Abstract
DNA double-strand breaks (DSBs) are the most lethal type of DNA damage induced by ionizing radiation or chemotherapeutic drugs used to eradicate cancer cells. The ability of cancer cells to effectively repair DSBs significantly influences the outcome of therapeutic regimens. Therefore, a new and important area of clinical cancer research is the development of DNA repair inhibitors that can be used as radio- or chemosensitizers. Non-homologus end-joining (NHEJ) is the predominant pathway for the repair of radiation-induced DSBs. A series of recent reports indicates that the epidermal growth factor receptor (EGFR) or its downstream components may modulate NHEJ through direct interaction with the DNA repair enzyme, DNA-dependent protein kinase (DNA-PK). As EGFR is overexpressed or activated in many cancers, these findings provide a compelling rationale for combining radiotherapy with therapies that block EGFR or its downstream signaling components. In this review, we delineate how these novel connections between a cell-surface receptor (EGFR) and a predominantly nuclear event (NHEJ) provide vulnerable nodes that can be selectively targeted to improve cancer therapy.
Introduction
DNA double-strand breaks and cancer therapy
DNA double-strand breaks (DSBs) are of paramount importance in the field of radiation oncology as these breaks are induced by ionizing radiation (IR) and most chemotherapeutic agents. Two distinct pathways exist in mammalian cells for the repair of DSBs – non-homolgous end-joining (NHEJ) and homologous recombination (HR). The choice of repair pathway actually used depends upon the cell cycle stage, with NHEJ being operative in all phases of the cell cycle and HR being functional only during the S/G2 phases when a sister chromatid is available for repair. The DNA damaging agent used for therapy also influences the choice between NHEJ and HR, i.e., certain agents induce breaks that occur during the process of DNA replication and such breaks are preferentially repaired by HR. Hence, radio- and chemo-therapeutic outcomes would depend, to a significant extent, upon the robustness of these two repair pathways in tumor cells, an understanding of which can be used to develop therapeutic strategies that are tailored to target a specific type of tumor.
An excellent case in point is that of Brca1/2-null breast and ovarian cancers that are defective in HR and, thus, acutely sensitive to PARP-inhibitors that generate secondary replication-associated DSBs. This concept of “synthetic lethality” [1] has been expounded upon in other reviews in this issue (see reviews by Powell and Chalmers). The focus of our review, however, is on NHEJ. Herein, we discuss how the NHEJ repair process can be modulated by oncogenic events during carcinogenesis and how this link between activated oncogenic signaling and NHEJ may constitute the proverbial “Achilles heel of cancer” [2] that could be targeted for therapy.
The rationale for targeting tumors with DNA repair inhibitors
In the simplest of terms, the rationale for selectively targeting tumor cells with inhibitors that hamper DNA repair is that this would allow smaller doses of radiation or chemotherapeutic agents to be used, thereby reducing the side-effects of therapy while allowing greater tumor control. However, for a radiosensitizer to be efficacious, it must exert a greater effect on tumor cells compared to normal cells. Logically, this is a feasible proposition for the following reasons:
In general, most cancer cells carry a greater burden of endogenous DSBs due to aberrant hyperproliferation [3]. These cells, as such, should be more susceptible to DNA repair inhibitors even in the absence of radiation as they must, perforce, repair endogenous DSBs on an ongoing basis. Moreover, the greater load of endogenous DSBs in these cancer cells would also render them more susceptible than normal cells to radiation.
Cancer cells are sometimes deficient in a specific DNA repair pathway [1] rendering them more reliant on an alternate repair pathway. When the alternate pathway is inhibited, the cancer cells would be specifically impaired in the repair of DSBs.
Cancer cells might have heightened DNA repair or damage-responsive mechanism(s) on which they may be over-dependent (perhaps, to deal with endogenous DNA damage), a phenomenon termed “non-oncogene addiction” [4].
In such a scenario, specifically targeting the over-activated DNA repair pathway may result in a greater radiosensitizing effect on cancer cells relative to normal cells. This concept is exemplified by glioblastomas with EGFR amplification or PTEN loss. These brain tumors may be more efficient at DSB repair by NHEJ due to cross-talk between the PI3K-Akt-1 signaling pathway and the DNA repair enzyme, DNA-PKcs (DNA-dependent protein kinase, catalytic subunit) [5, 6]. Such tumors may, therefore, be more susceptible to inhibition of NHEJ either by targeting EGFR or by directly targeting DNA-PKcs. These exciting new concepts are the major focus of this review. Herein, we will first describe the NHEJ repair pathway and the EGFR signaling cascade and then delineate the novel connection between EGFR signaling and NHEJ, describing how this connection could be subverted for efficient radiotherapy.
DSB repair by non-homologous end-joining (NHEJ)
Non-homologous end-joining (NHEJ) is one of the major pathways for the repair of IR-induced DSBs in mammalian cells [7, 8]. During NHEJ, the two DNA ends are simply ligated together, often after limited end processing. End processing during NHEJ can lead to loss of sequence information around the break; hence, NHEJ, unlike HR, is an error-prone process. Regardless, NHEJ is the predominant mechanism for the repair of radiation-induced DSBs in quiescent cells and in dividing cells in the G1 (Gap 1) phase of the cell cycle. Indeed, NHEJ can, a priori, function robustly in all phases of the mammalian cell cycle, making this pathway a very important factor in the response of tumor cells to therapy with radiation or radiomimetic agents.
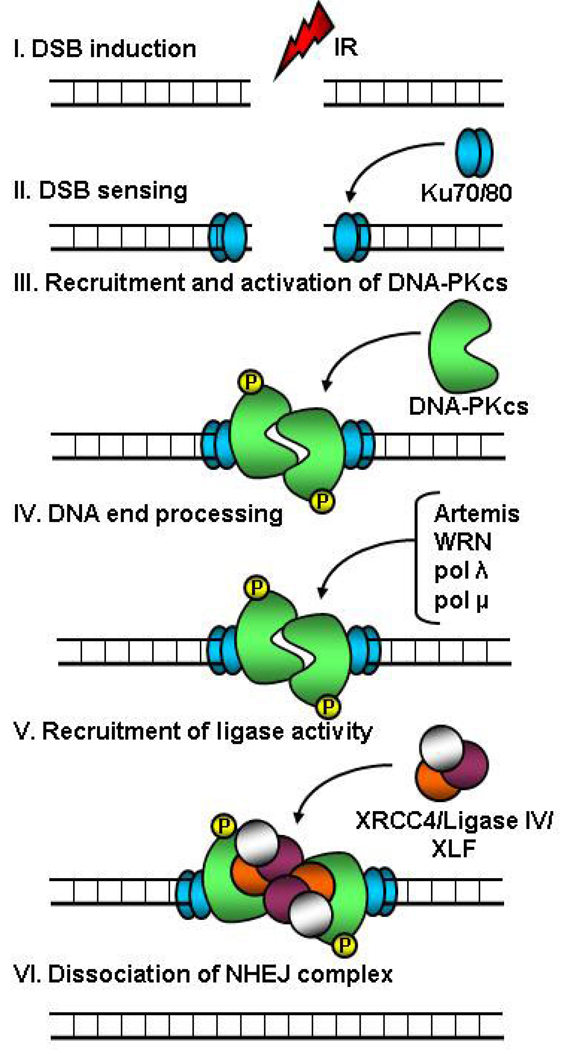
The core components of the NHEJ apparatus are Ku (Ku70/80 heterodimer), DNA-PKcs, XRCC4 (X-ray repair complementing defective repair in Chinese-hamster cells 4), DNA ligase IV, and XLF (XRCC4-like factor, also called Cernunnos). Figure 1 depicts a simplified scheme of events as they occur during NHEJ. It is important to point out here that the exact choreography of events at a break and the precise nature of the protein sub-complexes that participate in the repair process may be flexible and these aspects of NHEJ remain to be completely elucidated in vivo. However, it is quite clear that DNA-end binding by Ku, a heterodimer of two subunits of 70 kDa and 80 kDa, is the initiating event in NHEJ and serves to tether the DNA ends together. This is followed by the inward translocation of Ku allowing recruitment of DNA-PKcs to the extreme DNA termini; the resulting complex of Ku and DNA-PKcs is called DNA-PK. DNA-PKcs is an approximately 470-kDa serine/threonine kinase belonging to the PI3KK (phosphatidylinositol-3 kinase-related kinases) family [9]. The crystal structure of DNA-PKcs was elucidated very recently, revealing an open cradle-shaped ring (containing the DNA binding domain) with a “crown” (containing the kinase domain) [10]. Such an open structure would presumably allow DNA binding as well as enough flexibility for interactions with other proteins.
Figure 1. Simplified schematic depicting steps in the non-homologous end-joining (NHEJ) pathway of DNA double-strand break (DSB) repair.
DSBs induced by ionizing radiation (IR) or chemotherapeutic agents are recognized by the Ku70/80 heterodimer which then recruits DNA-PKcs (DNA-dependent protein kinase, catalytic subunit) resulting in activation of the latter. Activated DNA-PKcs phosphorylates itself and other proteins involved in repair or damage signaling. Following DNA-end processing by nucleases (such as Artemis and WRN) and DNA polymerases (such as pol λ and pol μ), the ends are ligated together by a complex of XRCC4 (X-ray repair complementing defective repair in Chinese-hamster cells 4), DNA ligase IV, and XLF (XRCC4-like factor, also called Cernunnos). The exact choreography of events at a break and the precise nature of the protein sub-complexes that participate in the repair process may be flexible. As the kinase activity of DNA-PKcs is essential for NHEJ, small molecule inhibitors that bind to and block the kinase domain of DNA-PKcs are very potent radiosensitizers.
The recruitment of DNA-PKcs to breaks serves to form a “synaptic complex” bridging the two ends together and also triggers the protein kinase activity of DNA-PKcs. Activated DNA-PKcs phosphorylates itself (both in cis and in trans) [11, 12] as well as other proteins participating in repair or damage signaling [9, 13, 14]. While the precise functions of these DNA-PKcs-mediated phosphorylation events are still not completely understood, it is postulated that autophosphorylation of DNA-PKcs triggers conformational changes and/or dissociation of DNA-PKcs which makes the DNA ends available to other factors participating in DNA end-processing or ligation [8]. Many nucleases as well as DNA polymerases have been implicated in the end-processing step. These include nucleases such as Artemis and WRN that remove DNA overhangs and polymerases such as pol λ and μ that “fill-in” gaps in DNA, the objective being to generate DNA ends that are ligation-compatible.
Finally, the ligation step in NHEJ is carried out by DNA ligase IV. Ligase IV and XRCC4 form a ligation complex wherein XRCC4 serves to stabilize Ligase IV and stimulate its joining activity. XLF/Cernunnos, a recently identified player in the ligation process, has a stimulatory effect on the XRCC4-Ligase IV complex in certain situations [8]. It is not yet clear as to exactly when and how the NHEJ apparatus is disassembled once repair is complete.
In addition to the classical NHEJ pathway just described, alternate NHEJ pathways (that do not require Ku or DNA-PKcs) exist in mammalian cells and these have been reviewed elsewhere [15]. It is important to point out here that while the exact function(s) of DNA-PKcs-mediated phosphorylations have not been completely elucidated, the kinase activity of DNA-PKcs is essential for NHEJ [16]. Hence, small molecule inhibitors that bind to and block the kinase domain of DNA-PKcs exert a powerful radiosensitizing effect [17]. Hopefully, it will be possible to use some of these DNA-PKcs inhibitors for cancer therapy in the future.
Apart from DNA-PKcs, two other kinases belonging to the PI3KK family also respond directly to DSBs but in a different capacity – ataxia telangiectasia mutated (ATM) and ATM and Rad3 related (ATR). ATM is activated directly by DSBs [18] while ATR is activated by DSBs that have been resected to generate regions of single-stranded DNA in the S and G2 phases of the cell cycle [19]. Both kinases primarily serve to enforce cell cycle checkpoints upon DNA damage and this involves a very intricate network of signaling cascades collectively called the DNA damage response (DDR) [20]. In addition to implementing cell cycle checkpoints, ATM has been reported to promote the repair of a small subset (about 10%) of DNA breaks (presumably, complex breaks or those associated with condensed regions of chromatin) [21]. Hence, ATM-deficient cells exhibit a subtle but distinct DSB repair defect and are acutely radiation sensitive [18]. Therefore, small molecule inhibitors binding to the kinase domain of ATM also have a strong radiosensitizing effect [17], similar to DNA-PKcs inhibitors. This approach of radiosensitization using ATM inhibitors will be discussed further at the end of this review.
EGFR signaling in normal and cancer cells
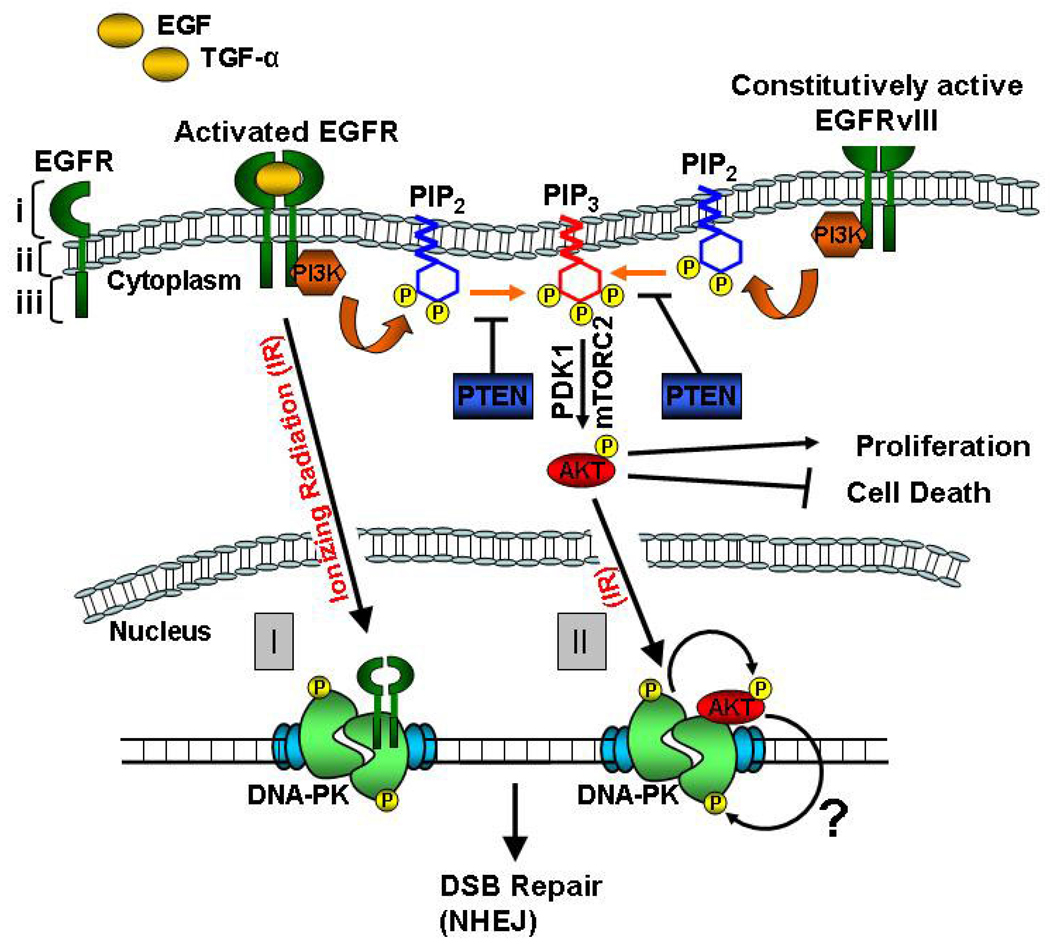
The EGFR is a 170-kDa cell surface receptor tyrosine kinase (RTK) that plays a pivotal role in cell proliferation, cell migration, and cell survival. EGFR belongs to the type I subfamily of RTKs, also called the ERBB tyrosine kinase receptors [22]. The other members of this subfamily are ERBB2 (HER2 or NEU), ERBB3 (HER3), and ERBB4 (HER4) [23]. EGFR is a transmembrane glycoprotein consisting of an extracelluar ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain (TKD) [24] (Figure 2). The ligands of EGFR include epidermal growth factor (EGF) and transforming growth factor-α (TGF-α). Ligand binding induces dimerization of EGFR with itself or with another ERBB family member resulting in intermolecular phosphorylation of key tyrosine residues in the TKD. This triggers downstream signaling events resulting in activation of: 1) phosphatidylinositol 3-kinase (PI3K)-Akt-1 pathway, 2) Ras/RAF/mitogen-activated protein kinase (MAPK)/extracellular signal regulated (ERK) pathway, and 3) signal transducer and activation of transcription (STAT) pathway [24].
Figure 2. Simplified schematic depicting connections between epidermal growth factor receptor (EGFR) signaling and non-homologous end-joining (NHEJ).
Membrane-bound wild type EGFR is activated after binding its ligands: epidermal growth factor (EGF) or transforming growth factor-α (TGF-α). The three domains of EGFR are highlighted with brackets: i (ligand-binding domain), ii (transmembrane domain), and iii (tyrosine kinase domain or TKD). Activated EGFR signals through the following pathways: 1) phosphatidylinositol 3-kinase (PI3K)-Akt-1 pathway, 2) Ras/RAF/mitogen-activated protein kinase (MAPK)/extracellular signal regulated (ERK) pathway, and 3) signal transducer and activation of transcription (STAT) pathway (only the PI3K-Akt-1 pathway is shown for simplicity). The two commonest categories of mutations observed in EGFR are: 1) Somatic “gain-of-function” mutations in the TKD, seen prominently in lung cancers (not shown), and 2) deletion mutations in the extracellular domain, seen commonly in brain cancers (EGFRvIII). EGFRvIII is constitutively active and signals preferentially via the PI3K-Akt-1 pathway. In this pathway, activated phosphatidylinositol kinase (PI3K) phosphorylates phosphatidylinositol-4,5-biphosphate (PIP2) to generate phosphatidylinositol-3,4,5-triphosphate (PIP3). PIP3 anchors Akt-1 to the plasma membrane, where it is phosphorylated by mTOR complex 2 (mTORC2) and 3-phosphoinositide-dependent kinase 1 (PDK1). Activated Akt-1 phosphorylates a plethora of downstream targets resulting in enhanced proliferation and decreased cell death. The PTEN tumor suppressor reverses PIP3 back to PIP2, negatively regulating PI3K-Akt-1 signaling. In response to ionizing radiation (IR), wild type EGFR can translocate into the nucleus and interact with DNA-PKcs (DNA-dependent protein kinase, catalytic subunit) stimulating its DNA repair activity (I). However, EGFR with somatic mutations in the TKD is defective in nuclear translocation and has a radiosensitizing effect (not shown). PI3K-Akt-1 signaling triggered by EGFR or EGFRvIII can also promote DSB repair (II). In response to IR, Akt-1 translocates into the nucleus and interacts with DNA-PKcs. DNA-PKcs phosphorylates Akt-1 in response to IR promoting survival (curved arrow) and we speculate that reciprocal phosphorylation of DNA-PKcs by Akt-1 (?) might promote DSB repair by NHEJ. As EGFR signaling promotes NHEJ, inhibitors of EGFR and Akt-1 can serve as effective radiosensitizers.
The net effect of EGFR activation is inhibition of apoptosis, increased cell proliferation, angiogenesis, and promotion of tumorigenesis. Therefore, it is not surprising that EGFR is frequently mutated and/or expressed at elevated levels in multiple cancers [24], including brain and lung cancers. Of particular relevance to this review are the two commonest categories of mutations observed in EGFR: 1) Somatic mutations in the TKD, seen prominently in lung cancers, and 2) deletion mutations in the extracellular domain, seen commonly in brain cancers.
EGFR is frequently over-expressed in non-small cell lung carcinoma (NSCLC) and approximately 12.5% of NSCLCs express EGFR with somatic mutations in the TKD, which are of two kinds – missense mutations or small in-frame deletions – both in the ATP-binding cleft of the TKD [25]. Collectively, these appear to be “gain-of-function” mutations conferring significantly enhanced ligand-dependent activity of the mutant receptor. EGFR is also overexpressed in about 50% of glioblastoma multiforme (GBM) tumors and, of these, about half express a mutant version of the receptor with a large deletion in the extra-cellular domain called EGFRvIII [26]. Although EGFRvIII is deficient in ligand binding, it is constitutively active and its expression promotes malignant growth and is associated with poor prognosis.
A novel connection between EGFR signaling and DSB repair
While the role of EGFR in transmembrane signaling is very well established, a spate of recent reports indicates that the receptor and/or its downstream components may have novel nuclear functions in addition to their canonical roles in the cytoplasm [27]. Of these novel functions, the role of EGFR in promoting the repair of radiation-induced DSBs is of particular importance, especially in the context of radiotherapy of tumors with EGFR amplification and/or mutations. In retrospect, this connection between EGFR and DSB repair is not completely surprising as a direct correlation has, for a long time, been known to exist between EGFR expression levels and radiation resistance of cells [28] and murine tumors [29]. This correlation was subsequently extended to human head and neck carcinomas where a clear inverse relationship was demonstrated between EGFR expression levels and response to radiotherapy [30].
It was also known for some time that radiation induces rapid and transient phosphorylation of EGFR [31], hinting at the possibility that the receptor might play an important role in the cellar response to radiation-induced DNA damage. More recently, it was clearly demonstrated by the Rodemann group that radiation induces nuclear relocalization of EGFR and that nuclear EGFR interacts with and augments the activity of DNA-PKcs, a key enzyme in the NHEJ pathway of DSB repair [32] (Figure 2). The connection between the wild type EGFR receptor and DNA-PKcs was subsequently extended to mutant and deleted versions of the receptor specific to NSCLCs and GBMs, respectively.
Interestingly, in the case of NSCLCs with somatic mutations in the TKD of EGFR, it was found that these mutant forms of EGFR actually attenuate DSB repair and render lung cancer cells as well as primary human bronchial epithelial cells sensitive to IR [33]. It was subsequently demonstrated that the radiosensitizing effect of mutant EGFR stems from an inability of the mutant version to translocate into the nucleus in response to IR [34].
In the context of GBMs, we and the Valerie group demonstrated that the constitutively-active EGFRvIII considerably enhances the repair of radiation-induced DSBs in genetically-defined murine astrocytes, human glioma lines, as well as in orthotopic mouse brain tumor models [5, 6]. Our results, showing hyperactivation of DNA-PKcs, upon expression of EGFRvIII, provided a molecular basis for the enormous radioresistance of GBMs, which are amongst the most lethal of all cancers. In sum, the exact effect of EGFR on DSB repair appears to be context-dependent and varies with the kind of mutation and type of cancer.
Akt-1: a linchpin kinase connecting EGFR signaling with NHEJ?
While the wild type EGFR receptor has been shown to interact with DNA-PKcs, it is not yet clear as to how this interaction might actually promote the activation of DNA-PKcs. In our studies using GBM-relevant cells and tumors, we failed to observe any interaction between EGFRvIII and DNA-PKcs that could provide a basis for the observed hyperactivation of DNA-PKcs. Therefore, it is possible that, at least in the context of GBMs, signaling from EGFRvIII, rather than a direct physical association of the receptor with DNA-PKcs, might underlie proficient DSB repair and radioresistance.
Several lines of evidence indicate that EGFRvIII preferentially activates the PI3K-Akt-1 pathway [35] that is implicated in most major mechanisms of radioresistance. Akt-1 (also known as protein kinase B) is a serine/threonine kinase that is a downstream target of RTK signaling [36]. Activated RTKs (such as EGFRvIII) activate phosphatidylinositol kinase (PI3K) which, in turn, phosphorylates phosphatidylinositol-4,5-biphosphate (PIP2) to generate phosphatidylinositol-3,4,5-triphosphate (PIP3) (Figure 2). PIP3 anchors Akt-1 to the plasma membrane, where it is fully activated by phosphorylation at Ser-473 by mTOR complex 2 (mTORC2) and subsequent phosphorylation at Thr-308 by 3-phosphoinositide-dependent kinase 1 (PDK1). The PTEN tumor suppressor reverses PIP3 back to PIP2 thereby exerting a powerful dampening effect on the PI3K-Akt-1 pathway. Akt-1 has over 100 nonredundant substrates whose phosphorylation leads to the activation of anti-apoptotic and pro-proliferative pathways [36].
Interestingly, recent reports indicate that activated Akt-1 may also play a role in the repair of DSBs. Ionizing radiation stimulates the activation of Akt-1 and its phosphorylation at threonine 308 and serine 473 in a PDK1 and DNA-PKcs dependent manner [37, 38]. It was demonstrated by the Maity group that radioresistance conferred by PI3K overexpression can be mitigated by protease inhibitors that downregulate Akt-1 activation [39]. Dampening of PI3K-Akt-1 signaling using small-molecule inhibitors impairs DSB repair in GBM [40] and breast cancer [41] cells while siRNA-mediated Akt-1 knockdown impairs DSB repair in lung cancer cells [42] resulting in radiation sensitivity. Conversely, hyperactivation of the PI3K-Akt-1 pathway due to deletion of PTEN promotes DSB repair and radiation survival [40]. We find that a PI3K inhibitor can abrogate the proficient DSB repair conferred by EGFRvIII overexpression and that hyperactivation of Akt-1 can mimic the effects of EGFRvIII overexpression on DSB repair in astrocytes [6].
A close association between Akt-1 and DNA-PKcs may be inferred from a recent report demonstrating that Akt-1 translocates to the nucleus upon irradiation and associates with DNA-PKcs at the sites of DSBs; subsequent phosphorylation of Akt-1 by DNA-PKcs promotes survival [37, 38]. We observe strikingly high levels of radiation-induced Akt-1 nuclear translocation in EGFRvIII-expressing orthotopic tumors (our unpublished data). As activation of DNA-PKcs involves its phosphorylation on serine/threonine residues [7, 9], it is conceivable that hyperactivation of DNA-PKcs by EGFRvIII may occur via phosphorylation of DNA-PKcs by Akt-1 (a serine/threonine kinase).
An interaction between Akt-1 and DDR proteins is not without precedent. Akt-1 can directly phosphorylate Chk1 (on Ser-280) [43] and Brca1 (on Thr-509) [44]. Also, cells and mice deficient in Akt-1 show attenuated induction of p53 and p21 in response to IR [37, 38]. Most importantly, a large-scale network analysis of DDR proteins carried out by the Elledge group clearly indicates that “the DDR network is likely to intersect with the RTK-PI3K-Akt-1 pathway at many points” [45]. It is, therefore, plausible that the increase in radioresistance of glioblastomas conferred by EGFRvIII may be executed via the PI3K-Akt-1 pathway rather than by a direct physical association between the receptor and DNA-PKcs. Hopefully, a thorough mechanistic understanding of the connection between EGFR signaling and NHEJ will lead to the development of more effective radiosensitizing strategies.
Inhibiting NHEJ by targeting EGFR: current clinical approaches
Given the pro-survival and pro-proliferative effects of EGFR signaling, the receptor has been extensively targeted for therapy, the rationale being that tumors highly dependent upon these pathways should be susceptible to EGFR inhibition (the “oncogene addiction” principle [2]). Given the novel connection between EGFR signaling and DSB repair, the use of anti-EGFR strategies for radiosensitization also makes intuitive sense.
EGFR-targeting approaches have typically involved either small molecules that inhibit receptor tyrosine kinase activity or monoclonal antibodies that block ligand-receptor interactions [46]. EGFR-specific tyrosine kinase inhibitors (TKIs) that are currently in clinical use include Erlotinib, Gefitinib, and Lapatinib. Monoclonal antibodies targeting EGFR include Panitumumab and Cetuximab. Clinical evidence suggests that the two classes of anti-EGFR agents (TKIs versus anti-EGFR antibodies) may function differently in patients [47]. First, TKIs function better as single agent therapies but are inefficient when combined with chemotherapy while Cetuximab produces only a modest benefit as a single agent therapy but significantly augments the effects of chemotherapy or radiotherapy. Second, there are distinct functional differences between these two agents. TKI-based therapy reversibly inactivates the receptor kinase and down-regulates EGFR-mediated downstream signaling. On the other hand, in addition to blocking receptor kinase activity and downstream signaling, Cetuximab treatment induces receptor internalization, receptor degradation, and long-term EGFR downregulation and also inhibits the repair of radiation-induced DNA damage.
Cetuximab (IMC-C225, Erbitux) is a chimeric mouse/human monoclonal antibody that binds the ligand-binding domain of EGFR, blocking growth factor binding, receptor activation and consequent signal transduction events. It binds specifically to EGFR on both normal and tumor cells with greater affinity than naturally occurring EGFR ligands [48]. Cetuximab has been approved for treatment of locally advanced head and neck squamous cell carcinoma (HNSCC) and metastatic colorectal cancer. Harari and colleagues demonstrated that Cetuximab can enhance the radiosensitivity of a HNSCC cell line both in vitro as well as in vivo in xenografts implanted in nude mice [49]. Other groups subsequently showed that Cetuximab as well as TKIs could radiosensitize a diverse range of cell types (including HNSCC, colon, ovarian, NSCLC, breast, and brain cancer lines) both in vitro and in vivo indicating the usefulness of EGFR inhibition as a radiosensitizing strategy.
Cetuximab was shown by several groups to block the nuclear import of EGFR resulting in an EGFR-DNA-PKcs complex that was sequestered in the cytoplasm and thereby rendered dysfunctional [32, 50]. These and other results, showing inhibition of DNA-PKcs activation and DSB repair with Cetuximab treatment and also with TKIs, provided a mechanistic basis for the radiosensitizing effect of EGFR inhibitors. Clinically, in a landmark study by Bonner and colleagues, the combination of Cetuximab and radiation was found to be more effective compared to radiation alone for the treatment of locally advanced HNSCC [51]. The success of this study offers the hope that tumors with heightened EGFR signaling may be specifically sensitized to radiation with anti-EGFR approaches and several trials are currently underway where patients are being treated with radiation and cisplatin with or without Cetuximab.
It is important to point out here that not all tumors overexpressing EGFR respond equally well to anti-EGFR therapy [24]. This is not surprising though as the effectiveness of anti-EGFR therapy can be compromised by factors such as co-activation of other RTKs that impinge upon common downstream signaling pathways or acquisition of secondary mutations in the EGFR kinase domain that prevent binding of TKIs as happens often in NSCLCs. Moreover, activation of downstream PI3K-Akt-1 signaling can occur by mechanisms other than receptor amplification or activation, including mutation or amplification of PI3K, amplification of Akt-1, activation of upstream oncogenes such as Ras, or loss of PTEN [52]. In a study of glioblastoma patients treated with radiation in conjunction with EGFR inhibitors, it became apparent that patients who responded best to this regimen expressed both EGFRvIII and PTEN [53]. It is likely that tumors that had lost PTEN were not as dependent upon EGFRvIII for Akt-1 activation and were thus refractory to EGFR inhibition. Therefore, it may be prudent to use Akt-1 activation (visualized by immunohistochemical staining of phosphorylated Akt-1 in biopsy or resection specimens [54]) as prognostic factors (in addition to EGFR status) and to develop anti-Akt-1 strategies that can be used in combination with radiotherapy in the clinic.
Directly blocking NHEJ: DNA-PKcs inhibitors provide hope for the future
While anti-EGFR approaches are already being used in the clinic, other approaches targeting NHEJ directly are currently in development. Given the central role of DNA-PKcs in DSB repair and the extreme radiosensitivity of DNA-PKcs-null cells, it is quite logical to presume that small-molecule inhibitors of DNA-PKcs could also be very useful in the clinic when combined with chemo- or radiotherapy. Indeed, because of the similarity of the kinase domain of DNA-PKcs (and other PI3KKs) to the kinase domain of PI3K, established PI3K inhibitors, LY294002 and wortmannin, can inhibit PI3KKs to varying degrees [55]. However, their clinical applicability as radiosensitizers has been hampered by their lack of selectivity and toxicity issues.
Interestingly, LY294002 was recently used as a template for the development of more specific DNA-PKcs inhibitors by medicinal chemistry. One of the first such compounds was NU7026, a specific DNA-PKcs inhibitor that could function as a radiosensitizer in vitro [56]. Further compound elaboration resulted in the identification of a more potent and specific inhibitor of DNA-PKcs. This compound, NU7441, has an IC50 of 13 nM and was shown to be an effective chemosensitizer in vivo in a human colon cancer xenograft model [57]. Several other compounds have been shown to inhibit DNA-PKcs and these have been extensively reviewed [58, 59]. These include, vanillin (a plant-derived natural compound that can inhibit DNA-PK activity), SU11752 (a chemically synthesized competitive inhibitor that binds to the ATP-binding pocket of DNA-PKcs), and IC87361 (a morphilin derivative with anti-tumor activity in mouse models).
Another potent radiosensitizer that was developed using LY294002 as a template was the ATM-specfic inhibitor KU-55933 [17]. An improved analogue of KU-55933, KU-60019 was recently reported to be even more effective at blocking ATM-mediated DDR events [60]. ATM is a particularly attractive target for radiosensitization as it plays important roles in cell cycle checkpoint implementation [18] as well as in DSB repair [21]. Thus, ATM inhibitors, by attenuating both processes, should exert a great degree of radio- or chemo-sensitization as is seen with KU-55933 in vitro [17].
While these compounds are being developed primarily for the purpose of sensitizing tumors to radiation or chemotherapy, it is theoretically possible that these agents could be used for monotherapy given the greater burden of endogenous DSBs in pre-cancerous and cancerous lesions [3]. It is hoped that with further refinement, derivatives of some of these compounds will enter into clinical trials within the next 5–10 years.
DNA repair inhibition and cancer therapy: pitfalls and promises
The development of the compounds and strategies described in this review holds great promise for the future of cancer therapy. However, optimism surrounding the development of these NHEJ-inhibiting approaches should be tempered with a certain degree of pragmatism. For these compounds to be effective as sensitizers, they must be significantly more toxic to tumors compared to normal tissues in patients. One can certainly be hopeful, though, that certain inherent features of cancer cells (such as endogenous DSBs or abrogated repair pathways) should render tumors more susceptible to these compounds with the caveat that some of these tumors might eventually develop resistance mechanisms as seen with the use of TKIs in NSCLCs. The development of methods to deliver these compounds directly to tumors combined with technological advances that allow fine-focused radiation delivery should certainly increase tumor response while minimizing normal tissue toxicity. A greater molecular understanding of the novel connections between oncogenic signaling and DSB repair should fuel the development of even more refined radiosensitizing approaches in the future.
Acknowledgements
SB is supported by grants from NASA (NNA05CS97G and NNX10AE08G) and from the Cancer Prevention and Research Institute of Texas (RP100644). CN is supported by a grant from NIH (CA129364). We thank Cristel V. Camacho for critically reading the manuscript and for generating the illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin SA, Lord CJ, Ashworth A. DNA repair deficiency as a therapeutic target in cancer. Curr Opin Genet Dev. 2008;18(1):80–86. doi: 10.1016/j.gde.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297(5578):63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 3.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319(5868):1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136(5):823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golding SE, et al. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8(8):730–738. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee B, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69(10):4252–4259. doi: 10.1158/0008-5472.CAN-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 2006;5(9–10):1042–1048. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417(3):639–650. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burma S, Chen DJ. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair (Amst) 2004;3(8–9):909–918. doi: 10.1016/j.dnarep.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Sibanda BL, Chirgadze DY, Blundell TL. Crystal structure of DNA-PKcs reveals a large open-ring cradle comprised of HEAT repeats. Nature. 463(7277):118–121. doi: 10.1038/nature08648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen BP, et al. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. J Biol Chem. 2005;280(15):14709–14715. doi: 10.1074/jbc.M408827200. [DOI] [PubMed] [Google Scholar]
- 12.Meek K, et al. trans Autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining. Mol Cell Biol. 2007;27(10):3881–3890. doi: 10.1128/MCB.02366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee B, et al. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair (Amst) 2006;5(5):575–590. doi: 10.1016/j.dnarep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Tomimatsu N, Mukherjee B, Burma S. Distinct roles of ATR and DNA-PKcs in triggering DNA damage responses in ATM-deficient cells. EMBO Rep. 2009 doi: 10.1038/embor.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iliakis G. Backup pathways of NHEJ in cells of higher eukaryotes: cell cycle dependence. Radiother Oncol. 2009;92(3):310–315. doi: 10.1016/j.radonc.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Kurimasa A, et al. Requirement for the kinase activity of human DNA-dependent protein kinase catalytic subunit in DNA strand break rejoining. Mol Cell Biol. 1999;19(5):3877–3884. doi: 10.1128/mcb.19.5.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickson I, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64(24):9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 18.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9(10):759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 19.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28(5):739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Goodarzi AA, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31(2):167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol. 2007;19(2):124–134. doi: 10.1016/j.ceb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Sergina NV, Moasser MM. The HER family and cancer: emerging molecular mechanisms and therapeutic targets. Trends Mol Med. 2007;13(12):527–534. doi: 10.1016/j.molmed.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyati MK, et al. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6(11):876–885. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 26.Furnari FB, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 27.Massie C, Mills IG. The developing role of receptors and adaptors. Nat Rev Cancer. 2006;6(5):403–409. doi: 10.1038/nrc1882. [DOI] [PubMed] [Google Scholar]
- 28.Sheridan MT, et al. Potential indicators of radiosensitivity in squamous cell carcinoma of the head and neck. Radiat Oncol Investig. 1997;5(4):180–186. doi: 10.1002/(SICI)1520-6823(1997)5:4<180::AID-ROI3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 29.Akimoto T, et al. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5(10):2884–2890. [PubMed] [Google Scholar]
- 30.Ang KK, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–7356. [PubMed] [Google Scholar]
- 31.Schmidt-Ullrich RK, et al. Radiation-induced autophosphorylation of epidermal growth factor receptor in human malignant mammary and squamous epithelial cells. Radiat Res. 1996;145(1):81–85. [PubMed] [Google Scholar]
- 32.Dittmann K, Mayer C, Rodemann HP. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother Oncol. 2005;76(2):157–161. doi: 10.1016/j.radonc.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Das AK, et al. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006;66(19):9601–9608. doi: 10.1158/0008-5472.CAN-06-2627. [DOI] [PubMed] [Google Scholar]
- 34.Das AK, et al. Somatic mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) abrogate EGFR-mediated radioprotection in non-small cell lung carcinoma. Cancer Res. 2007;67(11):5267–5274. doi: 10.1158/0008-5472.CAN-07-0242. [DOI] [PubMed] [Google Scholar]
- 35.Li B, et al. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23(26):4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 36.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boehme KA, Kulikov R, Blattner C. p53 stabilization in response to DNA damage requires Akt/PKB and DNA-PK. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0703423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bozulic L, et al. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30(2):203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Gupta AK, et al. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65(18):8256–8265. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 40.Kao GD, et al. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007;282(29):21206–21212. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedmann B, et al. Modulation of DNA repair in vitro after treatment with chemotherapeutic agents by the epidermal growth factor receptor inhibitor gefitinib (ZD1839) Clin Cancer Res. 2004;10(19):6476–6486. doi: 10.1158/1078-0432.CCR-04-0586. [DOI] [PubMed] [Google Scholar]
- 42.Toulany M, et al. Targeting of AKT1 enhances radiation toxicity of human tumor cells by inhibiting DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Ther. 2008;7(7):1772–1781. doi: 10.1158/1535-7163.MCT-07-2200. [DOI] [PubMed] [Google Scholar]
- 43.Puc J, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7(2):193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Altiok S, et al. Heregulin induces phosphorylation of BRCA1 through phosphatidylinositol 3-Kinase/AKT in breast cancer cells. J Biol Chem. 1999;274(45):32274–32278. doi: 10.1074/jbc.274.45.32274. [DOI] [PubMed] [Google Scholar]
- 45.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316(5828):1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 46.Dutta PR, Maity A. Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Cancer Lett. 2007;254(2):165–177. doi: 10.1016/j.canlet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerber DE. EGFR Inhibition in the Treatment of Non-Small Cell Lung Cancer. Drug Dev Res. 2008;69(6):359–372. doi: 10.1002/ddr.20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham J, Muhsin M, Kirkpatrick P. Cetuximab. Nat Rev Drug Discov. 2004;3(7):549–550. doi: 10.1038/nrd1445. [DOI] [PubMed] [Google Scholar]
- 49.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6(6):2166–2174. [PubMed] [Google Scholar]
- 50.Bandyopadhyay D, et al. Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells. J Biol Chem. 1998;273(3):1568–1573. doi: 10.1074/jbc.273.3.1568. [DOI] [PubMed] [Google Scholar]
- 51.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 52.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9(3):288–296. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 53.Mellinghoff IK, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 54.Lim J, et al. Prognostic value of activated Akt expression in oral squamous cell carcinoma. J Clin Pathol. 2005;58(11):1199–1205. doi: 10.1136/jcp.2004.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarkaria JN, et al. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58(19):4375–4382. [PubMed] [Google Scholar]
- 56.Willmore E, et al. A novel DNA-dependent protein kinase inhibitor, NU7026, potentiates the cytotoxicity of topoisomerase II poisons used in the treatment of leukemia. Blood. 2004;103(12):4659–4665. doi: 10.1182/blood-2003-07-2527. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, et al. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006;66(10):5354–5362. doi: 10.1158/0008-5472.CAN-05-4275. [DOI] [PubMed] [Google Scholar]
- 58.Bolderson E, et al. Recent advances in cancer therapy targeting proteins involved in DNA double-strand break repair. Clin Cancer Res. 2009;15(20):6314–6320. doi: 10.1158/1078-0432.CCR-09-0096. [DOI] [PubMed] [Google Scholar]
- 59.Helleday T, et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 60.Golding SE, et al. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol Cancer Ther. 2009;8(10):2894–2902. doi: 10.1158/1535-7163.MCT-09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]