Abstract
BACKGROUND AND PURPOSE
Calcitonin gene-related peptide (CGRP) is widely distributed in the trigeminovascular system and released from sensory fibres of the cranial dura mater upon noxious stimulation. Such release may be a mechanism underlying migraine headache. Based on data from guinea pig basilar artery preparations, we have here studied CGRP release and uptake in an organ preparation of the hemisected rat skull.
EXPERIMENTAL APPROACH
CGRP release from the cranial dura was quantified by a commercial enzyme-linked immunoassay. CGRP was depleted using repetitive challenges of capsaicin. After incubating the tissue with CGRP for 20 min and extensive washing, another capsaicin challenge was performed. Immunohistochemistry was used to visualize CGRP immunofluorescence in dural nerve fibres.
KEY RESULTS
Capsaicin-induced CGRP release was attenuated by the transient receptor potential vanilloid receptor type I antagonist capsazepine or by Ca2+-free solutions. After the CGRP-depleted preparation had been exposed to exogenous CGRP, capsaicin-induced CGRP release was increased compared to the challenge just prior to incubation. CGRP uptake was not influenced by Ca2+-free solutions. Olcegepant and CGRP8–37 (CGRP receptor antagonists) did not affect uptake of CGRP. However, a monoclonal CGRP-binding antibody decreased CGRP uptake significantly. Release of CGRP after incubation was attenuated by Ca2+-free solutions and by capsazepine. Immunohistochemical assays indicated a weak trend towards CGRP uptake in rat dura mater.
CONCLUSION AND IMPLICATIONS
We have presented evidence for CGRP uptake in nerves and its re-release in rat dura mater. This may have implications for the pathophysiology and treatment of migraine.
Keywords: CGRP, CGRP uptake, dura mater, capsaicin, olcegepant, Ca2+-free solution
Introduction
Calcitonin gene-related peptide (CGRP) is a naturally occurring 37 amino acid peptide belonging to the calcitonin family and is widely distributed in the central and peripheral nervous systems (Juaneda et al., 2000; Brain and Grant, 2004). Dural and pial vessels are richly supplied with CGRP-containing nerve terminals originating from all divisions of the trigeminal nerve (McCulloch et al., 1986; Suzuki et al., 1989; Lennerz et al., 2008). The CGRP receptor calcitonin receptor-like receptor (CALCRL) is a G protein-coupled receptor related to the calcitonin receptor (nomenclature follows Alexander et al., 2009). It is linked to a receptor activity-modifying protein type I (RAMP1) that is essential for functional activity (Alexander et al., 2009). CGRP receptor proteins have been localized in the cranial vasculature, the trigeminal ganglion and in the trigeminal nucleus caudalis by immunohistochemistry (Uddman et al., 1999; Ma et al., 2003; Lennerz et al., 2008), and binding sites for CGRP have been found in many other brain areas (van Rossum et al., 1997). CGRP is stored in vesicles of C and Aδ fibres and released via exocytosis in response to chemical or electrical stimulation (McCulloch et al., 1986; Jansen et al., 1990; Kummer, 1992). CGRP infusion causes migraine-like headaches in migraineurs (Lassen et al., 2002) and CGRP receptor antagonists are effective in the treatment of migraine attacks (Olesen et al., 2004; Ho et al., 2008). Thus, CGRP seems to play a role in migraine and thus it is important to understand the factors that determine CGRP homeostasis in physiological as well as in pathological conditions.
Peptide signalling molecules including CGRP are metabolized in the extracellular space and are not thought to be recycled by uptake. However, in one of our previous studies (Sams-Nielsen et al., 2001), capsaicin, a transient receptor potential vanilloid receptor type I (TRPV1) agonist, was used to successively elicit relaxations in pre-contracted guinea-pig artery by releasing CGRP. There was a gradual decrease in the magnitude of relaxations during successive challenges, which could have been due to progressive neuropeptide depletion. These functional responses to capsaicin recovered after incubation with exogenous CGRP, suggesting the possibility of CGRP uptake into neuronal stores.
In the present study, we measured the CGRP released from dura mater encephali using the hemisected rat skull model and enzyme-linked immunosorbent assay (elisa). We also explored the mechanism of CGRP release, after exposure to exogenous CGRP. We believe our data indicated a putative re-uptake of endogenous CGRP being released from sensory nerve terminals. Significant uptake of CGRP was observed, which was blocked by simultaneous incubation with antibodies directed against CGRP, but not by CGRP receptor antagonists. The release of CGRP induced by capsaicin, subsequent to the uptake, was sensitive to extracellular Ca2+ concentration and capsazepine. The existence of a CGRP uptake process would add a new dimension to our understanding of the physiological and pathological role of this neuropeptide.
Methods
Experimental set up
All animal care and experimental procedures were in accordance with domestic guidelines and regulations for animal care and treatment. The study protocol was approved by The Danish Animal Experimentation Inspectorate (file: 2004/561–850 and 2009/561–1664). Male Sprague–Dawley rats used for the present study were purchased from Taconic Europe (Tornbjergvej 40, Ejby, Denmark). They were housed under a standard light and dark cycle and given free access to food and water. A total of 110 rats (body weight 300–430 g) were used for the study.
The rats were killed by CO2 inhalation, decapitated and prepared as described earlier (Ebersberger et al., 1999). Briefly, the skull was cut mid-sagittally and the brain halves were carefully removed while the dura mater was left attached to the skull. The two skull halves with intact dura mater were continuously superfused at room temperature for a minimum of 30 min with 500 mL of synthetic interstitial fluid (SIF) of the following composition (in mM): 108 NaCl, 3.48 KCl, 3.5 MgSO4, 26 NaHCO3, 11.7 NaH2PO4, 1.5 CaCl2, 9.6 Na+ gluconate, 5.55 glucose and 7.6 sucrose, pH 7.4; and gassed with carbogen (95% O2 and 5% CO2). Both the skull halves were placed on separate platforms and placed in a humid chamber above a water bath to maintain a temperature of 37°C. The cavities were washed (using a micropipette) without touching the dura mater five times with 350 µL SIF, the solution covering the supratentorial dura mater but not the posterior fossa. As a standard protocol, 200 µL of the sample was collected as control after the fifth wash. This control sample provided us with the basal CGRP release in each animal. Although there was some variation in the basal CGRP level obtained from different animals, the CGRP levels detected in the two skull halves obtained from the same animal were similar. Comparing the basal release in the two skull halves to each other using the non-parametric paired Wilcoxon matched-pairs signed rank test showed no significant difference between skull halves (P > 0.05). Thus, it was possible to use one skull half as a control for the other half, reducing experimental variations.
Effect of capsaicin on CGRP release
Capsaicin was added at increasing concentrations from 1 nM to 1 µM to the skull cavities and SIF was collected after 10 min incubation for analysis. Pilot experiments showed that this incubation time with capsaicin was optimal in releasing reproducible amounts of CGRP. In case of experiments with antagonists, the antagonist was allowed to equilibrate within the skull cavity for 10 min before the addition of a standard concentration of capsaicin (100 nM). To study the role of extracellular Ca2+ in capsaicin-mediated release of CGRP, a similar SIF without CaCl2 was used. The TRPV1 antagonist capsazepine was used to block capsaicin responses at 1 and 10 µM concentrations.
CGRP depletion and CGRP uptake
For CGRP depletion, four consecutive challenges of capsaicin (100 nM) were applied before incubation with CGRP (100 nM) for 20 min. This was followed by 12 washes with SIF at intervals of 10 min, in order to remove exogenous CGRP. Then another control sample was taken, followed by another 100 nM capsaicin challenge. In order to confirm the concentration dependence of CGRP uptake, 10 nM and 1 µM of CGRP were also used in uptake experiments. We also used a higher concentration of capsaicin (1 µM) to deplete CGRP, but after this concentration there was no significant uptake.
To explore whether the capsaicin-stimulated increase in CGRP release after CGRP incubation was due to re-uptake from exogenous CGRP or due to the mobilization from endogenous CGRP pools, we incubated skull cavities with vehicle for 20 min. In a separate protocol, we also used eight successive 60 mM KCl challenges to deplete CGRP and followed the same protocol for CGRP uptake, as described with capsaicin.
Blockade of CGRP uptake and release of CGRP after uptake
The CGRP receptor (CALCRL + RAMP1) (Alexander et al., 2009) antagonist olcegepant (10 µM) and CGRP8–37 (10 µM) were added 10 min prior to CGRP and were allowed a 20 min incubation to study the role of CGRP receptors in CGRP uptake. In order to confirm that CGRP was being taken up, we incubated CGRP along with a humanized monoclonal CGRP-binding antibody that was raised in rabbit (Edvinsson et al., 2007) in the skull at increasing concentrations (12 and 48 µg·mL−1). The CGRP antibody scavenges CGRP, and this complex is unable to activate CGRP receptors (Juhl et al., 2007). These experiments were carried out in a paired manner with one skull half acting as a control for the treated half. Capsazepine (1 or 10 µM) was incubated for 10 min before challenging with capsaicin. Capsazepine and Ca2+-free SIF were used to evaluate the role of the TRPV1 receptors and extracellular Ca2+, respectively, in the release of exogenously mobilized CGRP. TRPV1 receptors are susceptible to desensitization (Mandadi et al., 2004) and the CGRP depletion protocol with multiple capsaicin challenges can lead to such a phenomenon. Therefore we adopted a different experimental design, in which four successive capsaicin challenges were followed by three KCl challenges, as KCl challenges have been reported to reverse desensitization as CGRP-mediated effects were restored after KCl challenges (Sheykhzade and Nyborg, 1998).
CGRP uptake was also examined in hemisected skulls not subjected to CGRP depletion with capsaicin. After the collection of the first basal sample, the skull halves were incubated with 100 nM CGRP for 20 min and then washed as described previously to rinse out exogenous CGRP. After collection of the second basal sample, CGRP release was induced with 30 nM capsaicin.
Measurement of CGRP levels
For measurement of the immunoreactive CGRP content in the samples, elisa kits (SPIbio, Paris, France) were used. In order to prevent CGRP degradation, the samples were immediately transferred to a vial containing elisa buffer with peptidase inhibitors. Samples were stored at −20°C and analysed within 1 week. The antibody in the immunoreactive CGRP (iCGRP) elisa kit is directed against human CGRP α/β but has 100% cross-reactivity to rat and mouse CGRP. The iCGRP detection limit is about 2 pg·mL−1. The protocol provided with the kit was carried out in detail. In short, the addition of 100 µL sample was followed by addition of 100 µL of the tracer (provided with the kit). Reaction was incubated at 4°C for 16–20 h. After carefully washing off the supernatant, wells were incubated with 200 µL of Ellman's reagent. The wells were covered with an aluminium sheet and placed in a dark room for 60 min at room temperature before measuring the optical density, which is directly proportional to the CGRP content of the sample. The optical density was measured at 410 nm using a microplate photometer (Tecan, infinite M200, software SW Magellan v.6.3, Männedorf, Switzerland). Standards with defined CGRP concentrations were treated accordingly and used to calibrate the curve of optical density. Blanks without CGRP were used as a control to exclude possible false-positive measurements.
Immunohistochemistry
After CGRP was depleted with capsaicin or depleted followed by incubation with exogenous CGRP as described above, some skulls with adhering dura mater were filled with paraformaldehyde (4%) in phosphate buffer for 2 h to fix the dura mater. For controls, other preparations were fixed without capsaicin challenge.
The fixed dura mater was carefully separated from the skull and pre-incubated with a solution of 5% normal goat serum (Dianova, Hamburg, Germany) containing 0.5% Triton X-100 and 1% bovine serum albumin (BSA) (Sigma-Aldrich, Steinheim, Germany) in cold phosphate-buffered saline (PBS) for 2 h. After rinsing in PBS (10 min), the whole mount was incubated with a solution of primary antibodies directed against CGRP (rabbit anti-α-CGRP in PBS, 1:1000; Peninsula T-4032, Bachem AG, Bubendorf, Switzerland) with 1% BSA and 0.5% Triton X-100 overnight in the refrigerator. After extensive rinsing in PBS (five times, several hours) the tissue was incubated with the secondary Cy3-conjugated antibody directed against rabbit IgG (goat anti-rabbit serum in PBS, 1:200; Dianova 111-165-144) with 1% BSA and 0.5% Triton X-100 for 2 h in the dark. The specificity of the primary and secondary antibodies has been tested in previous experiments in rat dura mater (Lennerz et al., 2008). After rinsing in PBS (five times) the stained dura mater was flattened by radial incisions, placed on a slide and cover-slipped with PBS-glycerin (pH 8.6).
Stainings were observed with a Leica Aristoplan epifluorescence microscope (Leica, Bensheim, Germany) using filter block N2.1 for red emission (ALEXA Fluor® 555, Leica Microsystems, Wetzlar, Germany) connected to a CCT camera (Spot RT, Visitron Systems, Puchheim, Germany). Images of 1600 × 1200 pixels were obtained with Spot advanced (Visitron Systems) at a fixed magnification and stored in an 8 bit grey-level TIFF file. For quantification of fluorescent structures 12 images were randomly selected from different parts of the each whole mount preparation. Photo-Paint X3 (Corel, Dublin, Ireland) was used to enhance contrast and intensity of the fluorescence and to eliminate fluorescent artefacts like mast cells (see Figure 8A). Image acquisition and processing was carried out without knowledge of the pre-treatment of preparations. The immunofluorescence was quantified using the software ImageJ (version 1.43, http://rsbweb.nih.gov/ij/download.html). After adjusting the threshold of grey density (auto threshold) to generate a black and white image (nerve fibres in black), the Analyze Particles function was used to determine the mean area and light intensity (inverted grey density) of fluorescent structures with a minimal size of 4 pixels. Mean values of these data were calculated for each whole mount.
Figure 8.

Fluorescence images of calcitonin gene-related peptide (CGRP) immunoreactive nerve fibers in the rat dura mater. Compared to an untreated control dura (A, panel a) the nerve fibers show a clear ‘string of pearls’ appearance after depletion of CGRP with capsaicin (B, panel b), which may indicate local clustering of CGRP within the axon. After incubation with CGRP (C, panel c) immunopositive varicosities seem to be more prominent as compared to depleted nerve fibers incubated with vehicle (B, panel b). Mast cells cross-reacting with the antibody as seen in the control (lower right in A) are no longer visible after capsaicin treatment. Magnification is the same in all images, size bar 100 µm. On the right panel, three equally magnified (3×) sections (a–c) of the corresponding left panels (A–C) (n = 4).
Data analysis
The released CGRP (measured as immunoreactive CGRP in the samples) is expressed in pg·mL−1, given as mean values ± SEM. Absorbance was recorded and values were calculated through an interpolation method using an equation derived from the standard curve. The blank values were subtracted from the sample values. The EC50 value was calculated from capsaicin-mediated CGRP release. anova (Kruskal–Wallis test) was conducted, followed by Dunnett's multiple comparison test on various concentration-response values to determine whether the responses were significantly different from the control. Wilcoxon matched-pairs test was used for non-parametric analysis of paired data and the Mann–Whitney U-test for analysis of non-paired data. Differences were considered significant if P < 0.05. GraphPad Prism (GraphPad Prism Software Inc., San Diego, CA, USA) or Statistica 7 (StatSoft, Tulsa, OK, USA) was used for statistical analysis.
Materials
Rat αCGRP and rat CGRP8–37 were obtained from NeoMPS (Strasbourg, France) and olcegepant ([[R-(R,(R*,S*)]-N-[2-[[5amino-1-[[4-(4-pyridinyl)-1-piperazinyl]-carbonyl] pentyl] amino]-1-[3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)-,1-Piperidinecarboxamide]) was kindly provided by Prof Lars Edvinsson. The humanized monoclonal CGRP-binding antibody raised in rabbit was obtained from Rinat Neuroscience, Palo Alto, CA, USA. All test substances, except capsaicin and capsazepine, were dissolved in isotonic saline (0.9% NaCl) and stored at −20°C. Capsaicin (Sigma-Aldrich, Schnelldorf, Germany) was dissolved in 90% ethanol as a stock solution of 0.01 M and further dilutions were prepared in SIF. Capsazepine (Sigma-Aldrich, Schnelldorf) was dissolved in methanol to obtain a 10−2 M stock and further dilutions were done in 99% ethanol. Prior to use, the samples were diluted in SIF to the final concentration.
Results
Effect of capsaicin on CGRP release
Basal CGRP levels in the skull halves were 13.54 ± 1.2 pg·mL−1 without any treatment. Capsaicin (1 nM–1 µM) increased CGRP release from the skull cavities in a concentration-dependent manner (Figure 1A). The increase in CGRP release was significant at 100 nM capsaicin and higher concentrations. At the highest concentration of capsaicin (1 µM) there was a more than five-fold increase, compared to the release at the basal level. Capsazepine (1–10 µM) blocked capsaicin-mediated CGRP release in a concentration-dependent manner (Figure 1B). CGRP release following capsaicin (100 nM) was not attenuated in presence of olcegepant (10 µM) (control: 250 ± 40 pg·mL−1; with olcegepant: 299 ± 26 pg·mL−1, n = 6). In Ca2+-free SIF, the basal and capsaicin-induced CGRP release was significantly decreased (Figure 2).
Figure 1.

Calcitonin gene-related peptide (CGRP) release in response to capsaicin stimulation (10 min) at different concentrations in rat hemisected skull (A) and effect of capsazepine incubated in the skull for 10 min before capsaicin-induced CGRP release (B). **P < 0.01 and ***P < 0.0001, significantly different from basal value; ##P < 0.01, significantly different from capsaicin without capsazepine. One-way anova followed by Dunnett's multiple comparison test (n = 5−11) (A) and the non-parametric paired Wilcoxon signed-rank test was used (n = 4−7) (B).
Figure 2.

Effect of Ca2+-free synthetic interstitial fluid (SIF) on basal calcitonin gene-related peptide (CGRP) levels (A) and on capsaicin-induced CGRP release (B). For statistical analysis the non-parametric paired Wilcoxon signed-rank test was used. **P < 0.01 and ***P < 0.0001, significantly different from normal SIF group (n = 10−12).
Interestingly, in studies of capsaicin-mediated CGRP release in the presence of CGRP8–37 (10 µM), we could not detect the expected increase in CGRP levels after the capsaicin challenge (data not shown). To understand this observation we incubated exogenous CGRP directly in the elisa plate with CGRP8–37. We found a concentration-dependent decrease in the CGRP signal with increasing concentrations of CGRP8–37. CGRP8–37, which lacks the N-terminal amino acids of the native peptide, cross-reacts with the CGRP assay and thus interfered with CGRP detection and diminished its signal.
CGRP depletion and CGRP re-uptake
In initial experiments, we used capsaicin 1 µM for depletion of CGRP, and the second exposure to capsaicin led to significantly lower release of CGRP (from 200 ± 33 to 36 ± 9 pg·mL−1, respectively, n = 6). After the third capsaicin challenge, we incubated with CGRP 10 nM or 100 nM. In neither case was there a significant recovery of capsaicin-induced CGRP release after incubation. Subsequently, we used four consecutive challenges of capsaicin (100 nM) for depletion. The amount of released CGRP dropped from 106 ± 21 pg·mL−1 at the first challenge to 20 ± 1 pg·mL−1 in the fourth challenge (Figure 3A). After incubation with CGRP (100 nM) for 20 min and extensive washing, the basal CGRP release was restored (no difference from the initial basal value). The subsequent capsaicin challenge caused a significant increase (P < 0.05) in CGRP release to 52 ± 8 pg·mL−1, which was 163 ± 47% more than the CGRP release after the fourth capsaicin challenge. Incubation with vehicle (SIF) over a similar time was not followed by a significant increase in CGRP release induced by capsaicin (Figure 3B). In addition to 100 nM, we also used 10 nM and 1 µM CGRP for incubation. With 10 nM CGRP there was no effect, whereas incubation with 1 µM CGRP was followed by an elevated CGRP release (153 ± 15%, n = 6) evoked by capsaicin. As there was no difference in CGRP release following incubation at CGRP concentrations higher than 100 nM, we opted for this concentration in further experiments. We assume that at this concentration part of the CGRP was taken up into the nerve endings, leading to an elevated release induced by the next capsaicin challenge.
Figure 3.

Calcitonin gene-related peptide (CGRP) release in response to four successive challenges of capsaicin (100 nM). This was followed by incubation with exogenous CGRP 100 nM (A) or synthetic interstitial fluid (SIF) (B). After extensive washing, a fifth capsaicin challenge was used to test if CGRP was taken up. For statistical analysis the non-parametric paired Wilcoxon signed-rank test was used. **P < 0.01 and ***P < 0.0001, significantly different from basal CGRP levels; $P < 0.05 and $$P < 0.01, significantly different from first challenge; #P < 0.05, significantly different from fourth challenge (n = 6).
Experiments with a depolarizing solution of 60 mM KCl indicated that four challenges as performed previously with capsaicin were not sufficient but eight successive challenges with KCl did deplete CGRP. After a subsequent 20 min incubation even with the vehicle (SIF), there was a significant increase in KCl-induced CGRP release (22 ± 3 pg·mL−1) as compared to the response to the eighth KCl challenge immediately before incubation (12 ± 1 pg·mL−1, n = 6 each). This may indicate that CGRP is being mobilized from endogenous pools but not taken up, as indicated in the experiments with capsaicin. In order to investigate if there is a difference in CGRP depletion induced by capsaicin and that induced by KCl, the skulls were either depleted by four capsaicin challenges followed by a KCl challenge or by eight KCl challenges followed by capsaicin. When KCl was given after capsaicin-induced CGRP depletion, no difference in CGRP release was found between the last capsaicin challenge and the KCl challenge (Figure 4A). In contrast, when capsaicin was given following depletion by eight KCl challenges there was a significant increase (229 ± 25%) in CGRP release as compared to the eighth KCl challenge (Figure 4B).
Figure 4.
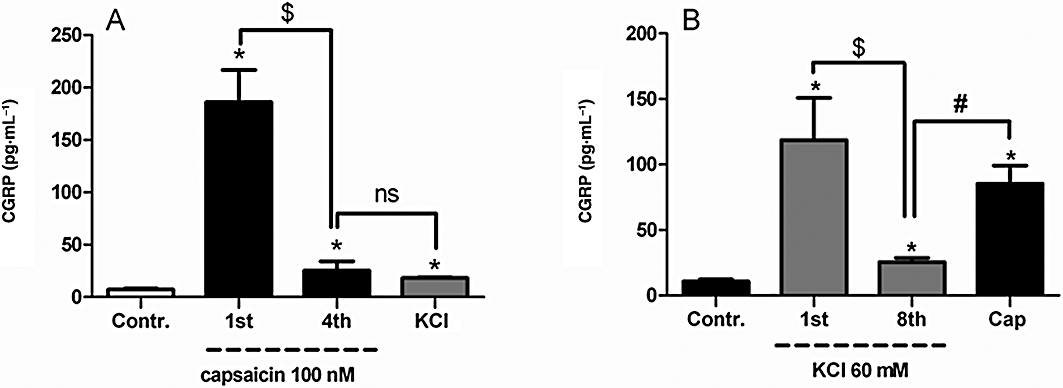
Calcitonin gene-related peptide (CGRP) release in response to four successive capsaicin (100 nM) challenges to deplete CGRP followed by one KCl (60 mM) challenge (A), or eight successive KCl (60 mM) challenges to deplete CGRP followed by one capsaicin (100 nM) challenge (B). For statistical analysis the non-parametric paired Wilcoxon signed-rank test was used. *P < 0.05, significantly different from basal CGRP levels; $P < 0.05, significantly different from first capsaicin or first KCl challenge; #P < 0.05, significantly different from fourth capsaicin or eighth KCl challenge (n = 6). Contr., control; ns, not significant.
Blockade of CGRP re-uptake and conditions of CGRP release after re-uptake
When the CGRP receptor antagonists – olcegepant or CGRP8–37– were incubated together with CGRP, there was still a significant increase in capsaicin-evoked CGRP release. After olcegepant incubation with CGRP there was a significant (P < 0.05) increase in capsaicin-induced CGRP release from 33 ± 3 to 54 ± 5 pg·mL−1n = 6, and similarly for CGRP8–37 the release increased from 20 ± 4 to 40 ± 6 pg·mL−1. In contrast, when the CGRP antibody (12 µg·mL−1 or 48 µg·mL−1) was incubated along with CGRP, a concentration-dependent suppression of the capsaicin-evoked CGRP increase was observed (Figure 5). After CGRP incubation in Ca2+-free SIF, there was no difference in CGRP release as compared to control (Figure 6A). Following CGRP depletion with capsaicin and subsequent incubation with CGRP, the release of CGRP induced by capsaicin was completely blocked when the challenge was performed in calcium-free SIF (Figure 6B). However, the CGRP release in response to capsaicin after CGRP incubation was not blocked by capsazepine (10 µM). The capsaicin challenge before incubation released 24 ± 1 pg·mL−1 CGRP, while subsequent to CGRP incubation the increase (61 ± 4 pg·mL−1, n = 8) was significant, even in the presence of capsazepine. In order to investigate if the lack of capsazepine-induced blockade was due to desensitization caused by multiple capsaicin challenges (Mandadi et al., 2004), another protocol was designed. Four capsaicin (100 nM) challenges (challenges 1–4) were followed by three KCl (60 mM) challenges (challenges 5–7) before CGRP incubation. The subsequent eighth challenge induced by capsaicin increased CGRP release, which was significantly blocked by capsazepine (Figure 7).
Figure 5.

Effect of simultaneous incubation of the calcitonin gene-related peptide (CGRP) antibody (Ab) 12 µg·mL−1 (A) and 48 µg·mL−1 (B) with CGRP (100 nM) on capsaicin-induced CGRP release subsequent to uptake. For statistical analysis the non-parametric paired Wilcoxon signed-rank test was used. #P < 0.05, significantly different from fourth challenge and *P < 0.05, significantly different from fifth capsaicin challenge in absence of antibody incubation (n = 6−8). ns, not significant.
Figure 6.

Effect of calcitonin gene-related peptide (CGRP) incubation in Ca2+-free synthetic interstitial fluid (SIF) on capsaicin-induced CGRP release in normal SIF (A) and capsaicin challenge in Ca2+-free SIF, subsequent to CGRP incubation in normal SIF (B). For statistical analysis the non-parametric paired Wilcoxon signed-rank test was used. ##P < 0.01 and ###P < 0.0001, significantly different from fourth challenge (n = 6−8).
Figure 7.

Effect of capsazepine (CPZ) on capsaicin (Caps)-induced calcitonin gene-related peptide (CGRP) release (eighth challenge) subsequent to depletion by four capsaicin challenges (1–4) and three KCl challenges (fifth to seventh) followed by 20 min incubation with CGRP and washing. For statistical analysis the non-parametric paired Wilcoxon signed-rank test was used. #P < 0.05, significantly different from the seventh challenge; *P < 0.05, significantly different from capsaicin challenge in absence of capsazepine (n = 6). ns, not significant.
In naïve skull halves (not depleted with capsaicin or KCl) there was a trend towards higher CGRP release (∼42%, n = 8) when incubated with CGRP as compared to their respective vehicle controls, although results did not reach significance.
Immunohistochemistry
CGRP immunoreactive fibres were abundantly observed in the dura mater, most of them running along dural blood vessels (Figure 8A, panel a). After four capsaicin challenges, the smooth appearance of the CGRP-immunofluorescence of the nerve fibres was transformed into a ‘string of pearls’ form and appeared sometimes weaker in fluorescence intensity than untreated segments (Figure 8B, panel b). After incubation with CGRP, subsequent to the fourth capsaicin challenge, the ‘string of pearls’ appearance seemed to be more pronounced (Figure 8C, panel c). Quantification of the fluorescence partly confirmed these impressions. The mean size of fluorescent particles decreased after capsaicin treatment; however, there was no significant reversal effect after CGRP incubation (Figure 9A). The mean intensity of fluorescence (i.e. inverse grey density) was not significantly different between the three groups, though after CGRP incubation the responses tended to return to control values (Figure 9B).
Figure 9.

Quantitative data on calcitonin gene-related peptide (CGRP) immunofluorescence in dura mater of control preparations and preparations treated with capsaicin or capsaicin and CGRP. Each mean (±SEM) is from measurements of 48 images in four dura halves. Average size of fluorescent particles (A) and intensity of fluorescence are shown as inverse grey density (B). *P < 0.05, significant difference between control and capsaicin-treated but not capsaicin-/CGRP-treated preparations (Mann–Whitney U-test).
Discussion
Uptake of neuropeptides into nerve fibres has not been reported earlier. In the present study we demonstrate CGRP re-uptake in rat meninges after CGRP depletion.
Effect of capsaicin on CGRP release
The noxious agent capsaicin, induced CGRP release in a concentration-dependent manner through activation of TRPV1 receptors, as was demonstrated by the TRPV1 antagonist capsazepine, which blocked this effect in agreement with our previous findings (Jansen et al., 1990; Jansen-Olesen et al., 1996). The release was significantly attenuated by Ca2+-free solutions, since opening of TRPV1, a non-selective cation channel, leads to an increase in intracellular Ca2+ only if the Ca2+ gradient is sufficiently high (Erdelyi et al., 1987).
CGRP depletion and CGRP uptake
In initial experiments, repeated challenges with 1 µM capsaicin caused a drastic decrease in CGRP release and, after incubation with CGRP, the following capsaicin challenge induced no significant increase in CGRP release. The high capsaicin concentrations are reported to change the membrane integrity/elasticity by altering the membrane lipid bi-layer (Lundbaek et al., 2005). In isolated porcine and human arteries, capsaicin-mediated relaxations at high capsaicin concentrations (>1 µM) were non-specific and were not mediated via CGRP, substance P or TRPV1 receptors (Gupta et al., 2007). In cat pial arterioles, capsaicin induced a dual response, a dilatation mediated via CGRP release, followed by a contraction mediated via another mechanism (Edvinsson et al., 1990). Rat meningeal blood flow increased when 100 nM capsaicin was applied topically, whereas higher concentrations – 1 and 10 µM – significantly decreased the blood flow (Dux et al., 2003). Therefore, caution should be advised when high concentrations (≥1 µM) of capsaicin are used. However, four consecutive 100 nM capsaicin challenges followed by CGRP incubation (10 nM–1000 nM) led to a significant concentration-dependent increase in CGRP release upon the fifth capsaicin challenge as compared to the fourth challenge. We assume that this phenomenon depends on an uptake of CGRP into the nerve endings.
Differences in CGRP depletion between capsaicin and KCl
After repeated exposure to capsaicin, KCl (60 mM) did not release further CGRP (see Figure 4A). However, after repeated exposure to KCl, capsaicin could still release a considerable amount of CGRP (see Figure 4B). This may suggest that CGRP is localized in two different pools: one sensitive to KCl and capsaicin and another only sensitive to capsaicin. KCl causes depolarization of the nerve fibres, thereby opening voltage-sensitive Ca2+ channels and inducing Ca2+ influx, which activates the CGRP release mechanism without opening TRPV1 receptor channels. Capsaicin activates TRPV1 receptor channels that are cation conducting without being dependent upon the initial membrane depolarization. Similarly, in rat spinal cord slices in continuous presence of KCl, capsaicin could still increase CGRP release, while reversing the stimulation did not yield an increase in CGRP release (Donnerer and Amann, 1990). An additional explanation for the partial recovery in CGRP release following depletion by KCl may be that in this case the TRPV1 receptor channels are not desensitized. After depletion of CGRP by capsaicin, CGRP release increased only when the skull cavities were incubated with exogenous CGRP, indicating that CGRP was taken up (see Figure 3A). The process of CGRP re-uptake is not likely to be based on passive diffusion due to difference in the CGRP gradient between the extra- and intracellular compartment, as the basal release of CGRP after incubation was similar to the first basal release. If it was a passive process, then the diffused CGRP would have leaked out when the concentration gradient was reversed by washing out the exogenous CGRP.
Interestingly, the KCl-induced CGRP release, subsequent to CGRP depletion with eight KCl challenges, was partially restored even without incubation with exogenous CGRP, again suggesting mobilization of CGRP from different pools. Indeed, our experiments also show that KCl-depleted dura mater still releases CGRP upon capsaicin challenge, whereas the reverse is not true (Figure 4). Structural evidence for a redistribution of CGRP in dural sensory nerves after electrical stimulation has previously been shown (Messlinger et al., 1995).
Blockade and putative conditions of CGRP re-uptake
Lack of inhibition of CGRP re-uptake by the CGRP receptor antagonists olcegepant and CGRP8–37 indicates that CGRP receptors are not involved in this process, in contrast to the previous functional studies done in guinea-pig basilar artery (Sams-Nielsen et al., 2001). Differences could be attributed to the 100-fold higher capsaicin concentration used in the earlier study or to the differences in tissue and species. The lack of a receptor-mediated uptake in our experiments is consistent with a recent study, where immunoreactivity for CALCRL and RAMP1 was located on Schwann cells but not on axons of sensory nerves innervating the rat dura mater (Lennerz et al., 2008). CGRP8–37 (the C-terminal fragment, non-cyclic analogue, of CGRP, lacking the disulphide-bonded loop) interferes with the CGRP-detecting elisa. Thus, CGRP8–37 in the elisa will decrease the final signal (Frobert et al., 1999; Denekas et al., 2006). This interaction of CGRP8–37 in the elisa led us to speculate on the putative amino acid sequences of CGRP involved in the uptake. As CGRP8–37 did not affect the CGRP re-uptake, it is unlikely that the antagonist itself was taken up. Hence the sequence and/or conformation of the parent peptide, CGRP, may be an important prerequisite for the transportation possibly underlying the uptake. Thus, these seven amino acids at the N-terminus or the cyclic structure of the CGRP peptide might be the key structural prerequisite for the putative CGRP uptake.
In initial studies, capsazepine, a TRPV1 receptor antagonist, was not able to attenuate the release of CGRP from replenished stores. These observations are in agreement with our earlier study (Sams-Nielsen et al., 2001), where capsazepine did not block the capsaicin responses, probably because capsazepine was not able to recognize the receptor in a desensitized state. Depolarization with KCl has been reported to restore the sensitivity to receptors (Sheykhzade and Nyborg, 1998). After KCl depolarization we found that capsazepine blocked the capsaicin-induced CGRP release following incubation with CGRP. These observations underline the fact that TRPV1 receptors in the present experimental preparation were also susceptible to desensitization and that they could be reactivated by KCl-induced depolarization. Thus, we conclude that CGRP is taken up in vesicles or pools which are sensitive to capsaicin, capsazepine as well as extracellular Ca2+, like the CGRP pools in naïve/non-depleted preparations.
Our immunohistochemical studies showed CGRP-positive nerve fibres in the rat dura mater. After depletion of CGRP with capsaicin, these nerve fibres show a ‘string of pearls’ appearance, unlike the continuous pattern observed with nerves in the untreated skull. This may indicate local clustering of CGRP within the axon, a phenomenon that was earlier described after electrical stimulation of rat dura mater (Messlinger et al., 1995). In addition, the intensity of the CGRP immunostaining was weaker after capsaicin treatment. Following incubation with CGRP, the immunopositive varicosities were more prominent. These observations suggest that CGRP is taken up into nerve fibres, seemingly into similar pools where endogenous CGRP was already present.
Quantification of the fluorescence showed that the size of the fluorescent particles decreased significantly after capsaicin treatment. After the incubation with exogenous CGRP, there was only a weak tendency for particle size to increase in this preparation. Measuring the intensity of fluorescence did not show significant differences for any of the treatments. However, there was a tendency towards a higher fluorescence density after capsaicin treatment that was reversed after incubation with CGRP.
Possible relevance of CGRP uptake
Uptake mechanisms are typical of non-peptide neurotransmitters. There are well-characterized transporters for di- and tripeptides (Leibach and Ganapathy, 1996; Meredith and Boyd, 2000) but not for larger peptides. Interestingly, an anandamide membrane transporter required for anandamide actions on TRPV1 receptors has been shown in trigeminal ganglion cultures (Price et al., 2005). Application of anandamide transport inhibitors significantly reduced the potency and efficacy of anandamide- and capsaicin-evoked CGRP release (Price et al., 2005). Our findings suggest that large peptides like CGRP are not only taken up, but can also be re-released. Such uptake may be relevant in terminating CGRP signalling by removing the peptide. It might also be relevant in order to reuse the peptide and thus reduce the need for new synthesis. Like anandamide membrane transporters, putative CGRP transporters may have an important role in controlling the pharmacological actions of CGRP.
Limitations of the study
There was variation between baseline CGRP releases from skull halves of individual rats, and this is most probably due to differences in the fibre density between animals. However, the CGRP release from the skull halves from the same animal were very similar and thus, one of these always served as a control for the other half. The relevance of our in situ model needs to be established under in vivo conditions and studies with human tissue are necessary in order to come to any conclusions on the relevance of our present findings for human disease. Our earlier findings (Sams-Nielsen et al., 2001) have, however, already provided similar results in a different model and a different species and it therefore appears likely that similar mechanisms are present in human tissues.
In conclusion, we provide evidence that CGRP can be taken up and subsequently released in response to chemical stimuli in a calcium-sensitive manner and we have summarized our finding schematically in Figure 10. This new phenomenon may have considerable influence on CGRP signalling. Elucidating the exact mechanisms and sites of uptake and how it can be used to manipulate CGRP release may be of interest in the treatment of neurovascular conditions like migraine and subarachnoid haemorrhage.
Figure 10.

Schematic presentation of putative calcitonin gene-related peptide (CGRP) reuptake into vesicles in dural sensory nerves through unknown transporter. This uptake was not mediated by pre-synaptic CGRP receptors because CGRP receptor antagonists were not able to block the uptake of exogenous CGRP. TRPV1, transient receptor potential vanilloid receptor type I.
Acknowledgments
This study was supported by Candy's Foundation, The Lundbeck Foundation as part of the Lundbeck Foundation Center for Neurovascular Signaling (LUCENS), The Aase and Ejnar Danielsen Foundation and the Danish Research Council. We gratefully acknowledge Prof Lars Edvinsson for providing olcegepant.
Glossary
Abbreviations
- BSA
bovine serum albumin
- CALCRL
calcitonin receptor-like receptor
- CGRP
calcitonin gene-related peptide
- CGRP8-37
C-terminal fragment of calcitonin gene-related peptide lacking disulphide bond
- elisa
enzyme-linked immunosorbent assay
- iCGRP
immunoreactive calcitonin gene-related peptide
- PBS
phosphate-buffered saline
- RAMP1
receptor activity-modifying protein type I
- SIF
synthetic interstitial fluid
- TRPV1
transient receptor potential vanilloid receptor type I
Conflict of interest
The authors state no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–10 as PowerPoint slide.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Denekas T, Troltzsch M, Vater A, Klussmann S, Messlinger K. Inhibition of stimulated meningeal blood flow by a calcitonin gene-related peptide binding mirror-image RNA oligonucleotide. Br J Pharmacol. 2006;148:536–543. doi: 10.1038/sj.bjp.0706742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnerer J, Amann R. Capsaicin-evoked neuropeptide release is not dependent on membrane potential changes. Neurosci Lett. 1990;18:331–334. doi: 10.1016/0304-3940(90)90686-4. [DOI] [PubMed] [Google Scholar]
- Dux M, Santha P, Jancso G. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J Physiol. 2003;552:859–867. doi: 10.1113/jphysiol.2003.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersberger A, Averbeck B, Messlinger K, Reeh PW. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience. 1999;89:901–907. doi: 10.1016/s0306-4522(98)00366-2. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Jansen I, Kingman TA, McCulloch J. Cerebrovascular responses to capsaicin in vitro and in situ. Br J Pharmacol. 1990;100:312–318. doi: 10.1111/j.1476-5381.1990.tb15801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8-37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol. 2007;150:633–640. doi: 10.1038/sj.bjp.0707134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdelyi L, Such G, Jancso G. Intracellular and voltage clamp studies of capsaicin induced effects on a sensory neuron model. Acta Physiol Hung. 1987;69:481–492. [PubMed] [Google Scholar]
- Frobert Y, Nevers MC, Amadesi S, Volland H, Brune P, Geppetti P, et al. A sensitive sandwich enzyme immunoassay for calcitonin gene-related peptide (CGRP): characterization and application. Peptides. 1999;20:275–284. doi: 10.1016/s0196-9781(98)00172-7. [DOI] [PubMed] [Google Scholar]
- Gupta S, Lozano-Cuenca J, Villalon CM, de Vries R, Garrelds IM, Avezaat CJ, et al. Pharmacological characterisation of capsaicin-induced relaxations in human and porcine isolated arteries. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:29–38. doi: 10.1007/s00210-007-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- Jansen I, Alafaci C, Uddman R, Edvinsson L. Evidence that calcitonin gene-related peptide contributes to the capsaicin-induced relaxation of guinea pig cerebral arteries. Regul Pept. 1990;31:167–178. doi: 10.1016/0167-0115(90)90003-f. [DOI] [PubMed] [Google Scholar]
- Jansen-Olesen I, Mortensen A, Edvinsson L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- Juaneda C, Dumont Y, Quirion R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol Sci. 2000;21:432–438. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- Juhl L, Edvinsson L, Olesen J, Jansen-Olesen I. Effect of two novel CGRP-binding compounds in a closed cranial window rat model. Eur J Pharmacol. 2007;567:117–124. doi: 10.1016/j.ejphar.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Kummer W. Ultrastructure of calcitonin gene-related peptide-immunoreactive nerve fibres in guinea-pig peribronchial ganglia. Regul Pept. 1992;37:135–142. doi: 10.1016/0167-0115(92)90662-e. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Leibach FH, Ganapathy V. Peptide transporters in the intestine and the kidney. Annu Rev Nutr. 1996;16:99–119. doi: 10.1146/annurev.nu.16.070196.000531. [DOI] [PubMed] [Google Scholar]
- Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- Lundbaek JA, Birn P, Tape SE, Toombes GE, Sogaard R, Koeppe RE, et al. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol Pharmacol. 2005;68:680–689. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 2003;120:677–694. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Uddman R, Kingman TA, Edvinsson L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A. 1986;83:5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium. 2004;35:471–478. doi: 10.1016/j.ceca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Meredith D, Boyd CA. Structure and function of eukaryotic peptide transporters. Cell Mol Life Sci. 2000;57:754–778. doi: 10.1007/s000180050040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messlinger K, Hanesch U, Kurosawa M, Pawlak M, Schmidt RF. Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can J Physiol Pharmacol. 1995;73:1020–1024. doi: 10.1139/y95-143. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Price TJ, Patwardhan AM, Flores CM, Hargreaves KM. A role for the anandamide membrane transporter in TRPV1-mediated neurosecretion from trigeminal sensory neurons. Neuropharmacology. 2005;49:25–39. doi: 10.1016/j.neuropharm.2005.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Sams-Nielsen A, Orskov C, Jansen-Olesen I. Pharmacological evidence for CGRP uptake into perivascular capsaicin sensitive nerve terminals. Br J Pharmacol. 2001;132:1145–1153. doi: 10.1038/sj.bjp.0703910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheykhzade M, Nyborg NC. Caliber dependent calcitonin gene-related peptide-induced relaxation in rat coronary arteries: effect of K+ on the tachyphylaxis. Eur J Pharmacol. 1998;351:53–59. doi: 10.1016/s0014-2999(98)00290-8. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular nerves storing substance P and calcitonin gene-related peptide in rat. Neuroscience. 1989;31:427–438. doi: 10.1016/0306-4522(89)90385-0. [DOI] [PubMed] [Google Scholar]
- Uddman R, Kato J, Lindgren P, Sundler F, Edvinsson L. Expression of calcitonin gene-related peptide-1 receptor mRNA in human tooth pulp and trigeminal ganglion. Arch Oral Biol. 1999;44:1–6. doi: 10.1016/s0003-9969(98)00102-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


