Abstract
This study elucidates the role of E6-associated protein, E6-AP (a dual function steroid hormone receptor coactivator and ubiquitin-protein ligase) in the regulation of PI3K-Akt signaling pathway, prostate gland growth and proliferation. Here, we report the generation of transgenic mice and prostate cancer cell line, LNCaP cells that overexpress E6-AP protein. Using these models we show that the levels of total Akt and phosphorylated Akt (active Akt) are increased in E6-AP overexpressing prostate gland and LNCaP cells suggesting that E6-AP regulates the PI3K-Akt signaling pathway. The prostate glands in our transgenic mice are ~20% larger and produce preneoplastic lesions at the age of 18 months. Our data also suggest that E6-AP modulates PI3K-Akt signaling pathway by both androgen-independent and -dependent mechanisms. In the androgen-independent mechanism, E6-AP modulates PI3K-Akt signaling by regulating the protein levels of RhoA, a small GTPase, which is a negative regulator of the Akt signaling pathway. Further, we show that E6-AP, a known coactivator of AR, amplifies the androgen-dependent activation of PI3K-Akt signaling pathway. In addition, we show that stable overexpression of E6-AP in prostate cancer cells results in increased cell size and proliferation. Overall our data suggests that E6-AP regulates both the positive and negative modulators of the PI3K-Akt pathway in prostate cells which results in increased prostate cell growth, proliferation and decreased apoptosis.
Introduction
E3 ubiquitin-protein ligase enzyme, E6-associated protein (E6-AP), is a novel dual function steroid hormone receptor coactivator [1, 2]. E6-AP not only interacts with and enhances the hormone-dependent transcriptional activities of various steroid hormone receptors, including androgen receptor (AR), but also is a member of the E3 class of functionally related ubiquitin-protein ligases [3–5]. E3 ubiquitin-protein ligases have been proposed to play a major role in defining the substrate specificity of the ubiquitin-proteasome system. Protein ubiquitination involves two other classes of enzymes, the E1 ubiquitin-activating enzyme (UBA) and E2 ubiquitin-conjugating enzymes (UBCs). UBA first activates ubiquitin in an ATP-dependent manner, and the activated ubiquitin then forms a thioester bond between the carboxyl-terminal glycine residue of ubiquitin and a cysteine residue of the UBA. Next, ubiquitin is transferred from the E1 to one of several E2s (UBCs), preserving the high energy thioester bond. In some cases, ubiquitin is transferred directly from E2 to the target protein through an isopeptide bond between the ε-amino group of lysine residues of the target protein and the carboxy terminus of ubiquitin. In other instances, the transfer of ubiquitin from UBCs to target proteins proceeds through an E3 ubiquitin-protein ligase intermediate, such as E6-AP. Finally, the ubiquitin-tagged target proteins undergo degradation via the 26S proteasome pathway. Since, E6-AP acts both as a coactivator and an E3 ubiquitin-protein ligase, it is postulated that E6-AP serves to link two important and opposing activities in a cell, the transcription and the protein degradation [6, 7].
Previously, we have shown that the prostate gland is smaller in E6-AP knockout animals implying that E6-AP is required for the proper development and growth of prostate gland [1]. Furthermore, we also showed that protein levels of the components of phosphatidylinositol 3-kinase/protein kinase B (PI3K-Akt) signaling pathway are decreased in E6-AP knockout animals. In addition, we showed that E6-AP regulates the protein levels and transcriptional activity of AR in prostate cells, suggesting that E6-AP plays important roles in the cytoplasm in addition to acting as a coactivator in the nucleus. Since, the PI3K-Akt pathway has been described as a dominant growth survival pathway in prostate cells and elevated PI3K-Akt signaling is correlated with prostate cancer progression [8–12], the main focus of this study is to understand the effect of modulation of this pathway by E6-AP in prostate gland growth and proliferation in different model systems like E6-AP overexpressing transgenics and LNCaP prostate cancer cells.
Akt, also known as protein kinase B (PKB), is a proto-oncogene with a pleckstrin homology and serine/threonine kinase domains. The kinase activity of Akt is stimulated by a variety of extracellular stimuli, such as growth factors and cytokines leading to the phosphorylation and regulation of a wide spectrum of its substrates involved in multiple cellular processes, including cell survival, cell growth, cell differentiation, cell cycle progression, cell proliferation and cellular metabolism [13]. Akt is activated by PI3K, a heterodimer composed of a p85 regulatory and a p110 catalytic subunit. In response to a variety of stimuli, Akt is phosphorylated at Threonone-308 (Thr-308) by 3-phosphoinositide-dependent protein kinase-1 and at Serine-473 (Ser-473) by integrin-linked kinase. Increasing evidence indicates that Akt plays an important role in tumorigenesis [14–16]. The PI3K-Akt pathway which is normally activated by growth factors is also activated by steroid hormones like estrogens and androgens [17–20]. The activation of PI3K-Akt pathway by androgens results in increased cell growth and anti-apoptotic activity [17, 20]. Previously published studies indicate that androgen regulates PI3K-Akt pathway via the AR but independent of its transcriptional activity, suggesting an important role of androgen receptors within the cytoplasm. However, the role for coactivator proteins in the regulation of PI3K-Akt pathway was unknown until the recent finding that steroid receptor coactivator-3 (SRC-3) is able to induce PI3K-Akt pathway in an androgen-independent manner [21]. The induction of cell proliferation and growth by SRC-3 has been attributed to its ability to induce Akt activity and expression. Therefore steroid hormone receptor coactivator proteins in addition to their transcriptional activation can also contribute to growth and proliferation by modulating important growth signaling pathways in the cytoplasm, independent of transcription.
In order to elucidate the specific roles of E6-AP (a dual function steroid receptor coactivator) in the regulation of PI3K-Akt pathway, prostate cell growth and survival, we have generated transgenic mice which specifically overexpresses E6-AP in the prostate gland and also E6-AP stable LNCaP cells (here on referred as E6-AP-LNCaP) that overexpress exogenous E6-AP [1]. Our data from these models suggest that E6-AP is involved in modulation of PI3K-Akt signaling pathway, prostate cell growth and proliferation, prostate gland growth and tumorigenesis.
Materials and Methods
Cell culture
LNCaP (parental cell line) and E6-AP-stable cell line (E6-AP-LNCaP) were maintained in RPMI 1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum at 37°C in 5% CO2. E6-AP stable cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum at 37°C in 5% CO2 in the presence of Doxycycline (2ug/ml).
Generation of E6-AP transgenic mice
Transgenic vector containing wild-type E6-AP with Flag tag (MKbpAII/Pb-Flag-E6-AP) was constructed and digested with NotI and KpnI to generate vector free fragments, purified and was microinjected into fertilized C57BL6 one-cell embryos. The injected embryos were then implanted into the oviducts of pseudo-pregnant recipient mothers. The pups received from the transgenic facility were screened for the presence of E6-AP transgene by PCR. Three wild-type E6-AP transgenic lines were established.
siRNA transfection
E6-AP siRNAs were procured from Dharmacon (Chicago, IL) as ON-TARGET plus Smart Pools. Non-targeting siRNAs (siScramble) were used as negative controls. siRNAs were reverse transfected into LNCaP cells using Lipofectamine RNAimax (Invitrogen) as per the manufacturer’s protocol. Breifly, 50 nM siRNA was diluted in 0.5ml of OPTIMEM reduced serum medium (Invitrogen) along with 5ul of Lipofectamine RNAimax on each well of a 6 well plate. 1 × 105 cells in 8ml of antibiotic free RPMI 1640 was added to the transfection mixture. 24 hours later the media was replaced with phenol red free RPMI 1640 supplemented with 5% charcoal stripped FBS with antibiotics.
Western Blot Analysis
Cells were grown as indicated; afterward cells were harvested and washed in TEN buffer (40 mM Tris-HCl (pH 7.5), 1 mM EDTA, and 150 mM NaCl). Then cells were lysed in ice-cold RIPA buffer (20mM Tris pH 7.5, 150mM Nacl, 1% NP40, 0.5% Sodium deoxycholate, 1mM EDTA and 0.1% SDS) by pipetting up and down. Thereafter, cell lysates were placed on ice for 30 minutes and cleared by centrifugation at 12,000 × g for 20 minutes at 4°C. The supernatants were collected and frozen at −80°C until used for analysis. The protein concentrations of lysates were measured using the Bio-Rad protein assay kit.
Twenty-five to 50 μg of total protein from each sample was resolved on 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and transferred onto nitrocellulose membranes (Protran, Schleicher & Schuell, Inc., Keene, NH). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (20 mM Tris base (pH 7.5) and 150 mM NaCl) containing 0.05% Tween 20 (TBS-T), then probed with the primary antibody. The primary antibodies were diluted in 1% nonfat dry milk in TBS-T as indicated and used for immunoblotting: Anti-E6-AP (1:1000 dilution; Bethyl laboratories, Inc., Montgomery, TX); anti-total Akt (1:1200 dilution), anti-phosphoAkt (1:1000 dilution), anti-PI3-Kinase and p85 (1:1000 dilution), anti-caspase-3 (8G10; 1:1000 dilution), and anti-Bax (1:750 dilution; Cell Signaling Technology, Beverly, MA); anti-RhoA (26C4; 1:800 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); and anti-β-actin (1:10,000 dilution; Sigma-Aldrich Corp.). After washing in TBS-T, membranes were incubated with their appropriate horseradish peroxidase-conjugated secondary antibodies (Bio-Rad Laboratories, Inc.) and developed using an enhanced chemiluminescence detection system (Amersham Biosciences, Arlington Heights, IL) according to the instructions of the manufacturer and were exposed to X-Ray film (Phenix research products, Hayward, CA).
In Vitro Protein-Protein Interaction Assay
Radiolabeled RhoA protein was synthesized using a rabbit reticulocyte-coupled in vitro transcription and translation (TNT) kit in the presence of 35S-methionine according to the manufacturer’s recommendations (Promega Corp.). GST-E6-AP and control GST proteins were expressed in Escherichia coli DH-5 α cells and immobilized on glutathione-Sepharose beads. The glutathione-bound GST and GST-E6-AP were incubated with in vitro-synthesized RhoA G14V protein in NETN buffer (100 mM NaCl, 1 mM EDTA, 20 mM Tris (pH 8.0), and 0.5% Nonidet P-40) for 2–3 hours at room temperature or overnight at 4°C. After washing four times with NETN buffer, E6-AP-bound RhoA protein was isolated by boiling the Sepharose beads in 1× sodium dodecyl sulfate gel loading buffer and were separated on a 10% SDS-PAGE, followed by autoradiograpy.
Proliferation Assays
Cell proliferation was measured using TACS MTT assay (Trevigen, Inc., Gaithersburg, MD). Briefly, LNCaP and E6-AP-LNCaP cells were grown in 96-well cell culture plates at a density of 2×103 cells/well in 100 μl stripped and normal serum containing medium for 24 hours in 5% CO2 at 37°C. Every day, 10 μl of MTT reagent was added to each well, and the cells were further incubated for 4 hours at 37°C to allow the formation of a purple precipitate. The precipitates were dissolved by adding 100 μl of detergent reagent to each well and the plates were incubated in dark over night. The absorbance at 570 nm was measured using a microplate reader (Biorad laboratories, Hercules, CA). For siRNA experiments, cell proliferation was measured by counting total cell number by hemocytometer.
Statistical Analysis
Data were presented as mean ± SE from 3 independent experiments. Statistical analysis was carried out with Student’s t test. P < 0.05 was considered as statistically significant difference.
Results
Characterization of E6-AP overexpressing transgenic mice
Previous studies using E6-AP null mice have shown that E6-AP is required for normal prostate gland development. To test the effect of over-expression of wild-type E6-AP in the development of the normal prostate gland, we generated a new transgenic mouse model, which utilizes the prostate-specific rat probasin (rPB) promoter to target the expression of wild-type human E6-AP to the mouse prostate epithelium. The efficiency of expression of transgenes in prostate gland will be contingent upon both the sites of integration of the transgene and the number of integrated copies per genome. This was assayed using Western blot analysis of tissue-specific expression of the transgenes in a variety of organs such as brain, prostate gland, testes and seminal vesicle. As shown in Figure 1A and B, wild-type E6-AP is specifically over expressed in transgenic mice prostate glands when compared with non-transgenic littermate controls. Since, E6-AP over expression was not observed in other tissues of transgenic lines, this indicates that the transgene expression was specific to the prostate gland.
Figure 1.
Characterization of E6-AP transgenic mice. (A & B) Representative Western blots from two lines of E6-AP over expressing transgenic mice showing the expression levels of E6-AP in different tissues. E6-AP is specifically over expressed in the prostate glands of transgenic mice. (WT- Wild-type, Tr- Transgenic). (C) Wet weights of prostate glands from wild-type and E6-AP over expressing transgenic mice. The wet weights were normalized with body weights. Prostate glands from E6-AP overexpressing transgenics weigh ~20% more than wild-type prostate glands (*, P < 0.05, n=15). (D) Western blots showing levels of total and phospho Akt (p-Akt) are elevated in E6-AP over-expressing transgenic mice. (E) Western blots showing lobe specific expression of E6-AP, p-Akt and phospho GSK β (p-GSK β) in E6-AP over-expressing transgenic mice.
To study the consequences of overexpression of wild-type E6-AP in prostate gland development, the total prostate glands from non-transgenic and transgenic males (12 – 18 weeks old) were compared morphologically. As shown in Figure 1C, the wet weight of total prostate glands of wild-type E6-AP transgenic glands were approximately 20% more than their littermate controls. We have previously characterized E6-AP knockout prostate glands by analyzing the expression of various proteins like PI3K, Akt and RhoA (Khan et al., 2006). These studies revealed the possible mechanism by which E6-AP might influence the development of normal prostate gland. Now, it is of interest to know how over expression of wild-type E6-AP affects the expression of these proteins during prostate gland development. The levels of PI3K, total Akt and phosphorylated Akt (Ser 473) from prostate glands of wild-type E6-AP transgenic mice was compared with non transgenic mice (24 weeks) by Western blot analyses. Figure 1D shows that the levels of total and phosphorylated Akt are increased in Wild-type E6-AP transgenics compared with littermate controls.
Mice prostate is structurally divided into four pairs of lobes: anterior, dorsal, ventral and lateral. It has been reported that there are lobe specific differences in transgene expression driven by the probasin promoter. In addition to that there are lobe specific differences in certain biochemical and pathological conditions. Hence we wanted to know if there is a lobe specific expression of E6-AP in the transgenic mice. Prostate gland from transgenic and Wild-type mice was individually dissected into anterior, ventral and dorsolateral lobes, proteins were isolated and Western blots were performed. Figure 1E shows that E6-AP was over expressed in all the lobes, when compared with Wild-type littermates, indicating that transgene is expressed in all lobes. Even though, there was a difference in the basal level of expression of E6-AP among the different lobes, there was no difference in the extent of over expression of transgene among the different lobes. We also wanted to examine if there are lobe specific differences in Akt protein levels. Figure 1E shows the expression levels of Phosho-Akt are different in different prostate gland lobes. The p-Akt is over expressed in E6-AP transgenic lobes compared to the Wild-type gland. We also examined the levels of phosphorylated form of glycogen synthase kinase-3β (p-Gskβ), which is a target of activated Akt. We observed that p-Gskβ was also elevated in E6-AP transgenic prostate glands (Figure 1E).
Over expression of E6-AP in prostate glands leads to preneoplastic lesions
Initiation and progression of prostate cancer is a multistage process involving a characteristic lesion termed as prostate intraepithelial neoplasia (PIN), believed to be the precursor for the formation of prostate cancers. Since E6-AP transgenic mice did not give raise to palpable prostate tumors, we decided to perform a histological observation of the prostate glands. Prostate glands from >18 month old transgenic and Wild-type litter mate controls were dissected into individual lobes (n=10). These tissues were processed, embedded in paraffin and 5μ sections were sliced. These sectioned tissues were stained for haematoxylin and eosin to determine the morphological features. Figure 2 shows that E6-AP transgenic mice develop hyperplasic characteristics resembling PIN when compared with Wild-type litter mate controls (arrows). This finding suggests that over expression of E6-AP could result in excessive proliferation and formation of preneoplastic lesions, which resembles PIN. These lesions were found in 7 out of 10 transgenic mouse prostate glands, but none were found in Wild-type prostate glands.
Figure 2.
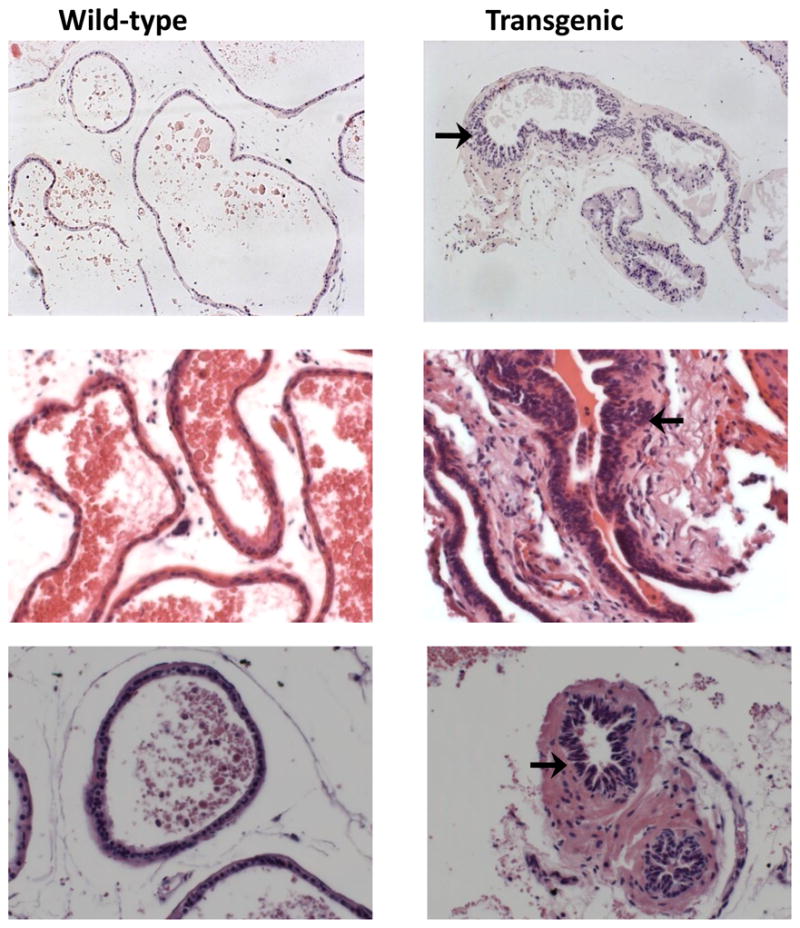
Histology of prostate glands from E6-AP transgenic mice. Representative sections from wild-type and transgenic mice stained for H & E. Precancerous lesions characteristic of PIN can be identified in transgenic sections (Arrows).
E6-AP modulates PI3K-Akt signaling
It is known that PI3K-Akt signaling pathway plays a central role in the development and progression of prostate cancer [14–16]. Our previous studies showed that PI3K-Akt signaling is down regulated in the prostate glands of E6-AP knockout mice [1]. In order to study the effect of over expression of E6-AP on PI3K-Akt signaling pathway, we utilized E6-AP-LNCaP cells and examined the expression levels of PI3K, total Akt and p-Akt (active Akt) and compared it with that of control parental LNCaP cells. Figure 3A shows that the levels of exogenously expressed Flag-E6-AP are less in uninduced condition (Dox treated cells) when compared to induced conditions (Dox untreated cells), indicating that E6-AP expression is regulated in Dox-dependent manner. Furthermore, E6-AP-LNCaP stable clones express high levels of E6-AP compared to control LNCaP cells. As shown in Figure 3B, the levels of total and p-Akt (Ser-473) is higher in the E6-AP-LNCaP cells compared to the control parental LNCaP cells. Similarly, the levels of PI3K are also high in E6-AP-LNCaP cells compared to that of untransfected control LNCaP cells (Figure 3C). However, under conditions where the expression of exogenous E6-AP (+Dox) is blocked, the levels of PI3K, total Akt and phospho-Akt are not significantly different from that of control LNCaP cells. The treatment of untransfected LNCaP cells with Dox does not significantly affect the levels of these proteins, which serves as a control. These experiments suggest that E6-AP is a key regulator of PI3K-Akt signaling pathway. These results are consistent with our previously published data from E6-AP knockout animals, suggesting that E6-AP is an important modulator of PI3K-Akt pathway in prostate glands. To test if E6-AP amplifies the hormone-dependent up-regulation of PI3K-Akt signaling, we cultured untransfected control LNCaP cells and E6-AP-LNCaP cells in 5% charcoal stripped medium supplemented with or without synthetic androgen R1881 for 24 hours. As shown in Figure 3D, treatment of control LNCaP cells with R1881 for 1h and 36h increases the levels of p-Akt and E6-AP further amplifies the hormone-dependent activation of Akt in E6-AP-LNCaP cells (Figure 3D).
Figure 3.
Over expression of E6-AP induces PI3K-Akt signaling pathway. (A) Western blots showing inducible expression of Flag-E6-AP in E6-AP-LNCaP stable clones. (B) Parental LNCaP cells and E6-AP-LNCaP cells were treated with or without Dox for 48 hours. Increased protein levels of total Akt and phosphorylated-Akt (p-Akt, Ser-473) were observed in E6-AP-LNCaP cells under E6-AP over expressing conditions (−Dox). β-actin was used as a loading control. (C) Parental LNCaP cells and E6-AP-LNCaP cells were treated with or without Dox for 48 hours. Increased protein levels of PI3K was observed in E6-AP-LNCaP cells under E6-AP over expressing conditions (−Dox). β-actin was used as a loading control. (D) Western blot analysis of p-Akt (Ser-473) levels from LNCaP cells and E6-AP-LNCaP stable cells grown in 5% charcoal stripped serum supplemented with or without synthetic androgen R1881 for 1h and 36h.
E6-AP regulates Akt activity via RhoA
Our previous studies indicate that small GTPase, RhoA, a negative regulator of Akt activity is increased in E6-AP knockout prostate glands [1]. We have also shown that inhibition of RhoA activity in prostate cancer cells increases Akt activity. Here we tested the levels of RhoA in our E6-AP-LNCaP cells, Figure 4A shows that the levels of RhoA are decreased under E6-AP induced conditions (− Dox) in our E6-AP-LNCaP cells. Since RhoA is a known target of the ubiquitin-proteasome pathway and its levels are decreased in E6-AP-LNCaP cells under E6-AP over expressing conditions, we hypothesized that, E6-AP interacts with RhoA, ubiquitinates it and induces its degradation via the ubiquitin proteasome pathway. To investigate whether RhoA levels are controlled by the ubiquitin-proteasome pathway in prostate cancer cells, we treated LNCaP cells with either vehicle DMSO or proteasome inhibitor, MG132. Figure 4B shows that RhoA protein levels are stabilized by MG132, indicating that RhoA protein levels in prostate cells are regulated by the ubiquitin-proteasome pathway. Since, E6-AP is an E3 ubiquitin-protein ligase enzyme, in order for E6-AP to act as a specific E3 ubiquitin-protein ligase for RhoA it should interact with RhoA. To test this possibility, we utilized glutathione-S-transferase (GST) pull down assay. 35S-Methionine-labeled RhoA protein was incubated with either control protein (GST only) or GST-E6-AP protein. Figure 4C depicts a significant interaction of RhoA with E6-AP suggesting that E6-AP may be a putative E3 ubiquitin-protein ligase for RhoA in prostate cells.
Figure 4.
Involvement of RhoA in E6-AP-mediated regulation of Akt activity. (A) Western blots showing that the levels of RhoA are decreased under E6-AP overexpressing conditions (−Dox) in E6-AP-LNCaP stable cells compared to that of parental LNCaP cells. (B) To determine if RhoA is target of the ubiquitin-protesome pathway in prostate cells, LNCaP cells were treated with DMSO or proteasome inhibitor, MG132 and cell lysates were examined for RhoA levels. Western blots showing RhoA stabilization with MG132 treatment. (C) In vitro interaction of E6-AP with RhoA. The human RhoA protein was synthesized in vitro using the TNT-coupled reticulocyte lysate system. RhoA protein was then incubated with GST-E6-AP fusion protein that was bounded to glutathione-Sepharose beads. The glutathione-bounded proteins were separated on SDS-PAGE, followed by autoradiography. Twenty percent of the TNT reaction was used as input, and GST alone was used as a negative control.
Over expression of E6-AP increases the cell growth and proliferation
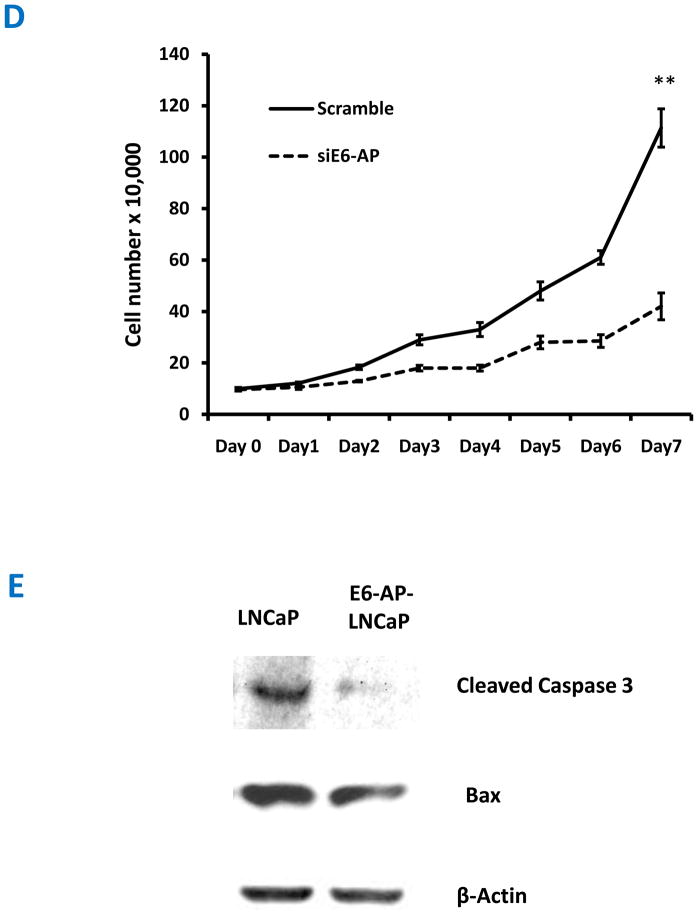
The members of the PI3K-Akt signaling pathway have been implicated in cell growth and proliferation. Since, over expression of E6-AP induces the PI3K-Akt pathway, we predicted that E6-AP-LNCaP cells will exhibit increased cell growth and proliferation compared to that of untransfected control LNCaP cells. As shown in Figure 5A, E6-AP-LNCaP cells that over express E6-AP exhibit changes in cell shape compared with control LNCaP cells. E6-AP-LNCaP cells lacks cell processes and also clump together. Furthermore, E6-AP stable LNCaP cells show increase in cell size compared to that of control LNCaP cells. In addition to cell shape, we also examined the effects of E6-AP overexpression on prostate cell cycle progression using propidium iodide staining of the DNA content of E6-AP-LNCaP cells and control LNCaP cells. As shown in Figure 5B, E6-AP-LNCaP cells have increased number of cells in S phase compared with that of control LNCaP cells. These data suggest that over expression of E6-AP leads to proliferation, which may be due to the upregulation of PI3K-Akt pathway. We also confirmed our results using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) growth assays. E6-AP-LNCaP cells and untransfected control LNCaP cells were grown for a period of 5 days in media containing either normal or charcoal stripped serum. Figure 5C shows a significant difference in proliferation of E6-AP-LNCaP cells compared with untransfected control LNCaP cells both in normal and stripped serum suggesting that over expression of E6-AP results in increased cell proliferation both in an androgen dependent and independent manner. These data confirms that over expression of E6-AP leads to prostate cell proliferation and growth, which correlates with our previous finding that loss of E6-AP leads to reduced prostate gland size and increased apoptosis. We also tested if loss of E6-AP could affect proliferation of LNCaP cells. siScramble or siE6-AP transfected LNCaP cells were starved without hormone for 72 hours and then treated with R1881 for 7 days and proliferation was assessed by enumerating the cell number at the end of each day. As shown in Figure 5D siScramble transfected cells proliferate normally in the presence of R1881, whereas siE6-AP transfected cells do no proliferate as siScramble transfected cells. This indicates that E6-AP is required for the normal proliferation of LNCaP cells.
Figure 5.
Exogenous overexpression of E6-AP enhances cell growth, proliferation and decreases apoptosis. (A) Morphology of E6-AP-LNCaP cells compared with parental control LNCaP cells, indicating the difference in cell size and shape. (B) E6-AP-LNCaP cells exhibit increased cell cycle progression. Parental LNCaP cells and E6-AP-LNCaP cells were grown for 72 h, harvested, fixed with 70% ethanol, stained with propidium iodide, and processed for FACS analysis. Data are plotted as the percentage of S phase cells. (*, P < .05) (C) MTT assay to measure cell proliferation. Parental LNCaP cells and E6-AP-LNCaP cells were grown in 96 well plates in the presence of normal and charcoal stripped serum and MTT assay was carried out on each day for 5 days. Error bars represent standard error of mean from three different measurements. (**, P < .01) (D) siRNA mediated knock down of E6-AP reduces the proliferation of LNCaP cells. 1 × 105 cells were plated in 6 well plates, transfected with either siScramble or siE6-AP, starved for 3 days without hormone and treated with R1881 for 7 days. Cell numbers were counted as a measure of proliferation. Error bars represent standard error of mean from three different experiments. (**, P < .01) (E) Overexpression of exogenous E6-AP in prostate cells protects cells against apoptosis. Parental LNCaP cells and E6-AP-LNCaP cells were grown in normal serum for twenty four hours and then treated with 100μM etoposide for another 6 hours. The cell lysates were analyzed by Western blots using anti-caspase 3 and anti-bax antibodies. E6-AP-LNCaP cells shows less amounts of proapoptotic proteins than parental LNCaP cells.
Our previous data from E6-AP knock-out mice shows that, loss of E6-AP leads to impaired prostate gland development. We also showed that this might be due to elevated apoptosis in E6-AP knockout prostate glands. It is of interest to see if an E6-AP over expressing condition provides a protective effect against apoptosis. To test this we cultured parental LNCaP cells and E6-AP-LNCaP cells and treated them with 100μM etoposide to induce apoptotic stress. Then cell lysates were probed for pro-apoptotic markers like caspase 3 and Bax using Western immunobloting. As shown in Figure 5E, the levels of cleaved caspase 3 and Bax are decreased in E6-AP-LNCaP cells compared to untransfected LNCaP cells indicating that E6-AP over expression provides the cells with survival advantage by protecting them from apoptosis.
Discussion
Coactivators enhance the transactivation functions of nuclear hormone receptors by means of their diverse array of enzymatic activities [22]. Recent studies have suggested that coactivators can also act as key regulators of signaling cascades, indicating that coactivators also possess functions that are distinct from their transcriptional activation functions [23]. E6-AP is an important member of the ubiquitin proteasome pathway and has also been characterized as a coactivator for several steroid hormone receptors including AR [2]. In our previous report, we have shown that loss of E6-AP affects the growth of the prostate gland [1]. We also identified the mechanisms by which E6-AP affects the prostate gland growth and we showed that E6-AP affects both the protein levels and functions of AR. In addition, we also demonstrated that the protein levels of PI3K, total Akt and p-Akt are decreased in E6-AP knockout prostate gland, suggesting that apart from its coactivation function, E6-AP may also modulate the functions of PI3K-Akt signaling pathway. In this study, we have utilized E6-AP over expressing transgenic mouse models to verify the observations from the E6-AP null mouse model. Here we show that over expression of E6-AP in the mouse prostate has a modest increase in wet weight of prostate gland. Additionally, prostate glands in these mice show elevated Akt levels and activity. Although not dramatic, our transgenic mouse model has the opposite phenotypic characteristics as that of E6-AP null mice. Also in this study, we provide evidence which suggests that E6-AP modulates the activity and functions of PI3K-Akt signaling and regulates prostate cell proliferation, prostate gland growth and tumorigenesis. Furthermore, our data also indicate that the components of the PI3K-Akt signaling pathway are elevated when E6-AP is over expressed in prostate cells.
Based on our data, we postulate that E6-AP regulates PI3K-Akt signaling by both androgen-independent and dependent mechanisms. In the androgen-independent mechanism, E6-AP enhances the basal protein levels and activity of the PI3K-Akt signaling pathway. The hormone-independent activation of PI3K-Akt signaling by E6-AP is consistent with previously published study which suggests that the steroid receptor coactivator, SRC-3 can stimulate cell growth by modulating the Akt signaling pathway in an androgen-independent manner, but it is still unknown how SRC-3 regulates Akt signaling [21, 24]. However, in this manuscript, we show the androgen-independent activation of PI3K-Akt signaling by E6-AP is through the down regulation of small GTPase, RhoA in prostate cancer cells. The idea that E6-AP regulates PI3K-Akt signaling and prostate cell growth by modulating the protein levels of RhoA is supported by our data which show that E6-AP interacts with RhoA and may potentially induce its degradation via the ubiquitin-proteasome pathway in prostate cells. Previously, we and others have shown that RhoA negatively regulates PI3K-Akt signaling pathway [1, 25]. It has also been shown that RhoA can promote prostate cancer cell apoptosis by inhibiting Akt signaling pathway via the protein kinase C zeta. Additionally, our previously published data also demonstrated that the levels of total RhoA and active RhoA are increased in E6-AP knockout prostate gland suggesting that RhoA negatively regulates the Akt pathway by inducing the inactivation of Akt in the absence of E6-AP, which subsequently alters prostate cell growth. This possibility was confirmed by the fact that the inhibition of RhoA activity increased the levels of phosphorylated (active) Akt. Consistent with this observation, we now show that the overexpression of E6-AP reduced the levels of RhoA which results in increased basal levels and activation of PI3K-Akt signaling, decreased apoptosis and increased prostate cell growth. E6-AP not only elevates the activity of constitutively active PI3K-Akt pathway in these cells, here, we show that E6-AP which is a known coactivator of the genomic functions of AR, also amplifies the androgen-dependent activation of PI3K-Akt signaling pathway. It is well-established that the PI3K-Akt signaling pathway is induced by androgens via AR [26] suggesting that in addition to ligand inducible transcriptional activity, AR in the cytoplasm also participate in the activation of a variety of signaling pathways including PI3K-Akt signaling pathway [17, 20, 27, 28]. Our data is consistent with and reinforces these findings, which suggest that androgens regulate PI3K-Akt signaling pathway.
Current studies have revealed that PI3K-Akt pathway regulates a variety of cellular functions including cell survival, cell growth, cell differentiation, cell proliferation, cell cycle progression and cellular metabolism [13]. One of the major functions of PI3K-Akt signaling in malignancy is promoting cancer cell survival. Cancer cells develop specific mechanisms to overcome apoptosis, or programmed cell death, which is a normal cellular function involved in the elimination of unnecessary or damaged cells. PI3K-Akt plays a central role in antiapoptotic pathways. Our data from E6-AP over expressing stable prostate cells also support the notion that PI3K-Akt signaling promotes cell growth and decrease apoptosis. Recently, it has been demonstrated that perturbations in the PI3K-Akt signaling are also significantly correlated with the progression of prostate cancer [14, 15]. In addition, it has been demonstrated that there is a direct synergy between AR and Akt signaling which is sufficient to initiate and promote prostate cancer growth and progression [16]. Since E6-AP acts as a coactivator of AR and modulator of PI3K-Akt and RhoA signaling, it may be a key contributor in the maintenance of this synergy between Akt and AR signaling. This might explain the increased proliferative rates, decreased apoptosis and increased tumorigenic potential in models that over express E6-AP.
In summary, we have demonstrated that E6-AP play vital roles in prostate cell proliferation, growth, apoptosis and potential cross talk between PI3K-Akt signaling, RhoA signaling and AR signaling pathways. Here, we provide evidence that E3 ubiquitin-protein ligase/steroid hormone receptor coactivator, E6-AP itself can modulate these cellular signaling pathways and may play a role in development of prostate tumors. Taken together, our studies identify E6-AP as a central protein that can integrate multiple signals into appropriate cellular responses. Furthermore, E6-AP through activation of the PI3K-Akt signaling pathway and their downstream effectors might play critical roles in many biological processes, especially in cell growth and tumorigenesis.
Acknowledgments
Grant Support: This work was supported by grants from the National Institute of Health (DK060907) to ZN and DOD predoctoral fellowship (PC060648) to SS
This work was supported by grants from the National Institute of Health (DK060907) to ZN and DOD predoctoral fellowship (PC060648) to SS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan OY, Fu G, Ismail A, Srinivasan S, Cao X, Tu Y, Lu S, Nawaz Z. Multifunction steroid receptor coactivator, E6-associated protein, is involved in development of the prostate gland. Mol Endocrinol. 2006;20:544–559. doi: 10.1210/me.2005-0110. [DOI] [PubMed] [Google Scholar]
- 2.Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O’Malley BW. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:1182–1189. doi: 10.1128/mcb.19.2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. Embo J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheffner M, Huibregtse JM, Howley PM. Identification of a human ubiquitin-conjugating enzyme that mediates the E6-AP-dependent ubiquitination of p53. Proc Natl Acad Sci U S A. 1994;91:8797–8801. doi: 10.1073/pnas.91.19.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 6.Dhananjayan SC, Ismail A, Nawaz Z. Ubiquitin and control of transcription. Essays Biochem. 2005;41:69–80. doi: 10.1042/EB0410069. [DOI] [PubMed] [Google Scholar]
- 7.Ismail A, Nawaz Z. Nuclear hormone receptor degradation and gene transcription: an update. IUBMB Life. 2005;57:483–490. doi: 10.1080/15216540500147163. [DOI] [PubMed] [Google Scholar]
- 8.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 9.Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–2556. [PubMed] [Google Scholar]
- 10.Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, Vessella RL, Neubauer BL. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275:24500–24505. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 11.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 12.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Ittmann MM, Ayala G, Tsai MJ, Amato RJ, Wheeler TM, Miles BJ, Kadmon D, Thompson TC. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005;8:108–118. doi: 10.1038/sj.pcan.4500776. [DOI] [PubMed] [Google Scholar]
- 14.Tokunaga E, Kataoka A, Kimura Y, Oki E, Mashino K, Nishida K, Koga T, Morita M, Kakeji Y, Baba H, Ohno S, Maehara Y. The association between Akt activation and resistance to hormone therapy in metastatic breast cancer. Eur J Cancer. 2006;42:629–635. doi: 10.1016/j.ejca.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–274. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Xin L, Teitell MA, Lawson DA, Kwon A, Mellinghoff IK, Witte ON. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci U S A. 2006;103:7789–7794. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 18.Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. Embo J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo RX, Wei LH, Tu Z, Sun PM, Wang JL, Zhao D, Li XP, Tang JM. 17 beta-estradiol activates PI3K/Akt signaling pathway by estrogen receptor (ER)-dependent and ER-independent mechanisms in endometrial cancer cells. J Steroid Biochem Mol Biol. 2006;99:9–18. doi: 10.1016/j.jsbmb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J Biol Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G, Hashimoto Y, Kwak I, Tsai SY, Tsai MJ. Role of the steroid receptor coactivator SRC-3 in cell growth. Mol Cell Biol. 2003;23:7742–7755. doi: 10.1128/MCB.23.21.7742-7755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan OY, Nawaz Z. Nuclear hormone receptor co-regulators. Curr Opin Drug Discov Devel. 2003;6:692–701. [PubMed] [Google Scholar]
- 23.Lonard DM, O’Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, Ittmann M, Tsai SY, Tsai MJ. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005;65:7976–7983. doi: 10.1158/0008-5472.CAN-04-4076. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh PM, Bedolla R, Mikhailova M, Kreisberg JI. RhoA-dependent murine prostate cancer cell proliferation and apoptosis: role of protein kinase Czeta. Cancer Res. 2002;62:2630–2636. [PubMed] [Google Scholar]
- 26.Mani A, Oh AS, Bowden ET, Lahusen T, Lorick KL, Weissman AM, Schlegel R, Wellstein A, Riegel AT. E6AP Mediates Regulated Proteasomal Degradation of the Nuclear Receptor Coactivator Amplified in Breast Cancer 1 in Immortalized Cells. Cancer Res. 2006;66:8680–8686. doi: 10.1158/0008-5472.CAN-06-0557. [DOI] [PubMed] [Google Scholar]
- 27.Freeman MR, Cinar B, Lu ML. Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol Metab. 2005;16:273–279. doi: 10.1016/j.tem.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]