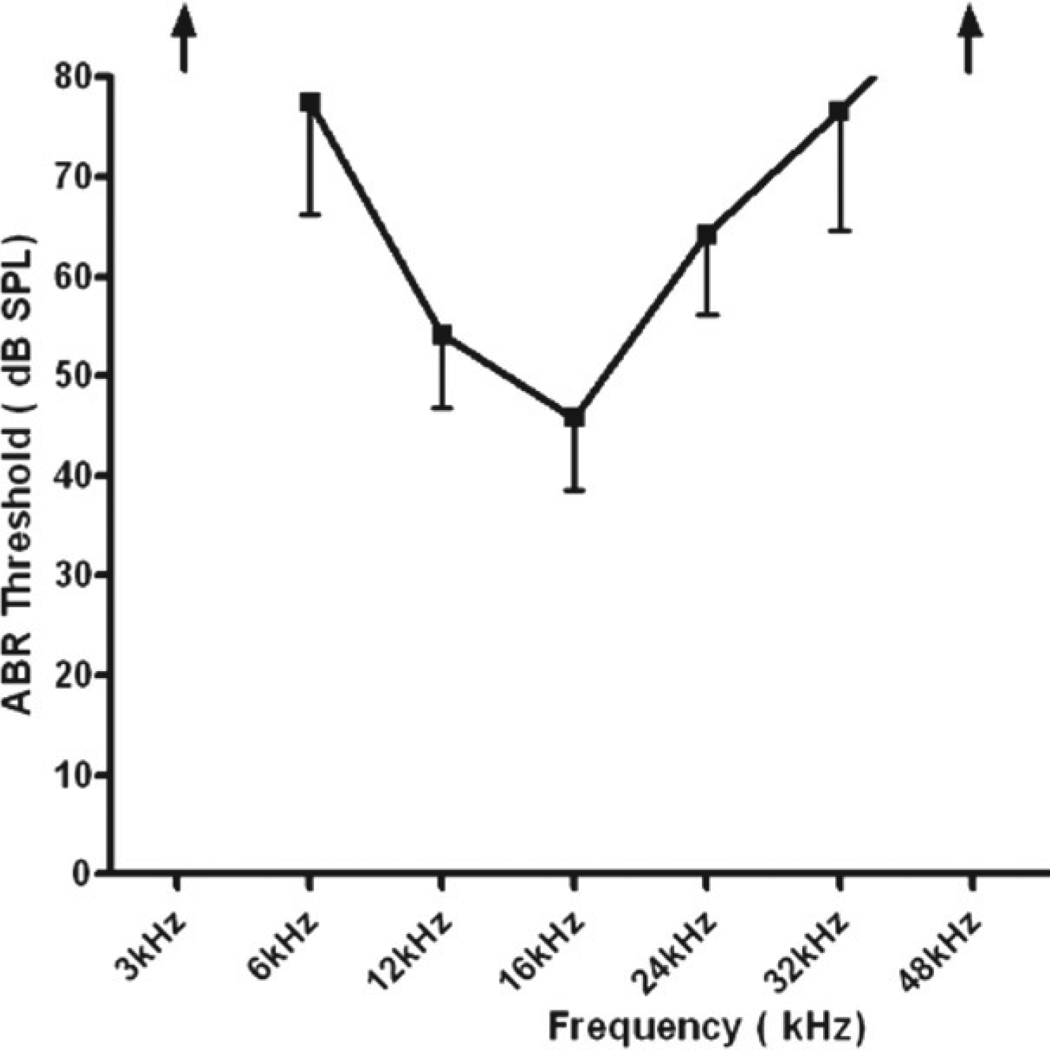
The ABR recordings confirmed that all of the aged mice had a moderate-to-severe, relatively flat hearing loss. Specifically, ABR thresholds were elevated by 25–50 dB relative to young adult CBA mice with normal hearing, with no significant differences between the males or females (Fig. 1). The hearing loss range was similar to that of previous studies of old CBA mice (Jacobson et al., 2003; Guimaraes et al., 2004).
Fig. 1.
Mean and standard deviation of ABR thresholds for the aged mice (3 females and 3 males, 26–28 mon old). Thresholds at 3 and 48 kHz were beyond the limits of the equipment. Thresholds between 6 and 32 kHz ranged from 45 to 74 dB SPL. The hearing losses varied from moderately-severe to profound with some individuals having greater hearing losses at both low and high frequencies.
Western blot analysis
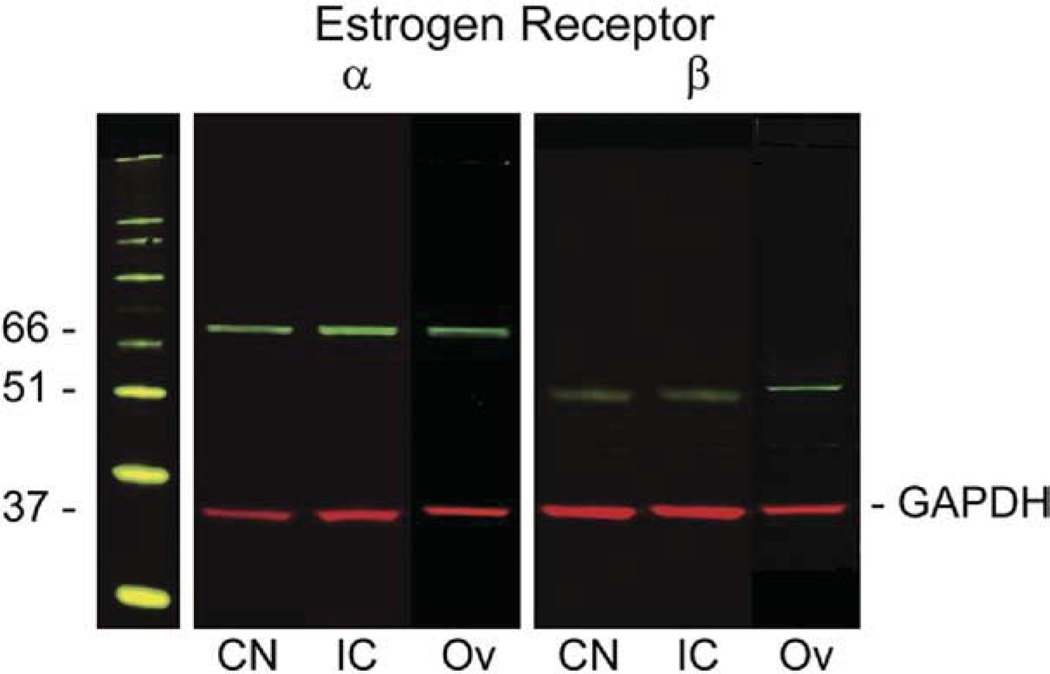
Immunoblotting experiments with the polyclonal antibodies against ERα and ERβ were used to control the specificity of the antibodies. Whole cell extracts from the mouse CN, inferior colliculus and ovary blotted with anti-ERα IgG revealed a band at approximately 66 kDa (Fig. 2). Anti-ERβ antiserum identified a band migrating at 51 kDa (Fig. 2). Expression of ERs in the CN and inferior colliculus was comparable to the expression levels in the ovary. Simultaneous incubation with anti-GAPDH IgG was used as a loading control and revealed a specific band at approximately 37 kDa.
Fig. 2.
Representative western blot showing the specificity of the antibodies detecting ERα and ERβ expression in whole cell extracts of mouse cochlear nucleus (CN), inferior colliculus (IC) and ovary (Ov). ERα was detected in CN (lane 1), IC (lane 2) and ovary (lane 3); ERβ was also found in CN (lane 4), IC (lane 5) and ovary (lane 6). Incubation of blots with either anti-ERα or anti-ERβ IgG detected the specific bands of 66 kDa in lanes 1–3 or 51 kDa in lanes 4–6 respectively. GAPDH (at 37 kDa in lanes 1–6) was used as a loading control. The molecular weight marker is seen on the left. 20µg of protein were loaded in each lane.
Distribution of ERα and ERβ immunoreactivity in the prepubertal mice
ERα
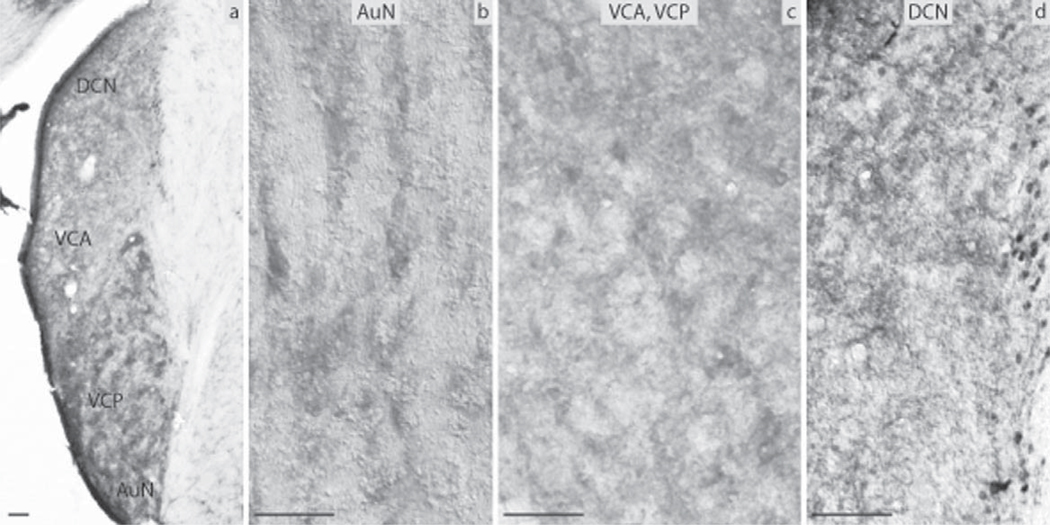
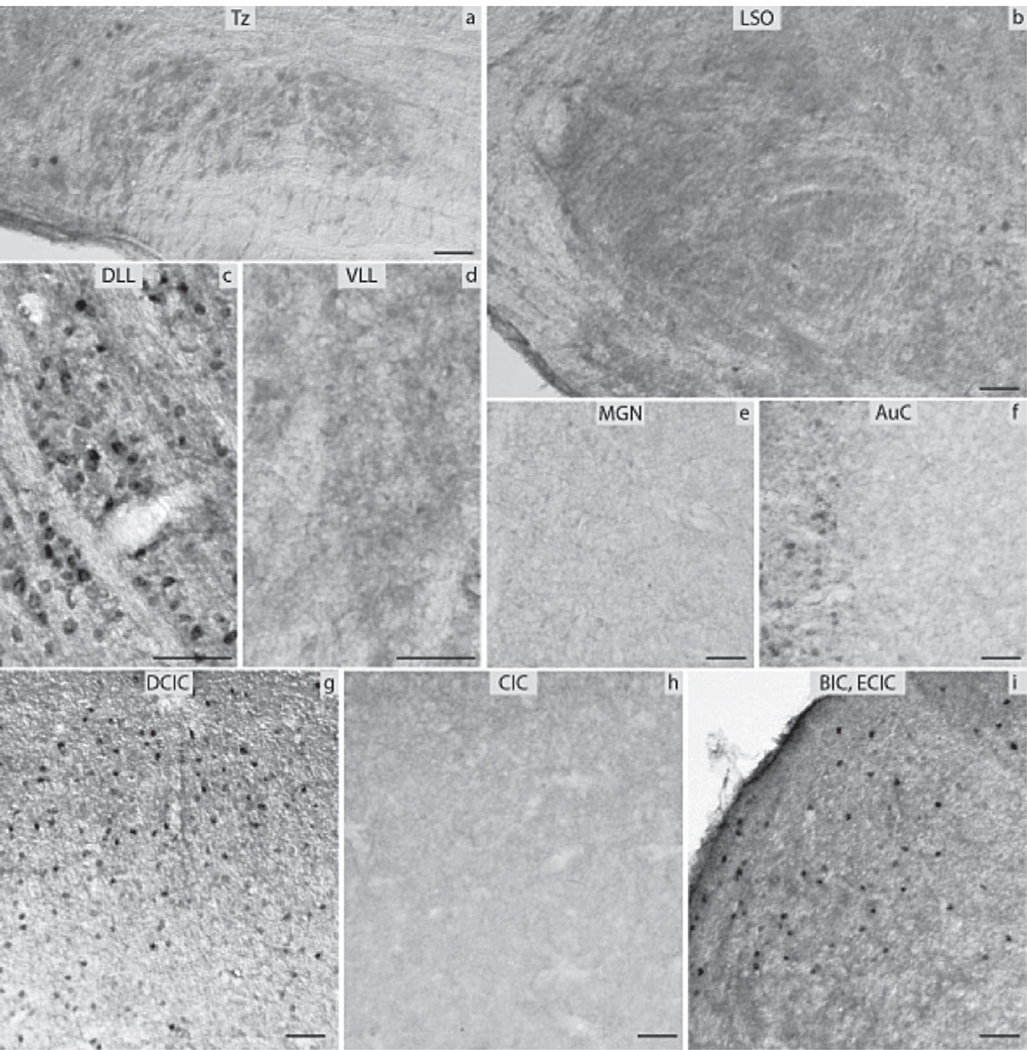
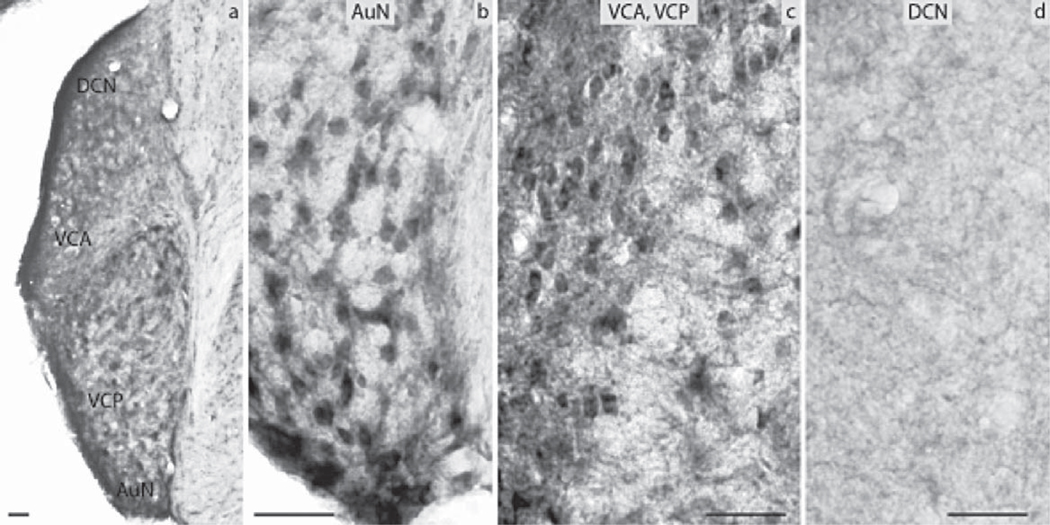
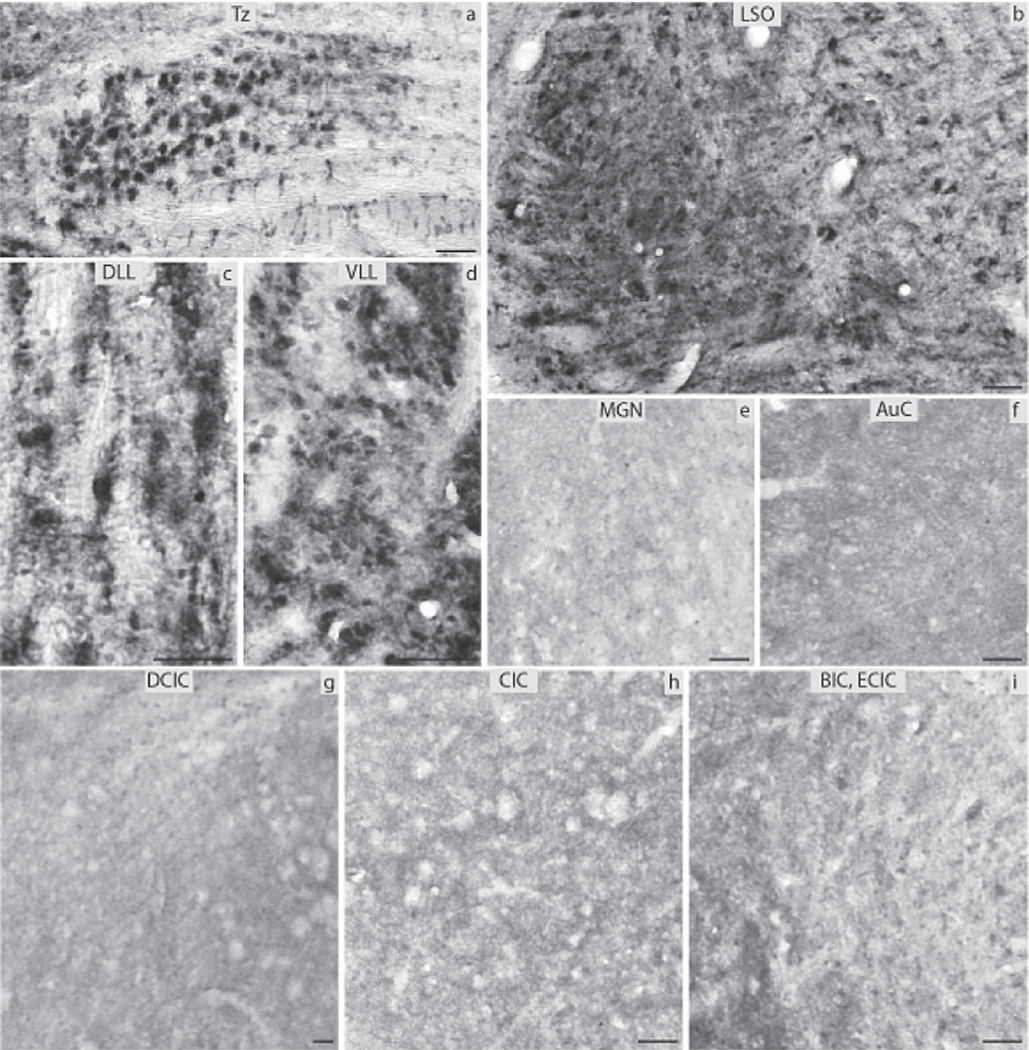
The subdivisions of the CN that were analyzed included the DCN, the VCP and the VCA as well as the AuN. The DCN was negative in its largest part, the granule cells though almost exclusively in the periphery of the nucleus showed moderate levels of immunoreactivity (Fig. 3d). The VCA and VCP were negative as was the AuN (Fig. 3b, c). All subnuclei of the SOC namely the Tz, LSO and MSO as well as the SPO, the LVPO and MVPO were all negative for ERα (Fig. 4a, b). The LL showed strong immunoreactivity in the DLL, in contrast to the VLL and the ILL nuclei that were negative (Fig. 4c, d). In the IC, the DCIC had a number of cells strongly positive (Fig. 4g). The CIC was negative (Fig. 4h). Immunoreactivity was obvious in the nuclei of the small neurons of the anterior parts of IC. The anterior part of the ECIC had a number of strongly positive cells forming a tract from the periaqueductal gray matter towards the BIC of the IC. The latter showed a number of cells with pronounced ERα immunoreactivity (Fig. 4i). All the ERα-immunopositive cells in the IC were mostly small neurons. The MGN was negative (Fig. 4e). Finally, the AuC had a few small neurons labeled in the deeper layers and some smaller cells in the more superficial layers (Fig. 4f).
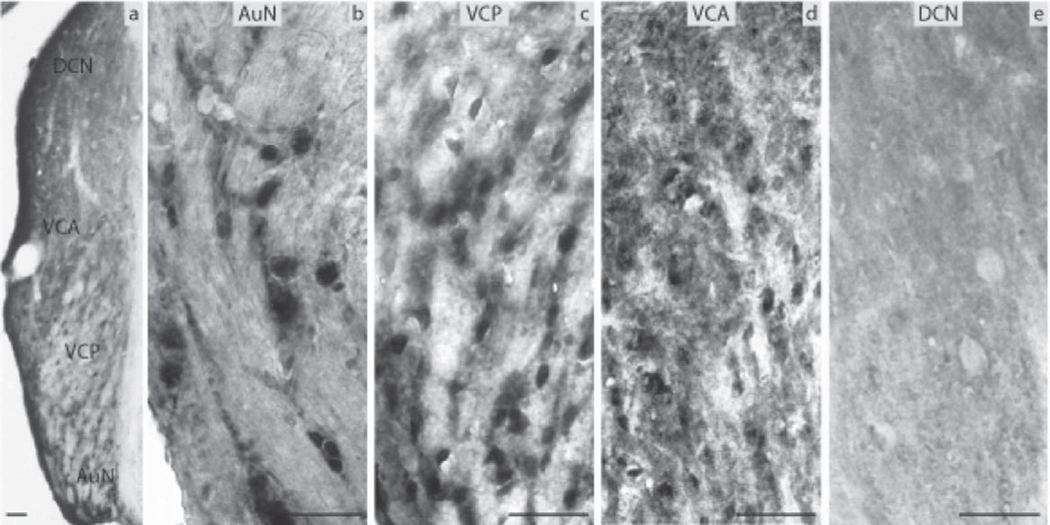
Fig. 3.
Estrogen receptor α (ERα) immunocytochemistry in prepubertal mice: (a) the auditory nerve nucleus (AuN) and the CN showing the dorsal cochlear nucleus (DCN), the anterior ventral cochlear nucleus (VCA), the posterior ventral cochlear nucleus (VCP). Higher magnification (20×) images showing the (b) AuN, (c) VCA and VCP, and (d) DCN with the granule cells in the periphery (scale bar=50 µm).
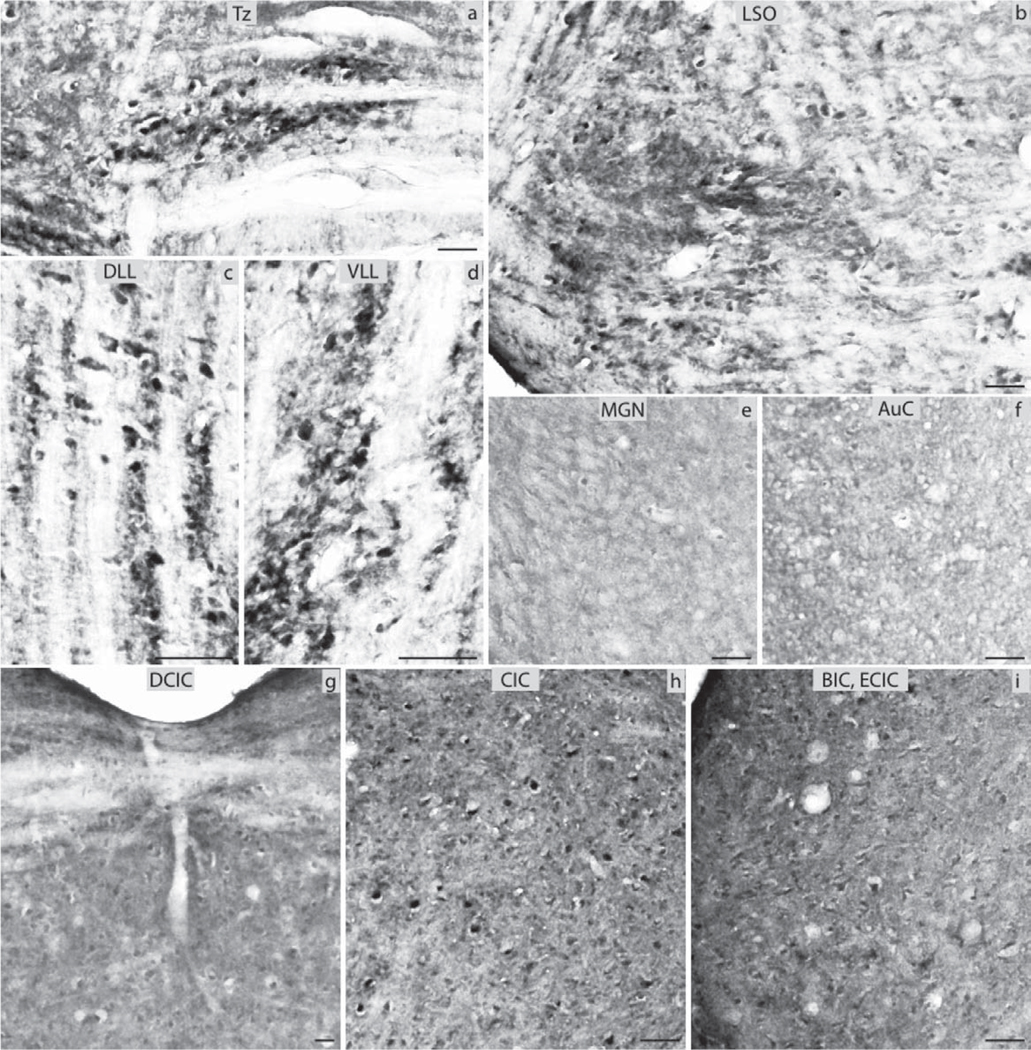
Fig. 4.
ERα immunocytochemistry throughout the central auditory pathway of prepubertal mice: (a) nucleus of the trapezoid body (Tz), (b) lateral superior olive (LSO), (c) dorsal nucleus of lateral lemniscus (DLL), (d) ventral nucleus of lateral lemniscus (VLL), (e) medial geniculate nucleus (MGN), (f) auditory cortex (AuC), (g) dorsal nucleus of inferior colliculus (DCIC), (h) central nucleus of inferior colliculus (CIC), (i) external nucleus of inferior colliculus (ECIC) and brachium of inferior colliculus (BIC) (scale bar=50 µm).
ERβ
A different pattern was found for ERβ in the CN. The DCN was negative without any immunoreactivity in the granule cells (Fig. 5d), whereas VCP and VCA were of medium and low immunoreactivity, respectively (Fig. 5c). The AuN was also positive (Fig. 5b). In the SOC the MNTB, LVPO, MVPO were all of medium immunoreactivity whereas the LSO, MSO and SPO were lightly stained (Fig. 6a, b). All three subdivisions of the lateral lemniscus (DLL, ILL, VLL) were lightly stained (Fig. 6c, d). All nuclei of the IC were negative (Fig. 6g–i) as well as the MGN (Fig. 6e) and the AuC (Fig. 6f).
Fig. 5.
Estrogen receptor β (ERβ) immunocytochemistry in prepubertal mice: (a) the auditory nerve nucleus (AuN) and the CN showing the dorsal cochlear nucleus (DCN), the anterior ventral cochlear nucleus (VCA), the posterior ventral cochlear nucleus (VCP). Higher magnification (20×) images showing the (b) AuN, (c) VCA and VCP, and (d) DCN (scale bar=50 µm).
Fig. 6.
ERβ immunocytochemistry throughout the central auditory pathway of prepubertal mice: (a) nucleus of the trapezoid body (Tz), (b) lateral superior olive (LSO), (c) dorsal nucleus of lateral lemniscus (DLL), (d) ventral nucleus of lateral lemniscus (VLL), (e) medial geniculate nucleus (MGN), (f) auditory cortex (AuC), (g) dorsal nucleus of inferior colliculus (DCIC), (h) central nucleus of inferior colliculus (CIC), (i) external nucleus of inferior colliculus (ECIC) and brachium of inferior colliculus (BIC) (scale bar=50 µm).
Distribution of ERα and ERβ immunoreactivity in the aged mice
ERα
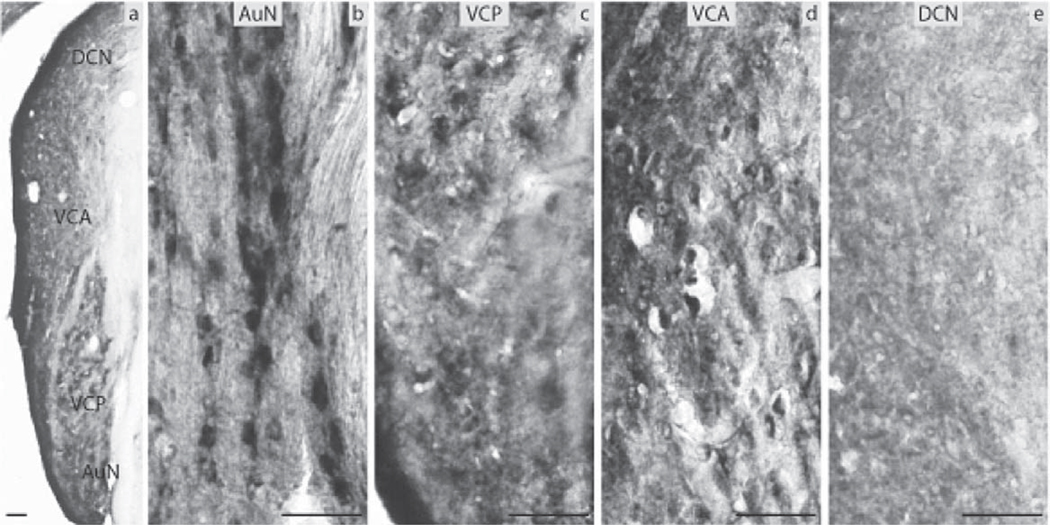
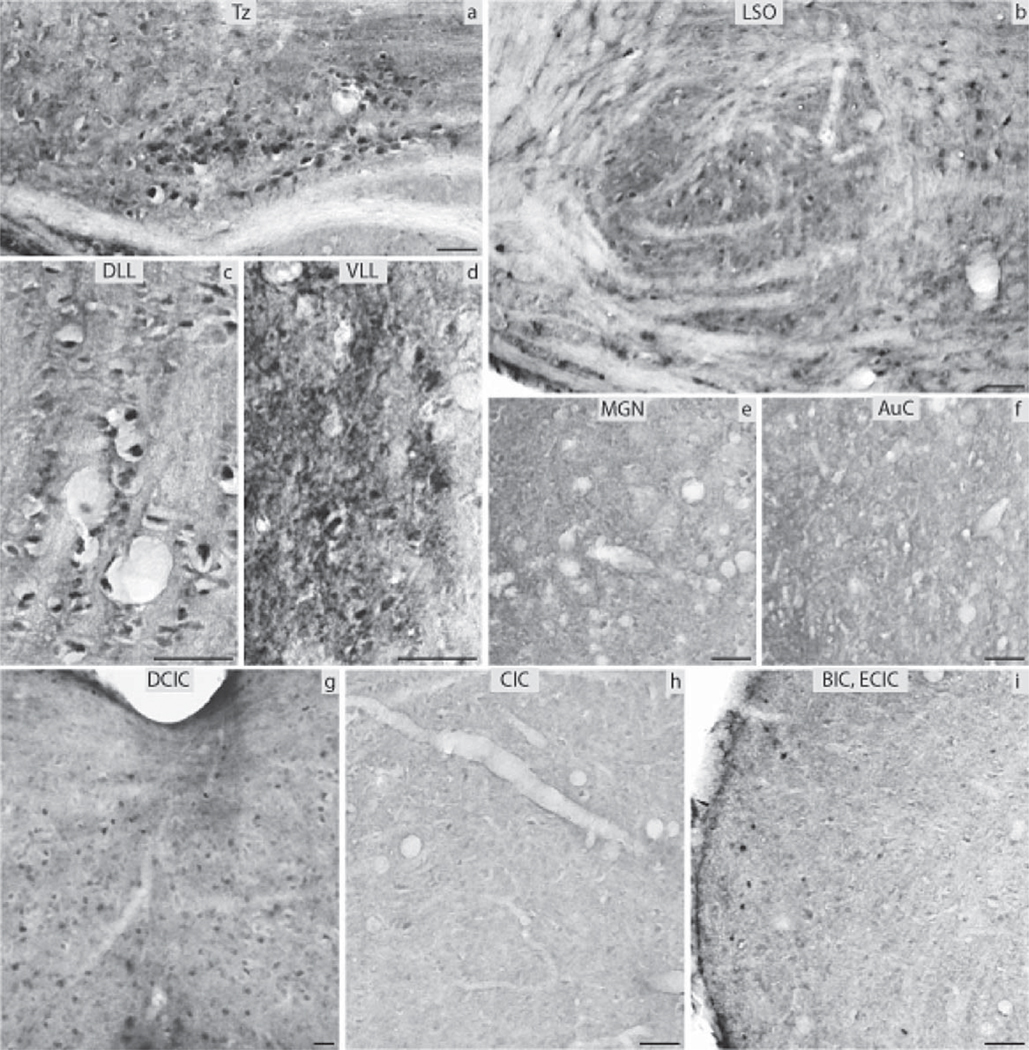
The DCN (Fig. 7e) and VCA (Fig. 7d) were negative for ERα in the aged group, and the VCP (Fig. 7c) showed immunoreactivity of low intensity. Medium intensity immunoreactivity was observed in the AuN adjacent to the VCN (Fig. 7b). The LSO and MSO were negative (Fig. 8b) while the Tz (Fig. 8a), SPO, MVPO and LVPO (Fig. 8b) had immunopositive neurons of medium intensity. The DLL was positive (Fig. 8c) whereas the ventral and inferior nuclei were negative (Fig. 8d). In the IC the CIC was negative (Fig. 8h) whereas some immunoreactivity was seen in the commissure of the DCIC (Fig. 8g). The ECIC and the BIC showed medium and strong immunoreactivity, respectively (Fig. 8i). The MGN and the AuC were negative (Fig. 8e, f). The variability of immunostaining of ERα regarding intensity and density among the aged animals was low.
Fig. 7.
ERα immunocytochemistry in aged mice: (a) the auditory nerve nucleus (AuN) and the CN showing the dorsal cochlear nucleus (DCN), the anterior ventral cochlear nucleus (VCA), the posterior ventral cochlear nucleus (VCP). Higher magnification (20×) images showing the (b) A, (c) VCP, (d) VCA, and (e) DCN (scale bar=50 µm).
Fig. 8.
ERα immunocytochemistry throughout the central auditory pathway of aged mice: (a) nucleus of the trapezoid body (Tz), (b) lateral superior olive (LSO), (c) dorsal nucleus of lateral lemniscus (DLL), (d) ventral nucleus of lateral lemniscus (VLL), (e) medial geniculate nucleus (MGN), (f) auditory cortex (AuC), (g) dorsal nucleus of inferior colliculus (DCIC), (h) central nucleus of inferior colliculus (CIC), (i) external nucleus of inferior colliculus (ECIC) and brachium of inferior colliculus (BIC) (scale bar=50 µm).
ERβ
In the aged male and female mice the DCN was negative for ERβ (Fig. 9e). Immunoreactivity of medium intensity was seen in the VCP and VCA (Fig. 9c, d). The AuN showed strong ERβ-immunoreactivity (Fig. 9b). All parts of the SOC were positive with the Tz and the periolivary nuclei showing slightly higher expression than the other regions (Fig. 10a, b). The DLL and VLL had some immunopositive cells of low intensity (Fig. 10c, d). Most divisions of the IC (CIC, ECIC and BIC) showed low intensity immunoreaction mainly in large neurons (Fig. 10g–i). The majority of these neurons were found in the CIC while the DCIC was negative. The MGN and the AuC were devoid of ERβ (Fig. 10e, f). The variability of immunostaining of ERβ regarding intensity and density among the aged animals was low.
Fig. 9.
ERβ immunocytochemistry in aged mice: (a) the auditory nerve nucleus (AuN) and the CN showing the dorsal cochlear nucleus (DCN), the anterior ventral cochlear nucleus (VCA), the posterior ventral cochlear nucleus (VCP). Higher magnification (20×) images showing the (b) AuN, (c) VCP, (d) VCA, and (e) DCN (scale bar=50 µm).
Fig. 10.
ERβ immunocytochemistry throughout the central auditory pathway of aged mice: (a) nucleus of the trapezoid body (Tz), (b) lateral superior olive (LSO), (c) dorsal nucleus of lateral lemniscus (DLL), (d) ventral nucleus of lateral lemniscus (VLL), (e) medial geniculate nucleus (MGN), (f) auditory cortex (AuC), (g) dorsal nucleus of inferior colliculus (DCIC), (h) central nucleus of inferior colliculus (CIC), (i) external nucleus of inferior colliculus (ECIC) and brachium of inferior colliculus (BIC) (scale bar=50 µm).
DISCUSSION
In the present study we document the distribution of estrogen receptors throughout the central auditory pathway in prepubertal and aged male and female mice. A variety of mapping studies using similar semi-quantitative methods as the present study have demonstrated that ERα and ERβ occupy anatomically distinct neuronal populations, with little overlap. In prepubertal mice a clear dichotomy was noted when comparing the immunocytochemical mapping of ERα and ERβ throughout the central auditory system. ERβ-positive neurons were found in the lower parts of the central auditory system whereas the majority of ERα was found in the higher parts. More specifically ERα-positive neurons were localized in the metencephalon while no staining was observed in the mesencephalon, diencephalon or the telencephalon. In contrast, ERα-positive neurons were found in the mesencephalon and the telencephalon. The only exception was that ERα immunoreactivity was found in the prepubertal DCN (metencephalon). Such a clear distinction in the expression of the two types of ERs between different levels of auditory processing in the brain has not been previously reported. However, despite the numerous mapping studies of estrogen receptors in the brain (Merchenthaler et al., 2004; Mitra et al., 2003; Vanderhorst et al., 2005) it must be noted that, to the best of our knowledge, mapping studies of estrogen receptors with focus on one CNS sensory or motor pathway have not previously been reported. Therefore it remains to be determined if other CNS pathways show clear distinctions in estrogen receptor labeling at different levels of the brain as shown here. The functional cause for this dichotomy could be related to this particular stage of maturation of the brain since this dichotomy in receptor mapping becomes less apparent with increasing age (compare Table 1 and Table 2). Moreover, after animals have entered puberty (6–9- weeks old) a distinct dichotomy in receptor mapping is not seen (Charitidi and Canlon, 2009). The granular cells of DCN show an interesting pattern of expression for ERα. ERα is clearly expressed in these cells only in the prepubertal animals whereas no expression is seen in the older animals. The functional role of ERα expression during this phase of maturation is not known. The granular cells of DCN have been suggested to play a role in the multimodal integration of auditory and nonauditory stimuli along the acoustic processing (Ryugo et al., 2003). Understanding the function of ERα expression during this phase of maturation would be of interest.
Table 1.
Distribution of cells in the prepubertal mouse central auditory system exhibiting either ERα or ERβ immunoreactivity
| Region | ERα | ERβ | ||
|---|---|---|---|---|
| Density | Intensity | Density | Intensity | |
| AuN | − | − | ++ | ++ |
| CN | ||||
| DCN | ++ | ++ | − | − |
| VCP | − | − | ++ | ++ |
| VCA | − | − | +++ | + |
| SOC | ||||
| LSO | − | − | + | + |
| MSO | − | − | + | + |
| SPO | − | − | + | + |
| Tz | − | − | +++ | ++ |
| LVPO | − | − | +++ | ++ |
| MVPO | − | − | +++ | ++ |
| LL | ||||
| DLL | +++ | ++++ | − | − |
| ILL | − | − | − | − |
| VLL | − | − | − | − |
| IC | ||||
| DCIC | ++ | ++++ | − | − |
| ECIC | ++ | ++++ | − | − |
| CIC | − | − | − | − |
| BIC | ++ | ++++ | − | − |
| MGN | − | − | − | − |
| AuC | + | + | − | − |
Distribution and relative density of immunoreactive cells: ++++ very abundant; +++ abundant; ++ present; + few; − not present. Intensity of label: ++++ very intense; +++ intense; ++ moderate; + weak; − not present.
Table 2.
Distribution of cells in the aged mouse central auditory system exhibiting either ERα or ERβ immunoreactivity
| Region | ERα | ERβ | ||
|---|---|---|---|---|
| Density | Intensity | Density | Intensity | |
| AuN | ++ | ++ | ++ | +++ |
| CN | ||||
| DCN | − | − | − | − |
| VCP | + | + | ++ | ++ |
| VCA | − | − | +++ | ++ |
| SOC | ||||
| LSO | − | − | ++ | + |
| MSO | − | − | ++ | + |
| SPO | ++ | + | ++ | + |
| Tz | ++ | + | +++ | ++ |
| LVPO | ++ | + | +++ | ++ |
| MVPO | ++ | + | +++ | ++ |
| LL | ||||
| DLL | +++ | ++ | ++ | + |
| ILL | − | − | − | − |
| VLL | − | − | + | + |
| IC | ||||
| DCIC | + | + | − | − |
| ECIC | ++ | ++ | + | + |
| CIC | − | − | + | + |
| BIC | ++ | +++ | + | + |
| MGN | − | − | − | − |
| AuC | − | − | − | − |
Distribution and relative density of immunoreactive cells: ++++ very abundant; +++ abundant; ++ present; + few; − not present. Intensity of label: ++++ very intense; +++ intense; ++ moderate; + weak; − not present.
In the aged animals a different pattern of estrogen receptor (ER) expression was found in comparison to the prepubertal mice in terms of location and overall intensity. In contrast, the aged animals demonstrated a similar pattern to the previously reported pattern of expression (Charitidi and Canlon, 2009) of the pubertal mice throughout the auditory pathway except for a reduced staining intensity in most of the regions. The areas showing the greatest reduction for ERα were the DCIC, ECIC and AuC; reduction of ERβ expression was not as distinct. The expression of ERα though was more widespread than ERβ, therefore changes are also expected to be more apparent for ERα. It is interesting to note that auditory brainstem thresholds in the prepubertal and pubertal mice are normal while they are significantly elevated in the aged male and female mice. It is well-known that auditory sensitivity in aged animals is progressively reduced and the overall reduction in the ER-immunopositive neurons could reflect the functional decline in hearing sensitivity. In some regions positively stained neurons were observed in aged animals when no staining was evident in the younger animals. This was particularly apparent in parts of the SOC and the VCA. One possible explanation for this upregulation of ERs in the aged animals could be a compensation for the loss of function in the aged neurons of these regions. Other areas with low expression levels CIC or with no expression for ERs (MGN) show no difference in their expression patterns between different age groups. Generally, the fact that there is no uniform change in the expression patterns of the two types of ERs with aging, shows that region-specific mechanisms regulate the age-related ER expression. Region-specific regulation of expression has been previously reported for aromatase (Ishunina et al., 2005) and androgen receptors (Feng et al., 2010). Further studies are needed to understand how the complexity of ER expression described here in the different age groups may be related to the maturation and plasticity of the central auditory system.
The present study compared prepubertal with aged mice in order to determine changes in ER expression with age in the auditory system. However it is difficult to distinguish whether these changes are due to maturation or aging when these two age groups are compared. Our previous study (Charitidi and Canlon, 2009) was performed in young sexually mature mice (6–9 weeks old) enabling us now to highlight the major differences that are due to maturation or aging. When ERα is compared between the prepubertal mice and the young sexually mature mice, significant changes are apparent in various regions (auditory cortex, parts of the SOC, VCP, DCN and the auditory nerve). In contrast, when comparing prepubertal to young adults for ERβ the immunoprofiles did not show significant changes due to maturation. When the young sexually mature mice are compared to the aged mice ERα shows a decrease in auditory cortex, BIC, ECIC, and DCIC, parts of the SOC and to some extent the auditory nerve. For ERβ the only major change that was found was an increased expression in the VCA. Thus, aging effects are more obvious for ERα than for ERβ, suggesting different functional roles that each receptor may play in the auditory system throughout aging.
Despite the fact that they were not quantified, the qualitative images of ERs in males and females were similar, suggesting that the distribution and expression levels of ERs in the central auditory system do not demonstrate sex dimorphisms. In order to explain this absence of sex differences in ER expression one should take into account the hormonal status of these animals. Both prepubertal and aged mice are not in their reproductive period and their gonads are not producing high amounts of sex steroid hormones. Estrogens and androgens circulate in low levels before puberty. In the same manner the gradual reduction of the gonads’ activity with progressive age results in low hormone levels in the aged animals. Thus, low levels of circulating hormones could account for minimizing possible sex differences in the expression patterns of ERs in these animals. However, in our previous report in which animals were in their puberty and therefore their estrogen levels were high, sex differences were also absent (Charitidi and Canlon, 2009). In a study demonstrating functional responses to acoustic trauma no sex-related differences were revealed in the auditory system of wild type mice or in mice lacking either ERα or ERβ (Meltser et al., 2008). Together, these findings suggest that processes other than circulating hormone levels contribute to the overall receptor expression. Local estrogen synthesis in the central auditory nuclei via aromatization could be one explanation. It has been shown that de novo synthesized estrogens in the brain play an important role in the imprinting and neural differentiation in both sexes, making the interpretation of estrogens action in the brain more complex (Bakker et al., 2006; Toran-Allerand, 1980; Gillies and McArthur, 2010). This does not mean that sex-specific events in different aspects of hearing are unaccountable. Sex differences could be sought during critical periods of development when the brain is under the influence of very high levels of hormones, especially androgens that push towards a “male” or a “female” brain. Permanent sex-dimorphic organization of the brain that takes place during development, results in sex-dimorphic cells which thereafter respond with a different sensitivity to gonadal hormones, and may or may not be related to ERs (Gillies and McArthur, 2010).
CONCLUSION
In conclusion, the purpose of this paper was to describe the expression of estrogen receptors in the main brain areas related to hearing in prepubertal and aged animals. The presence of estrogen receptors throughout the central auditory system in different periods of maturation suggests that estrogens directly affect the processing of hearing in both males and females by regulating the transcription of genes via their classical estrogen receptor pathway. Several studies show the neurotrophic and neuroprotective effect of estrogens throughout the CNS, including the auditory system (Meltser et al., 2008). Therefore it is of importance to understand the physiological actions and underlying mechanisms of estrogens in hearing in order to develop estrogen-based strategies to protect hearing.
Acknowledgments
Canlon and Charitidi were supported from the Swedish Research Council, Karolinska Institutet, Funds of Karolinska Institute, Tysta Skolan, KID funding. Frisina, Vasilyeva and Zhu were supported from NIH Grant P01 AG09524 from the National Institute on Aging, and NIH Grant P30 DC05409 from the National Institute on Deafness & Communication Disorders. The funding sources of this study were not involved in the study design, collection, analysis, or interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication. Special thanks to Agneta Viberg for technical assistance.
Abbreviations
- ABR
auditory brainstem response
- AuC
auditory cortex
- AuN
auditory nerve nucleus
- BIC
brachium of the IC
- CIC
central nucleus of the IC
- CN
cochlear nucleus
- DCIC
dorsal cortex of the IC
- DCN
dorsal cochlear nucleus
- DLL
dorsal nucleus of the lateral lemniscus
- ECIC
external cortex of the IC
- IC
inferior colliculus
- ILL
intermediate nucleus of the lateral lemniscus
- LL
lateral lemniscus
- LSO
lateral superior olive
- LVPO
lateroventral periolivary nucleus
- MGN
medial geniculate nucleus
- MSO
medial superior olive
- MVPO
medioventral periolivary nucleus
- PBS
phosphate buffer saline
- PFA
paraformaldehyde
- SOC
superior olivary complex
- SPL
sound pressure level
- SPO
superior paraolivary nucleus
- RT
room temperature
- Tz
nucleus of the trapezoid body
- VCA
ventral cochlear nucleus, anterior part
- VCN
ventral cochlear nucleus
- VCP
ventral cochlear nucleus, posterior part
- VLL
ventral nucleus of the lateral lemniscus
REFERENCES
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Agnello C, Garozzo A, Di Mari L, Serra A. Effects of patch or gel estrogen therapies on auditory brainstem response in surgically postmenopausal women: a prospective, randomized study. Fertil Steril. 2003a;79:556–561. doi: 10.1016/s0015-0282(02)04763-5. [DOI] [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Rugolo S, Intelisano G, Farina M, Cocuzza S, Serra A. Auditory brainstem response in premenopausal women taking oral contraceptives. Hum Reprod. 2003b;18:85–89. doi: 10.1093/humrep/deg003. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Canlon B. Estrogen receptors in the central auditory system of male and female mice. Neuroscience. 2009;165:923–933. doi: 10.1016/j.neuroscience.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Tahera Y, Canlon B. Functional responses of estrogen receptors in the male and female auditory system. Hear Res. 2009;252:71–78. doi: 10.1016/j.heares.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80:209–215. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Feng Y, Weijdegard B, Wang T, Egecioglu E, Fernandez-Rodriguez J, Huhtaniemi I, Stener-Victorin E, Billig H, Shao R. Spatiotemporal expression of androgen receptors in the female rat brain during the oestrous cycle and the impact of exogenous androgen administration: a comparison with gonadally intact males. Mol Cell Endocrinol. 2010;321:161–174. doi: 10.1016/j.mce.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 2008. [Google Scholar]
- Gillies GE, McArthur S. Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson’s disease: a contribution to sex-specific neuroprotection by estrogens. Horm Behav. 2010;57:23–34. doi: 10.1016/j.yhbeh.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Haggard M, Gaston JB. Changes in auditory perception in the menstrual cycle. Br J Audiol. 1978;12:105–118. doi: 10.3109/03005367809078862. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, van Beurden D, van der Meulen G, Unmehopa UA, Hol EM, Huitinga I, Swaab DF. Diminished aromatase immunoreactivity in the hypothalamus, but not in the basal forebrain nuclei in Alzheimer’s disease. Neurobiol Aging. 2005;26:173–194. doi: 10.1016/j.neurobiolaging.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Jacobson M, Kim S, Romney J, Zhu X, Frisina RD. Contralateral suppression of distortion-product otoacoustic emissions declines with age: a comparison of findings in CBA mice with human listeners. Laryngoscope. 2003;113:1707–1713. doi: 10.1097/00005537-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Jerger J, Johnson K. Interactions of age, gender, and sensorineural hearing loss on ABR latency. Ear Hear. 1988;9:168–176. doi: 10.1097/00003446-198808000-00002. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullar JS, Oesterle EC. Cellular targets of estrogen signaling in regeneration of inner ear sensory epithelia. Hear Res. 2009;252:61–70. doi: 10.1016/j.heares.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morse GG, House JW. Changes in Meniere’s disease responses as a function of the menstrual cycle. Nurs Res. 2001;50:286–292. doi: 10.1097/00006199-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biol Reprod. 1990;42:649–655. doi: 10.1095/biolreprod42.4.649. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Haenggeli CA, Doucet JR. Multimodal inputs to the granule cell domain of the cochlear nucleus. Exp Brain Res. 2003;153:477–485. doi: 10.1007/s00221-003-1605-3. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Thompson SK, Zhu X, Frisina RD. Estrogen blockade reduces auditory feedback in CBA mice. Otolaryngol Head Neck Surg. 2006;135:100–105. doi: 10.1016/j.otohns.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro. II. Morphological correlates and hormonal specificity. Brain Res. 1980;189:413–427. doi: 10.1016/0006-8993(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zutphen LFM, Baumans V, Beynen AC. Principles of laboratory animal science. Amsterdam: Elsevier; 2001. [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Wharton JA, Church GT. Influence of menopause on the auditory brainstem response. Audiology. 1990;29:196–201. doi: 10.3109/00206099009072850. [DOI] [PubMed] [Google Scholar]
- Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J Comp Neurol. 2009;512:688–701. doi: 10.1002/cne.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettel ML, Zhu X, O’Neill WE, Frisina RD. Age-related decline in Kv3.1b expression in the mouse auditory brainstem correlates with functional deficits in the medial olivocochlear efferent system. J Assoc Res Otolaryngol. 2007;8:280–293. doi: 10.1007/s10162-007-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Vasilyeva ON, Kim S, Jacobson M, Romney J, Waterman MS, Tuttle D, Frisina RD. Auditory efferent feedback system deficits precede age-related hearing loss: contralateral suppression of otoacoustic emissions in mice. J Comp Neurol. 2007;503:593–604. doi: 10.1002/cne.21402. [DOI] [PubMed] [Google Scholar]