Abstract
v-Rel is the acutely oncogenic member of the NF-κB family of transcription factors. Infection with retroviruses expressing v-Rel rapidly induces fatal lymphomas in birds and transforms primary lymphocytes and fibroblasts in vitro. We have previously shown that AP-1 transcriptional activity contributes to v-Rel-mediated transformation. While v-Rel increases the expression of these factors, their activity may also be induced through phosphorylation by the mitogen-activated protein (MAP) kinases. The expression of v-Rel results in the strong and sustained activation of the ERK and JNK MAPK pathways. This induction is critical for the v-Rel transformed phenotype, as suppression of MAPK activity with chemical inhibitors or siRNA severely impairs colony formation of v-Rel transformed lymphoid cell lines. However, signaling must be maintained within an optimal range in these cells, since strong additional activation of either pathway beyond the levels induced by v-Rel through the expression of constitutively active MAPK proteins attenuates the transformed phenotype. MAPK signaling also plays an important role in the initial transformation of primary spleen cells by v-Rel, although distinct requirements for MAPK activity at different stages of v-Rel-mediated transformation were identified. We also show that the ability of v-Rel to induce MAPK signaling more strongly than c-Rel contributes to its greater oncogenicity.
Keywords: v-Rel, NF-κB, transformation, oncogenesis, MAPK
Introduction
Rel/NF-κB signaling is involved in the regulation of key processes, including proliferation, cellular survival, inflammation, innate and adaptive immunity, and embryogenesis (Bonizzi and Karin, 2004; Dutta et al., 2006; Hayden and Ghosh, 2004). In most cells, NF-κB dimers are maintained in the cytoplasm through interaction with inhibitory IκB proteins. Activating signals cause degradation of IκB, releasing NF-κB dimers to the nucleus, where they regulate the transcription of numerous target genes. Aberrant NF-κB signaling has been implicated in numerous pathologies, including multiple stages of cancer (Basseres and Baldwin, 2006; Gilmore et al., 2004; Karin, 2006). v-rel, which arose from the viral transduction of the c-rel proto-oncogene, is the most strongly oncogenic member of the NF-κB family, and its expression rapidly transforms primary lymphoid and fibroblast cultures (Gilmore, 1999; Nehyba et al., 1997). v-Rel carries out transformation through the altered transcription of genes normally controlled by cellular NF-κB.
Previously, we have shown that the levels of AP-1 transcription factors (c-Jun, c-Fos, ATF-2) are increased in cells expressing v-Rel, and AP-1 transcriptional activity contributes to transformation by v-Rel [(Kralova et al., 1998), Liss et al., in press]. In addition to being regulated by transcription, AP-1 activity is also controlled by post-translational modification, primarily through phosphorylation by the mitogen-activated protein kinases (MAPKs). In this study, we report that MAPK signaling is elevated in cells expressing v-Rel and plays a critical role in v-Rel-mediated transformation. The major MAPK pathways include those that activate extracellular-regulated kinase (ERK), c-Jun amino-terminal kinase (JNK) and p38 signaling (Raman et al., 2007). In each pathway, a MAP kinase kinase kinase (MKKK) phosphorylates and activates a MAP kinase kinase (MKK), which in turn phosphorylates and activates the MAPK proteins. These cascades translate extracellular or stress stimuli into specific cellular actions by phosphorylating a range of substrates (Raman et al., 2007; Yoon and Seger, 2006). As important regulators of cellular proliferation and survival, MAPK pathways have been implicated in oncogenesis. ERK activation leads to transformation and blocks differentiation (Mansour et al., 1994; McCubrey et al., 2007; Robinson et al., 1998). The role of JNK and p38 signaling in tumorigenesis is less clear, since signaling can lead to transformation or apoptosis depending on cellular context (Dhillon et al., 2007; Whitmarsh and Davis, 2007).
In this report we demonstrate that activation of the ERK and JNK signaling pathways plays a crucial role in v-Rel transformation. The reduction of ERK or JNK activity in v-Rel transformed cells, through treatment with pharmacological MAPK pathway inhibitors or with MAPK-specific siRNAs, significantly decreased the anchorage-independent growth of these cells. Interestingly, experiments with constitutively active mutants of MAPK activators revealed that signaling must be maintained within an optimal range in v-Rel transformed cells, since strong additional MAPK activation also resulted in the attenuation of the transformed phenotype. In contrast, studies in primary spleen cells demonstrated that further elevated MAPK activity enhanced the transformation of these cells by v-Rel, thus identifying different requirements for MAPK signaling during initial and late stages of transformation by v-Rel. The colony formation of DT40 cells overexpressing c-Rel was enhanced by additional MAPK activation, indicating that MAPK signaling is an important contributor to NF-κB-mediated transformation in this model.
Results
ERK and JNK signaling is strongly activated by v-Rel
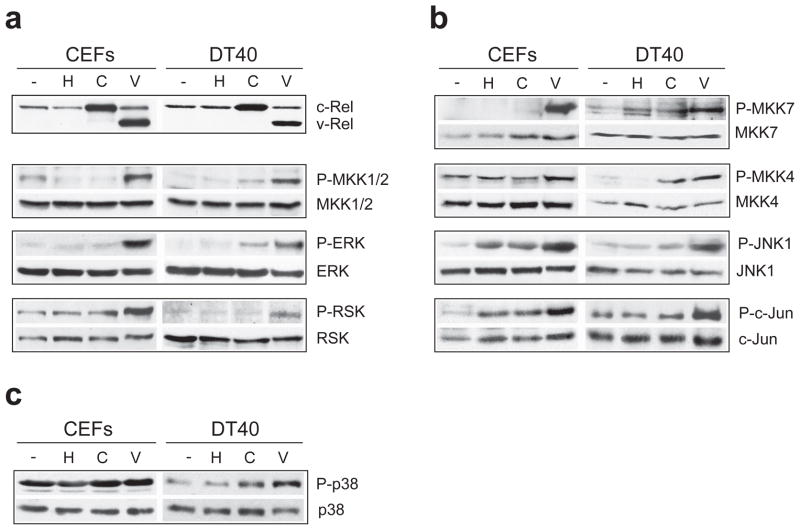
We examined the activation of the major MAPK cascades in cells expressing c-Rel or v-Rel. Chicken embryo fibroblasts (CEFs) and the avian B-cell line, DT40, were infected with helper virus alone (chick syntitial virus, CSV) or with retroviruses expressing c-Rel (REV-C) or v-Rel (REV-TW). Cell lysates were prepared following morphological transformation of cells expressing v-Rel. The activity of the MAPK pathway components was determined by measuring their phosphorylation status, including the levels of active, phosphorylated ERK, JNK, and p38. Cells expressing v-Rel exhibited high levels of ERK and JNK phosphorylation in both cell types relative to uninfected or CSV-infected cells, while total protein levels remained unchanged (Figure 1a, Figure 1b). In contrast, v-Rel activation of p38 was not as dramatic and was primarily limited to DT40 cells (Figure 1c). The phosphorylation of downstream targets of ERK (RSK) and JNK (c-Jun) correlated with the activation of their respective kinases in v-Rel-expressing cells. While v-Rel expression increased the total levels of c-Jun (1.5–1.6 fold) compared to uninfected cells, the levels of phosphorylated c-Jun normalized to total levels were also elevated (~1.7 fold). Further, the phosphorylation levels of the upstream kinases for ERK (MKK1/2) and JNK (MKK4/7) were also increased, thereby suggesting activation of the entire MAPK signaling cascades in cells expressing v-Rel. In comparison to v-Rel expression in these cells, the overexpression of c-Rel resulted in a smaller and sometimes non-detectable increase in MAPK phosphorylation at each level of these cascades, suggesting that a difference in MAPK activation contributes to the stronger oncogenicity of v-Rel. Similar data were obtained in the DT95 B-cell line (Supplemental Figure 1).
Figure 1.
Induction of the ERK and JNK MAPK pathways by v-Rel. Chicken embryo fibroblasts (CEFs) or DT40 cells were left uninfected (−) or were infected with helper virus alone (H) or with retroviruses expressing c-Rel (C) or v-Rel (V). Cell lysates were prepared 7–10 days later, when cells expressing v-Rel exhibited characteristic morphological changes. Western blot analysis examined the levels of total and phosphorylated protein for components of the (a) ERK, (b) JNK, and (c) p38 MAPK signaling cascades. The expression of c-Rel and v-Rel in these cells is shown in panel (a).
ERK and JNK activation is essential for the maintenance of the v-Rel transformed phenotype
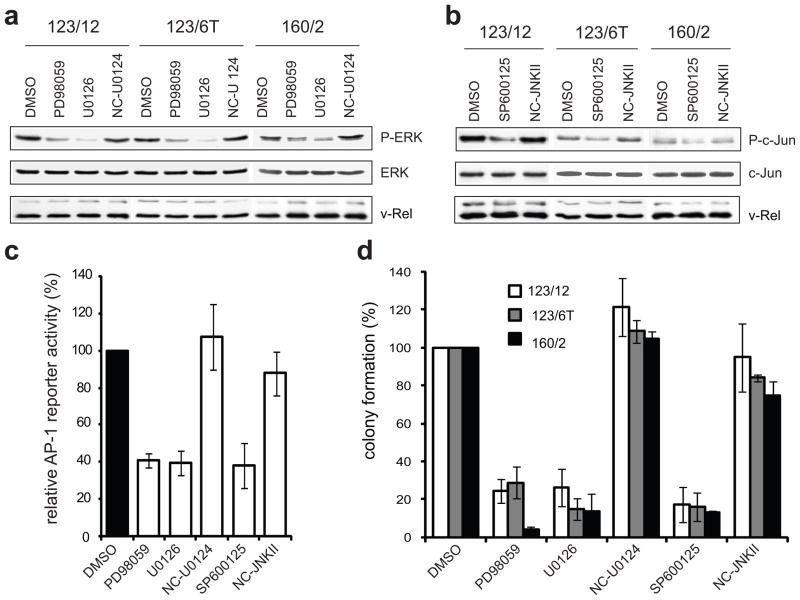
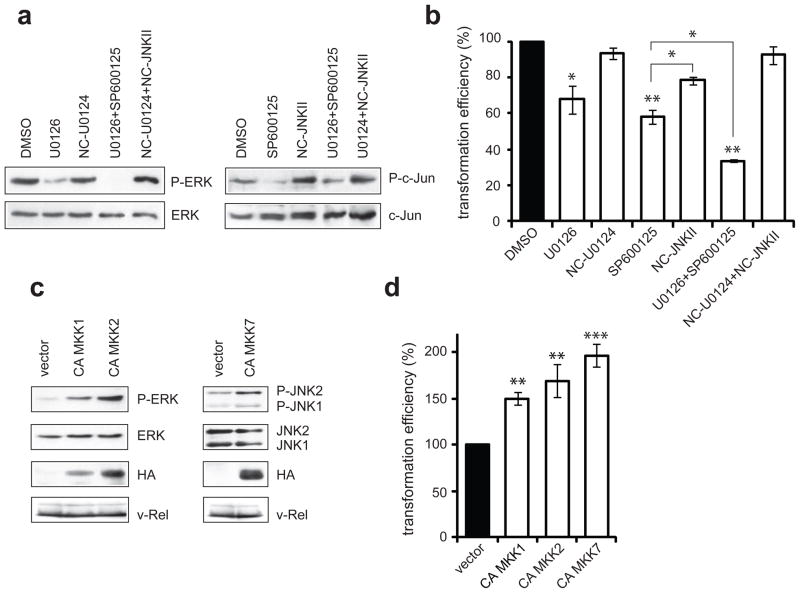
The importance of ERK and JNK signaling to the transformed phenotype of established v-Rel transformed cell lines was examined. MAPK activity was reduced through the use of pharmacological inhibitors, including MEK inhibitors (U0126 and PD98059) to block ERK activation and a JNK inhibitor (SP600125) to block JNK activity. Three histologically distinct v-Rel transformed lymphoid cell lines were selected, including a T-cell (160/2), B-cell (123/12), and non-B/non-T (123/6T) cell line. Cells were incubated in the presence of DMSO vehicle alone, MEK or JNK inhibitors, or their respective negative controls. Incubation with either MEK inhibitor caused significant reduction in ERK phosphorylation relative to treatment with the negative control (U0124) or DMSO (Figure 2a). Similarly, incubation with the JNK inhibitor reduced the levels of phosphorylated c-Jun in comparison to treatment with negative controls (Figure 2b). Total levels of ERK and c-Jun were not altered by any treatment. Importantly, inhibitor treatment did not affect the retroviral expression of v-Rel in any of these lineages.
Figure 2.
Inhibition of ERK and JNK pathways attenuates colony formation of v-Rel transformed cells. Three established v-Rel transformed cell lines of histologically distinct origin were utilized, including a T-cell (160/2), B-cell (123/12), and non-B/non-T (123/6T) cell line. (a) Cell lines were treated for one hour with carrier alone (DMSO), MEK inhibitors (PD98059, U0126), or negative control (U0124) at a 3 μM concentration. Cell lysates were prepared and levels of phosphorylated and total ERK were examined by Western blot. (b) Cell lines were treated for one hour with carrier alone (DMSO), JNK inhibitor (SP600125), or negative control (NC-JNK II) at a 3 μM concentration and cell lysates were examined by Western blot for phosphorylated and total c-Jun. (c) Reporter assays evaluated the effect of MAPK inhibitors on AP-1 activity in cells expressing v-Rel. CEFs were co-transfected with a pGL2 luciferase reporter (1 μg) containing multiple repeats of an AP-1 consensus site (Kralova et al., 1998), the Rc/RSV expression vector (1 μg) encoding v-Rel, and with the pRL-TK reporter vector (0.3 μg). Eight hours later, MAPK inhibitors or their respective negative controls were added to the media at a 10 μM concentration. Luciferase activity in cells treated with carrier alone (DMSO) was standardized to 100. The average and standard deviation for four independent experiments are shown. (d) Cell lines were incubated with inhibitors or negative controls (3 μM) for two days and plated into soft agar in the presence of the same concentration of inhibitors. Colonies were scored microscopically 10 days later and colony numbers for cells treated with carrier alone (DMSO) were standardized to 100. The average and standard deviation for four independent experiments are shown.
The effect of the MAPK inhibitors on v-Rel-induced AP-1 activity was evaluated using a luciferase reporter construct containing multiple consensus AP-1 binding sites. As we described previously, v-Rel strongly activates this reporter (Kralova et al., 1998), in part, through increased expression of c-Jun and c-Fos [(Fujii et al., 1996), Liss et al., in press]. Moreover, it was shown that MAPK phosphorylation of AP-1 factors contributes to their activity (Shaulian and Karin, 2002). Therefore, it was expected that activation of ERK and JNK signaling by v-Rel would contribute to AP-1 activation. To examine this possibility, CEF cultures were co-transfected with the AP-1 reporter construct and with vector encoding v-Rel or empty vector. Transfected cells were then incubated with MAPK inhibitors or negative controls. Both MEK and JNK inhibitors reduced reporter activation by v-Rel by ~60%, while negative controls had no significant effect (Figure 2c). These results provide evidence that the induction of MAPK signaling by v-Rel is important for v-Rel-mediated AP-1 activation.
To determine the role of MAPK activity in the maintenance of the phenotype of v-Rel transformation, the effect of MAPK inhibitor treatment on colony formation of the v-Rel transformed cell lines was examined. Cells were pre-treated with inhibitors or negative controls for 48 hours and plated into soft agar. Treatment of these cells with MAPK inhibitors for 10 days had little or no effect on cell viability or growth rate in liquid culture (less than 10%, data not shown). However, treatment of the cell lines with ERK and JNK pathway inhibitors resulted in a dramatic reduction in the number (70–90%) and size of colonies in soft agar in comparison to cells incubated with the negative controls (Figure 2d). In contrast, treatment of the v-Rel cell line, 123/12, with the p38 inhibitor did not have a significant effect on soft agar colony formation (Supplemental Figure 2). These experiments reveal a correlation between the specific activation of ERK and JNK MAPK signaling and the growth potential of v-Rel transformed cells in soft agar, whereas p38 signaling is dispensable for this process.
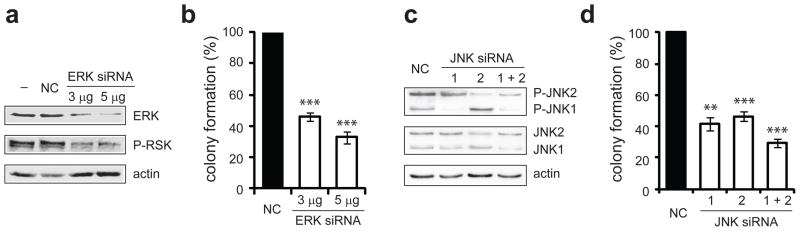
To investigate the importance of individual MAPK isoforms, we used a siRNA knockdown approach. In chicken, only one isoform of ERK is present, which shares the greatest homology with mammalian ERK2. In our experiments, the T-cell line (160/2) was electroporated with negative control siRNA or with increasing amounts of siRNA targeting ERK. Cells from the same electroporation population were plated into soft agar and harvested for protein. Western blot analysis showed a clear decrease in ERK protein levels (3 μg and 5 μg of ERK siRNA resulted in a 60% and 75% decrease in total ERK levels, respectively) (Figure 3a). This reduced level of protein corresponded with diminished ERK activity, as demonstrated by lowered phosphorylation of its downstream target, RSK. Moreover, cells transfected with ERK siRNA formed 2–3-fold fewer colonies than those receiving negative control siRNA (Figure 3b).
Figure 3.
ERK and JNK knockdown inhibits colony formation of v-Rel transformed cells. (a–b) The v-Rel transformed cell line, 160/2, was electroporated with siRNA targeting ERK (3 or 5 μg) or with negative control siRNA (3 μg). Cells from the same electroporation population were plated into soft agar 16 hours after transfection and harvested at 48 hours for protein. (a) The levels of total ERK, as well as the levels of phosphorylation of a downstream target, p90-RSK were examined by Western blot. (b) Soft agar colonies were scored microscopically 7–8 days after plating. Colony numbers from cells electroporated with negative control siRNA were standardized to 100. The average for four independent experiments is shown with standard error. (c–d) 160/2 cells were electroporated with negative control siRNA or with siRNA (1 μg) targeting JNK1, JNK2, or both. Cells were plated into soft agar and harvested for protein 16 hours after transfection. (c) Western blot analysis examined the levels of phosphorylated and total JNK1 and JNK2. (d) Soft agar colonies were scored microscopically 7–8 days after plating. Colony numbers from cells electroporated with negative control siRNA were standardized to 100. The average for four independent experiments is shown with standard error. Statistically significant differences in colony formation of cells electroporated with specific siRNA relative to negative control are indicated (**P<0.01, ***P<0.001).
Similar experiments were designed to specifically reduce the levels of individual JNK isoforms, since studies have demonstrated that JNK isoforms can have non-redundant functions (Bode and Dong, 2007). The chicken genome encodes two JNK proteins, JNK1 and JNK2, and siRNAs complementary to the mRNA of each of these isoforms were introduced into 160/2 cells, alone and in combination. Cells were harvested for protein, and Western blot analysis demonstrated that siRNA targeting JNK1 or JNK2 specifically reduced the phosphorylated and total levels of the appropriate JNK isoform (Figure 3c). In these experiments, the level of each phosphorylated JNK protein was decreased by 70–80%. Interestingly, the effect of siRNA on phosphorylated JNK was more substantial than on total protein levels, suggesting a complex regulation of JNK activation, which has been noted in other JNK siRNA experiments (Oleinik et al., 2007). Treatment of cells with the JNK siRNAs together resulted in a simultaneous reduction of active JNK1 and JNK2. Transfected cells were plated into soft agar and treatment of the v-Rel transformed cell line with either JNK siRNA alone caused a significant (55–60%) decrease in colony formation (Figure 3d), indicating that both JNK isoforms contribute to transformation by v-Rel. Treatment with the JNK siRNAs together resulted in a 70% reduction in colony numbers, slightly greater than with individual siRNAs. Thus, through selective reduction of the JNK isoforms, we determined that JNK1 and JNK2 each have an important and overlapping function in transformation by v-Rel. Although transfected siRNA persisted in cells for a relatively short time interval (~ 48 hours, data not shown), these results indicate that an initial block in MAPK signaling is sufficient to prevent colony formation in soft agar.
Requirement for ERK and JNK activation is specific for v-Rel transformation
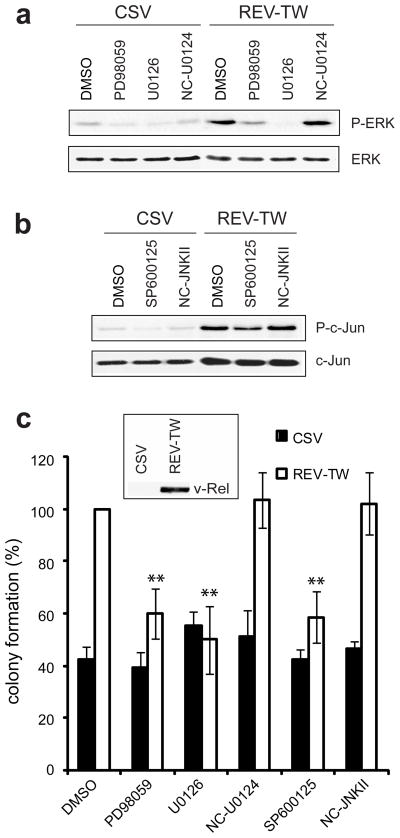
To further address the role of ERK and JNK activation in v-Rel transformation, experiments were performed in the DT40 B-cell line. These cells, although already transformed by the insertion of the avian leukosis virus long terminal repeat upstream of c-myc, are sensitive to v-Rel transformation. When expressing v-Rel, DT40 cells display altered morphology, become adherent within several days of infection, and have an increased rate of metabolism (data not shown). Furthermore, DT40 cells expressing v-Rel form colonies in soft agar twice as efficiently as CSV-infected cells (Figure 4, DMSO treated cells). The expression of v-Rel in DT40 cells also leads to an increase in the phosphorylation of ERK and JNK (Figure 1a, Figure 1b). Therefore, DT40 cells provide a useful model for examining the direct involvement of ERK and JNK activity in v-Rel-mediated transformation. DT40 cells infected with CSV alone or with retroviruses expressing v-Rel were incubated for one hour with ERK or JNK pathway inhibitors or appropriate negative controls. Cells were harvested for protein and plated into soft agar. Treatment with MAPK pathway inhibitors resulted in a decrease in the phosphorylation of ERK (Figure 4a) and c-Jun (Figure 4b) in both cell populations. Following six hours of inhibitor treatment, decreased MAPK activity was still apparent, while the levels of v-Rel were unchanged relative to controls (Supplemental Figure 3). In cells expressing v-Rel, treatment with ERK or JNK inhibitors, but not negative controls, resulted in a 50% decrease in growth in soft agar (Figure 4c), thus eliminating the v-Rel-mediated increase in colony formation. In contrast, there was no decrease in colony formation accompanying inhibitor treatment of CSV-infected cells. Treatment of either cell type with the p38 inhibitor did not affect colony formation (Supplemental Figure 2c), consistent with our previous results indicating that p38 activity is dispensable for the v-Rel transformed phenotype (Supplemental Figure 2b). In sum, the results in DT40 cells suggest that the requirement for ERK and JNK activation is specific to the v-Rel oncogene and is not a general requirement for transformation.
Figure 4.
ERK and JNK activation is a specific requirement for v-Rel transformation. DT40 cells were infected with helper virus alone (CSV) or with retroviruses expressing v-Rel (REV-TW). Cells were grown for 7–10 days, until those expressing v-Rel exhibited characteristic morphological changes. (a) Infected cells were treated for one hour with carrier alone (DMSO), MEK inhibitors (PD95089, U0126), or negative control (U0124) at a 3 μM concentration and cell lysates were examined for phosphorylated and total ERK. (b) Infected cells were treated for one hour with carrier alone (DMSO), JNK inhibitor (SP600125), or negative control (NC-JNK II) at a 3 μM concentration and cell lysates were examined for phosphorylated and total c-Jun. (c) Infected cells were incubated for one hour with inhibitors or negative controls (3 μM) and then plated into soft agar containing the same concentration of inhibitors. Colonies were scored microscopically after 7–8 days. Colony numbers for REV-TW infected cells treated with DMSO were standardized to 100. The average and standard deviation for four independent experiments are shown. Statistically significant differences in colony formation of REV-TW-infected cells treated with various inhibitors relative to those treated with DMSO are indicated (**P<0.01). v-Rel expression in REV-TW infected cells was verified by Western blot analysis, as shown in the inset of panel (c).
Constitutive ERK and JNK activity attenuates the v-Rel transformed phenotype
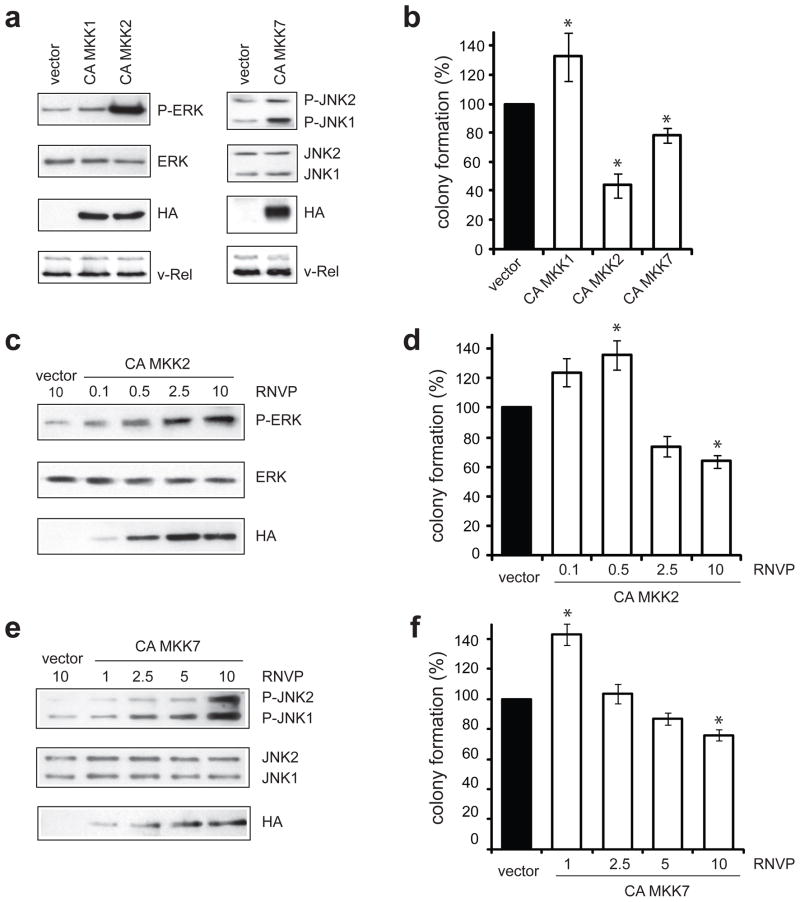
Experiments using MAPK inhibitors or siRNA to reduce ERK and JNK activity demonstrated that signaling from these pathways is required for the growth of v-Rel-transformed cells in soft agar. We further wanted to determine if the transformed phenotype of the v-Rel cell lines could be enhanced by elevating MAPK signaling to an even greater extent than the levels induced by v-Rel. ERK and JNK activity was increased through the ectopic expression of constitutively active mutants of upstream MAP kinase kinases (MKKs). We used constituitvely active (CA) MKK1 and CA MKK2 to further activate ERK (Mansour et al., 1994) and CA MKK7 for JNK activation (Holtmann et al., 1999). The appropriate activity of these human constructs in chicken cells was confirmed by determining the effect of their transient expression on ERK and JNK phosphorylation and on AP-1 reporter activity in chicken embryo fibroblasts (Supplemental Figure 4). CA MKK mutants were cloned into the DS vector, an RSV-based retroviral vector (Okuno et al., 1991), and viral stocks were prepared in CEFs. DS retroviruses were used to superinfect the v-Rel transformed T-cell line, 160/2, and cells were grown in liquid culture for five days. Expression of the HA-tagged constructs was verified by Western analysis (Figure 5a). Both CA MKK1 and CA MKK2 increased the levels of phosphorylated ERK. However, despite similar expression levels, CA MKK2 activated ERK much more strongly than CA MKK1 (30-fold increase by CA MKK2 compared to 2.5-fold increase by CA MKK1). CA MKK7 expression resulted in a modest (2–3 fold) increase in the levels of phosphorylated JNK1 and JNK2.
Figure 5.
Constitutive ERK and JNK activity attenuates colony formation of v-Rel transformed cells. (a) The v-Rel cell line (160/2) was infected with DS retroviruses encoding the CA MKK1, CA MKK2, and CA MKK7 mutants or with empty vector. Cells were infected with each virus with relative numbers of virus particles (RNVP) of 10. Western blot analysis demonstrated the expression of the CA MKK mutants by detection of the HA epitope located at the N-terminus of each protein and the levels of phosphorylated and total ERK in cells expressing CA MKK1 and CA MKK2 (left panels) and phosphorylated and total JNK in cells expressing CA MKK7 (right panels) relative to cells infected with empty DS virus. (b) Cells were plated into soft agar five days after infection and colonies were scored microscopically 7–9 days later. Colony numbers from cells infected with empty DS retroviruses were standardized to 100. The average of at least two independent experiments is shown with standard error. Statistically significant differences from cells infected with empty vector are indicated (*P<0.05). (c–f) Dose-dependent effect of ERK and JNK activity on colony formation of the v-Rel cell line (160/2). Cells were infected with empty DS retroviruses (RNVP of 10) or with increasing amounts of viruses encoding CA MKK2 or CA MKK7 and cell lysates were prepared. Western analysis examined (c) the levels of phosphorylated and total ERK in cells infected with virus expressing CA MKK2 (RNVP of 0.1, 0.5, 2.5, and 10) and (e) the levels of phosphorylated and total JNK in cells infected with virus expressing CA MKK7 (RNVP of 1, 2.5, 5, and 10) relative to control cells. (d,f) Infected cells were plated into soft agar and colonies were scored microscopically 10–14 days later. Colony numbers for cells infected with empty DS retroviruses were standardized to 100. In each panel, the average of at least two independent experiments is shown with standard error. Statistically significant differences from cells infected with empty vector are indicated (*P<0.05).
The effect of increased MAPK activity on the transformed phenotype of these cells was determined by plating cells in soft agar (Figure 5b). The small increase in ERK activity by CA MKK1 resulted in a slight, but significant increase in colony formation. Interestingly, expression of CA MKK2 that led to strong ERK activation caused a dramatic (50%) reduction in colony formation. Increase in JNK activity by CA MKK7 similarly resulted in a decrease (22%) in colony formation. These results suggest that excess activation of ERK by CA MKK2 and JNK by CA MKK7 in v-Rel transformed cells, instead of further promoting the oncogenic potential of v-Rel, is inhibitory to the growth of transformed cells in soft agar. Cells expressing CA MKK2 or CA MKK7 did not exhibit higher cell death or defects in cell cycle progression in liquid culture, although CA MKK7 expression resulted in increased sensitivity to apoptotic stimuli, as discussed later (data not shown).
The level of ERK and JNK activity that contributes to v-Rel transformation was further defined by examining the dose-dependent effect of elevated MAPK activity on colony formation of v-Rel transformed cells. For these experiments, 160/2 cells were infected with increasing numbers of virus particles up to the amount used in the experiments described above. Since even high expression of CA MKK1 did not strongly increase ERK activity, only CA MKK2 and CA MKK7 were used in these studies. The relative numbers of viral particles (RNVP) were increased in 4 different dilutions (0.1–10 for CA MKK2 and 1–10 for CA MKK7). Increasing viral concentration resulted in increased expression of the CA mutants and a gradual rise in ERK and JNK activity relative to control cells (Figure 5c, Figure 5e). Colony formation of cells infected with viruses expressing the CA MKK constructs was compared to that of cells infected with empty DS virus. Since infection with empty DS viruses at high and low concentrations had equivalent effects on colony formation (10–20% decrease in colony numbers compared to uninfected cells, data not shown), only results obtained with cells infected at the highest amount of control virus are shown. A small increase in the activity of ERK (<4 fold) and JNK (<2 fold) due to CA MKK expression (RNVP ≤ 1) tended to enhance colony formation (Figure 5d, Figure 5f). In contrast, marked activation (RNVP ≥ 2.5 for CA MKK2 and RNVP ≥ 5 for CA MKK7) resulted in reduced colony formation. These results indicate that the levels of ERK and JNK activation that promote v-Rel transformation occur within a limited range.
ERK and JNK signaling is important for the initiation of transformation by v-Rel
Cancer is commonly viewed as a multi-step process in which genetic changes that initially lead to malignant transformation are not necessarily the same as those contributing to tumor progression and metastasis. We have demonstrated an important role for ERK and JNK signaling in established v-Rel transformed cell lines (Figures 2 and 3). Experiments were also performed to examine the contribution of MAPK signaling to the initial stages of transformation by v-Rel, using the transformation of primary splenic lymphocytes as a model for this event. Spleen cells were infected with retroviruses expressing v-Rel (REV-TW). The following day, cells were incubated for one hour in the presence of ERK or JNK pathway inhibitors or the appropriate negative controls. A reduction in ERK phosphorylation was observed in cells incubated with MEK inhibitor (U0126) in comparison to cells exposed to the negative control (U0124) or vehicle (DMSO) alone (Figure 6a). Similarly, incubation of cells with the JNK inhibitor (SP600125) lowered c-Jun phosphorylation in comparison to cells treated with the negative control (NC-JNK II) or vehicle alone. Combined exposure to these inhibitors resulted in a simultaneous reduction in the levels of both phosphorylated ERK and c-Jun.
Figure 6.
Role of ERK and JNK signaling in the transformation of primary splenic lymphocytes by v-Rel. (a–b) Primary splenic lymphocytes were isolated from three week-old chickens and infected with retroviruses expressing v-Rel (REV-TW). (a) The next day, cells were treated for one hour with carrier alone (DMSO), with MAPK pathway inhibitors (MEK inhibitor, U0126; JNK inhibitor, SP600125) or their respective negative controls (U0124, NC-JNK II) singly, or with MAPK inhibitors or negative controls in combination. All treatments were performed at a 3 μM concentration of inhiibitor or control. Cell lysates were analyzed for phosphorylated and total ERK and c-Jun. (b) The day following infection, cells were incubated for six hours with inhibitors or negative controls at a 3 μM concentration and then plated into soft agar containing the same concentration of inhibitors. Colonies were scored microscopically 10–14 days later. Colony numbers for cells treated with carrier alone (DMSO) were standardized to 100. The average of at least three independent experiments is shown with standard error. Statistically significant differences from cells treated with DMSO are indicated (*P<0.05, **P<0.01). (c–d) Primary splenic lymphocytes were co-infected with retroviruses expressing v-Rel (REV-TW) and DS retroviruses encoding CA MKK1, CA MKK2, or CA MKK7 or empty vector. (c) Infections were expanded in liquid culture and cell lysates were prepared 10 days after infection. Western blots examined the expression of v-Rel and the CA MKK mutants. The levels of phosphorylated and total ERK in cells expressing CA MKK1 and CA MKK2 and phosphorylated and total JNK in cells expressing CA MKK7 relative to cells infected with empty DS viruses were also determined. (d) The day following infection, an aliquot of each infection was plated into soft agar and colonies were scored microscopically 10–14 days later. Colony numbers for cells infected with empty DS viruses were standardized to 100. The average of nine independent experiments is shown with standard error (*P<0.05, **P<0.01, ***P<0.001).
The effect of the MAPK inhibitors on the transformation efficiency of primary spleen cells by v-Rel was examined. Spleen cells infected with retroviruses expressing v-Rel were pre-treated for six hours with MAPK inhibitors or negative controls and plated into soft agar. Inhibition of ERK and JNK signaling resulted in significant reductions in colony formation (30% and 40%, respectively) relative to cells treated with the DMSO control (Figure 6b). Treatment with the JNK negative control also slightly impaired colony formation, but this effect was independent of JNK activity, since the levels of phosphorylated c-Jun in these cells were not lower than in DMSO-treated cells. Importantly, treatment with the JNK inhibitor resulted in a significant decrease in colony numbers when compared to negative control-treated cells. Spleen cells were also exposed to both MAPK inhibitors at the same time to examine whether ERK and JNK signaling act through overlapping or separate pathways. In these experiments, combined inhibitor treatment resulted in a 67% decrease in colony formation, while corresponding exposure to the negative controls had no effect. The decrease with combined inhibitor treatment was very significant when compared to DMSO-treated cells and was also significantly lower (P<0.05) than the reduction caused by JNK inhibitor treatment alone. Although the observed decreases in colony formation with single inhibitor treatment were not as substantial as in the established v-Rel cell lines (Figure 2d), the attenuation of transformation efficiency indicates that MAPK activity also plays a role in the early stages of transformation by v-Rel. Moreover, the results from combined inhibitor treatment indicate that ERK and JNK contribute to transformation through the regulation of largely separate downstream targets.
Complementary experiments were performed to determine whether further activation of ERK or JNK signaling could enhance the initiation of transformation by v-Rel. Spleen cells were co-infected with retroviruses expressing v-Rel (REV-TW) and DS retroviruses encoding the CA MKK constructs. Cells were expanded in liquid culture and whole cell lysates were prepared after 10 days. Expression of CA MKK1 and CA MKK2 increased the levels of phosphorylated ERK relative to control cells infected with the empty DS virus (Figure 6c). ERK activation by CA MKK2 was more efficient than that mediated by CA MKK1, perhaps as a result of the higher expression of CA MKK2. Expression of CA MKK7 increased the levels of phosphorylated JNK1 and JNK2 relative to control cells.
Spleen cells infected with retroviruses expressing v-Rel and the CA MKK mutants were plated into soft agar the day following infection. ERK activation by CA MKK1 and CA MKK2 increased colony formation relative to control cells by 1.5 and 1.8 fold, respectively (Figure 6d). JNK induction by CA MKK7 increased colony formation by 2 fold. Thus, further activation of ERK and JNK signaling enhances the oncogenic potential of v-Rel in primary splenic lymphocytes, illustrating the importance of MAPK signaling in initial stages of v-Rel transformation. In combination with the contrasting results obtained with CA MKK mutant expression in the established v-Rel transformed cell lines (Figure 5), the results in primary spleen cells indicate that there may be distinct requirements for MAPK activity at different stages of v-Rel-mediated transformation.
Enhanced activation of ERK and JNK signaling by v-Rel contributes to its stronger oncogenic potential compared to c-Rel
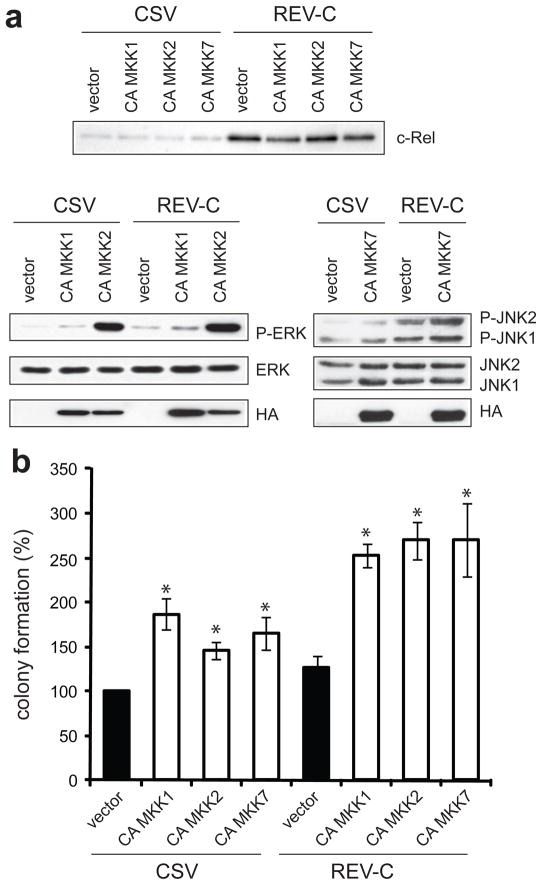
v-Rel is much more oncogenic than c-Rel. Spleen cells infected with retroviruses expressing v-Rel readily form colonies in soft agar, whereas cells overexpressing c-Rel can only grow in liquid culture. Our initial observations showed that v-Rel expression activates MAPK signaling to a much greater extent than c-Rel (Figure 1a, Figure 1b). To determine whether the difference in c-Rel and v-Rel oncogenicity results from their differential activation of MAPK signaling, we examined whether additional induction of MAPK activity in cells expressing c-Rel would enhace their ability to grow in soft agar. These experiments were performed in DT40 cells, in which expression of v-Rel results in a 2.3-fold increase in colony formation relative to CSV-infected cells (Figure 4c). DT40 cells were co-infected with helper virus (CSV) or with retroviruses expressing c-Rel (REV-C) and with DS retroviruses expressing the CA MKK mutants. Western analysis demonstrated c-Rel overexpression in REV-C infected cells and confirmed similar expression of the CA MKK constructs in all infections (Figure 7a). c-Rel overexpression alone caused a slight increase in MAPK activation. In both CSV- and REV-C-infected cells, expression of the CA MKK mutants resulted in elevated levels of ERK and JNK activity. Notably, when CA MKKs were expressed in REV-C-infected cells, the levels of ERK and JNK signaling were higher than in CSV-infected cells expressing the same MKK constructs. Moreover, CA MKK2 expression, either alone or in the context of c-Rel overexpression, resulted in stronger ERK activation than CA MKK1.
Figure 7.
CA MKK mutants enhance colony formation of DT40 cells infected with helper virus or retroviruses encoding c-Rel. DT40 cells were co-infected with CSV alone or with retroviruses expressing c-Rel (REV-C) as well as with retroviruses expressing the CA MKK mutants. Infections were expanded in liquid culture for 8–14 days and cells were harvested for protein and plated into soft agar. (a) The expression of c-Rel and the CA MKK mutants were examined by Western blot. The levels of phosphorylated and total ERK in cells expressing CA MKK1 and CA MKK2 and phosphorylated and total JNK in cells expressing CA MKK7 relative to cells infected with empty DS viruses were also determined. (b) Soft agar colonies were scored microscopically 7–9 days later. Colony numbers for cells infected with CSV and with empty DS retroviruses were standardized to 100. Statistically significant differences from cells infected with CSV and empty DS vector are indicated (*P<0.05). The average of at least three independent experiments is shown with standard error.
The effect of increased MAPK activity on colony formation was examined by plating infected cells from each population into soft agar. In DT40 cells that were infected with helper virus and constructs that express the CA MKK mutants, there was a 1.5–1.9-fold increase in relative transformation efficiency (Figure 7b). Thus, elevated MAPK activity by itself increased anchorage-independent growth of CSV-infected cells. The overexpression of c-Rel alone only weakly increased colony formation. In cells co-infected with viruses overexpressing c-Rel and CA MKK constructs, there was an average 2.5–2.7-fold increase in transformation efficiency relative to control cells. Therefore, MAPK activation was sufficient to increase colony formation in DT40 cells overexpressing c-Rel to levels obtained with v-Rel.
Discussion
v-Rel is acutely oncogenic, rapidly transforming multiple primary cell types and rendering them immortalized. The transcriptional activity of v-Rel is essential for its oncogenic potential, and its transforming ability is mediated by the altered expression of NF-κB-regulated genes involved in growth and protection from apoptosis. Thus, the v-Rel model system provides a valuable tool for delineating the mechanisms underlying multiple stages of NF-κB-mediated transformation. In this study, we demonstrate that the transformation of lymphoid and fibroblast cells by the v-rel oncogene results in marked and sustained activation of the ERK and JNK MAPK pathways (Figure 1).
Our results support the view that Rel-mediated cellular transformation and tumor progression are dependent on dysregulated mitogenic signaling. Activation of the ERK and JNK signaling pathways is crucial for v-Rel transformation, since blocking either pathway profoundly impaired the anchorage-independent growth of v-Rel transformed cells (Figure 2), while not affecting general growth in liquid culture. A similar effect was seen in all three cell lines tested, indicating that the contribution of ERK and JNK activity to transformation is independent of cell lineage derivation. Whereas previous studies have shown distinct functions for the JNK isoforms in tumorigenesis (Bost et al., 1999; Chen et al., 2001; Kennedy et al., 2003; Yang et al., 2003), the specific reduction of individual JNK isoforms in our siRNA studies demonstrated that JNK1 and JNK2 have overlapping functions in v-Rel transformation (Figure 3). We have also shown that MAPK activation is important during initial stages of lymphocyte transformation (Figure 6b). Although the effect on colony formation in this context was not as strong, these results indicate that both the initiation and maintenance of the v-Rel transformed phenotype are dependent, at least in part, on ERK and JNK activation.
A complete list of biological substrates of the ERK and JNK pathways that contribute to the v-Rel transformed phenotype remains to be determined. However, we have previously demonstrated the importance of AP-1 transactivation in transformation by v-Rel [(Kralova et al., 1998), Liss et al., in press]. Our current evidence indicates that MAPK signaling is responsbile for AP-1 activation by v-Rel (Figure 2c), and thus AP-1 activation is likely an important means by which MAPK signaling contributes to v-Rel transformation. Previous gene expression studies of MAPK signaling in tumor cells have identified multiple additional transcriptional targets (Nielsen et al., 2007; Potapova et al., 2002; Pratilas et al., 2009), indicating that AP-1-independent processes are also likely to have a role in transformation. Exposure of primary spleen cells to ERK and JNK pathway inhibitors together resulted in an almost additive decrease in transformation efficiency relative to cells exposed to these inhibitors singly (Figure 6B). These results suggest that these pathways mediate transformation, at least durnig intial stages, through the regulation of mostly separate, non-redundant dwonstream targets.
Interestingly, our experiments revealed that a very delicate balance of MAPK activation is required to maintain the v-Rel transformed state (Figure 5). The existence of thresholds in pathways required for transformation has previously been reported (Suzukawa et al., 2002). However, the prevailing model views constitutive ERK signaling as an important mediator of cancer, despite the lack of universally high ERK activity in tumor cells (Dhillon et al., 2007; Houben et al., 2008; Tsavachidou et al., 2004). Our experiments demonstrate that MAPK pathways must still be tightly regulated in tumor cells. It is conceivable that a relatively small increase in activity would be sufficient for the maintenance of transformation, since different signaling strength and duration are translated into distinct substrate selection and signaling outcomes in the MAPK pathways (Murphy and Blenis, 2006).
Previous studies have identified a negative effect of high-intensity ERK signaling on cell cycle progression (Courtois-Cox et al., 2008; Sewing et al., 1997; Woods et al., 1997), while CA MKK1 and CA MKK2 were demonstrated to have functional differences in tumor cell lines (Skarpen et al., 2008; Ussar and Voss, 2004). We examined the growth in liquid culture of v-Rel transformed cells with strongly elevated MAPK activity to determine if similar mechanisms might underlie their transformation defect. However, our studies revealed no difference in apoptotic index or cell cycle progression in cells expressing CA MKK2 or CA MKK7 relative to control cells or those expressing CA MKK1 (data not shown). Interestingly, exposure to apoptotic stress in cells with elevated JNK activity increased the induction of apoptosis (data not shown), consistent with the establishment of a pro-apoptotic state by JNK activity, rather than the automatic induction of cell death (Lin, 2003). Analogous experiments have not yet been performed with cells expressing the CA MKK2 mutant, and it is possible that a similar mechanism contributes to decreased colony formation by these cells. Alternatively, phosphorylation of targets not normally regulated by these kinases may result from their high expression and may be reponsible for the negative biological consequences of these mutatns.
While v-Rel expression increases the levels of phosphorylated ERK and JNK, it does not increase the total levels of these proteins (Figure 1). Overexpression of MAPK-activating cytokines or receptors has been detected in tumor cells (Blume-Jensen and Hunter, 2001; Dhillon et al., 2007), and NF-κB factors are known to directly regulate the expression of many of these factors (nf-kb.org). Our recent microarray studies of the v-Rel transcriptional program identified an increase in several MAPK-activating cytokines, including Interleukin-1β, CCL4, and NGF (Bargmann et al., manuscript in preparation). Interestingly, transient exposure of DT40 cells to conditioned media from DT40 cells expressing v-Rel but not from control cells resulted in increased ERK and JNK activation (data not shown). This indicates that altered cytokine production may indeed be a mechanism of MAPK activation by v-Rel. Consistent with our Western analysis (Figure 1), array studies did not reveal changes in the expression of most core MAPK signaling components. However, two upstream factors, Tpl2 (MAP3K8) and MAP4K4, exhibited increased expression in multiple v-Rel cell lineages and may represent an additional mechanism by which MAPK signaling is activated by v-Rel. Finally, increased total levels of the MAPK-activating Ras GTPase were found in cells expressing v-Rel and correlated with more active Ras (Liss et al., in press). Notably, expression of dominant negative Ras in v-Rel transformed cells resulted in decreased ERK activity and impaired colony formation. Thus, multiple mechanisms are likely to contribute to MAPK activation in v-Rel transformed cells. In contrast, microarray analysis indicated that only a limited number of the above factors, namely IL-1β, CCL4, and Tpl2, can be activated by c-Rel, although c-Rel-mediated increases in the transcription of these genes were generally significantly less than those resulting from v-Rel expression [(Bunting et al., 2007; Gupta et al., 2008), Bargmann et al., in preparation]. This observation may explain the stronger activation of MAPK signaling by v-Rel relative to c-Rel.
In contrast to the results obtained in v-Rel transformed cells, the expression of the CA MKK mutants enhanced the initiation of v-Rel transformation in spleen cells. Thus, there may be distinct requirements for MAPK activity during different stages of v-Rel-mediated transformation, with a more stringent requirement for a particular level of MAPK activity in the maintenance of transformation. Although distinct gene expression patterns have been correlated with different stages of tumor progression (Bubendorf et al., 1999; Corzo et al., 2006; Karakosta et al., 2005), differences in MAPK activity have not previously been noted. Considering that studies have not examined the effect of further MAPK activation on tumor cells, our observations might reflect a more common phenomenon than is represented in the literature. Interestingly, CA MKK7 expression primarily resulted in activation of the JNK1 isoform in a v-Rel cell line (Figure 5a, Figure 5e), while mainly JNK2 was activated in primary spleen cells (Figure 6c). Thus, although the JNK isoforms seem to contribute equally to the maintenance of v-Rel transformation (Figure 3d), the preferential additional activation of specific JNK isoforms may explain the opposing effects of CA MKK7 expression on v-Rel transformation in primary spleen cells and the established cell line. This finding is consistent with previous studies that identify JNK2 as the major isoform that contributes to tumorigenesis (Bode and Dong, 2007).
The v-Rel oncogene acquired a higher oncogenic potential relative to c-Rel as a result of deletion events and multiple mutations (Gilmore, 1999; Gilmore et al., 2004). Herein, we demostrate that the ability of v-Rel to activate ERK and JNK pathways to a greater extent than c-Rel contributes to its stronger oncogenic potential. The additional activation of these pathways by CA MKK mutants enhanced the growth in soft agar of DT40 cells expressing c-Rel. These results strongly implicate ERK and JNK activity in v-Rel transformation and suggest that these signaling pathways may cooperate with aberrant cellular NF-κB activation in the pathogenesis of lymhoproliferative disorders.
Materials and Methods
General cell culture techniques
Cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 5% chicken serum (Invitrogen, Carlsbad, CA), and 1% penicillin-streptomycin (1×106 U Penicillin G, 5 g Streptomycin in 1 L PBS). All cells described were grown at 37°C and 8% CO2.
Reagents
Antibodies for phosphorylated and total MAPK proteins were obtained from Cell Signaling Technologies (Danvers, MA) and Santa Cruz Biotechnologies (Santa Cruz, CA). MAPK inhibitors and negative controls were obtained from EMD Biosciences.
Construction of retroviral and expression vectors
HA-tagged CA MKK1 and CA MKK2 were a gift from the laboratory of Natalie Ahn (Mansour et al., 1994). CA MKK7 was constructed using a MKK7-JNK1 fusion construct provided by the laboratory of Aming Lin (Holtmann et al., 1999; Zheng et al., 1999). CA MKK mutants were cloned into the pDS retroviral vectors (Okuno et al., 1991).
Preparation of retroviral stocks
Viruses were made as previously described (Majid et al., 2006). Briefly, CEFs were plated at 6 × 105 cells per 60 mm tissue culture dish 24 hours prior to transfection. Cells were transfected with retroviral vectors using a calcium phosphate precipitate technique. CEF cultures were expanded and virus was harvested through the collection of supernatant fluids. Virus titers were determined by dot-blot hybridization analysis (Nelson et al., 1998).
Western blot analysis
Proteins in whole cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred overnight to a nitrocellulose membrane. Western blots were performed as described previously (Majid et al., 2006). To strip blots, membranes were washed four times in TBST, incubated in stripping solution (625 mM Tris, pH 6.8, 2% SDS, and 0.7% β-mercaptoethanol for 30 minutes at 50°C, and washed an additional four times with TBST.
Reporter assays
CEFs were plated 24 hours before transfection at 7–8 × 105 cells per 60 mm dish. Cells were transfected by a modified calcium-phosphate method (Kralova et al., 1996). Cells were harvested 32–36 hours after transfection and luciferase activity was analyzed with the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Readings were normalized by Renilla luciferase activity.
siRNA experiments
For JNK1, a small interfering (siRNA) was used that was slightly modified from one employed for the knockdown of human JNK1 (Oleinik et al., 2007). The sequence used was 5′-CCAAGUGAUUCAGAUGGAGCUAGA-3′ and 5′-UAGCUCCAUCUGAAUCACUUGGUU-3′. This sequence corresponds to nucleotide 348 with respect to the start codon. The sequence used for JNK2 was 5′-AUGAAUUCUGCUGAGGCGUU-3′ and 5′-CGCCCUCAGCAGAAUUCAUUU-3′, which corresponds to nucleotide 730 relative to the start codon. The sequence used for ERK2 was 5′-CAAAGUUCGAGUUGCUAUAUU-3′ and 5′-UAUAGCAACUCGAACUUUGUU-3 ′, corresponding to nucleotide 165 relative to the start codon. siRNA constructs were chemically synthesized by Dharmacon (Boulder, CO). Negative control siRNA was Silencer Negative Control #1 siRNA (Ambion, Austin, TX).
v-Rel transformed cells was transfected with siRNA by electroporation. Cells (1.5 × 105) were transfected in siPORT electroporation buffer according to the manufacturer’s instructions (Ambion). Electroporations were carried out at 300 kV and 1 μF.
Colony formation assays
Cell lines were plated into soft agar as previously described (Majid et al., 2006). Cells (6 × 103 for DT40 cultures, 6 × 104 for the v-Rel transformed cell lines) were suspended in plating media and divided equally between three 60 mm tissue culture dishes. Media for plating DT40 cells contained different amounts of certain components than in previous experiments, including 4 ml fetal bovine serum, 1 ml chicken serum, and 17.5 ml of 1.1% Noble agar (Becton Dickinson, Sparks, MD). Inhibitors or negative controls were added to the media just prior to plating. P-values for differences in colony formation relative to controls were determined by two-tailed Student’s t-tests.
In vitro transformation assays
Spleen cells were isolated as described previously (Majid et al., 2006). REV-TW viruses were diluted in normal growth media to a final concentration of 1 × 105 virus particles/ml, and DS viruses were added to a final concentration of 1 × 106 virus particles/ml. Cells were plated in soft agar 18–24 hours after infection by adding plating media and dividing the cell suspension into three 60 mm tissue culture dishes. MAPK inhibitors or negative controls were added to the plating media just prior to plating.
Supplementary Material
Acknowledgments
We would like to thank the laboratories of Natalie Ahn and Aming Lin for generously providing the CA MKK constructs.
This work was supported by Public Health Service grants CA33192 and CA098151 from the National Cancer Institute. Jarmila Kralova was supported, in part, by grant KAN200200651 from the Grant Agency of the Academy of Sciences of the Czech Republic.
We also thank R. Hrdlicková and J. Nehyba for their critical reading of the manuscript.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary information accompanies this paper on the Oncogene website (http://www.nature.com/onc).
References
- Basseres DS, Baldwin AS. Nuclear factor- B and inhibitor of B kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–30. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46:591–8. doi: 10.1002/mc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF- B activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bost F, McKay R, Bost M, Potapova O, Dean NM, Mercola D. The Jun kinase 2 isoform is preferentially required for epidermal growth factor-induced transformation of human A549 lung carcinoma cells. Mol Cell Biol. 1999;19:1938–49. doi: 10.1128/mcb.19.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–6. [PubMed] [Google Scholar]
- Bunting K, Rao S, Hardy K, Woltring D, Denyer GS, Wang J, et al. Genome-wide analysis of gene expression in T cells to identify targets of the NF- B transcription factor c-Rel. J Immunol. 2007;178:7097–109. doi: 10.4049/jimmunol.178.11.7097. [DOI] [PubMed] [Google Scholar]
- Chen N, Nomura M, She QB, Ma WY, Bode AM, Wang L, et al. Suppression of skin tumorigenesis in c-Jun NH2-terminal kinase-2-deficient mice. Cancer Res. 2001;61:3908–12. [PubMed] [Google Scholar]
- Corzo C, Corominas JM, Tusquets I, Salido M, Bellet M, Fabregat X, et al. The MYC oncogene in breast cancer progression: from benign epithelium to invasive carcinoma. Cancer Genet Cytogenet. 2006;165:151–6. doi: 10.1016/j.cancergencyto.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–9. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF- B. Oncogene. 2006;25:6800–16. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- Fujii M, Minamino T, Nomura M, Miyamoto KI, Tanaka J, Seiki M. Selective activation of the proto-oncogene c-jun promoter by the transforming protein v-Rel. Oncogene. 1996;12:2193–202. [PubMed] [Google Scholar]
- Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene. 1999;18:6925–37. doi: 10.1038/sj.onc.1203222. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Kalaitzidis D, Liang MC, Starczynowski DT. The c-Rel transcription factor and B-cell proliferation: a deal with the devil. Oncogene. 2004;23:2275–86. doi: 10.1038/sj.onc.1207410. [DOI] [PubMed] [Google Scholar]
- Gupta N, Delrow J, Drawid A, Sengupta AM, Fan G, Gelinas C. Repression of B-cell linker (BLNK) and B-cell adaptor for phosphoinositide 3-kinase (BCAP) is important for lymphocyte transformation by rel proteins. Cancer Res. 2008;68:808–14. doi: 10.1158/0008-5472.CAN-07-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF- B. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Holtmann H, Winzen R, Holland P, Eickemeier S, Hoffmann E, Wallach D, et al. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol Cell Biol. 1999;19:6742–53. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben R, Vetter-Kauczok CS, Ortmann S, Rapp UR, Broecker EB, Becker JC. Phospho-ERK staining is a poor indicator of the mutational status of BRAF and NRAS in human melanoma. J Invest Dermatol. 2008;128:2003–12. doi: 10.1038/jid.2008.30. [DOI] [PubMed] [Google Scholar]
- Karakosta A, Golias C, Charalabopoulos A, Peschos D, Batistatou A, Charalabopoulos K. Genetic models of human cancer as a multistep process. Paradigm models of colorectal cancer, breast cancer, and chronic myelogenous and acute lymphoblastic leukaemia. J Exp Clin Cancer Res. 2005;24:505–14. [PubMed] [Google Scholar]
- Karin M. Nuclear factor- B in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Sluss HK, Jones SN, Bar-Sagi D, Flavell RA, Davis RJ. Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev. 2003;17:629–37. doi: 10.1101/gad.1062903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralova J, Liss AS, Bargmann W, Bose HR., Jr AP-1 factors play an important role in transformation induced by the v-rel oncogene. Mol Cell Biol. 1998;18:2997–3009. doi: 10.1128/mcb.18.5.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralova J, Schatzle JD, Liss AS, Bargmann W, Bose HR., Jr Synergistic stimulation of avian I Ba transcription by rel and fos/jun factors. Oncogene. 1996;12:2595–604. [PubMed] [Google Scholar]
- Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- Liss AS, Tiwari R, Kralova J, Bose HR., Jr Cell transformation by v-Rel reveals distinct roles of AP-1 family members in oncogenesis by Rel/NF- B. 2010 doi: 10.1038/onc.2010.239. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid SM, Liss AS, You M, Bose HR., Jr The suppression of SH3BGRL is important for v-Rel-mediated transformation. Oncogene. 2006;25:756–68. doi: 10.1038/sj.onc.1209107. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–70. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–75. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Nehyba J, Hrdlicková R, Bose HR., Jr Differences in B DNA-binding properties of v-Rel and c-Rel are the result of oncogenic mutations in three distinct functional regions of the Rel protein. Oncogene. 1997;14:2881–97. doi: 10.1038/sj.onc.1201150. [DOI] [PubMed] [Google Scholar]
- Nelson DM, Wahlfors JJ, Chen L, Onodera M, Morgan RA. Characterization of diverse viral vector preparations, using a simple and rapid whole-virion dot-blot method. Hum Gene Ther. 1998;9:2401–5. doi: 10.1089/hum.1998.9.16-2401. [DOI] [PubMed] [Google Scholar]
- Nielsen C, Thastrup J, Bottzauw T, Jaattela M, Kallunki T. c-Jun NH2-terminal kinase 2 is required for Ras transformation independently of activator protein 1. Cancer Res. 2007;67:178–85. doi: 10.1158/0008-5472.CAN-06-2801. [DOI] [PubMed] [Google Scholar]
- Okuno H, Suzuki T, Yoshida T, Hashimoto Y, Curran T, Iba H. Inhibition of jun transformation by a mutated fos gene: design of an anti-oncogene. Oncogene. 1991;6:1491–7. [PubMed] [Google Scholar]
- Oleinik NV, Krupenko NI, Krupenko SA. Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway. Oncogene. 2007;26:7222–30. doi: 10.1038/sj.onc.1210526. [DOI] [PubMed] [Google Scholar]
- Potapova O, Anisimov SV, Gorospe M, Dougherty RH, Gaarde WA, Boheler KR, et al. Targets of c-Jun NH2-terminal kinase 2-mediated tumor growth regulation revealed by serial analysis of gene expression. Cancer Res. 2002;62:3257–63. [PubMed] [Google Scholar]
- Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, et al. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci USA. 2009;106:4519–24. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Stippec SA, Goldsmith E, White MA, Cobb MH. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol. 1998;8:1141–50. doi: 10.1016/s0960-9822(07)00485-x. [DOI] [PubMed] [Google Scholar]
- Sewing A, Wiseman B, Lloyd AC, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–97. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–6. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Skarpen E, Flinder LI, Rosseland CM, Orstavik S, Wierød L, Oksvold MP, et al. MEK1 and MEK2 regulate distinct functions by sorting ERK2 to different intracellular compartments. FASEB J. 2008;22:466–76. doi: 10.1096/fj.07-8650com. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Weber TJ, Colburn NH. AP-1, NF- B, and ERK activation thresholds for promotion of neoplastic transformation in the mouse epidermal JB6 model. Environ Health Perspect. 2002;110:865–70. doi: 10.1289/ehp.02110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavachidou D, Coleman ML, Athanasiadis G, Li S, Licht JD, Olson MF, et al. SPRY2 is an inhibitor of the ras/extracellular signal-regulated kinase pathway in melanocytes and melanoma cells with wild-type BRAF but not with the V599E mutant. Cancer Res. 2004;64:5556–9. doi: 10.1158/0008-5472.CAN-04-1669. [DOI] [PubMed] [Google Scholar]
- Ussar S, Voss T. MEK1 and MEK2, different regulators of the G1/S transition. J Biol Chem. 2004;279:43861–9. doi: 10.1074/jbc.M406240200. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26:3172–84. doi: 10.1038/sj.onc.1210410. [DOI] [PubMed] [Google Scholar]
- Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Bost F, Charbono W, Dean N, McKay R, Rhim JS, et al. c-Jun NH2-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res. 2003;9:391–401. [PubMed] [Google Scholar]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- Zheng C, Xiang J, Hunter T, Lin A. The JNKK2-JNK1 fusion protein acts as a constitutively active c-Jun kinase that stimulates c-Jun transcription activity. J Biol Chem. 1999;274:28966–71. doi: 10.1074/jbc.274.41.28966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.