Abstract
Summary
A meta-analysis of studies was conducted involving 24,511 participants with 7,864 fractures in which polymorphisms in the 5′ flank of COL1A1 (rs1107946, rs2412298, and rs1800012) were related to osteoporosis phenotypes. Polymorphisms of all three sites were associated with BMD, and rs1800012 was associated with fracture but effect sizes were modest.
Introduction and hypothesis
Polymorphisms in the 5′ flank of COL1A1 gene have been implicated as genetic markers for susceptibility to osteoporosis, but previous studies have yielded conflicting results.
Methods
We conducted a meta-analysis of 32 studies including 24,511 participants and 7,864 fractures in which alleles at the -1997G/T (rs1107946), -1663in/delT (rs2412298), and Sp1 binding site polymorphisms (rs1800012) of COL1A1 had been related to bone mineral density (BMD) or fracture.
Results
For the Sp1 polymorphism, BMD values in TT homozygotes were 0.13 units [95% CI, 0.03 to 0.24] lower at the spine (p=0.01) and 0.16 units [0.10 to 0.23] lower at the hip (p = 1 × 10−6) than GG homozygotes. Clinical fractures were 1.31-fold [1.04–1.65] increased in TT homozygotes (p=0.02) and vertebral fractures were 1.34-fold [1.01–1.77] increased (p=0.04). We also observed associations between spine BMD and allelic variants at the -1997G/T (p=0.05) and the -1663indelT (p=0.009) sites. We found no association between alleles at the -1997G/T or -1663indelT sites and fracture but power was limited.
Conclusions
The COL1A1 Sp1 polymorphism is associated with a modest reduction in BMD and an increased risk of fracture, although we cannot fully exclude the possibility that the results may have been influenced by publication bias. Further studies are required to fully evaluate the contribution of the -1997G/T and -1663in/delT sites to these phenotypes and to determine if they interact with the Sp1 polymorphism to regulate susceptibility to osteoporosis.
Keywords: BMD, COL1A1, Fracture, Genetic, Meta-analysis, Osteoporosis, Polymorphism
Introduction
Osteoporosis is a common disease characterized by low bone mass, micro-architectural deterioration of bone tissue and enhanced bone fragility which leads to an increased incidence of fracture. It is now well established that genetic factors play a major role in regulating bone mineral density (BMD) [1] other determinants of fracture risk [2] and fracture itself [3]. Recently, genome-wide association studies (GWAS) have been successful in identifying several common variants that are significantly associated with BMD and with fracture risk [4–7]. Candidate gene association studies have also been used to identify genetic variants that are associated with BMD and fractures [8–12]. An important limitation of many candidate gene studies has been the fact that the samples sizes were limited. This has resulted in the publication of many false negative results due to lack of statistical power and also many false positive results which could not been replicated in subsequent studies [13, 14]. Candidate gene studies can sometimes yield useful information however, since the GWAS techniques currently available do not capture all variants within candidate genes, especially rare variants [15]. In this regard, polymorphisms of the COL1A1 gene which have previously been associated with osteoporosis in several studies [8, 10, 16] are poorly captured by the single-nucleotide polymorphism (SNP) used in the recent GWAS studies of osteoporosis [13].
Over recent years, meta-analysis has been employed to validate associations between genetic variants and phenotype in complex diseases such as osteoporosis, and has been applied to candidate gene studies [16–18], linkage studies [19], and GWAS studies [4]. Five meta-analyses of the COL1A1 gene have been conducted so far in relation to BMD or fracture but all were limited to studies of the Sp1 binding site polymorphism within intron 1 of COL1A1 (rs1800012) [8, 16, 20–22]. Since these studies were published, two further polymorphisms have been identified in the 5′ flank of the COL1A1 gene at positions -1997G/T (rs1107946) and -1663ins/delT (rs2412298) which have been associated with BMD [23]. Moreover, several further studies of the Sp1 polymorphism have been published that were not included in previous meta-analyses [24–32]. In view of this, the aim of the present meta-analysis was to re-evaluate the effect of the Sp1 binding site polymorphism in relation to BMD and osteoporotic fractures and for the first time to conduct a meta-analysis of the -1997G/T and -1663in/delT polymorphism in relation to BMD and fracture.
Methods
Retrieval of studies and data extraction
Association studies in which any of the three COL1A1 polymorphisms have been studied in relation to BMD and/or fractures were identified by electronic searches of MEDLINE between 1996 and 2009, using several search terms including “collagen”, “COL1A1”, “polymorphism”, “genetics”, “BMD”, “fracture”, “Sp1” (rs1800012), “-1997G/T” (rs1107946), and “-1663 ins/delT” (rs2412298). The studies were included in the meta-analysis provided that the study outcomes included BMD or fracture and complete genotype data were provided either in the paper or by communication with the corresponding author, when data or important details thereof were not available in the published paper (N=4). We recorded the unadjusted mean BMD and standard deviation for each genotype at lumbar spine and femoral neck, and the number of individuals in each genotype group with or without fracture in each study.
Statistical analysis
Data were analyzed using Review Manager (version 5) and Stata 10. Both random and fixed effects were considered for the analysis. In fixed effect models it is assumed that the true effect of the genetic risk is the same in each study. The random effects model incorporates the between-study heterogeneity and allows the risk allele effects for each study to vary around some overall average effect [33]. In the absence of heterogeneity, the fixed and the random effects coincide. Between-study heterogeneity was assessed by the Q statistic (Cochrane’s Q) which is considered significant for p<0.1. The heterogeneity was quantified by the I2 metric and its 95% confidence intervals were calculated [34]. Values for I2 can range from 0% to 100% and it is usually considered small, moderate large, and very large for values of 1–24%, 25–49%, 50–74%, and >75%, respectively [30]. For the BMD analysis, we calculated the standardized mean difference in BMD values in different genotype groups (equivalent to BMD Z-score values) based on the actual BMD values reported in the different studies. For the analysis of fractures, we computed the natural logarithms of the odds ratios from individual studies in order to compute the summary effect sizes. In order to identify any potential small-study effect (whether small studies yield more spectacular results), we performed the Egger test for BMD [35] and Harbord test for fractures [36]. Moreover, we applied the Ioannidis–Trikalinos test [37] to examine whether there was an excess of single studies with nominally significant results. All of these tests probe the possibility of biases in the accumulated evidence.
Thresholds for statistical significance
Throughout the manuscript, we present unadjusted p values and odds ratios, but since we performed multiple analyses of related phenotypes and SNP we estimated the adjusted significance threshold based on the following calculations. Since the three SNP studied are in strong linkage disequilibrium with each other (D′ value 0.90 [24]), we estimated that this constituted 1.1 independent tests. Similarly, we analyzed BMD at two sites (hip and spine), which are known to be correlated (r=0.6 [24]) which equates to 1.6 independent tests. Combining these data with the analysis of fracture amounts to 3.76 independent tests giving an adjusted threshold for significance of p=0.013.
Results
Studies included in the meta-analysis
Sixty studies were identified in the initial search. We excluded studies which simply recorded the prevalence of COL1A1 alleles in different populations (n=1); studies of children (age<15-years old, n=5); studies on diseases other than osteoporosis (n=1); and studies where there was no information on either BMD or fractures (n=16). We also identified studies in which subsets of populations had been published upon more than one time (n=5) and selected only one study for analysis so that duplicate samples were not included. Following these exclusions, 32 eligible studies with a total of 25,411 subjects were selected for the analysis as summarized in Table 1. The minor allele frequencies were similar in the control subjects from the different populations. For the Sp1 polymorphism the frequency of the T allele (mean±SD) was 0.19±0.05 with a range of 0.07 in the study of Selezneva et al. [31] to 0.32 in the study of Braga et al. [38]. For the -1663 indelT polymorphism, corresponding values were 0.20±0.01 with a range of 0.20 in the study of Husted et al. [29] to 0.22 in the study of Bustamante et al. [27]. For the -1997G/T polymorphism, values were 0.20±0.01 with a range of 0.13 in the study of Bustamante et al. [27] to 0.38 in the study of Yamada et al. [25]. Of all the studies listed, prospective genotyping was performed so far as we are aware, only for the GENOMOS study [16].
Table 1.
Studies included in the meta-analysis
| Study | Location | Mean age (range) | Study design (n) | Gender/number | Fracture type | SNP studied |
|---|---|---|---|---|---|---|
| Aerssens [42] | Belgium | 76.0 (69–87) | Case-control, (135/239) | F/374 | hip | Sp1 |
| Alvarez-Hernandez [47] | Spain | 64.0 (50–86) | Population-based | M/156 | vert, osteoporotic | Sp1 |
| Ashford [48] | UK | 79.9 (>75) | Population-based | F/314 | vert | Sp1 |
| Bernad [49] | Spain | 59.5 (51–67) | Clinic referrals | F/319 | vert, wrist | Sp1 |
| Braga [38] | Italy | 63.5 (63–64) | Clinic referrals | F/715 | any | Sp1 |
| Braga [50] | Italy | 58.4 (50–60) | Population-based | M/253 | – | Sp1 |
| Bustamante [27] | Spain | 54.7 (46–63) | Population-based | F/719 | – | Sp1, -1997, -1663 |
| Efstathiadou [51] | Greece | 54.2 (47–61) | Clinic referrals | F/154 | – | Sp1 |
| Garnero [52] | France | 38.9 (34–46) | Population-based | F/220 | – | Sp1 |
| Gerhem [28] | Sweden | 75.2 (75) | Population-based | F/964 | Any fracture | Sp1 |
| Grant [53] | UK | 61.3 (50–70) | Case-control | F/299 | vert | Sp1 |
| Harris [54] | USA | 70.2 (>65) | Intervention | M/108, F/135 | – | Sp1 |
| Heegaard [55] | Demark | 50.9 (45–54) | Population-based | F/133 | vert | Sp1 |
| Husted [29] | Demark | 60.1 (45–73) | Case-control, (291/283) | M/120, F/454 | vert | Sp1, -1997, -1663 |
| Hustmyer [56] | USA | 33.9 (21–49) | Case-control, (56/78) | M/4, F/130 | vert | Sp1 |
| Keen [57] | UK | 54.3 (45–64) | Case-control, (55/130) | F/185 | osteoporotic | Sp1 |
| Liden [58] | Sweden | 67.7 (58–77) | Case-control, (64/72) | F/136 | vert | Sp1 |
| McGuigan [59] | UK | 70.8 (69–75) | Case-control, (93/88) | F/181 | vert | Sp1 |
| McGuigan [60] | UK | 64.2 (56–77) | Clinic referrals | M/156, F/185 | – | Sp1 |
| Mezquita-Raya [61] | Spain | 60.9 (53–70) | Case-control, (43/101) | F/144 | vert | Sp1 |
| Nguyen [30] | Australia | 70.0 (63–77) | Population-based | F/677 | hip | Sp1 |
| Peris [62] | Spain | 48.3 (31–71) | Case-control, (35/60) | M/95 | – | Sp1 |
| Roux [63] | France | 63.1 (45–90) | Case-control, (110/107) | F/217 | – | Sp1 |
| Ralston [16] | GENOMOS | 63.7 (20–80) | multi-center | M/4,776, F/7,759 | any, vert, non-vert | Sp1 |
| Selezneva [31] | Russia | N/A (50–70) | Clinic referrals | F/124 | osteoporotic | Sp1, -1997, -1663 |
| Sowers [64] | USA | 37.3 (28–48) | Population-based | F/259 | – | Sp1 |
| Stewart [24] | UK | 54.8 (45–54) | Population-based | F/3,270 | – | Sp1, -1997, -1663 |
| Valimaki [43] | Finland | 89.0 (85–98) | Population-based | F/601 | hip | Sp1 |
| van Pottelbergh [65] | Belgium | 75.0 (71–86) | Population-based | M/352 | – | Sp1 |
| Weichetova [32] | Czech Republic | 60.5 (45–70) | Case-control, (183/178) | F/361 | wrist | Sp1 |
| Yamada [25] | Japan | 59.2 (45–65) | Population-based | M/1,126, F/1,100 | – | Sp1, -1997 |
| Yazdanpanah [26] | Netherlands | 68.0 (60–74) | Population-based | M/2,452, F/3,374 | vert, non-vert | Sp1, -1997 |
Details of the studies included are shown
Values for age are mean (range) in years
Number of cases and controls are indicated in the study design column
Vert vertebral fracture, non-vert non vertebral fracture, M male, F female
Association between Sp1 polymorphism and BMD
For the Sp1 polymorphism (rs1800012), 26 studies were identified with a total of 24,511 participants (6,584 men and 17,927 women) for which hip BMD had been measured and 23,757 participants (5,843 men and 17,914 women) for which spine BMD has been measured. Random and fixed effect model estimates from analyses including all subjects are presented in Table 2. At the lumbar spine, individuals with the TT genotype had BMD values 0.13 units [95% CI, 0.03 to 0.24] lower than GG homozygotes under a random effects model (p = 0.01). At the femoral neck TT homozygotes had BMD values 0.16 units [95% CI, 0.10 to 0.23] lower than GG homozygotes under a random effects model (p = 1 × 10−6). Nominally, significant results were also obtained under a recessive model (TT vs. GG+GT) at lumbar spine (p=0.03) and femoral neck (p = 3 × 10−6).
Table 2.
Meta-analysis of Sp1 polymorphism in relation to spine and hip BMD in the whole study population
| Studies/N | Comparison | Fixed effect model
|
Random effects model
|
|||
|---|---|---|---|---|---|---|
| Lumbar spine BMD | P | Lumbar spine BMD | P | I2 (95% CI) | ||
| 26/22,764 | GG vs. GT | 0.03 (0, 0.06) | 0.04 | 0.03 (0, 0.07) | 0.05 | 19 (0–44) |
| 26/16,446 | GG vs. TT | 0.10 (0.04, 0.17) | 0.002 | 0.13 (0.03, 0.24) | 0.01 | 50 (30–65) |
| 26/23,757 | GG vs. GT+TT | 0.04 (0.01, 0.06) | 0.006 | 0.04 (0.01, 0.08) | 0.01 | 22 (0–47) |
| 26/23,757 | GG+GT vs. TT | 0.08 (0.02, 0.14) | 0.01 | 0.08 (0.01, 0.16) | 0.03 | 16 (0–42) |
| Femoral neck BMD | Femoral neck BMD | |||||
| 26/23,522 | GG vs. GT | 0.01 (−0.01, 0.04) | 0.30 | 0.01 (−0.02, 0.05) | 0.42 | 23 (0–47) |
| 26/17,048 | GG vs. TT | 0.16 (0.10, 0.23) | 1×10−6 | 0.16 (0.10, 0.23) | 1×10−6 | 0 (0–35) |
| 26/24,511 | GG vs. GT+TT | 0.03 (0, 0.06) | 0.02 | 0.03 (0, 0.07) | 0.09 | 30 (0–51) |
| 26/24,511 | GG+GT vs. TT | 0.15 (0.09, 0.22) | 3×10−6 | 0.15 (0.09, 0.22) | 3×10−6 | 0 (0–35) |
The number of eligible studies and total number of participants is indicated for each comparison
Values are standardized mean difference (95% CI)
Positive values denote higher BMD values in the first listed genotype group of the comparison
The p values shown have not been corrected for multiple testing
I2 Heterogeneity
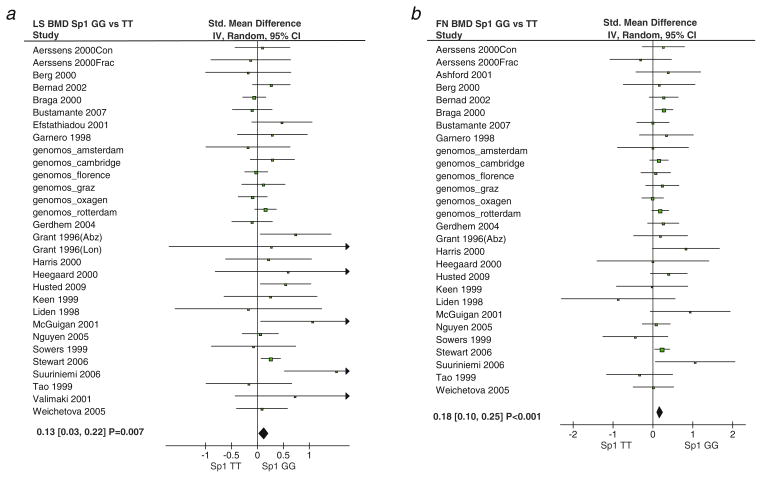
Gender specific analysis showed that females who were homozygous for the T/T genotype had lumbar spine BMD values 0.13 units [95% CI, 0.03 to 0.22] lower than G/G homozygotes (p=0.007). Similarly, BMD values at the femoral neck in TT homozygotes were 0.18 units [95% CI, 0.10 to 0.25] lower than GG homozygotes under a random effects model (p<0.001; Fig. 1). There were no significant differences in BMD values for males at the lumbar spine or femoral neck (not shown). All of the effect sizes were very similar in the fixed effects model, because the between-study heterogeneity was modest with I2 values ranging from 16% to 50% for lumbar spine BMD and 0% to 30% for femoral neck BMD (Table 2).
Fig. 1.
Meta-analysis of Sp1 polymorphism and association with BMD in females. Panel a Sp1 GG homozygotes versus TT homozygotes for lumbar spine BMD. Panel b Sp1 GG homozygotes versus TT homozygotes for femoral neck BMD. Each study is shown as the point estimate of the standardized mean difference with 95% confidence intervals as analyzed using a random effects model. The diamond shows the overall effect. Where the diamond lies to the right of the vertical line this indicates a higher BMD value in the GG genotype compared with the TT genotype group. The p values shown have not been corrected for multiple testing
Association between Sp1 polymorphism and fractures
Fracture data was analyzed in 20 studies including 13,870 females and 5,056 males. Within these studies, there were 7,864 clinical fractures and 2,531 vertebral fractures. The results are summarized in Table 3. The GG genotype was associated with reduced risk for all fractures under a random effects model with an odds ratio of 0.89 [95% CI, 0.82 to 0.97], (p=0.01), whereas TT homozygotes had an increased risk for all fractures: 1.31 [95% CI, 1.04 to 1.65] (p=0.02) and vertebral fracture: 1.34 [95% CI, 1.01 to 1.77] (p=0.04). The I2 estimates ranged from 43% to 53% for all fractures and 19% to 62% for vertebral fractures (Table 3).
Table 3.
Meta-analysis of the Sp1 polymorphism in relation to fracture in the whole study population
| Studies/N | Comparison | Fixed effect model
|
Random effects model
|
|||
|---|---|---|---|---|---|---|
| All fractures | P | All fractures | P | I2(95% CI) | ||
| 20/28,352 | Sp1 GG vs. GT+TT | 0.94 (0.89, 0.99) | 0.03 | 0.89 (0.82, 0.97) | 0.01 | 43 (13–62) |
| 17/24,218 | Sp1 GT vs. GG+TT | 1.05 (0.99, 1.12) | 0.13 | 1.10 (0.99, 1.22) | 0.08 | 53 (30–69) |
| 17/27,444 | Sp1 TT vs. GG+GT | 1.20 (1.04, 1.39) | 0.01 | 1.31 (1.04, 1.65) | 0.02 | 51 (27–67) |
| Vertebral fracture | Vertebral fracture | |||||
| 10/17,029 | Sp1 GG vs. GT+TT | 0.94 (0.86, 1.04) | 0.23 | 0.93 (0.79, 1.09) | 0.38 | 58 (34–74) |
| 8/16,646 | Sp1 GT vs. GG+TT | 1.04 (0.94, 1.15) | 0.46 | 1.08 (0.90, 1.29) | 0.42 | 62 (39–76) |
| 8/16,646 | Sp1 TT vs. GG+GT | 1.38 (1.10, 1.74) | 0.006 | 1.34 (1.01, 1.77) | 0.04 | 19 (0–52) |
The number of eligible studies and total number of participants is indicated for each comparison
Values are odds ratio (95% CI)
Values greater than 1.0 denote an increased risk of fracture for the first listed genotype group of the comparison compared with the other groups
The p values shown have not been corrected for multiple testing
I2 Heterogeneity
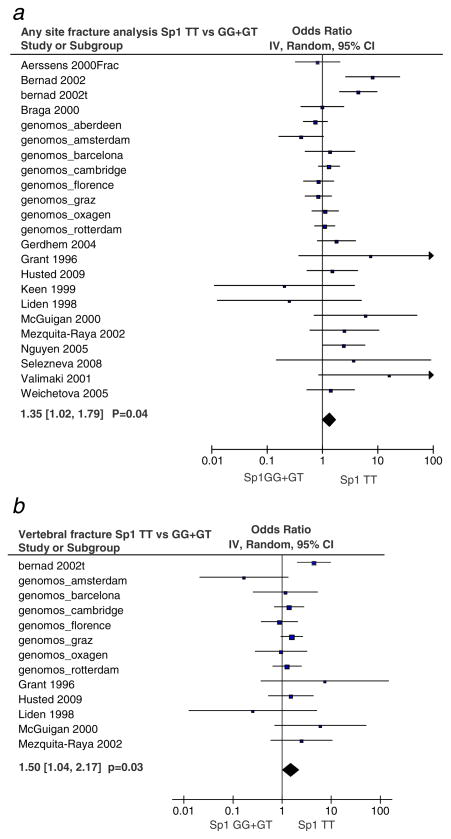
Gender specific analysis revealed that female TT homozygotes had a 1.35-fold [95% CI, 1.02 to 1.79] increased risk for clinical fractures at any site compared with the GG and GT genotypes (p=0.04) (Fig. 2a). For vertebral fracture, female TT homozygotes had a 1.50-fold [95% CI, 1.04 to 2.17] (p=0.03) increased risk compared with GT and GG genotypes (Fig. 2b). No association with fracture was observed for males (not shown).
Fig. 2.
Meta-analysis for Sp1 polymorphism and association with fracture in females. Odds ratio (OR) for fracture is reported with 95% confidence intervals as analyzed using a random effects model for a all fractures or b vertebral fracture. The diamond shows the overall risk and where it lies towards the right of the vertical line, this indicates an increased risk of fracture associated with the genotype. The p values shown have not been corrected for multiple testing
Allele based analysis showed that Sp1 T allele was associated with a 1.13-fold increased risk for all fractures [95% CI, 1.04 to 1.23] (p=0.003). Gender specific analysis showed no significant association with fracture in males. In females, the Sp1 T allele was associated with a 1.18-fold increased risk for all fractures [95% CI, 1.06 to 1.30] (p= 0.002) and a 1.19-fold increased risk for vertebral fracture [95% CI, 1.01 to 1.40] (p=0.03).
Association between promoter polymorphisms, BMD, and fractures
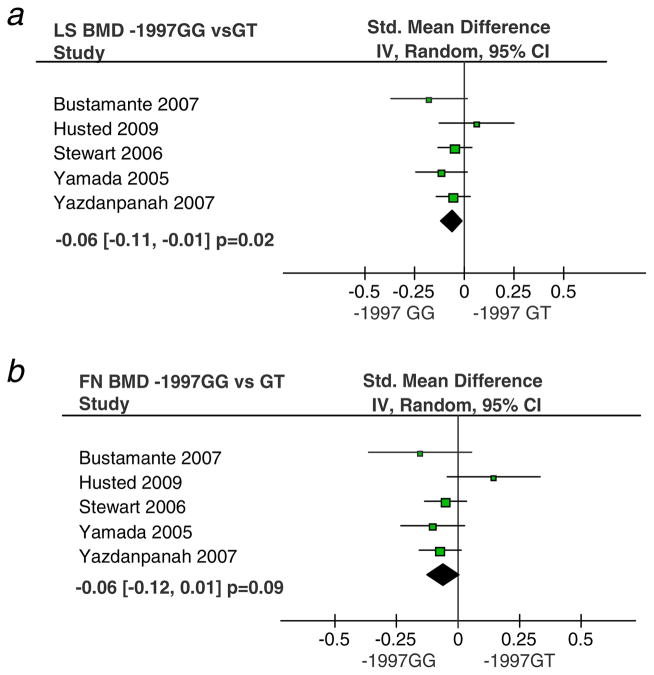
There were fewer eligible studies for analysis of associations between the promoter polymorphisms and osteoporosis-related phenotypes. For the -1997G/T polymorphism (rs1107946) we identified five eligible studies which together included 8,257 women and 5,706 men. There was no significant association between this polymorphism and BMD overall with the exception of the comparison between GG homozygotes and carriers of the T allele where the association was borderline significant under a fixed effects model (Table 4). Gender specific analysis showed a significant association between the -1997G/T polymorphism in females as depicted in Fig. 3. Accordingly, BMD values were 0.06 units [95% CI, 0.01 to 0.11] lower in female -1997 G/G homozygotes as compared with G/T heterozygotes at the lumbar spine (p=0.02; Fig. 3a). A similar difference was observed at the femoral neck (Fig. 4b), although it did not reach statistical significance (p=0.09). Similar results were obtained under a recessive model at lumbar spine (p=0.02). There was no association with BMD in males although power was limited in view of the small number of males studied for this polymorphism.
Table 4.
Meta-analysis of promoter polymorphisms in relation to BMD in the whole study population
| Studies/N | Comparison | Fixed effect model
|
Random effects model
|
||||
|---|---|---|---|---|---|---|---|
| Lumbar spine BMD | P | Lumbar spine BMD | P | I2 (95%CI) | |||
| -1997G/T | 5/11,232 | GG vs. GT | −0.04 (−0.08,−0.00) | 0.06 | −0.04 (−0.09, 0.01) | 0.16 | 28 (0–68) |
| 5/8,480 | GG vs. TT | −0.05 (−0.15,0.04) | 0.30 | −0.03 (−0.17, 0.10) | 0.62 | 37 (0–72) | |
| 5/11,750 | GG vs. GT+TT | −0.04 (−0.08,−0.00) | 0.05 | −0.04 (−0.09, 0.01) | 0.14 | 29 (0–68) | |
| 5/11,750 | TT vs. GG+GT | 0.05 (−0.05,0.14) | 0.33 | 0.03 (−0.08, 0.15) | 0.55 | 24 (0–65) | |
| -1663in/delT | 3/3,975 | insT/insT vs. insT/delT | 0.03 (−0.04,0.10) | 0.36 | 0.03 (−0.04, 0.10) | 0.36 | 0 (0–85) |
| 3/2,927 | insT/insT vs. delT/delT | 0.16 (0.02, 0.31) | 0.03 | 0.23 (−0.04, 0.49) | 0.09 | 43 (0–81) | |
| 3/4,183 | Dominanta | −0.06 (−0.12,0.01) | 0.09 | −0.06 (−0.12, 0.01) | 0.09 | 0 (0–85) | |
| 3/4,183 | Recessiveb | −0.19 (−0.33,−0.05) | 0.006 | −0.30 (−0.63, 0.03) | 0.07 | 41(0–80) | |
| Femoral neck BMD | Femoral neck BMD | ||||||
| -1997G/T | 5/11,143 | GG vs. GT | −0.04 (−0.08, 0.01) | 0.09 | −0.03 (−0.09, 0.02) | 0.23 | 28 (0–68) |
| 5/8,409 | GG vs. TT | −0.01 (−0.11,0.08) | 0.77 | −0.01 (−0.13, 0.11) | 0.91 | 25 (0–66) | |
| 5/11,657 | GG vs. GT+TT | −0.04 (−0.08, 0.01) | 0.09 | −0.03 (−0.08, 0.03) | 0.32 | 36 (0–72) | |
| 5/11,657 | TT vs. GG+GT | −0.01 (−0.10, 0.08) | 0.88 | −0.01 (−0.12, 0.10) | 0.87 | 20 (0–62) | |
| -1663in/delT | 3/3,892 | insT/insT vs. insT/delT | 0.03 (−0.04,0.09) | 0.46 | 0.03 (−0.04,0.09) | 0.46 | 0 (0–85) |
| 3/2,862 | insT/insT vs. delT/delT | 0.13 (−0.01,0.28) | 0.07 | 0.13 (−0.04, 0.30) | 0.12 | 5 (0–85) | |
| 3/4,085 | Dominanta | −0.04 (−0.10,0.03) | 0.26 | −0.04 (−0.10,0.03) | 0.26 | 0 (0–85) | |
| 3/4,085 | Recessiveb | −0.12 (−0.27,0.02) | 0.09 | −0.12 (−0.29, 0.04) | 0.14 | 6 (0–85) | |
Values are standardized mean difference (95% CI)
Positive values denote higher BMD values in the first listed genotype group of the comparison
The p values shown have not been corrected for multiple testing
I2: heterogeneity
Dominant model: insT/delT+delT/delT vs. insT/insT
Recessive model: delT/delT vs. insT/insT+insT/delT
Fig. 3.
Association between -1997 G/T polymorphism and BMD in females. Comparisons are shown in panel a for lumbar spine BMD and panel b for femoral neck BMD. Each study is shown as the point estimate of the standardized mean difference with 95% confidence intervals as analyzed using a random effects model. Diamonds which lie to the left of the vertical line indicates a reduced BMD in the -1997 GG genotype compared with G/T and TT genotype groups. The p values shown have not been corrected for multiple testing
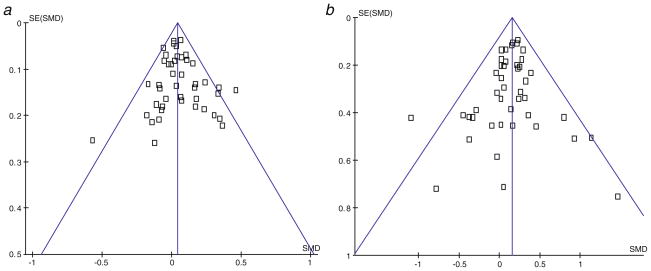
Fig. 4.
Funnel plots of spine and hip BMD in the whole study population. a Funnel plot of lumbar spine BMD in whole study population. b Funnel plot of femoral neck BMD in whole study population. Standardized mean difference is plotted on the horizontal axis and its standard error on the vertical axis
For the -1663ins/delT polymorphism (rs2412298) there were only three eligible studies which together included 3,999 women and 184 men. As shown in Table 4, under a fixed effects model there was a significant association at the lumbar spine where BMD values in -1663 delT/delT homozygotes were 0.16 units [95% CI, 0.02 to 0.31] lower than insT/insT homozygotes (p=0.03). Corresponding values under a recessive model were 0.19 [0.05 to 0.33] (p=0.006). The significance was also present in females, but not in males at either site. However, the significance disappeared under a random effects model due to modest heterogeneity (I2 =43%). There was no significant association between either -1997G/T or -1663ins/delT and fracture (data not shown) but fracture data were only available in three published studies.
Evaluation of bias
For all analyses, both Egger and Harbord tests revealed no evidence of publication bias and examples of funnel plots for lumbar spine BMD and femoral neck BMD in the whole study population are shown in Fig. 4. Furthermore, each of the meta-analyses shown in Figs. 1, 2, and 3 had between zero and five single studies with nominally significant results, and thus there was no suggestion that there were too many single studies with significant results.
Discussion
This study extends the observations made in previous meta-analyses of the COL1A1 Sp1 polymorphism [8, 16, 20–22] and provides the only meta-analysis of the -1997G/T and -1663ins/delT polymorphisms. We confirmed the association previously reported between the Sp1 polymorphism, BMD, and osteoporotic fractures and found that this was strongest with vertebral fracture. Under a recessive model of inheritance, the effect size was 0.08 units at lumbar spine and 0.15 units at femoral neck, which is very similar to the results previously reported by Mann et al. [20] who reported that spine BMD values in TT homozygotes were 0.09 units lower than in GG homozygotes and reported that femoral neck BMD values in TT homozygotes were 0.19 units lower than in GG homozygotes. The reasons which underlie the smaller effect size at the lumbar spine as compared with the femoral neck are unclear but could possibly reflect the fact that spine BMD values in older subjects are confounded by coexisting problems such as osteoarthritis, degenerative disk disease, and aortic calcification. All of these factors would be expected to reduce power to detect genotype-related difference in BMD at this site. Although recent GWAS studies have detected a greater number of significant hits at the spine than the hip [4] it should be noted that most of the populations included in these studies were younger than those included in this meta-analysis. Our study included some cohorts of young people, but most of the study populations had an average age above 60 years and many subjects were aged greater than 70 years where DEXA examination of the spine can give misleading results due to the factors mentioned above.
However, in this study we found no difference in BMD at femoral neck between GG homozygotes and G/T heterozygotes which was in agreement with what was reported in the GENOMOS meta-analysis [16] but not with the results of a previous meta-analysis [20]. The modest increase in risk for fractures at any site found in this study was driven mainly by the vertebral fracture and most of the significant associations were observed in females. This could indicate that genetic variation at the COL1A1 locus influences susceptibility to osteoporosis in a gender specific manner as has been demonstrated in linkage studies of mice [39], and in human linkage studies [40]. However, another perhaps more likely possibility is that the lack of significant associations in men was due to reduced power given that only 26% of the study population were male.
We also observed a significant association between the -1997G/T polymorphism and BMD in females but we found no significant associations between the -1663ins/delT polymorphisms and BMD and neither polymorphism was associated with fracture. However this could be due to the fact that we had reduced power to evaluate these outcomes as the promoter polymorphisms have been much less widely studied than the Sp1 polymorphism.
We and others have previously presented evidence to suggest that the three polymorphisms studied here interact with each other to regulate COL1A1 gene transcription by modifying gene expression and transcription factor binding [8, 41]. There is also evidence to suggest that allelic variants at the Sp1 binding site polymorphism adversely affect bone quality [8, 41] which is consistent with the data presented here in which the observed increase in vertebral fracture risk for Sp1 female TT homozygote (50%) was greater than the risk predicted by the modest genotype-specific reduction in spine BMD (13%). Some previous studies have shown positive associations between the alleles at the Sp1 polymorphism and hip fracture [30, 42, 43], and in one study, a rare haplotype defined by all three polymorphisms was strongly associated with hip fracture, although these were very highly selected patients and the sample size was small [44]. It would be of interest to perform a meta-analysis of COL1A1 haplotypes in relation to BMD and osteoporotic fracture, but only three studies had been performed where haplotype data were available. More studies on the relationship between COL1A1 haplotypes and osteoporosis-associated phenotypes including fracture would therefore be of great interest.
We made a meticulous effort to identify all relevant data; but publication bias, population stratification within individual studies, and other reporting biases are a threat for the validity of significant associations emerging in the literature and we cannot completely exclude the possibility that one or more of these biases may have been operative in this study. The tests that assess the existence of small-study effects were also not significant indicating that there is no evidence that the smaller studies had biased the results or that there were too many single studies with nominally significant results. While bias still cannot be fully excluded, the validity of the Sp1 associations reported here is corroborated by the concordant results of a prospective meta-analysis which should be immune to reporting biases [16] The associations we report here are relatively weak and explain a very small fraction of the heritability of BMD and fracture risk.
The association with BMD reported here falls short of the accepted threshold for genome-wide significance although the p values for association with fracture and the effect size reported here are in keeping with those previously reported by recent GWAS studies [4]. Using a modest prior for the credibility of these associations [45, 46], the available data suggest that the Sp1 G/T associations are very likely to be genuine, while the other two polymorphisms still lack strong evidence. Further research will be required to fully define the role that variants in the COL1A1 gene play as genetic determinant of osteoporosis. Evaluation of the role that the polymorphisms described here play in osteoporosis has been impaired by the fact that they are not efficiently tagged by the markers used in the GWAS performed so far in the osteoporosis field [13]. For example the best r2 value between the Sp1 polymorphism and the 6 SNP analyzed from the COL1A1 region in the GWAS of Richards and colleagues [5] was 5% with the rs2586471 in the 3′ flank of the gene (Jin and Ralston, unpublished data)
It is possible however that emerging initiatives such as the 1000 Genomes Project (www.1000genomes.org) will allow us evaluate the role of the COL1A1 alleles described here in populations that have already undergone GWAS. Even if the results of this were to be positive however, our findings indicate that at best, the SNP we studied in COL1A1 account for only a small proportion of the genetic risk of osteoporosis.
Acknowledgments
This study was supported in part by grants from the European Commission (GENOMOS; QLK6-CT-2002-02629) and from the Arthritis Research Campaign (15389).
Footnotes
Conflicts of interest SHR holds patents on the use of COL1A1 genotyping as a diagnostic test for susceptibility to osteoporosis.
Contributor Information
H. Jin, Rheumatic Disease Unit, Molecular Medicine Centre, Institute of Genetics and Molecular Medicine, Western General Hospital, University of Edinburgh, Edinburgh EH4 2XU, UK
E. Evangelou, Clinical and Molecular Epidemiology Unit, Department of Hygiene and Epidemiology, University of Ioannina School of Medicine, Ioannina, Greece
J. P. A. Ioannidis, Clinical and Molecular Epidemiology Unit, Department of Hygiene and Epidemiology, University of Ioannina School of Medicine, Ioannina, Greece. Biomedical Research Institute, Foundation for Research and Technology-Hellas, Ioannina, Greece. Center for Genetic Epidemiology and Modeling, ICRHPS, and Tufts Clinical and Translational Science Institute, Tufts University School of Medicine, Boston, USA
S. H. Ralston, Email: stuart.ralston@ed.ac.uk, Rheumatic Disease Unit, Molecular Medicine Centre, Institute of Genetics and Molecular Medicine, Western General Hospital, University of Edinburgh, Edinburgh EH4 2XU, UK
References
- 1.Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res. 1997;12:2076–2081. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- 2.Knapp KM, Andrew T, MacGregor AJ, Blake GM, Fogelman I, Spector TD. An investigation of unique and shared gene effects on speed of sound and bone density using axial transmission quantitative ultrasound and DXA in twins. J Bone Miner Res. 2003;18:1525–1530. doi: 10.1359/jbmr.2003.18.8.1525. [DOI] [PubMed] [Google Scholar]
- 3.Michaelsson K, Melhus H, Ferm H, Ahlbom A, Pedersen NL. Genetic liability to fractures in the elderly. Arch Intern Med. 2005;165:1825–1830. doi: 10.1001/archinte.165.16.1825. [DOI] [PubMed] [Google Scholar]
- 4.Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41:1119–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Snorradottir S, Center JR, Nguyen TV, Alexandersen P, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K. New sequence variants associated with bone mineral density. Nat Genet. 2009;41:15–17. doi: 10.1038/ng.284. [DOI] [PubMed] [Google Scholar]
- 7.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Center JR, Nguyen TV, Bagger Y, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K. Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008;358:2355–2365. doi: 10.1056/NEJMoa0801197. [DOI] [PubMed] [Google Scholar]
- 8.Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP, Aspden RM, Ralston SH. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest. 2001;107:899–907. doi: 10.1172/JCI10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang Y, van Meurs JB, d’Alesio A, Jhamai M, Zhao H, Rivadeneira F, Hofman A, van Leeuwen JP, Jehan F, Pols HA, Uitterlinden AG. Promoter and 3′-untranslated-region haplotypes in the vitamin D receptor gene predispose to osteoporotic fracture: the Rotterdam study. Am J Hum Genet. 2005;77:807–823. doi: 10.1086/497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uitterlinden AG, Burger H, Huang Q, Yue F, McGuigan FEA, Grant SFA, Hofman A, van Leeuwen JPTM, Pols HAP, Ralston SH. Relation of alleles of the collagen type I α 1 gene to bone density and risk of osteoporotic fractures in postmenopausal women. N Engl J Med. 1998;338:1016–1022. doi: 10.1056/NEJM199804093381502. [DOI] [PubMed] [Google Scholar]
- 11.van Meurs JB, Trikalinos TA, Ralston SH, Balcells S, Brandi ML, Brixen K, Kiel DP, Langdahl BL, Lips P, Ljunggren O, Lorenc R, Obermayer-Pietsch B, Ohlsson C, Pettersson U, Reid DM, Rousseau F, Scollen S, Van HW, Agueda L, Akesson K, Benevolenskaya LI, Ferrari SL, Hallmans G, Hofman A, Husted LB, Kruk M, Kaptoge S, Karasik D, Karlsson MK, Lorentzon M, Masi L, McGuigan FE, Mellstrom D, Mosekilde L, Nogues X, Pols HA, Reeve J, Renner W, Rivadeneira F, van Schoor NM, Weber K, Ioannidis JP, Uitterlinden AG. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:1277–1290. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langdahl BL, Uitterlinden AG, Ralston SH, Trikalinos TA, Balcells S, Brandi ML, Scollen S, Lips P, Lorenc R, Obermayer-Pietsch B, Reid DM, Armas JB, Arp PP, Bassiti A, Bustamante M, Husted LB, Carey AH, Perez CR, Dobnig H, Dunning AM, Fahrleitner-Pammer A, Falchetti A, Karczmarewicz E, Kruk M, van Leeuwen JP, Masi L, van Meurs JB, Mangion J, McGuigan FE, Mellibovsky L, Mosekilde L, Nogues X, Pols HA, Reeve J, Renner W, Rivadeneira F, van Schoor NM, Ioannidis JP. Large-scale analysis of association between polymorphisms in the transforming growth factor beta 1 gene (TGFB1) and osteoporosis: The GENOMOS study. Bone. 2008;42:969–981. doi: 10.1016/j.bone.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Zillikens MC, Wilson SG, Mullin BH, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra BA, Pols HA, Sigurdsson G, Thorsteinsdottir U, Soranzo N, Williams FM, Zhou Y, Ralston SH, Thorleifsson G, van Duijn CM, Kiel DP, Stefansson K, Uitterlinden AG, Ioannidis JP, Spector TD. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151:528–537. doi: 10.7326/0003-4819-151-8-200910200-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kryukov GV, Shpunt A, Stamatoyannopoulos JA, Sunyaev SR. Power of deep, allexon resequencing for discovery of human trait genes. Proc Natl Acad Sci USA. 2009;106:3871–3876. doi: 10.1073/pnas.0812824106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ralston SH, Uitterlinden AG, Brandi ML, Balcells S, Langdahl BL, Lips P, Lorenc R, Obermayer-Pietsch B, Scollen S, Bustamante M, Husted LB, Carey AH, Diez-Perez A, Dunning AM, Falchetti A, Karczmarewicz E, Kruk M, van Leeuwen JPTM, Meurs JB, Mangion J, McGuigan FE, Mellibovsky L, Monte FD, Pols HA, Reeve J, Reid DM, Renner W, Rivadeneira F, Schoor NM, Sherlock RE, Ioannidis JP. Large-scale evidence for the effect of the COLIA1 Sp1 polymorphism on osteoporosis outcomes: the GENOMOS study. PLoS Med. 2006;3:e90. doi: 10.1371/journal.pmed.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trikalinos TA, Salanti G, Zintzaras E, Ioannidis JP. Meta-analysis methods. Adv Genet. 2008;60:311–334. doi: 10.1016/S0065-2660(07)00413-0. [DOI] [PubMed] [Google Scholar]
- 18.Uitterlinden AG, Ralston SH, Brandi ML, Carey AH, Grinberg D, Langdahl BL, Lips P, Lorenc R, Obermayer-Pietsch B, Reeve J, Reid DM, Amidei A, Bassiti A, Bustamante M, Husted LB, ez-Perez A, Dobnig H, Dunning AM, Enjuanes A, Fahrleitner-Pammer A, Fang Y, Karczmarewicz E, Kruk M, van Leeuwen JP, Mavilia C, van Meurs JB, Mangion J, McGuigan FE, Pols HA, Renner W, Rivadeneira F, van Schoor NM, Scollen S, Sherlock RE, Ioannidis JP. The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med. 2006;145:255–264. doi: 10.7326/0003-4819-145-4-200608150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Ioannidis JP, Ng MY, Sham PC, Zintzaras E, Lewis CM, Deng HW, Econs MJ, Karasik D, Devoto M, Kammerer CM, Spector T, Andrew T, Cupples LA, Duncan EL, Foroud T, Kiel DP, Koller D, Langdahl B, Mitchell BD, Peacock M, Recker R, Shen H, Sol-Church K, Spotila LD, Uitterlinden AG, Wilson SG, Kung AW, Ralston SH. Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J Bone Miner Res. 2007;22:173–183. doi: 10.1359/jbmr.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann V, Ralston SH. Meta-analysis of COL1A1 Sp1 polymorphism in relation to bone mineral density and osteoporotic fracture. Bone. 2003;32:711–717. doi: 10.1016/s8756-3282(03)00087-5. [DOI] [PubMed] [Google Scholar]
- 21.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 22.Efstathiadou Z, Tsatsoulis A, Ioannidis JP. Association of collagen Ialpha 1 Sp1 polymorphism with the risk of prevalent fractures: a meta-analysis. J Bone Miner Res. 2001;16:1586–1592. doi: 10.1359/jbmr.2001.16.9.1586. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Giralt N, Nogues X, Enjuanes A, Puig J, Mellibovsky L, Bay-Jensen A, Carreras R, Balcells S, Diez-Perez A, Grinberg D. Two new single nucleotide polymorphisms in the COLIA1 upstream regulatory region and their relationship with bone mineral density. J Bone Miner Res. 2002;17:384–393. doi: 10.1359/jbmr.2002.17.3.384. [DOI] [PubMed] [Google Scholar]
- 24.Stewart TL, Jin H, McGuigan FE, Albagha OM, Garcia-Giralt N, Bassiti A, Grinberg D, Balcells S, Reid DM, Ralston SH. Haplotypes defined by promoter and intron 1 polymorphisms of the COLIA1 gene regulate bone mineral density in women. J Clin Endocrinol Metab. 2006;91:3575–3583. doi: 10.1210/jc.2005-2651. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Ando F, Niino N, Shimokata H. Association of a -1997G–>T polymorphism of the collagen I alpha1 gene with bone mineral density in postmenopausal Japanese women. Hum Biol. 2005;77:27–36. doi: 10.1353/hub.2005.0035. [DOI] [PubMed] [Google Scholar]
- 26.Yazdanpanah N, Rivadeneira F, van Meurs JB, Zillikens MC, Arp P, Hofman A, van Duijn CM, Pols HA, Uitterlinden AG. The -1997 G/T and Sp1 polymorphisms in the collagen type I alpha1 (COLIA1) gene in relation to changes in femoral neck bone mineral density and the risk of fracture in the elderly: the Rotterdam study. Calcif Tissue Int. 2007;81:18–25. doi: 10.1007/s00223-007-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bustamante M, Nogues X, Enjuanes A, Elosua R, Garcia-Giralt N, Perez-Edo L, Caceres E, Carreras R, Mellibovsky L, Balcells S, ez-Perez A, Grinberg D. COL1A1, ESR1, VDR and TGFB1 polymorphisms and haplotypes in relation to BMD in Spanish postmenopausal women. Osteoporos Int. 2007;18:235–243. doi: 10.1007/s00198-006-0225-8. [DOI] [PubMed] [Google Scholar]
- 28.Gerdhem P, Brandstrom H, Stiger F, Obrant K, Melhus H, Ljunggren O, Kindmark A, Akesson K. Association of the collagen type 1 (COL1A 1) Sp1 binding site polymorphism to femoral neck bone mineral density and wrist fracture in 1044 elderly Swedish women. Calcif Tissue Int. 2004;74:264–269. doi: 10.1007/s00223-002-2159-2. [DOI] [PubMed] [Google Scholar]
- 29.Husted LB, Harslof T, Gonzalez-Bofill N, Schmitz A, Carstens M, Stenkjaer L, Langdahl BL. Haplotypes of promoter and intron 1 polymorphisms in the COLIA1 gene are associated with increased risk of osteoporosis. Calcif Tissue Int. 2009;84:85–96. doi: 10.1007/s00223-008-9199-1. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TV, Esteban LM, White CP, Grant SF, Center JR, Gardiner EM, Eisman JA. Contribution of the collagen I alpha1 and vitamin D receptor genes to the risk of hip fracture in elderly women. J Clin Endocrinol Metab. 2005;90:6575–6579. doi: 10.1210/jc.2005-1153. [DOI] [PubMed] [Google Scholar]
- 31.Selezneva LI, Khusainova RI, Nurlygaianov RZ, Fazlyeva EA, Usenko KP, Lesniak OM, Khucnutdinova EK. Association of polymorphisms and haplotypes in the 5′ region of COLIA1 gene with the risk of osteoporotic fractures in Russian women from Volga-Ural region. Genetika. 2008;44:219–225. [PubMed] [Google Scholar]
- 32.Weichetova M, Stepan JJ, Haas T, Michalska D. The risk of Colles’ fracture is associated with the collagen I alpha1 Sp1 polymorphism and ultrasound transmission velocity in the calcaneus only in heavier postmenopausal women. Calcif Tissue Int. 2005;76:98–106. doi: 10.1007/s00223-004-0134-9. [DOI] [PubMed] [Google Scholar]
- 33.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 34.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. Br Med J. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test [see comments] Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 37.Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4:245–253. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 38.Braga V, Mottes M, Mirandola S, Lisi V, Malerba G, Sarton L, Bianchi G, Gatti D, Rossini M, Bianchini D, Adami S. Association of CTR and COLIA1 alleles with BMD values in peri- and postmenopausal women. Calcif Tissue Int. 2000;67:361–366. doi: 10.1007/s002230001160. [DOI] [PubMed] [Google Scholar]
- 39.Orwoll ES, Belknap JK, Klein RF. Gender specificity in the genetic determinants of peak bone mass. J Bone Miner Res. 2001;16:1962–1971. doi: 10.1359/jbmr.2001.16.11.1962. [DOI] [PubMed] [Google Scholar]
- 40.Ralston SH, Galwey N, Mackay I, Albagha OM, Cardon L, Compston JE, Cooper C, Duncan E, Keen R, Langdahl B, McLellan A, O’Riordan J, Pols HA, Reid DM, Uitterlinden AG, Wass J, Bennett ST. Loci for regulation of bone mineral density in men and women identified by genome wide linkage scan: the FAMOS study. Hum Mol Genet. 2005;14:943–951. doi: 10.1093/hmg/ddi088. [DOI] [PubMed] [Google Scholar]
- 41.Jin H, Van’t Hof RJ, Albagha OM, Ralston SH. Promoter and intron 1 polymorphisms of COL1A1 interact to regulate transcription and susceptibility to osteoporosis. Hum Mol Genet. 2009;18:2729–2738. doi: 10.1093/hmg/ddp205. [DOI] [PubMed] [Google Scholar]
- 42.Aerssens J, Dequeker J, Peeters J, Breemans S, Broos P, Boonen S. Polymorphisms of the VDR, ER and COLIA1 genes and osteoporotic hip fracture in elderly postmenopausal women. Osteoporos Int. 2000;11:583–591. doi: 10.1007/s001980070079. [DOI] [PubMed] [Google Scholar]
- 43.Valimaki S, Tahtela R, Kainulainen K, Laitinen K, Loyttyniemi E, Sulkava R, Valimaki M, Kontula K. Relation of collagen type I alpha 1 (COLIA1) and vitamin D receptor genotypes to bone mass, turnover, and fractures in early postmenopausal women and to hip fractures in elderly people. Eur J Intern Med. 2001;12:48–56. doi: 10.1016/s0953-6205(00)00137-0. [DOI] [PubMed] [Google Scholar]
- 44.Jin H, Stewart TL, Hof RV, Reid DM, Aspden RM, Ralston S. A rare haplotype in the upstream regulatory region of COL1A1 is associated with reduced bone quality and hip fracture. J Bone Miner Res. 2009;24:448–454. doi: 10.1359/jbmr.081111. [DOI] [PubMed] [Google Scholar]
- 45.Ioannidis JP. Effect of formal statistical significance on the credibility of observational associations. Am J Epidemiol. 2008;168:374–383. doi: 10.1093/aje/kwn156. [DOI] [PubMed] [Google Scholar]
- 46.Wacholder S, Chanock S, Garcia-Closas M, El GL, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvarez-Hernandez D, Naves M, Diaz-Lopez JB, Gomez C, Santamaria I, Cannata-Andia JB. Influence of polymorphisms in VDR and COLIA1 genes on the risk of osteoporotic fractures in aged men. Kidney Int. 2003;63:S14–S18. doi: 10.1046/j.1523-1755.63.s85.5.x. [DOI] [PubMed] [Google Scholar]
- 48.Ashford RU, Luchetti M, McCloskey EV, Gray RL, Pande KC, Dey A, Kayan K, Ralston SH, Kanis JA. Studies of bone density, quantitative ultrasound, and vertebral fractures in relation to collagen type I alpha 1 alleles in elderly women. Calcif Tissue Int. 2001;68:348–351. doi: 10.1007/s002230010010. [DOI] [PubMed] [Google Scholar]
- 49.Bernad M, Martinez ME, Escalona M, Gonzalez ML, Gonzalez C, Garces MV, Del Campo MT, Martin ME, Madero R, Carreno L. Polymorphism in the type I collagen (COLIA1) gene and risk of fractures in postmenopausal women. Bone. 2002;30:223–228. doi: 10.1016/s8756-3282(01)00639-1. [DOI] [PubMed] [Google Scholar]
- 50.Braga V, Sangalli A, Malerba G, Mottes M, Mirandola S, Gatti D, Rossini M, Zamboni M, Adami S. Relationship among VDR (BsmI and FokI), COLIA1, and CTR polymorphisms with bone mass, bone turnover markers, and sex hormones in men. Calcif Tissue Int. 2002;70:457–462. doi: 10.1007/s00223-001-1088-9. [DOI] [PubMed] [Google Scholar]
- 51.Efstathiadou Z, Kranas V, Ioannidis JP, Georgiou I, Tsatsoulis A. The Sp1 COLIA1 gene polymorphism, and not vitamin D receptor or estrogen receptor gene polymorphisms, determines bone mineral density in postmenopausal Greek women. Osteoporos Int. 2001;12:326–331. doi: 10.1007/s001980170123. [DOI] [PubMed] [Google Scholar]
- 52.Garnero P, Borel O, Grant SFA, Ralston SH, Delmas PD. Collagen I α 1 polymorphism, bone mass and bone turnover in healthy French pre-menopausal women: the OFELY study. J Bone Miner Res. 1998;13:813–818. doi: 10.1359/jbmr.1998.13.5.813. [DOI] [PubMed] [Google Scholar]
- 53.Grant SFA, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporosis associated with a polymorphic Sp1 site in the collagen type I alpha 1 gene. Nat Genet. 1996;14:203–205. doi: 10.1038/ng1096-203. [DOI] [PubMed] [Google Scholar]
- 54.Harris SS, Patel MS, Cole DE, Dawson-Hughes B. Associations of the collagen type I alpha 1 Sp1 polymorphism with five-year rates of bone loss in older adults. Calcif Tissue Int. 2000;66:268–271. doi: 10.1007/pl00005842. [DOI] [PubMed] [Google Scholar]
- 55.Heegaard A, Jorgensen HL, Vestergaard AW, Hassager C, Ralston SH. Lack of influence of collagen type I alpha 1 Sp1 binding site polymorphism on the rate of bone loss in a cohort of postmenopausal Danish women followed for 18 years. Calcif Tissue Int. 2000;66:409–413. doi: 10.1007/s002230010083. [DOI] [PubMed] [Google Scholar]
- 56.Hustmyer FG, Lui G, Johnston CC, Christian J, Peacock M. Polymorphism at an Sp1 binding site of COLIA1 and bone mineral density in pre-menopausal female twins and elderly fracture patients. Osteoporos Int. 1999;9:346–350. doi: 10.1007/s001980050157. [DOI] [PubMed] [Google Scholar]
- 57.Keen RW, Woodford-Richens KL, Grant SF, Ralston SH, Lanchbury JS, Spector TD. Association of polymorphism at the type I collagen (COL1A1) locus with reduced bone mineral density, increased fracture risk, and increased collagen turnover. Arthritis Rheum. 1999;42:285–290. doi: 10.1002/1529-0131(199902)42:2<285::AID-ANR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 58.Liden M, Wilen B, Ljunghall S, Melhus H. Polymorphism at the Sp 1 binding site in the collagen type I alpha 1 gene does not predict bone mineral density in postmenopausal women in sweden. Calcif Tissue Int. 1998;63:293–295. doi: 10.1007/s002239900529. [DOI] [PubMed] [Google Scholar]
- 59.McGuigan FE, Reid DM, Ralston SH. Susceptibility to osteoporotic fracture is determined by allelic variation at the Sp1 site, rather than other polymorphic sites at the COL1A1 locus. Osteoporos Int. 2000;11:338–343. doi: 10.1007/s001980070123. [DOI] [PubMed] [Google Scholar]
- 60.McGuigan FEA, Armbrecht G, Smith R, Felsenberg D, Reid DM, Ralston SH. Prediction of osteoporotic fractures by bone densitometry and COLIA1 genotyping: a prospective, population-based study in men and women. Osteoporos Int. 2001;12:91–96. doi: 10.1007/s001980170139. [DOI] [PubMed] [Google Scholar]
- 61.Mezquita-Raya P, Munoz-Torres M, de Dios LJ, Lopez-Rodriguez F, Quesada JM, Luque-Recio F, Escobar-Jimenez F. Performance of COLIA1 polymorphism and bone turnover markers to identify postmenopausal women with prevalent vertebral fractures. Osteoporos Int. 2002;13:506–512. doi: 10.1007/s001980200062. [DOI] [PubMed] [Google Scholar]
- 62.Peris P, Alvarez L, Oriola J, Guanabens N, Monegal A, De Osaba MJ, Jo J, Pons F, Ballesta AM, Munoz-Gomez J. Collagen type Ialpha1 gene polymorphism in idiopathic osteoporosis in men. Rheumatology (Oxford) 2000;39:1222–1225. doi: 10.1093/rheumatology/39.11.1222. [DOI] [PubMed] [Google Scholar]
- 63.Roux C, Dougados M, Abel L, Mercier G, Lucotte G. Association of a polymorphism in the collagen I α 1 gene with osteoporosis in French women. Arthritis Rheum. 1998;41:187–188. doi: 10.1002/1529-0131(199801)41:1<187::AID-ART32>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 64.Sowers M, Willing M, Burns T, Deschenes S, Hollis B, Crutchfield M, Jannausch M. Genetic markers, bone mineral density and serum osteocalcin levels. J Bone Miner Res. 1999;14:1411–1419. doi: 10.1359/jbmr.1999.14.8.1411. [DOI] [PubMed] [Google Scholar]
- 65.Van Pottelbergh I, Goemaere S, Nuytinck L, De PA, Kaufman JM. Association of the type I collagen alpha1 Sp1 polymorphism, bone density and upper limb muscle strength in community-dwelling elderly men. Osteoporos Int. 2001;12:895–901. doi: 10.1007/s001980170043. [DOI] [PubMed] [Google Scholar]