Abstract
We tested the ability of two plasticity-promoting approaches to enhance recovery in a mouse model of incomplete spinal cord injury (SCI). Genetically, we reduced myelin-mediated inhibition of neural plasticity through Nogo66-receptor (NgR) gene deletion. Behaviorally, we utilized a novel multimodal exercise training paradigm. Adult mice of wild-type or NgR-null genotype were subjected to partial lateral hemisection (LHx) at C3-C4 with the intent of producing anatomically and functionally mild deficits. Exercise training or control treatment proceeded for 14 weeks. Behavioral outcomes were assessed prior to tract tracing and histological analysis. Genotype and training exerted differing effects on performance; training improved performance on a test related to the training regimen (task-specific benefit), whereas genotype also improved performance on more generalized behaviors (task-non-specific benefit). There were no significant histological differences across genotype or training assignment with regard to lesion size or axonal tract staining. Thus either NgR gene deletion or exercise training benefits mice with mild cervical spinal injury. In this lesion model, the effects of NgR deletion and training were not synergistic for the tasks assessed. Further work is required to optimize the interaction between pharmacological and physical interventions for SCI.
Key words: incomplete spinal cord injury, Nogo, Nogo receptor, plasticity, rehabilitation, task specificity
Introduction
Most spinal cord injuries (SCI) are anatomically incomplete (Sherwood et al., 1992). Even in cases with complete motor and sensory loss below the lesion, a spared fraction of intact fibers may provide a substrate for recovery (Kakulas, 1999). Endogenous neural plasticity allows spared brainstem and spinal cord pathways to mediate detour signals from lesioned corticospinal tract (CST) fibers (Bareyre et al., 2004; Courtine et al., 2008). In addition to ongoing efforts to improve regrowth of severed spinal cord fibers, we aimed to exploit this potential of spared fiber plasticity to mediate recovery from SCI.
One approach to maximizing neural plasticity is to target myelin-associated inhibitors of axonal growth such as Nogo, MAG, and OMgp. Genetic or pharmacological interference with these and other myelin-associated inhibitors improves recovery in various models of central nervous system injury (Cafferty et al., 2010; Cafferty and Strittmatter, 2006; Freund et al., 2007; Lee et al., 2004; Li et al., 2004; Liu et al., 2006; McGee and Strittmatter, 2003; Schwab, 2004). An antibody targeted against the human Nogo-A protein is now in a Phase I clinical trial for acute SCI (Novartis ATI355, clinicaltrials.gov #NCT00406016).
Another approach to improving neural plasticity is through exercise-based SCI rehabilitation, a safe and more easily accessible pathway to clinical application. To optimize exercise therapy for clinical use, methods need to be standardized and dosages need to be titrated (Hart et al., 2008). Therefore, further work is required in animal models. Weight-supported gait training represents one of the most popularly studied and applied exercise treatments for SCI (Behrman et al., 2006; Wernig et al., 1995). This approach is promising in preclinical studies, but has not shown clinical efficacy for overground walking (Barbeau and Rossignol, 1987; Dobkin et al., 2006, 2007; Goldshmit et al., 2008; Wolpaw, 2006). Weight-supported gait training tends to focus on training intrinsic spinal pattern-generating circuitry below the lesion, while paying less attention to improving supraspinal control. Exercise treatments focusing on more rostral elements of the central nervous system have been more widely studied in the field of stroke rehabilitation. A variety of skilled training paradigms have been reported with encouraging results. However, benefits of skilled training have generally been limited to tasks similar to the training exercises (Dobkin, 2007; Garcia-Alias et al., 2009; Girgis et al., 2007; Grasso et al., 2004; Keurzi et al., 2010; Magnuson et al., 2009). This phenomenon of task-specificity limits the generalization of training benefits to other everyday tasks. Whether a different approach to exercise training, or a multifactorial approach combining exercise and other treatment modalities, can overcome task-specific limitations remains to be determined.
In the context of multifactorial approaches, we designed exercise apparatus to induce random balance perturbations simultaneously as rodents perform skilled gripping and reaching tasks. We applied this training regimen to adult mice of either wild-type (WT) or NogoReceptor1 (NgR)-null genotype that had received an incomplete cervical partial lateral hemisection (LHx) at C3-C4. Lesions were intentionally mild to allow injured mice to participate in the training regimen.
We tested three hypotheses: (1) NgR deletion improves recovery in this model; (2) multifactorial exercise training improves recovery in this model; and (3) the combination of genotype and training further improves recovery. We report that genotype and training produce distinct benefits in functional recovery after incomplete cervical SCI. As with other training regimens, our multifactorial training paradigm produced task-specific benefits. NgR deletion produced task-non-specific benefits on several tests. The combination of training and NgR deletion did not synergistically improve recovery, but may accelerate recovery in a task-specific outcome. Neither genotype nor training significantly affected the extensive regrowth of serotonergic fibers caudal to the LHx. We discuss the implications for future studies of multifactorial approaches to recovery from SCI.
Methods
Animals
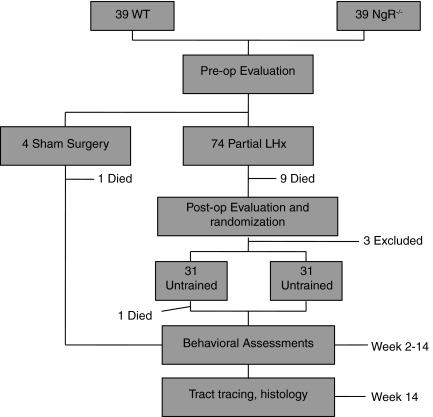
Adult (3-month-old) male and female C57/BL6 mice of either wild-type or NgR-null genotype (Kim et al., 2004) were bred and housed in standard-sized cages with ad libitum food and water and a 12-h light-dark cycle. The NgR-null allele had been backcrossed to C56/BL6 for greater than 10 generations. Females were housed up to four per cage; males were housed individually. See Figure 1 for an experimental outline.
FIG. 1.
Experimental outline. A total of 78 mice underwent preoperative acclimation, baseline evaluation, and surgery. A total of 14 mice dropped out over the course of the experiment (11 deaths and 3 exclusions described in the results section). Partial lateral hemisection (LHx) mice were randomized to trained or untrained groups after the 1-week evaluations, then underwent training and serial evaluation during weeks 2–14. Corticospinal tracts were anterogradely labeled with dextran amines prior to perfusion and histological analysis (WT, wild-type; NgR–/–, NogoReceptor1-null).
Surgery
All surgical procedures and postoperative care were performed in accordance with the guidelines of the Yale Institutional Animal Care and Use Committee. Partial lateral hemisections were performed on the left side at the C3-C4 level as follows. The mice were anesthetized with intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg). The surgical site was shaved and swabbed with povidone-iodine and alcohol. A midline incision was made over the cervical region, followed by dissection and exposure of the second through fifth cervical vertebrae. A C3 laminectomy was then performed, with surgical foam used to control bleeding. The dura was carefully removed, followed by application of xylocaine-soaked surgical foam directly over the C3-C4 segments of the cord. Left-sided hemisection was then performed using a microknife (FST 10318-14), to incise from the midline dorsal vessel to the lateral aspect of the cord. Care was taken to avoid severing the midline dorsal vessel and other midline portions of the cord; thus the lesions spared all or most of the dorsal CST (Fig. 2). Sham surgeries consisted of laminectomy without dura removal or cord sectioning. After bleeding was controlled, subcutaneous tissues were anesthetized with xylocaine, and the skin was sutured with 4-0 polyglactin 910.
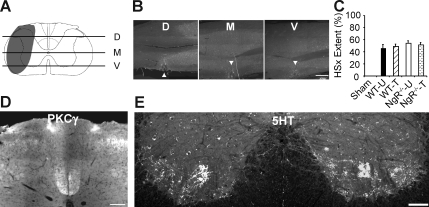
FIG. 2.
Partial lateral hemisection results in consistent lesions with minimal long-term effect on corticospinal tract (CST) or serotonergic tracts. (A) The extent of a typical partial lateral hemisection (LHx) depicted in gray. (B) Representative horizontal sections of the lesion site taken at the levels of the lines shown in A (D, dorsal; M, medial; V, ventral). Horizontal sections were stained for glial fibrillary acidic protein. Lesion site is denoted with arrowhead (scale bar = 500 μm). (C) The extent of lesioned tissue in LHx mice, expressed as a percentage of the hemicord lesioned over the dorsal, medial, and ventral planes, was similar across genotype and training assignment. Numbers of mice per group: sham (Sham, 3); WT-untrained (WT-U, 10); WT-trained (WT-T, 14); NgR–/–-untrained (NgR–/–-U,15); NgR–/–-trained (NgR–/–-T,12). (D) Representative lumbar transverse section stained for PKC-γ, indicating that the CST was not affected by partial LHx (scale bar = 100 μm). (E) Representative lumbar cord transverse section stained for 5-hydroxytryptamine (5-HT). Serotonergic innervation was not significantly reduced on the lesioned side of the cord 14 weeks after LHx (scale bar = 100 μm; WT, wild-type; NgR–/–, NogoReceptor1-null).
In the immediate postoperative period, mice were placed in a recovery cage kept at 37°C, and given a subcutaneous dose of ampicillin and buprenorphine. The mice were returned to their home cages once they had fully recovered from anesthesia. Mice received ampicillin/buprenorphine injections, as well as bladder expression twice daily, for the next 48 h. Though most mice were able to freely void by this time point, bladders continued to be checked daily and expressed when necessary.
Two weeks prior to sacrifice, both CSTs were anterogradely traced using dextran amines. Biotinylated dextran amine (BDA; Invitrogen, Carlsbad, CA) was injected into the left sensorimotor cortex, and Oregon-Green dextran amine (OGDA; Invitrogen) was injected into the right sensorimotor cortex. A total of 1.5 μL was infused at five sites total within each sensorimotor cortex (coordinates from the bregma: 1.0 mm anterior/0.5 mm lateral, 1.0 mm anterior/1.5 mm lateral, 0.5 mm anterior/1.0 mm lateral, 1.0 mm posterior/0.5 mm lateral, and 1.0 mm posterior/1.5 mm lateral). The animals received postoperative ampicillin twice daily for 48 h.
Training
In the nutating mesh exercise, groups of 8–10 mice were placed on a wire grid (29 × 39 cm, 1.25-cm squares, 0.75 mm gauge) fitted onto a lab bench nutator that continuously tilts in three axes in a regular pattern. In the suspended mesh tube exercise, groups of 2–3 mice were placed in wire mesh cylinders (60 cm length, 6 cm diameter, 2.5 × 3.5-cm hexagonal pattern, 0.75 mm gauge) that were suspended by an elastic band at the center. Thus the mesh tube continuously shifted on its elastic fulcrum in unpredictable fashion as the mice moved and climbed within the cylinders. These two types of apparatus as used in a typical training session are shown in Supplementary Video 1 (supplementary video files are available at www.liebertonline.com/neu). Each exercise session consisted of 1 h per apparatus. Untrained mice were brought to the training room for the same period, handled outside of their cages at the same frequency, and placed in groups of 7–10 in large smooth-surfaced containers to provide similar exposure to both human handling and social interaction with non-cagemates. Training began at 11–12 days post-injury, and continued 5–6 days per week until 14 weeks post-injury. At the initiation of training, the mice spent 56 ± 6% of their time ambulating on the suspended mesh tube, and 49 ± 6% on the nutator (mean ± SEM; n = 16). There was no indication that this fraction changed significantly over the training period. The time spent ambulating in the two types of wire apparatus serves as an indicator of the effort directed at task-specific training.
Behavioral evaluation
All evaluations were performed blinded to genotype and training assignment. The mice were acclimated to each test for four or five sessions before pre-operative assessment. They were then assessed periodically post-SCI as described below.
Open field behavior (4 days, 7 days, and bi-weekly thereafter)
The mice were assessed in a circular pen with a smooth surface and a radius of 45 cm. We used the Basso mouse scale (BMS) to measure hindlimb function (Basso et al., 2006), and a variation of the BMS to measure forelimb function (BMS-FL, see Table 1). The BMS-FL was devised based on observations of a prior cohort of mice that had undergone partial cervical LHx. BMS-FL scores range from 0 (non-weight-bearing) to 4 (normal). A score of 3 indicates grossly normal forelimb stepping, but a preference to use the non-injured forelimb for rearing and reaching actions; a score of 2 indicates the impaired forepaw is held in a closed or fisted posture; a score of 1 indicates frequent steps taken on the dorsal forepaw surface, or “skiing” along the plantar forepaw without lifting it in discrete steps. Most animals scored 2 or 3 points on this scale.
Table 1.
Basso Mouse Scale Forelimb Score
| Points | |
|---|---|
| 0 | Non-weight bearing; drags limb |
| 1 | Dorsal stepping; “skiing” limb |
| 2 | Digits in closed posture |
| 3 | Reaching and rearing preference |
| 4 | Normal |
The impaired forelimb was scored during open-field ambulation on a 5-point scale as shown above.
Rotarod (1 week, 9 weeks, and 13 weeks)
The rotarod apparatus (Columbus Instruments, Columbus, OH) was set at a baseline speed of 2 rpm, with acceleration at 0.2 rpm/sec. Latency in seconds for the animal to fall off the rotating drum was recorded. Each session included five consecutive trials, with the average fall latency calculated from the best three trials per animal.
Grip strength (7 days and 13 weeks)
Each individual forelimb was tested for tensile grip strength by pulling on a triangular wire attached to a Chatillon DFE-002 force gauge (Chatillon Force Measurement Systems, Largo, FL). The mice were held by the scruff of the back and neck, parallel to the benchtop, at an angle to expose one forelimb at a time. Eight pulls were performed for each forelimb. The highest single recording and the two lowest recordings were excluded. The mean of the five remaining recordings was determined, then the mean of the left (impaired) forelimb was divided by the mean of the right forelimb and multiplied by 100 to determine impaired forelimb strength as a percentage of the unimpaired limb.
Treadmill (7 days and 14 weeks)
A multiple-lane treadmill (Panlab LE8710M; Harvard Apparatus, Holliston, MA) was set to accelerate in four linear phases over 4 min: 5–11 cm/sec over minute 1; 12–20 cm/sec over minute 2; 22–30 cm/sec over minute 3; and 31–40 cm/sec over minute 4. Latency in seconds to falling off the end of the treadmill was recorded, with a maximum of 240 sec. Average latency was determined from the best three out of four trials.
Inclined grid (7 days, 8 weeks, and 14 weeks)
A metallic wire grid (2 mm gauge) composed of 2.5 × 2.5-cm squares was set at an incline of 22.5°. The mice were videotaped as they climbed the inclined grid over a length of 42.5 cm (lane width 8 cm). The videos were scored for errors made during four consecutive climbs up the grid. Any slip of a limb below the plane of the grid was scored as 2 error points; any slip that caused the animal's trunk to fall against the grid was scored as 3 error points. Error points for each limb were summed over four runs. See Supplementary Videos 3 and 4 for pre-operative and post-operative grid walking, respectively (supplementary video files are available at www.liebertonline.com/neu).
Composite score at 7 days
Composite scores were determined based on the results of open-field testing, rotarod latency, treadmill persistence, grip strength, and inclined mesh performance. To account for the importance of general ambulation, cumulative open-field scores were double-weighted in the composite score calculation. The final formula used was:
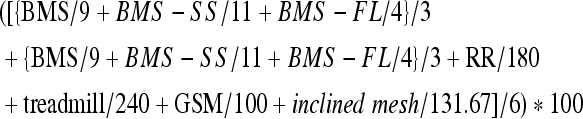 |
where BMS = Basso mouse scale score; BMS-SS = Basso mouse scale subscore; BMS-FL = Basso mouse scale-forelimb score; RR = rotarod latency; and GSM = grip strength measurement.
Histological analysis
Tissue preparation
The animals were transcardially perfused with PBS followed by 4% paraformaldehyde in PBS. The dissected spinal cords were post-fixed in 4% paraformaldehyde/PBS overnight. Tissue blocks were embedded in gelatin prior to vibrating microtome sectioning. We prepared horizontal sections of the lesioned cervical cord (30 μm thick, 2 mm rostral and caudal to the lesion), and transverse sections (30 μm thick) of the caudal cervical and caudal lumbar cord.
Staining
The sections were treated with hydrogen peroxide, blocked with 10% donkey serum, then incubated overnight with primary antibody (rabbit anti-PKC-γ 1:400; Santa Cruz Biotechnology, Santa Cruz, CA and rabbit anti-5-HT 1:16,000; Immunostar, Hudson, WI). BDA signal was processed using avidin-biotin complex (Vector Laboratories, Burlingame, CA), and tyramide signal amplification (Perkin-Elmer, Waltham, MA). Labeled secondary antibodies or mouse Cy3-conjugated anti-glial fibrillary acidic protein (GFAP) were obtained from Invitrogen and Sigma-Aldrich (St. Louis, MO), respectively.
Lesion analysis
Horizontal sections of the lesioned area were stained for GFAP, then scored as the extent of full hemisection in the dorsal, middle, and ventral planes by an observer blinded to genotype and training assignment. The graph in Figure 2C depicts the average value per animal among the three planes, grouped by genotype and training assignment.
5-Hydroxytryptamine (5-HT) analysis
Ventral horn 5-HT at the caudal cervical and caudal lumbar levels was quantified using Image J software (National Institutes of Health). 5-HT immunoreactivity was thresholded at 1.5 times the mean image intensity. The fibers were then skeletonized. The length of 5-HT fibers per ventral horn was determined on each side for 3–5 sections per level per animal.
Statistical analysis
As noted in the introduction, we tested three different hypotheses: (1) NgR deletion improves recovery; (2) training improves recovery; and (3) the combination of NgR deletion and training interact to improve recovery. To test hypothesis 1, we performed Student's t tests comparing untrained WT versus untrained NgR-null groups. To test hypothesis 2, we performed Student's t tests comparing untrained versus trained WT groups. To test hypothesis 3, we performed two-way analysis of variance (ANOVA) testing for interaction between genotype and training.
All data are presented as mean ± SEM. In the figures, statistical significance is indicated in the legends: WT-trained in comparison to WT-untrained; NgR–/–-untrained in comparison to WT-untrained; and two-way ANOVA test for interaction of genotype and training in the NgR–/–-trained group. One asterisk indicates p < 0.05, two asterisks indicate p < 0.01, and NS indicates non-significant.
For the 1-week post-lesion behavioral assessments, one-way ANOVAs were applied to the sham-lesioned, WT-lesioned, and NgR-null-lesioned groups and genders. Post-hoc pairwise comparisons were performed via Tukey's method whenever between-group effects reached statistical significance.
Results
Partial cervical lateral hemisection causes reproducible mild anatomical lesions
We aimed to avoid severing the dorsal midline spinal vessel during LHx. This resulted in sparing the dorsal CST, as well as portions of ventromedial areas of the cord containing vestibulospinal, reticulospinal, and ventral CST fibers (Brosamle and Schwab, 1997; Maier et al., 2009). To quantify lesion size, horizontal sections of the lesioned cervical cord were stained for GFAP. Lesion extent was determined in the dorsal, medial, and ventral planes as depicted in Figure 2. There were no significant differences between groups in cumulative lesion extent (as a percentage of total hemisection).
We traced both CSTs with anterograde dextran amine tracers prior to sacrifice. We also stained transverse sections of the spinal cord for PKC-γ, a marker of CST fibers (Mori et al., 1990). Not surprisingly, the dorsal CST was largely intact on the lesioned as well as the unlesioned side of the cord caudal to LHx (Fig. 2). We also stained transverse sections for 5-HT reactivity to mark descending serotonergic tracts. Surprisingly, 5-HT immunoreactivity caudal to the lesion was at least as high in LHx animals as in sham-lesioned animals (Fig. 2). Though not statistically significant, the LHx groups tended to have higher 5-HT immunoreactivity than sham animals in both sides of the caudal cervical cord (Supplementary Fig. 1; Supplementary Data are available online at www.liebertonline.com/neu). Thus in all groups a combination of sparing, sprouting, or regeneration prevented any net deficit in corticospinal or raphespinal fibers.
Partial cervical lateral hemisection causes reproducible mild behavioral deficits
Mice of either genotype performed similarly on all behavioral tests prior to lesion (Table 2). Shortly after LHx, the mice tended to take dorsal weight-bearing steps on their left forelimbs, with several mice unable to bear weight on their left forelimb. Steps with the left hindlimb were weight-bearing, but frequently dorsal and externally rotated. Most mice had disrupted left-right coordination, with fewer steps taken on the injured side. The tail always deviated toward the left, frequently dragging or hitting against the ground (see Supplementary Video 2; supplementary video files are available at www.liebertonline.com/neu).
Table 2.
Behavioral Testing at Baseline and 1 Week after Partial LHx
| Baseline | 7 Days post-SCI | ||
|---|---|---|---|
| Sham | BMS | 9.00 ± 0.00 | 8.67 ± 0.33 |
| BMS-SS | 11.00 ± 0.00 | 10.67 ± 0.33 | |
| BMS-FL | 4.00 ± 0.00 | 4.00 ± 0.00 | |
| TM | 231.33 ± 8.67 | 211.60 ± 28.44 | |
| RR | 122.89 ± 8.73 | 158.86 ± 21.01 | |
| GS | 101.55 ± 1.35 | 115.60 ± 5.56 | |
| MESH | 0.67 ± 0.11 | 4.67 ± 1.56 | |
| WT LHx | BMS | 9.00 ± 0.00 | 6.69 ± 0.22** |
| BMS-SS | 11.00 ± 0.00 | 7.93 ± 0.33** | |
| BMS-FL | 4.00 ± 0.00 | 2.41 ± 0.17** | |
| TM | 204.68 ± 8.62 | 154.87 ± 9.18** | |
| RR | 114.92 ± 4.44 | 76.15 ± 6.63** | |
| GS | 97.22 ± 2.31 | 69.56 ± 4.55** | |
| MESH | 1.66 ± 0.53 | 40.62 ± 7.57** | |
| NgR–/– LHx | BMS | 9.00 ± 0.00 | 6.59 ± 0.17** |
| BMS-SS | 11.00 ± 0.00 | 7.84 ± 0.24** | |
| BMS-FL | 4.00 ± 0.00 | 2.25 ± 0.11** | |
| TM | 203.31 ± 5.43 | 151.68 ± 6.13** | |
| RR | 118.77 ± 4.08 | 93.99 ± 4.32** | |
| GS | 96.51 ± 2.40 | 73.87 ± 4.39** | |
| MESH | 2.07 ± 0.43 | 48.93 ± 5.25** |
indicates a difference from baseline with p value < 0.01.
WT and NgR–/– mice performed similarly on a battery of tests at baseline. One week post-LHx, NgR–/– mice performed better than WT mice on the rotarod test, but otherwise both genotypes displayed similar mild deficits. Asterisks denote statistical significance between baseline and one-week results.
WT, wild-type; NgR–/–, NogoReceptor1-null; LHx, partial lateral hemisection; BMS, Basso mouse scale; BMS-FL, Basso mouse scale-forelimb score; BMS-SS, Basso mouse scale subscore; MESH, inclined mesh, TR, treadmill; RR, rotarod; GS, grip strength; SCI, spinal cord injury.
Open-field ambulation was formally assessed using the Basso mouse scale (BMS) to evaluate hindlimb performance, and a variation of the BMS to evaluate forelimb performance (Table 1). At 7 days in the open field, similar mild deficits were observed in both male and female WT and NgR-null genotypes. Typically, the left forepaw was held in a clenched posture, the left hindpaw remained externally rotated but able to bear weight on the plantar surface, and the tail remained leftward deviated. Likewise, compared to baseline performance, LHx mice displayed mild to moderate deficits in all functional tests at 1 week (Table 2).
Of note, females persisted significantly longer on the treadmill than males; males tended to perform slightly better on the rotarod than females (data not shown). However, overall performance of males and females was similar (see composite scores below). NgR-null mice performed better on the rotarod than WT mice at 7 days post-LHx, consistent with the early recovery seen in more severe thoracic injury models (Cafferty et al., 2007; GrandPre and Strittmatter, 2002; Kim et al., 2004; Li et al., 2004, 2005; Wang et al., 2006). Otherwise, genotype did not affect behavioral performance at 7 days.
Post-LHx composite scores were derived from the 7-day results of open-field locomotion, rotarod, treadmill endurance, grip-strength, and inclined-mesh performance. LHx mouse composite scores were moderately lower than sham-lesioned mice, and the scores were nearly identical across genotype and gender (Fig. 3D).
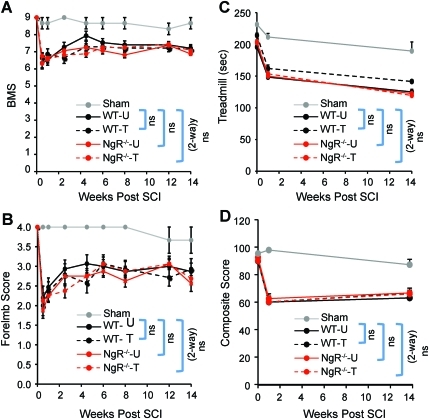
FIG. 3.
Mild deficits in open-field ambulation, treadmill locomotion, and overall composite scores were not significantly affected by genotype or training over time. (A) Scores on the Basso mouse scale (BMS). (B) Scores on the BMS-Forelimb Score. (C) Persistence on the accelerating treadmill. (D) Composite scores calculated from performance in the open-field, rotarod, treadmill, grip-strength, and inclined grid tests as described in the methods section. Among injured mice, there were no significant differences across genotype or training. Numbers of mice per group: sham (Sham, 3); WT-untrained (WT-U, 15); WT-trained (WT-T, 14); NgR–/–-untrained (NgR–/–-U,16); NgR–/–-trained (NgR–/–-T,16; SCI, spinal cord injury; NgR, NogoReceptor1). Color image is available online at www.liebertonline.com/neu.
Training assignment and protocol
Following the 7-day evaluations, the mice were randomly assigned to trained or untrained groups. Three mice were excluded from further analysis due to behavioral deficits that were either too mild (2) or too severe (1). The overall scores of each group were equally weighted by subgrouping mice with similar composite scores prior to randomization. Sham-lesioned mice remained untrained. The final numbers of mice in each group were as follows: sham (3); WT-untrained (15); WT-trained (14); NgR-null-untrained (16); and NgR-null-trained (16).
Training began at 11–12 days post-LHx. Briefly, trained mice were placed in groups of 8–10 on nutating wire platforms for 1 h, followed by placement in groups of 2–3 in suspended wire mesh cylinders for 1 h, for a total of 2 h per day (see Supplementary Video 1; supplementary video files are available at www.liebertonline.com/neu). Untrained mice were transported and handled outside of their cages at the same frequency. Training continued 5–6 days per week for 14 weeks.
Mild deficits in open-field and treadmill ambulation were not affected by genotype or training
As soon as 2 weeks post-surgery, LHx mice had recovered significantly in open-field ambulation. By 4 weeks post-surgery, all groups of mice reached a plateau of slightly more than 7 on the BMS, and slightly less than 3 on the BMS-FL scales (Fig. 3A and B). Neither genotype nor training significantly affected BMS or BMS-FL scores over the course of the 14-week training period. As another measure of general ambulation, we also tested the persistence of mice on an accelerating treadmill. LHx mice displayed mildly reduced treadmill persistence throughout the experiment; interestingly, persistence did not improve over time (Fig. 3C). As with the open-field ambulation, neither genotype nor training significantly affected treadmill persistence.
Training improved performance on a task-specific skilled climbing test
Both daily training exercises involved grasping and maintaining balance on a fine wire mesh. The behavioral test most related to the training exercises involved climbing an inclined wire grid. Given the similarity of the inclined grid test to the training paradigm, performance on the inclined grid represents a task-specific test of training effects. The results are depicted in Figure 4. All groups of LHx mice improved over time. Training increased the magnitude and the rate of improvement in performance by either genotype; this effect already trended toward significance at 8 weeks, with further improvement seen by 14 weeks. More errors were made by the left hindlimb (Fig. 4B) than the left forelimb (Fig. 4A); consequently, the magnitude of the training effect was correspondingly higher for the left hindlimb. Furthermore, despite making vastly fewer errors on the uninjured right side, training improved performance on the right side as well as it did on the injured left side (Fig. 4C).
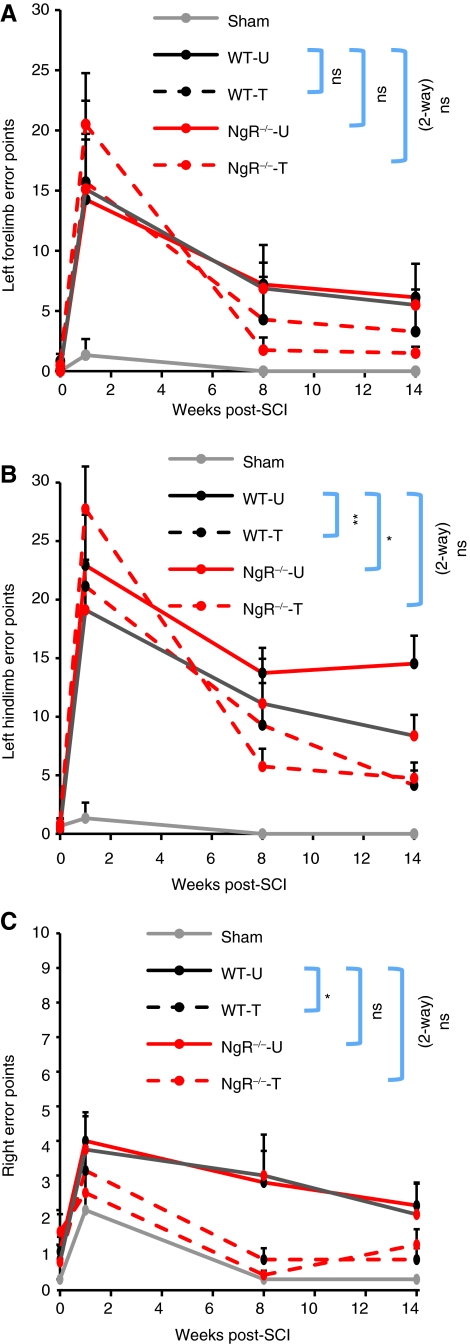
FIG. 4.
Training improved performance on the inclined grid. Footfall errors were recorded over four traverses up an inclined grid. (A) Left forelimb error points. (B) Left hindlimb error points. (C) Right error points. Training significantly reduced hindlimb errors. NgR–/– genotype also significantly reduced errors by the left hindlimb. There was a trend towards a positive interaction of training and NgR–/– genotype to increase the rate of recovery of impaired limbs at 8 weeks (WT-U, WT-untrained; WT-T, WT-trained; NgR–/–-U, NgR–/–-untrained; NgR–/–-T, NgR–/–-trained; NgR, NogoReceptor1). Color image is available online at www.liebertonline.com/neu.
Independently of training, NgR deletion alone significantly improved left hindlimb performance at the 14-week time point (Fig. 4B). Furthermore, the combination of NgR deletion and training tended to speed the rate of improved left hindlimb performance. At 8 weeks, trained NgR-null mice had 5.75 ± 1.52 error points, whereas trained WT mice had 9.29 ± 3.59 error points, and untrained WT mice had 13.73 ± 2.15 error points. This improvement at 8 weeks was more impressive because the trained NgR-null group had higher overall error rates at 7 days post-LHx, prior to assignment to trained and untrained groups.
NgR deletion improved performance on two task-non-specific tests
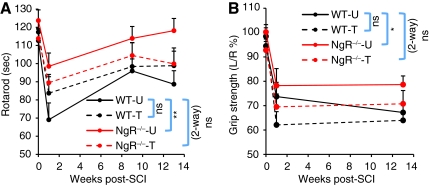
Maintaining balance on the rotarod represents a task-non-specific measure of coordination, whereas grip strength represents a task-non-specific measure of strength. As early as 7 days post-injury, NgR-null mice showed improved rotarod performance (Fig. 5A). Compared to untrained WT mice, the benefit of NgR deletion increased further by 13 weeks post-surgery. Interestingly, whereas training tended to improve the performance of WT mice at 13 weeks, trained NgR-null mice tended to perform worse on the rotarod than their untrained counterparts. On the grip-strength task, NgR-null mice had increased injured forepaw strength at 13 weeks (Fig. 5B). Training had no significant effect on grip strength in either genotype. Thus genotype, but not training, conferred task-non-specific benefits in the rotarod and grip-strength tasks.
FIG. 5.
NgR deletion improved performance in rotarod endurance and grip strength. (A) Mean latency to falling off an accelerating rotarod. (B) Grip strength of the injured left forelimb expressed as a percentage of the uninjured right forelimb. Compared to untrained WT mice, untrained NgR–/– mice performed significantly better on the rotarod at 1 and 13 weeks post-SCI, as well as displaying improved strength of the injured left forepaw at 13 weeks post-SCI (WT-U, WT-untrained; WT-T, WT-trained; NgR–/–-U, NgR–/–-untrained; NgR–/–-T, NgR–/–-trained; NgR, NogoReceptor1; SCI, spinal cord injury; WT, wild-type). Color image is available online at www.liebertonline.com/neu.
Discussion
Pharmacologically or genetically reducing myelin-mediated inhibition of neural growth provides beneficial effects in the context of SCI and various other types of CNS injury (Cafferty et al., 2010; Cafferty and Strittmatter, 2006; Freund et al., 2007; Kim et al., 2003, 2004; Lee et al., 2004; McGee et al., 2005; Raineteau et al., 1999; Simonen et al., 2003). Clinically, physical therapy remains the mainstay of care today, and will certainly be used in combination with any new therapies for SCI applied in the future. In this study, we aimed to test a myelin-targeted approach and a novel exercise rehabilitation paradigm, either alone or in combination, for their ability to affect recovery from mild cervical SCI. We found that genotype and training affected different aspects of recovery; training improved performance in a task-specific test of function, whereas NgR deletion also improved performance in task-non-specific tests of function. Thus the major finding is the independent and distinct nature of these two interventions.
Using incomplete cervical lateral hemisection to study SCI recovery
The partial lateral hemisection (LHx) applied in the current study resulted in damage to the dorsolateral funiculus (DLF), the dorsal horn (DH), and portions of the ventral horn (VH) and ventrolateral funiculus (VLF). The DLF contains tracts important for hindlimb coordination (the dorsal spinocerebellar tract), and skilled forelimb movement (lateral CST and rubrospinal tract) (Kuchler et al., 2002; Muir et al., 2007; Schucht et al., 2002). The VLF includes reticulospinal and propriospinal fibers that have been shown to correlate with locomotor recovery after incomplete SCI (Ballermann and Fouad, 2006; Schucht et al., 2002; You et al., 2003).
One advantage of cervical partial LHx relative to thoracic dorsal hemisection or contusion injuries is that unilateral cervical injury allows study of both forelimb and hindlimb recovery, with internal comparison of affected and unaffected sides. Furthermore, the mild behavioral deficits produced by partial LHx allow animals to participate in strenuous physical rehabilitation protocols. Additionally, in conjunction with a prolonged period between injury and sacrifice, partial LHx spares enough neural tissue to allow significant recovery through endogenous plasticity (Bareyre et al., 2004; Courtine et al., 2008). Finally, unlike the selective CST ablation mediated by pyramidotomy, partial cervical LHx affects fibers of multiple tracts, more closely mimicking the effects of clinical SCI.
The fact that the partial LHx lesions reported here spared the dorsal CST and other para-midline fibers led to several disadvantages. Clinically, the CST is of high importance in human patients due to its primary role in mediating skilled motor movements, and since it was largely spared in our study, it is more difficult to draw conclusions about the recovery of skilled movements after human SCI. Histologically, it is more difficult to label brainstem-origin tracts with high specificity using anterograde tracing. Conversely, the easily traced CST was largely unaffected by LHx. We therefore lost the opportunity to analyze the effects of genotype or training on anatomical plasticity of the tracts most affected by partial LHx. Furthermore, the relatively mild effects of partial LHx on open-field and treadmill ambulation led to a small statistical window in which to detect functional differences among treatment groups. It should also be noted that our small group of sham-lesioned WT animals was untrained; we do not know if our training regimen improves performance of intact animals.
Neuroanatomical findings
As mentioned above, the incomplete LHx lesions reported here disrupted large portions of the DLF and VLF. These types of lesions have been shown to disrupt serotonergic tracts and their ipsilateral terminations caudal to the injury (Bullitt and Light, 1989; Hylden et al., 1985). We were therefore surprised to find upon analyzing the cords of mice sacrificed 4 months after LHx that lesioned animals had equivalent levels of ventral horn 5-HT immunoreactivity caudal to the injury relative to sham-lesioned animals. It is difficult to discern the relative contribution of fiber sparing, severed fiber regeneration, and compensatory sprouting of uninjured fibers, to the increase in 5-HT immunoreactivity seen after LHx. However, the trend toward a greater increase in serotonergic fiber growth contralateral to the lesion suggests compensatory sprouting of unlesioned fibers over time. We cannot make similar inferences regarding pathways such as the vestibulospinal and reticulospinal tracts because we did not anterogradely or retrogradely trace them to obtain direct anatomical evidence of their plasticity in the partial LHx model.
Task-non-specific benefit of NgR deletion
NgR deletion improved outcome in two functional tasks unrelated to the training paradigm: rotarod performance and grip strength. This task-non-specific improvement is in agreement with previous investigations of the effect of NgR deletion in more severe models of CNS injury. For example, NgR-null mice subjected to cortical photolytic strokes showed improved post-injury rotarod performance and skilled food pellet retrieval relative to WT mice (Lee et al., 2004). NgR-null mice subjected to thoracic dorsal over-hemisection (DHx) injury showed improved hindlimb open-field locomotion relative to WT mice (Kim et al., 2004). Studies from our and other labs have also shown beneficial effects of pharmacological interference with the Nogo-NgR pathway on multiple functional parameters after CNS injuries (Fang et al., 2010; GrandPre and Strittmatter, 2002; Li et al., 2004, 2005; Liebscher et al., 2005; Raineteau et al., 1999; Seymour et al., 2005; Wang et al., 2006). In the current study, NgR deletion did not affect performance in certain task-non-specific measures such as open-field and treadmill ambulation. However, given the very mild deficits in open-field and treadmill ambulation produced by partial LHx, this lack of benefit is likely to reflect a “ceiling” effect for targeting myelin-mediated inhibition.
Task-specific functional benefit of training
Both training exercises required mice to grip and maintain balance on a mobile wire mesh. The behavioral test most closely related to the training paradigm involved climbing a non-mobile inclined grid. Therefore, performance on the inclined grid serves as a measure of the task-specific effects of training. As expected, trained mice of either genotype performed significantly better on the inclined grid than their untrained counterparts. Training did not improve performance on other behavioral tests. This lack of broader benefit is consistent with the task-specific benefits conferred by training in a variety of animal models and human patients with stroke and SCI (Garcia-Alias et al., 2009; Grasso et al., 2004; Keurzi et al., 2010; Magnuson et al., 2009; Smith et al., 2006).
Training's task-specific effect on grid climbing applied not only to the limbs ipsilateral to the spinal lesion, but to the contralateral limbs as well. Several explanations may underlie the improved performance of uninjured limbs with training. One, training may improve performance of injured and uninjured limbs simply through a practice effect. Two, training may affect plasticity of sensorimotor tracts with commissures rostral and/or caudal to the lesion. Three, training may affect signaling within the distributed locomotor central pattern-generating circuitry itself. None of these possible explanations are mutually exclusive.
Interestingly, training improved hindlimb performance more dramatically than forelimb performance. NgR deletion alone also improved lesioned hindlimb performance, and showed a trend toward an increase in the rate of overall hindlimb improvement in combination with training. Several possible explanations may contribute to the greater responsiveness of the hindlimbs to both interventions. One, proper forelimb placement on the inclined grid requires visuomotor skill, depending partly on the CST. Proper hindlimb placement requires more proprioceptive skill, depending largely on tracts in the DLF (Metz et al., 2000; Muir et al., 2007). Since the dorsolateral funiculus was proportionally more damaged by partial LHx (Fig. 2), training would be expected to more significantly improve performance of the affected hindlimb than the forelimb. Additionally, plasticity of cervical/forelimb neural circuitry may have been limited by factors such as inflammation and scarring in proximity to the lesion. Furthermore, the lumbar locomotor central pattern generator may possess greater intrinsic plasticity than cervical circuits. Again, these possibilities are not mutually exclusive.
In contrast to our findings, mice undergoing voluntary running on a runged surface after thoracic contusion injury performed worse than mice given a smooth-surface running wheel during the recovery period (Engesser-Cesar et al., 2005). They hypothesized that the runged surface may have caused subtle harm to injured mice during attempted ambulation. Though we did not directly compare a runged surface to a smooth surface in our training paradigm, we found no evidence for injury or other detrimental effects in trained mice.
Training goals: Multimodal plasticity, synergism, and task-non-specificity
In the context of anatomically incomplete SCI, the distributed organization of brainstem and intrinsic spinal cord circuits confers a greater ability to maintain innervation distal to the lesion, as opposed to more anatomically focal tracts such as the CST (Jankowska et al., 2006; Matsuyama et al., 2004). However, to establish conscious control of these brainstem and spinal circuits, connections are needed with cortical-origin pathways. Activity-based therapy, whether through exercise or more invasive means, has clearly demonstrated the ability to improve connectivity and function after a variety of CNS injuries (Ahmed and Wieraszko, 2008; Brus-Ramer et al., 2007; Carmel et al., 2010; Courtine et al., 2009; Minassian et al., 2007; Sadowsky and McDonald, 2009). In an effort to induce these types of connections non-invasively, we designed a training regimen to simultaneously invoke skilled forelimb use (CST) and postural reflexes (vestibulospinal, reticulospinal, and other brainstem tracts). The nutating mesh task accomplished this through predictably sequenced motion in three axes. In contrast, the suspended mesh tube task forced mice to maneuver an unpredictably rocking, bouncing, and twisting apparatus. Both tasks involved gripping and ambulating on a fine wire mesh.
To the best of our knowledge, this multimodal training approach represents a novel combination of exercises simultaneously stimulating fine motor and postural reflex pathways. This approach aims to exploit Hebbian principles to strengthen latent or alternative connections between fine motor pathways originating in the motor cortex and postural/vestibular/locomotor pathways originating in the brainstem and spinal cord. These newly formed or activated connections could provide a route for detour pathways around a lesion and recovery of supraspinal control of intrinsic spinal circuitry (Bareyre et al., 2004; Courtine et al., 2008; Jankowska and Edgley, 2006; Raineteau and Schwab, 2001). The fact that the tracts most affected by partial LHx were not specifically traced in the current study prevented us from obtaining direct anatomical evidence for plasticity of these circuits.
Recent studies have analyzed the effects of combining exercise rehabilitation with other enhancers of neural plasticity in animal models of SCI. Chondroitinase ABC, which removes components of astrocytic scars, was examined in combination with either skilled reach training or general locomotor training in a rat C4 dorsal funiculus lesion model (Garcia-Alias et al., 2009). Chondroitinase ABC independently improved CST sprouting. Skilled training improved manual dexterity, but did not improve general locomotion behavior. Conversely, general locomotion training improved locomotion, but actually worsened performance in skilled reaching tasks. In a rat thoracic T-lesion model, an antibody against Nogo-A was tested in combination with treadmill training (Maier et al., 2009). Either anti-Nogo-A or training improved performance on a climbing task, but the two treatments did not synergize. Indeed, anti-Nogo-A and locomotor training appeared to counteract each other in their effects on gait kinematics.
Thus, the search continues for combinations of treatments that lead to synergistic, task-non-specific improvements in recovery from SCI. In recent studies of rats with complete thoracic cord transection, a combination of serotonergic agonists, epidural electrical stimulation, and locomotor training resulted in nearly normal gait kinematics (Courtine et al., 2009). The combined effect of these interventions outweighed their individual effects. In a rat stroke model, the combination of skilled motor training and intraventricular administration of an NgR antagonist resulted in additive effects on recovery of skilled reaching, but did not improve recovery on a grid-walking test (Fang et al., 2010).
Remaining issues to be addressed include defining the optimal dosage and type of training exercise; the persistence of training benefits; the utility of training in more severe injury models; and coordinating training with other treatment modalities to produce synergistic benefits in a broad range of functional tasks.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
We thank Yiguang Fu for dedicated technical assistance. We thank Haakon B. Nygaard for assisting with data blinding. We thank William B.J. Cafferty, Philip Duffy, and Xingxing Wang for training in surgical technique and histological analysis. This work was supported by grants from the National Institute of Neurological Disorders and Stroke, the Falk Medical Research Trust, the Christopher and Dana Reeve Foundation, and the Wings for Life Foundation.
Author Disclosure Statement
S.M.S. is a co-founder of Axerion Therapeutics, which seeks to develop NgR antagonist therapy for CNS injury.
References
- Ahmed Z. Wieraszko A. Combined effects of acrobatic exercise and magnetic stimulation on the functional recovery after spinal cord lesions. J. Neurotrauma. 2008;25:1257–1269. doi: 10.1089/neu.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann M. Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur. J. Neurosci. 2006;23:1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Bareyre F.M. Kerschensteiner M. Raineteau O. Mettenleiter T.C. Weinmann O. Schwab M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Fisher L.C. Anderson A.J. Jakeman L.B. McTigue D.M. Popovich P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Behrman A.L. Bowden M.G. Nair P.M. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys. Ther. 2006;86:1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- Brosamle C. Schwab M.E. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J. Comp. Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M. Carmel J.B. Chakrabarty S. Martin J.H. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J. Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E. Light A.R. Intraspinal course of descending serotoninergic pathways innervating the rodent dorsal horn and lamina X. J. Comp. Neurol. 1989;286:231–242. doi: 10.1002/cne.902860208. [DOI] [PubMed] [Google Scholar]
- Cafferty W.B. Duffy P. Huebner E. Strittmatter S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty W.B. Strittmatter S.M. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J. Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty W.B. Yang S.H. Duffy P.J. Li S. Strittmatter S.M. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J. Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel J.B. Berrol L.J. Bros-Ramer M. Martin J.H. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J. Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G. Gerasimenko Y. van den Brand R. Yew A. Musienko P. Zhong H. Song B. Ao Y. Ichiyama R.M. Lavrov I., et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G. Song B. Roy R.R. Zhong H. Herrmann J.E. Ao Y. Qi J. Edgerton V.R. Sofroniew M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B. Apple D. Barbeau H. Basso M. Behrman A. Deforge D. Ditunno J. Dudley G. Elashoff R. Fugate L., et al. Weight-supported treadmill vs. over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B. Barbeau H. Deforge D. Ditunno J. Elashoff R. Apple D. Basso M. Behrman A. Fugate L. Harkema S., et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized spinal cord injury locomotor trial. Neurorehabilitation Neural Repair. 2007;21:25–35. doi: 10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B.H. Confounders in Rehabilitation Trials of Task-Oriented Training: Lessons From the Designs of the EXCITE and SCILT Multicenter Trials. Neurorehabilitation Neural Repair. 2007;21:3–13. doi: 10.1177/1545968306297329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engesser-Cesar C. Anderson A.J. Basso D.M. Edgerton V.R. Cotman C.W. Voluntary wheel running improves recovery from a moderate spinal cord injury. J. Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Fang P.C. Barbay S. Plautz E.J. Hoover E. Strittmatter S.M. Nudo R.J. Combination of NEP 1-40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544–549. doi: 10.1161/STROKEAHA.109.572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P. Wannier T. Schmidlin E. Bloch J. Mir A. Schwab M.E. Rouiller E.M. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J. Comp. Neurol. 2007;502:644–659. doi: 10.1002/cne.21321. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G. Barkhuysen S. Buckle M. Fawcett J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Girgis J. Merrett D. Kirkland S. Metz G.A. Verge V. Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y. Lythgo N. Galea M.P. Turnley A.M. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J. Neurotrauma. 2008;25:449–465. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- GrandPre T. Li S. Strittmatter S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Grasso R. Ivanenko Y.P. Zago M. Molinari M. Scivoletto G. Lacquaniti F. Recovery of forward stepping in spinal cord injured patients does not transfer to untrained backward stepping. Exp. Brain Res. 2004;157:377–382. doi: 10.1007/s00221-004-1973-3. [DOI] [PubMed] [Google Scholar]
- Hart T. Fann J.R. Novack T.A. The dilemma of the control condition in experience-based cognitive and behavioural treatment research. Neuropsychol. Rehabil. 2008;18:1–21. doi: 10.1080/09602010601082359. [DOI] [PubMed] [Google Scholar]
- Hylden J.L. Ruda M.A. Hayashi H. Dubner R. Descending serotonergic fibers in the dorsolateral and ventral funiculi of cat spinal cord. Neurosci. Lett. 1985;62:299–304. doi: 10.1016/0304-3940(85)90565-8. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Edgley S.A. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Stecina K. Cabaj A. Pettersson L.G. Edgley S.A. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J. Physiol. 2006;575:527–541. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakulas B.A. The applied neuropathology of human spinal cord injury. Spinal Cord. 1999;37:79–88. doi: 10.1038/sj.sc.3100807. [DOI] [PubMed] [Google Scholar]
- Keurzi J. Brown E.H. Shum-Siu A. Siu A. Burke D. Morehouse J. Smith R.R. Magnuson D.S. Task-specificity vs. Ceiling Effect: Step-training in shallow water after spinal cord injury. Exp. Neurol. 2010 doi: 10.1016/j.expneurol.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E. Li S. GrandPre T. Qiu D. Strittmatter S.M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kim J.E. Liu B.P. Park J.H. Strittmatter S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kuchler M. Fouad K. Weinmann O. Schwab M.E. Raineteau O. Red nucleus projections to distinct motor neuron pools in the rat spinal cord. J. Comp. Neurol. 2002;448:349–359. doi: 10.1002/cne.10259. [DOI] [PubMed] [Google Scholar]
- Lee J.K. Kim J.E. Sivula M. Strittmatter S.M. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Kim J.E. Budel S. Hampton T.G. Strittmatter S.M. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol. Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Liu B.P. Budel S. Li M. Ji B. Walus L. Li W. Jirik A. Rabacchi S. Choi E., et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T. Schnell L. Schnell D. Scholl J. Schneider R. Gullo M. Fouad K. Mir A. Rausch M. Kindler D., et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann. Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Liu B.P. Cafferty W.B. Budel S.O. Strittmatter S.M. Extracellular regulators of axonal growth in the adult central nervous system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson D.S. Smith R.R. Brown E.H. Enzmann G. Angeli C. Quesada P.M. Burke D. Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabilitation Neural Repair. 2009;23:535–545. doi: 10.1177/1545968308331147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier I.C. Ichiyama R.M. Courtine G. Schnell L. Lavrov I. Edgerton V.R. Schwab M.E. Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132:1426–1440. doi: 10.1093/brain/awp085. [DOI] [PubMed] [Google Scholar]
- Matsuyama K. Mori F. Nakajima K. Drew T. Aoki M. Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog. Brain Res. 2004;143:239–249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- McGee A.W. Strittmatter S.M. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- McGee A.W. Yang Y. Fischer Q.S. Daw N.W. Strittmatter S.M. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz G.A. Merkler D. Dietz V. Schwab M.E. Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- Minassian K. Persy I. Rattay F. Pinter M.M. Kern H. Dimitrijevic M.R. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum. Mov. Sci. 2007;26:275–295. doi: 10.1016/j.humov.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Mori M. Kose A. Tsujino T. Tanaka C. Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J. Comp. Neurol. 1990;299:167–177. doi: 10.1002/cne.902990204. [DOI] [PubMed] [Google Scholar]
- Muir G.D. Webb A.A. Kanagal S. Taylor L. Dorsolateral cervical spinal injury differentially affects forelimb and hindlimb action in rats. Eur. J. Neurosci. 2007;25:1501–1510. doi: 10.1111/j.1460-9568.2007.05411.x. [DOI] [PubMed] [Google Scholar]
- Raineteau O. Schwab M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Raineteau O. Z'Graggen W.J. Thallmair M. Schwab M.E. Sprouting and regeneration after pyramidotomy and blockade of the myelin-associated neurite growth inhibitors NI 35/250 in adult rats. Eur. J. Neurosci. 1999;11:1486–1490. doi: 10.1046/j.1460-9568.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- Sadowsky C.L. McDonald J.W. Activity-based restorative therapies: concepts and applications in spinal cord injury-related neurorehabilitation. Dev. Disabil. Res. Rev. 2009;15:112–116. doi: 10.1002/ddrr.61. [DOI] [PubMed] [Google Scholar]
- Schucht P. Raineteau O. Schwab M.E. Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Schwab M.E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Seymour A.B. Andrews E.M. Tsai S.Y. Markus T.M. Bollnow M.R. Brenneman M.M. O'Brien T.E. Castro A.J. Schwab M.E. Kartje G.L. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J. Cereb. Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- Sherwood A.M. Dimitrijevic M.R. McKay W.B. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J. Neurol. Sci. 1992;110:90–98. doi: 10.1016/0022-510x(92)90014-c. [DOI] [PubMed] [Google Scholar]
- Simonen M. Pedersen V. Weinmann O. Schnell L. Buss A. Ledermann B. Christ F. Sansig G. van der Putten H. Schwab M.E. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Smith R.R. Shum-Siu A. Baltzley R. Bunger M. Baldini A. Burke D.A. Magnuson D.S. Effects of swimming on functional recovery after incomplete spinal cord injury in rats. J. Neurotrauma. 2006;23:908–919. doi: 10.1089/neu.2006.23.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Baughman K.W. Basso D.M. Strittmatter S.M. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann. Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A. Muller S. Nanassy A. Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur. J. Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Wolpaw J.R. Treadmill training after spinal cord injury: good but not better. Neurology. 2006;66:466–467. doi: 10.1212/01.wnl.0000203915.14930.b4. [DOI] [PubMed] [Google Scholar]
- You S.W. Chen B.Y. Liu H.L. Lang B. Xia J.L. Jiao X.Y. Ju G. Spontaneous recovery of locomotion induced by remaining fibers after spinal cord transection in adult rats. Restor. Neurol. Neurosci. 2003;21:39–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.