Abstract
Competing visions for health reform in the United States (US) and renewed interest in health technology assessment (HTA) have led to fierce national debates about the appropriateness of rationing. Because of a limited supply of organs, kidney transplantation has always required rationing and overt discussion of the ethics that guide it. The field of transplantation, however, has also contended recently with internal calls for a new rationing system. The aim of the Life Years from Transplantation (LYFT) proposal is to allocate kidneys to patients who obtain the greatest survival benefit from transplantation. As a result, it would lengthen the lives of kidney transplant recipients but restrict the ability of older Americans to obtain a transplant. The debate around the LYFT proposal reveals the ethical and policy challenges of identifying which patients should receive a treatment based on the results of cost-effectiveness and other HTA studies. In this article, we argue that attempts to use HTA for health care rationing are likely to disadvantage older patients. We propose guiding principles to help ensure that resources such as kidneys are justly allocated across the life span.
Background
Finite resources and an expanding cohort of older adults have been cited by President Obama, Congressional leadership, and corporations as evidence of the pressing need to reform health care in the US.1-5 Policy debates have often focused on whether reforms will ultimately involve the use of health technology assessment (HTA), such as cost- and comparative-effectiveness studies, to restrict access to expensive and scarce therapies.6, 7 Opponents have denounced such reforms as a covert mechanism for age-based rationing and even “death panels” for the elderly.8 Despite retorts by the White House that rationing is anathema to American health care,9 this response misses the fact that in certain areas of medicine, rationing has been explicit for decades.
Since its inception, the field of kidney transplantation has struggled with the problem that there are fewer kidneys than the number of people who need them. Recently, the United Network for Organ Sharing (UNOS), which regulates US organ transplantation, proposed sweeping changes in kidney allocation based on HTA analyses. The Life Years from Transplantation (LYFT) proposal aimed to improve survival for kidney transplant recipients in part by decreasing access to transplantation for older adults.10, 11 The proposal faced sharp criticism because of ethical concerns about age-rationing, but also because the LYFT system has limited accuracy in predicting which patients derive the greatest benefit from transplantation.12 The demise of this attempt to change how kidneys are rationed raises compelling questions about the design and implementation of HTA in health policy. Is it ethically defensible to use age to ration medical resources such as kidney transplants? If age is used, what principles should guide the development of a rationing system that respects the rights of older individuals?
The need to better allocate a scarce resource
A number of pressing issues motivated UNOS to transform the process for rationing kidneys: the ever-rising waiting time for a kidney, the rapid growth in the number of older individuals eligible for a kidney transplant, and the decreased effectiveness of transplantation in these patients as compared to younger patients. In particular, UNOS representatives argued that the devastating mortality rate with end-stage renal disease (ESRD) creates an imperative to get as much life out of each transplanted organ as possible (Table 1).10
Table 1.
Criticisms of the current system for kidney allocation that motivated the development of the Life Years from Transplant (LYFT) proposal10
| 1) Waiting time for a kidney transplant has increased greatly |
| 2) The current system does not seek to maximize survival for patients on the kidney transplant waiting list |
| a. Mortality and morbidity for wait-listed patients are high |
| 3) In the current system, mismatches between expected patient survival and kidney allograft survival are common |
| a. Many functioning kidney transplants are lost because the recipient dies; this problem is most common among older recipients |
Developed decades ago, the existing system to allocate kidney transplants to adults with ESRD has primarily emphasized fairness according to a single principle: the longer a person has waited for a kidney, the more priority he or she has to receive one.13 The system does not take into account how long someone is projected to live before or after receiving a kidney transplant. As a result, higher priority could be given to transplanting a 65 year-old on dialysis with diabetes and extensive vascular disease who has accumulated more waiting time than a more recently listed 30 year-old with no comorbidities except ESRD.
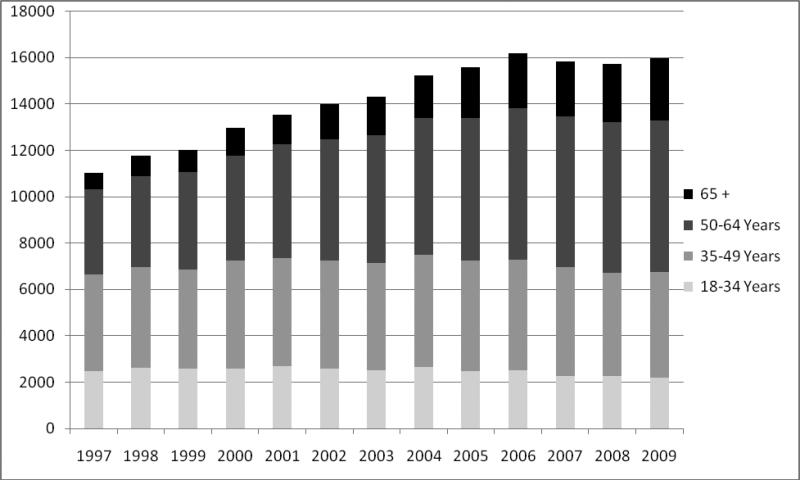
This system arguably worked when the number of older adults receiving transplants was relatively small. But recent trends have transformed the demographics of renal transplantation and contributed to dissatisfaction with the current method of kidney allocation. The epidemics of diabetes, hypertension and obesity have led to tremendous growth in the number of ESRD patients, especially among older adults. This, in turn, has greatly increased the demand for a kidney transplant, such that many patients die before a kidney becomes available. The waiting list for kidney transplantation more than doubled from 30,010 candidates in 1997 to over 83,000 candidates in 2009. During the same period, the proportion of kidney transplant recipients 65 years or older increased from 6.5% to over 16% (Figure 1).14 Twenty years ago, transplantation of patients aged 60 and older was rare. Today, centers commonly accept patients in their 70s, and even patients over 80 years have received kidney transplants.15, 16
Figure 1.
Increasing age of US kidney transplant recipients14
The rising age of kidney transplant recipients also raised the concern that donor kidneys are not effectively allocated, or even wasted. A number of studies have shown that older adults with ESRD live longer with a kidney transplant than they would have lived on dialysis and therefore derive a benefit as individuals.17 From a societal perspective, however, the comparative survival benefit derived from a transplant is greater among young recipients. Specifically, younger patients gain more additional years of life from kidney transplantation (compared to dialysis) than older patients do. Older kidney transplant recipients are more likely to die while their transplanted kidneys still function—an outcome that some view as a waste of valuable organs.10 Seen from the perspective of HTA, a kidney transplanted into a younger person provides greater returns in terms of survival benefit, quality of life, and cost of therapy per year of life gained than a kidney transplanted into an older person.18
The Life Years From Transplant proposal: maximizing the life gained from each kidney
In 2007, UNOS proposed a solution. Instead of using waiting time to assign priority for a kidney transplant, UNOS would assign priority based on the additional projected years of survival a patient would obtain from a transplant.11, 12 In research simulations used to derive the LYFT system, older age proved to be a powerful predictor of relative survival benefit. As a result, older adults would receive lower scores and lower priority for a kidney.11 This, in turn, means that the LYFT system probably would increase the net survival of transplant recipients and thus transplanted kidneys would last longer. The LYFT system could decrease average waiting times for a kidney (as long as four years in many regions) because less patients with failed transplants would return to the waiting list. Unfortunately, these achievements come at a cost: as patients get older, their access to transplantation decreases.19
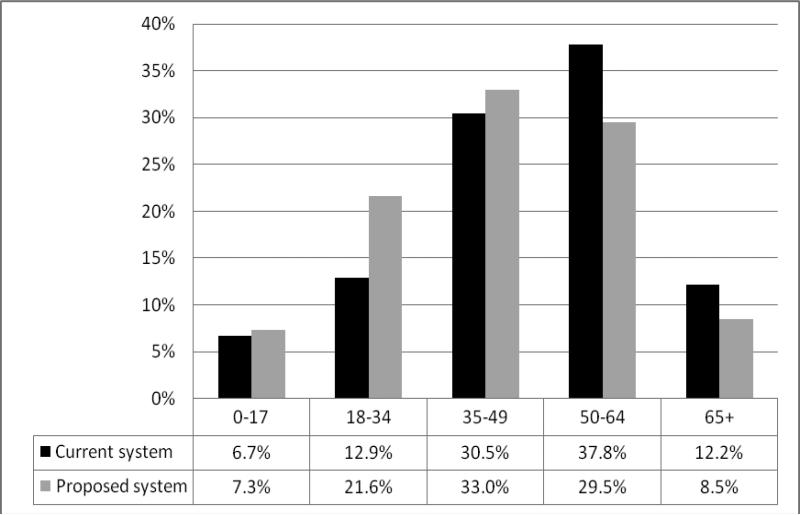
The basic formula to determine the transplant survival benefit for an individual—his or her “LYFT score”—is straightforward. Projected years of survival on dialysis are subtracted from projected years after a kidney transplant. (There is also an adjustment reflecting lower quality of life on dialysis, omitted here for simplicity). The consequences of LYFT for different transplant candidates would be profound. For example, suppose the 65 year-old diabetic candidate cited earlier would live 1 year on dialysis and 4 years with a transplant. He would have a LYFT score of 3 and lower priority for a transplant than the 30 year-old candidate, who has the same blood type but a LYFT score of 9.11 In contrast, under the existing system, the 65 year-old would receive the kidney if he had waited longer. Figure 2 shows how the LYFT proposal would shift kidney transplants to younger patients.
Figure 2.
The LYFT proposal would shift kidney allograft allocation toward younger patients19
The use of age to assign priority for transplantation has obvious benefits. Chronological age is difficult to falsify. Physicians, patients and policy-makers understand what it means. However, using age as a criterion to allocate a scarce resource such as donor kidneys presents challenges. Although increasing age is associated with increasing probabilities of disease, age itself is not a disease.20 Another limitation of the LYFT model is that it does not adequately distinguish between variations in health among individuals of the same age, whether young or old. At a given age, some older adults have marked functional impairment and thus greater risk of death while others have maintained function and vitality. From the perspective of a highly functional older individual, the LYFT system is unfair.
Analytical frameworks for rationing care with health technology assessment such as LYFT
In order to show how the LYFT analyses relate to contemporary debates about health reform, it is useful to define HTA and how, given its place in the recently passed “health care legislation,” HTA will be employed as a tool to promote efficiency. HTA encompasses comparative effectiveness, cost-effectiveness and cost-benefit studies; all provide information about outcomes with different treatment strategies. These studies can also be used to determine access to medical care based on findings that one approach to care is superior.18, 21 HTA typically involves comparing the outcomes (e.g. survival) of different treatments for the same patient. The LYFT analysis instead compared the same treatment (a kidney transplant) for different patients. However, the output of the LYFT analysis—a ratio of cost to health outcome—is also what a cost-effectiveness analysis provides. With kidney allocation, the “cost” is the limited resource of a donated organ, while cost-effectiveness analysis commonly expresses the cost in dollars.
The focus on HTA by President Obama and others has attracted strident criticism because of fears that administrative panels – within the federal government, quasi-government agencies or insurance companies – will ultimately use this research to decide which treatments are acceptable and for whom.22, 23 As US health care legislation is implemented, it is unclear what role and powers that the new US board tasked with funding and interpreting HTA will have.24 Yet, such HTA-driven rationing is already implemented through diverse areas of the medical system including the Veterans Health Administration and private pharmacy plans.25-27 It is used in other Western nations (such as the United Kingdom with its National Institute for Health and Clinical Excellence (NICE) board).18 Given that medical costs will continue to pose a substantial economic burden, HTA-driven rationing will play a role in the delivery of US health care in some form. And as with LYFT, concerns will persist that administrative panels won't allocate treatments in a way that respects the circumstances of individual patients, especially the elderly.
How the LYFT proposal informs future debates about the ethics of using age to assess treatment benefit
We believe that using age to guide access to care can be acceptable, but the rationing system must respect principles that safeguard the rights of older individuals. Before describing these principles, we review the standard ethical arguments supporting age as a criterion to allocate treatments, and illustrate their shortcomings in the context of the LYFT proposal and scientific advances in geriatric medicine.
Despite the high-profile use of the word “rationing” to accuse others of ethical failings in health reform debates,8 the use of age to ration treatment has gained legitimacy in medical ethics. The LYFT system conforms to utilitarian theory to optimize outcomes for the greatest number of ESRD patients (in this case, the outcome of mortality).13 Contemporary ethicists such as Daniel Callahan and Ezekiel Emanuel have also developed ideas about why limiting health care among older people is reasonable and humane.28-30 Callahan argued that the high price of medical innovations has made it increasingly difficult for nations such as the US to fund universal medical care. He suggested that biomedical research offers a false promise of longer and better life for everyone, and that care for the elderly (who benefit less from medical technology) should instead focus on dignity and relief of suffering.28
Ezekiel Emanuel, now Special Advisor for Health Policy in the White House, developed the “complete lives” principle of distributing health care resources. This principle holds that rationing health care by age is not utilitarian but egalitarian because it maximizes the possibility that every person could experience the different stages of life. Hence, the elderly should have less priority for kidney transplants or other treatments because they have already enjoyed “a complete life.” Emanuel has identified areas as diverse as vaccination, intensive care admissions and transplantation where withholding therapies from older adults may be considered. Unlike the HTA approach that led to the LYFT formula, Emanuel does not focus primarily on whether older patients derive less relative therapeutic benefit than younger patients do, but instead argues that directing health care resources to the young gives them a greater chance of being able to reach all of life's stages.29
Arguments based on utility or the life-cycle approach are vulnerable to the criticism that allocating therapies by age doesn't respect the rights of older adults as individuals. While extreme old age is arguably a “complete life,” over the course of the last century, the concept of what “old” means has shifted.16, 31 Today, some patients in their seventies thrive after kidney transplantation, while others engage in vital activities such as running for President of the United States. The notion of a ‘natural’ lifespan is a cultural construct as well as a biological reality.20
To date, the LYFT proposal has been rejected by transplant leaders because it does not adequately address the fact that although age adds value in predicting survival, it is too indirect a means to capture the health of older adults.12 The LYFT formula predicts individual survival using only the co-morbidities of diabetes, prior transplant, cause of kidney disease and body mass index. Recipient age contributes nearly 25% to an individual's LYFT score.11 By lumping together transplant candidates of similar ages, this proposal does not take advantage of a substantial body of literature on healthy aging.
A key insight of geriatric medicine is that a tally of co-morbidities and age is not sufficient to represent the health of older adults. Measures of function are essential. Functional status and physical activity may reveal the cumulative impact of comorbidities, and capture the effects of age-related syndromes such as frailty. For example, the Vulnerable Elders Survey-13 (VES-13) of adults 65 years or older measured difficulties performing everyday activities such as walking, shopping, and managing personal finances. The VES-13 had a high degree of accuracy in predicting two-year mortality or new disability.32 As shown in Appendix 1, a number of other studies have advanced the methodological sophistication of risk models that predict survival among older individuals.33-36 These risk models have not been validated in ESRD, but provide kidney transplant researchers a template to design and validate methods to predict mortality among kidney transplant patients.
The lack of inclusion of functional status and other measures of health in the LYFT formula stems from problems with the data used. The architects of the LYFT system used the best dataset available to them—registry data on ESRD patients from the Organ Procurement and Transplantation Network (OPTN).11 While powerful, these data have serious flaws. OPTN data lack high quality information on functional status, changes in health over time, and co-morbidities. For instance, the three physical function fields are coarse, ranging from “No limitations” to “Wheelchair bound.”37 Similarly, the OPTN does not record prior cardiovascular events or results of cardiac tests, but instead provides a restricted range of fields related to the presence of “angina.” This limited characterization of cardiac status is especially important because cardiovascular events are the leading cause of death in ESRD, and because prior studies suggest that inclusion of co-morbidities improves the prediction of survival for transplant patients.38, 39 Not surprisingly, the LYFT formula has limited accuracy in modeling survival benefit from transplantation.11, 12
This accuracy problem is an important lesson to proponents of using HTA to determine when certain treatments – from kidney transplants to ICU beds – should be approved and who should get them.6 The debate over LYFT reveals how challenging it will be to identify those patients who benefit the most from a scarce or expensive treatment. The devil is in the dataset.
We believe, however, that making use of age as one factor along with other informative health data to predict health outcomes and ration treatments is ethically defensible. Below, we summarize principles for developing sound policies that use age as a criterion to select patients.
A framework for evaluating age-rationing of treatments and a proposed research agenda
The successful use of HTA in health policy will depend on guiding principles acceptable to physicians, policy-makers and the public. Kidney allocation provides a useful example of the kind of research agenda that is needed to determine which patients derive the most benefit from an intervention. This agenda matters not only for sharing kidneys, but also to a larger national audience looking to see whether transplantation will lead the way in treating older adults fairly while employing the tools of HTA.
First, as with the development of LYFT, the process of using HTA to identify which patients receive a therapy should be transparent and objective. The datasets and analyses used by policy makers need to be publicly available. The people who perform these analyses should be free of conflicts of interest.
Second, age-rationing of therapies should only be considered in situations where the costs of not doing so are unacceptably high. Although the determination of an unacceptably high cost may be contentious, we believe that important benchmarks would include large differences in survival (i.e. measurable in years) or substantial reductions in suffering from receiving a therapy. For instance, in kidney transplantation, the cost of maintaining the current system is a far greater number of deaths among ESRD patients than if age-rationing were implemented through LYFT.
Third, as we argued earlier, proposals like LYFT that involve age-rationing should be judged by whether they distinguish adequately between individuals of the same age. The analysis of which patients benefit from therapy should be based on a dataset with detailed assessment of co-morbidities and functional status. The HTA research should report whether the inclusion of these data diminishes the predictive value of age.
The field of renal transplantation needs research into what factors explain differences in mortality among older transplant candidates. In addition to functional status data, the predictive ability of LYFT should be re-examined with the addition of validated data about prior myocardial infarction, stroke, peripheral vascular disease, malignancies and tobacco use.40, 41 This research will require new, prospective data collection by transplant centers, and UNOS is committed to this long-term process. In the interim, this research might take advantage of large datasets generated by dialysis corporations and major health care organizations, or data from non-US countries that have accurate information on co-morbidities and medical testing. Among both dialysis patients and kidney transplant candidates, there is wide variation in physical function.42, 43 Transplant leaders committed to driving better survival through more efficient kidney allocation need to study whether this variation affects transplant endpoints.
Lastly, this research agenda would be enriched by empirical assessment of how transplant candidates themselves believe that organ rationing should take place. Minimal information exists, for instance, about whether kidney transplant candidates would consider a system that matches projected kidney allograft survival to patient survival to be more acceptable than a system based primarily on waiting time.
Weighing the burdens of collecting more information about transplant candidates
The policy implications of this research agenda will depend on the burdens imposed on transplant centers. More detailed clinical assessments of transplant candidates will consume resources that should be reimbursed by health insurance. On the other hand, in the current system, costs are higher when patients with co-morbidities and poor functional status suffer peri-operative complications with prolonged hospital stays. Centers could see a financial upside if changes in organ allocation led to selection of patients who recover rapidly from transplant surgery.
The UNOS Kidney Committee also cited concerns that clinical variables used to generate the LYFT score should not be easily falsifiable. For instance, if peripheral vascular disease were a component of LYFT, a patient's foot amputation might be withheld from UNOS to improve the patient's priority. To some extent, this concern over data reliability could be addressed by center audits.
Lastly, the barriers to completing the many steps to transplant eligibility are well described.44 Creating new steps will require justification that the effort and expense don't slow the already cumbersome process of being accepted to the waiting list.
Conclusions
The LYFT proposal to use age for rationing kidneys has substantial relevance to contemporary debates about the role of HTA in allocating expensive or scarce treatments. Broad criticisms of any health-care rationing ignore the ethical imperatives created by limited resources such as organ transplants. Were it implemented, the LYFT system could drive important gains in survival from renal transplantation at the cost of disadvantaging older transplant candidates. The failure of the LYFT proposal to be accepted, however, reveals the challenge of finding high quality data to predict which patients benefit most from treatment. When rationing care includes an age-criterion, the process of selecting candidates for therapies must respect principles that safeguard older patients. The process must be transparent and the high costs of not rationing care efficiently must be evident. Lastly, the rationing system must account for clinically significant differences in health between older individuals.
Acknowledgments
Funding sources: Dr. Reese is supported by K23 - DK078688-01, and by a geriatric nephrology grant from the American Society of Nephrology and the Association of Specialty Professors. Dr. Karlawish is supported by a Robert Wood Johnson Investigator Award in Health Policy Research.
Sponsor's Role: The study had no sponsor.
The authors thank representatives from the United Network for Organ Sharing for approving the use of Figure 2.
Appendix
Appendix 1.
| Carey | Inouye | Lee | Walter | ||
|---|---|---|---|---|---|
| Population | Community dwelling age >=70 years | Hospitalized, age >=65 years (validation) | Community dwelling, age >50 years | Hospitalized, age >=70years | |
| Risk Factorsa | |||||
| Demographic: | Age | 76-80 (1) | *Age not significant & not in the index. |
60-64 (1) | *Age not significant & not in the index. |
| >80 (2) | 65-69 (2) | ||||
| 70-74 (3) | |||||
| 75-79 (4) | |||||
| 80-84 (5) | |||||
| >85 (7) | |||||
| Gender | Male (2) | Male (2) | Male (1) | ||
| Comorbidities: | Diagnoses | High-risk diagnoses (0-3) | |||
| Diabetes | Any (1) | ||||
| Cancer | Any (2) | Solitary (3) Metastatic (8) |
|||
| Lung disease | Any (2) | ||||
| Heart failure | Any (2) | Any (2) | |||
| Current tobacco use | Any (2) | ||||
| BMI | < 25 (1) | ||||
| Labs: | Albumin | <= 3.5 mg/dl (0-1) | 3.0-3.4 g/dl (1) | ||
| <3.0 g/dl (2) | |||||
| Creatinine | > 1.5 mg/dl (0-1) | > 3.0 mg/dl (2) | |||
| Functional: | ADLs | Dependence bathing (1) |
Dependence bathing (2) |
||
| Number of dependent ADLs |
1 - 4 (2) | ||||
| All 5 (5) | |||||
| IADLs | Dependence in grocery shopping (2) |
||||
| Managing finances (2) |
|||||
| Physical function | Limited ability to walk (2) |
Limited ability to walk (0-1) |
Limited ability to walk (2) |
||
| Difficulty moving heavy objects (1) |
Difficulty moving heavy objects (1) |
||||
| Cognition: | Dementia | Dementia (0-1 points) | |||
| Outcome | 2 year mortality | 1 year mortality | 4 year mortality | 1 year mortality | |
| Risk Groups | low (0-2 points) | group I (0-1 points) | 0-5 points | 0-1 points | |
| middle (3-6 points) | group II (2 points) | 6-9 points | 2-3 points | ||
| high (>=7 points) | group III (3 points) | 10-13 points | 4-6 points | ||
| group IV (>=4 points) | >=14 points | >6 points |
Points awarded to each clinical factor are noted in parentheses.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Author Contributions: All authors participated in the manuscript's concept and design. Dr. Reese drafted the manuscript and all the co-authors participated in revision.
References
- 1.Marmor T, Oberlander J, White J. The Obama Administration's Options for Health Care Cost Control: Hope vs. Reality. Ann Intern Med. 2009 doi: 10.7326/0003-4819-150-7-200904070-00114. [DOI] [PubMed] [Google Scholar]
- 2.Lubitz J, Cai L, Kramarow E, Lentzner H. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349:1048–1055. doi: 10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 3.Obama B. Cutting Costs and Covering America: A 21st Century Health Care System. Democratic National Committee; May 27, 2007. URL: http://www.barackobama.com/2007/05/29/cutting_costs_and_covering_ame.php. [Google Scholar]
- 4.Lieberman J. Healthy Americans Act. Congressional Record, November. 2007;13 URL: http://www.govtrack.us/congress/record.xpd?id=110-s20071113-32. [Google Scholar]
- 5.Rideout J. The Impact on Healthcare Costs for American Companies. Vol. 3. Heathcare Technology; 2007. pp. 2–5. [Google Scholar]
- 6.Daschle T. Critical: What We Can Do About the Health-Care Crisis. New York: St. Martin's Press. 2008 [Google Scholar]
- 7.Congressional Budget Office Research on the Comparative Effectiveness of Medical Treatments. 2007 Dec; [Google Scholar]
- 8.Palin S. Obama and the Bureaucratization of Health Care; The president's proposals would give unelected officials life-and-death rationing powers. Wall Street Journal. 2009 Sep 8; http://online.wsj.com/article/SB10001424052970203440104574400581157986024.html.
- 9.Obama B. New York Times; Sep 9, 2009. Health Care Speech to Congress. http://www.nytimes.com/2009/09/10/us/politics/10obama.text.html. [Google Scholar]
- 10.OPTN . Public Forum: Kidney Allocation Policy Development. Dallas, TX: Feb 8, 2007. http://www.optn.org/SharedContentDocuments/KidneyAllocationSlides_Reduced.pdf. [Google Scholar]
- 11.Wolfe RA, McCullough KP, Schaubel DE, et al. Calculating life years from transplant (LYFT): methods for kidney and kidney-pancreas candidates. Am J Transplant. 2008;8:997–1011. doi: 10.1111/j.1600-6143.2008.02177.x. [DOI] [PubMed] [Google Scholar]
- 12.American Society of Transplant Surgeons [1/15/2009];Proposed Kidney Allocation Concepts - ASTS Response. http://www.asts.org/Advocacy/Regulatory04.aspx.
- 13.Shapiro ME. The development of new allocation policy for deceased donor kidneys. Curr Opin Nephrol Hypertens. 2007;16:512–515. doi: 10.1097/MNH.0b013e3282f08638. [DOI] [PubMed] [Google Scholar]
- 14. [3/15/2010];United Network for Organ Sharing. http://www.unos.org/data/data_resources.asp.
- 15.Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069–1074. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 16.Belitsky P. Can old be too old for kidney transplantation? Transplantation. 2007;83:1015–1016. doi: 10.1097/01.tp.0000259649.97241.05. [DOI] [PubMed] [Google Scholar]
- 17.Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Semin Nephrol. 2009;29:621–635. doi: 10.1016/j.semnephrol.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson G. Comparative Clinical Effectiveness and Cost-Effectiveness Research: Background, History and Overview. Congressional Research Service. 2007 URL: http://aging.senate.gov/crs/medicare6.pdf.
- 19.OPTN/UNOS Kidney Transplantation Committee [5/4/2009];Kidney Allocation Concepts: Request for Information. 2008 Sep; URL: http://optn.transplant.hrsa.gov/SharedContentDocuments/KidneyAllocationSystem--RequestForInformation.pdf.
- 20.Caplan AL. In: Is Aging a Disease? in Applied Ethics. Chadwick R, Schroeder D, editors. Routledge; London: 2002. [Google Scholar]
- 21.Goodman CS. HTA 101: Introduction to Health Technology Assessment. United States National Library of Medicine; 2004. URL: http://www.nlm.nih.gov/nichsr/hta101/ta101_c1.html. [Google Scholar]
- 22.Pipes SC. Obama Will Ration Your Health Care. Wall Street Journal. 2009 Dec 30; http://online.wsj.com/article/SB123060332638041525.html.
- 23.Healy B. Comparative Effectiveness: Is Obama Really Calling for Rationing? US News and World Report. 2009 URL: http://www.usnews.com/health/blogs/heart-to-heart/2009/03/18/comparative-effectiveness-is-obama-really-calling-for-rationing.html.
- 24.United States Congress; Patient Protection and Affordable Care Act, H.R. 3590. URL: http://democrats.senate.gov/reform/patient-protection-affordable-care-act-as-passed.pdf. [Google Scholar]
- 25.Aspinall SL, Good CB, Glassman PA, Valentino MA. The evolving use of cost-effectiveness analysis in formulary management within the Department of Veterans Affairs. Med Care. 2005;43:20–26. doi: 10.1097/01.mlr.0000170004.17480.49. [DOI] [PubMed] [Google Scholar]
- 26.Neumann PJ. The arrival of economic evidence in managed care formulary decisions: the unsolicited request process. Med Care. 2005;43:27–32. doi: 10.1097/01.mlr.0000170005.09751.b4. [DOI] [PubMed] [Google Scholar]
- 27.Information on cost-effectiveness: an essential product of a national comparative effectiveness program. Ann Intern Med. 2008;148:956–961. doi: 10.7326/0003-4819-148-12-200806170-00222. [DOI] [PubMed] [Google Scholar]
- 28.Callahan D. Setting Limits: Medical Goals in an Aging Society. Simon & Schuster; New York: 1987. [PubMed] [Google Scholar]
- 29.Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009;373:423–431. doi: 10.1016/S0140-6736(09)60137-9. [DOI] [PubMed] [Google Scholar]
- 30.Williams A. Intergenerational equity: an exploration of the 'fair innings' argument. Health Econ. 1997;6:117–132. doi: 10.1002/(sici)1099-1050(199703)6:2<117::aid-hec256>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 32.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 33.Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19:1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inouye SK, Bogardus ST, Jr., Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41:70–83. doi: 10.1097/01.MLR.0000039829.60382.12. [DOI] [PubMed] [Google Scholar]
- 35.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 37.United Network for Organ Sharing [1/12/2009];Adult Kidney Transplant Candidate Registration Worksheet. http://www.unos.org/SharedContentDocuments/Kidney-Adult_Transplant_Candidate_Registration.pdf.
- 38.Weinhandl ED, Snyder JJ, Israni AK, Kasiske BL. Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance. Am J Transplant. 2009;9:506–516. doi: 10.1111/j.1600-6143.2008.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machnicki G, Pinsky B, Takemoto S, et al. Predictive ability of pretransplant comorbidities to predict long-term graft loss and death. Am J Transplant. 2009;9:494–505. doi: 10.1111/j.1600-6143.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- 40.Cardinal H, Hebert MJ, Rahme E, et al. Modifiable factors predicting patient survival in elderly kidney transplant recipients. Kidney Int. 2005;68:345–351. doi: 10.1111/j.1523-1755.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- 41.Kauffman HM, McBride MA, Cors CS, Roza AM, Wynn JJ. Early mortality rates in older kidney recipients with comorbid risk factors. Transplantation. 2007;83:404–410. doi: 10.1097/01.tp.0000251780.01031.81. [DOI] [PubMed] [Google Scholar]
- 42.Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41:1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 43.Hartmann EL, Kitzman D, Rocco M, et al. Physical Function in Older Candidates for Renal Transplantation: An Impaired Population. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. Jama. 1998;280:1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]