Abstract
The pattern recognition molecules Nod1 and Nod2 play important roles in intestinal homeostasis; however, how these proteins impact on the development of inflammation during bacterial colitis has not been examined. In the streptomycin-treated mouse model of Salmonella colitis, we found that mice deficient for both Nod1 and Nod2 had attenuated inflammatory pathology, reduced levels of inflammatory cytokines, and increased colonization of the mucosal tissue. Nod1 and Nod2 from both hematopoietic and nonhematopoietic sources contributed to the pathology, and all phenotypes were recapitulated in mice deficient for the signaling adaptor protein Rip2. However, the influence of Rip2 was strictly dependent on infection conditions that favored expression of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system (TTSS), as Rip2 was dispensable for inflammation when mice were infected with bacteria grown under conditions that promoted expression of the SPI-1 TTSS. Thus, Nod1 and Nod2 can modulate inflammation and mediate efficient clearance of bacteria from the mucosal tissue during Salmonella colitis, but their role is dependent on the expression of the SPI-2 TTSS.
The first step in initiating an inflammatory response to a microbial pathogen is to recognize the presence of an infection. Pattern recognition molecules (PRMs) serve this role by monitoring for the presence of microbe-associated molecular patterns (MAMPs) in extracellular or intracellular locations and triggering inflammation when MAMPs are detected by specific cell types or within normally microbe-free environments of the host (26). One family of extracellular PRMs is the Toll-like receptor (TLR) family, whose members include TLR4 and TLR5, which recognize bacterial lipopolysaccharide and flagellin, respectively (22, 25). When a TLR interacts with its specific ligand, MyD88-dependent signaling cascades that result in activation of NF-κB are initiated, leading to the transcription of genes encoding proinflammatory proteins. Another group of PRMs is the nucleotide-binding and oligomerization domain (NOD)-like receptor (NLR) family of intracellular receptors, which includes Nod1 and Nod2, which recognize unique MAMPs found in bacterial peptidoglycan (4, 10, 11, 14, 39, 42). Nod1 recognizes d-glutamyl-meso-diaminopimelic acid, a molecule found predominantly in peptidoglycan from Gram-negative bacteria (5, 16), while Nod2 recognizes muramyl dipeptide (MDP), a motif common to both Gram-negative and Gram-positive bacteria (17, 24). Upon recognition of their specific ligands, Nod1 and Nod2 associate with a common adaptor protein called Rip2 (or RICK) and initiate a signal transduction cascade that leads to expression of NF-κB-dependent proinflammatory mediators (23, 38).
A specific role for Nod1 and Nod2 in regulating intestinal inflammation is evidenced by the association of mutations in Nod1 and Nod2 with inflammatory bowel disease (IBD) (4, 10, 11, 14). In particular, Nod2 has received much attention within the research community due to its association with Crohn's disease. A Crohn's disease-associated frameshift mutation in Nod2 results in loss of NF-κB activation in response to MDP (2, 6, 17, 24, 29). However, the reasons why inactivation of Nod2 can result in chronic colitis remain largely speculative. IBD is thought to be a bacterium-driven inflammatory response; therefore, it is important to understand the function of risk factors such as Nod1 and Nod2 in the context of bacterial colitis. Although several studies have shown that Nod1 and Nod2 modulate inflammatory responses to various bacterial pathogens in both in vitro and in vivo infection models (14), surprisingly little is known about their specific roles during infectious colitis. Therefore, the investigation of the role of Nod1 and Nod2 in a bacterial colitis model is likely to provide valuable insights into the etiology of IBD.
In humans Salmonella enterica serovar Typhimurium infection causes acute colitis; however, in mice the same organism causes a systemic disease with little or no intestinal inflammation. Interestingly, pretreatment with the antibiotic streptomycin alters the infection in mice so that S. enterica serovar Typhimurium causes acute colitis. This streptomycin-pretreated mouse model has been useful for dissecting both bacterial and host factors that modulate inflammation (18). For example, the roles of Salmonella pathogenicity island 1 (SPI-1)- and Salmonella pathogenicity island 2 (SPI-2)-encoded type III secretion systems (TTSS) have been investigated in mice that are lacking MyD88, the adaptor protein that regulates NF-κB in response to TLR signaling (20). Through these studies, it was determined that MyD88 signaling was not essential for mediating inflammation when streptomycin-pretreated mice were infected with wild-type (WT) S. enterica serovar Typhimurium (encoding both the SPI-1 and SPI-2 TTSS). However, MyD88 signaling mediated inflammation when mice were infected with a strain of S. enterica serovar Typhimurium that lacks the SPI-1 TTSS.
Using the streptomycin-pretreated mouse model, we investigated the role of Nod1 and Nod2 in S. enterica serovar Typhimurium colitis. Although mice deficient in either Nod1 or Nod2 did not display any significant changes in inflammatory pathology, mice deficient for both Nod1 and Nod2 (double-knockout [DKO] mice) had significantly reduced inflammation and cytokine production in response to infection. The reduced inflammation resulted in increased bacterial burden in the inflamed tissue but did not affect translocation to systemic sites or shedding in feces. Furthermore, Nod1 and Nod2 signaling from both hematopoietic and nonhematopoietic cells was found to contribute to the pathology. Although another group reported that Rip2-deficient mice are not impaired for inflammation in this model (3), all phenotypes that we observed in DKO mice were identical in Rip2-deficient mice. We therefore investigated whether bacterial growth conditions could affect the outcome of the infection as a possible explanation for this discrepancy. We found that Rip2 mediated inflammation when mice were infected with Salmonella cultures that were grown under conditions that favor SPI-2 TTSS expression whereas Rip2 was dispensable for inflammation when mice were infected with cultures that favor SPI-1 TTSS expression. Thus, Nod1 and Nod2 can play an important role in modulating the intensity of inflammation during Salmonella colitis but the influence of Nod1 and Nod2 on colitis depends upon the expression of the SPI-1 and SPI-2 TTSS.
MATERIALS AND METHODS
Mice.
C57BL/6, Nod1−/−, Nod2−/−, Nod1 Nod2−/− (DKO), and Rip2−/− mice were bred and housed under specific-pathogen-free conditions in the animal facility of the Center for Cellular and Biomolecular Research, Toronto, Canada. Nod1−/− mice were originally from Millennium Pharmaceuticals, Nod2−/− mice were from Marco Giovaninni and Jean-Pierre Hugot, and Rip2−/− mice were from Richard Flavell. Mutant mice were backcrossed at least 10 generations. All animal experiments were approved by the Animal Ethics Review Committee of the University of Toronto. Rip2−/− mice and WT littermates were obtained by crossing heterozygous mice and using PCR for genotyping.
Bacterial infections.
For infections, mice were fasted for 3 h and then orally administered 20 mg of streptomycin. Twenty-one hours after streptomycin treatment, mice were again fasted for 3 h and then orally infected with either 5 × 107 or 1 × 103 CFU of SL1344, a streptomycin-resistant strain of Salmonella enterica serovar Typhimurium. Except where indicated, overnight cultures were used for infections and were washed with phosphate-buffered saline (PBS) and then diluted to the desired CFU level based on optical density readings at 600 nm. For SPI-1-inducing conditions, overnight cultures were diluted 1:33 and grown at 37°C with aeration for 3 h prior to dilution to the desired CFU concentration. For SPI-2-inducing conditions, overnight cultures were used. These growth conditions have previously been shown to allow for specific activity of the SPI-1 and SPI-2 TTSS (13, 15).
Pathological scoring.
Cecum samples were collected after mice were sacrificed, and the distal half (containing the cecal patch) was fixed in 10% formalin and then stained with hematoxylin and eosin (H/E) at the Toronto Center of Phenogenomics by standard histological staining procedures. Some samples were also stained with Alcian Blue to visualize mucus. H/E-stained cecum samples were then analyzed by a pathologist specializing in intestinal inflammation who was blinded to the experimental conditions. The scoring system was based on one that was previously published that was slightly modified due to differences in microscopy instrumentation and also to improve the dynamic range and the empirical nature of the scoring system. Edema and epithelial erosion were scored as previously described (1). Neutrophil recruitment was scored by calculating the average number of neutrophils present from 10 microscopic fields of a given sample. The average neutrophil count was then divided by 120 (the maximum average) and then multiplied by 4 (see Table S1 in the supplemental material for raw polymorphonuclear leukocyte [PMN] counts and corresponding scores). The goblet cell depletion score was calculated by counting the average number of goblet cells present in 10 microscopic fields of a sample, this average was divided by 65 (the average number of goblet cells present in uninfected samples), the log of the resulting fraction was multiplied by −1.5, and the maximum value for the goblet score was capped at 4 (if the average number of goblet cells was 0, then a score of 4 was given) (see Table S2 for raw goblet cell counts and corresponding scores). The resulting neutrophil and goblet scores were similar to those already published with the additional benefit that the score is continuous.
Cytokine ELISAs.
Serum samples were collected from mice, allowed to coagulate at room temperature for 2 h, and then centrifuged to remove red blood cells and coagulated material. The half of the cecum proximal to the ileum was collected, the contents were removed, and then the tissue was placed in PBS, weighed, and homogenized using a rotor homogenizer. A small sample (10 μl) was removed to count CFU, and then the samples were centrifuged to remove nonsoluble material and the supernatants were collected to use in enzyme-linked immunosorbent assays (ELISAs). Serum samples and cecum samples were diluted (beginning with a 1:5 dilution), and ELISAs (R and D Systems) were used to measure keratinocyte-derived chemokine (KC) and interleukin-1β (IL-1β) levels, which were normalized to tissue weight for cecal tissue samples (detection threshold for serum, ∼80 pg/ml; detection threshold for cecal tissue, ∼500 pg/g).
Bacterial load quantification.
The liver, spleen, mesenteric lymph node, and fecal pellets were collected from infected mice and placed in PBS containing 1% Triton X-100 and then homogenized using a rotor homogenizer. Cecal tissue samples were collected as described above, and a small (10-μl) sample was diluted in PBS containing 1% Triton X-100. Samples were serially diluted in PBS and plated on MacConkey agar containing 50 μg/ml streptomycin.
Mouse chimeras.
WT or DKO mice were lethally irradiated with 1,000 cGy of ionizing radiation, and 24 h later these mice were reconstituted with bone marrow from the tibia and femurs of either WT or DKO mice and allowed to reconstitute for 6 to 8 weeks prior to any experimental manipulation.
RESULTS
Nod1 and Nod2 have redundant roles in Salmonella colitis.
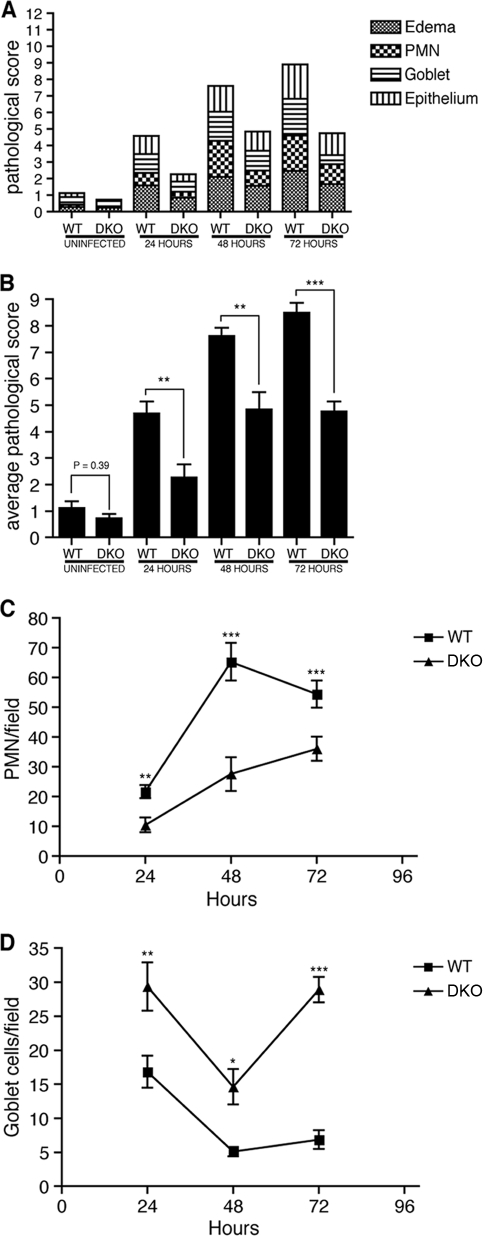
To investigate the role of Nod1 and Nod2 in Salmonella colitis, wild-type (WT), Nod1-deficient, and Nod2-deficient mice as well as mice deficient for both Nod1 and Nod2 (DKO) were treated with streptomycin and infected with 5 × 107 CFU of the streptomycin-resistant S. enterica serovar Typhimurium strain SL1344. Mice were sacrificed at 24-hour intervals following infection (Table 1 shows numbers of mice used for each time point and group), and then their ceca were removed and stained for histological analysis. Samples were analyzed for neutrophil accumulation, goblet cell depletion, edema, and epithelial erosion and were scored using a previously established scoring system (1) (see Materials and Methods). Although neither Nod1- nor Nod2-deficient mice had any significant changes in pathology (see Fig. S1 in the supplemental material), DKO mice had reduced inflammation from 24 to 72 h after infection. This was reflected by decreased neutrophil recruitment and goblet cell depletion as well as overall pathological score in infected DKO mice compared to WT mice (Fig. 1). Thus, Nod1 and Nod2 modulate the intensity of inflammation during Salmonella colitis and either protein is sufficient to complement the lack of the other.
TABLE 1.
| Duration of infection with SL1344 | No. of mice with genotype: |
||||
|---|---|---|---|---|---|
| WT | Nod1−/− | Nod2−/− | DKO | Rip2 | |
| Uninfected | 21 | 16 | |||
| 24 h | 30 | 14 | 12 | 14 | |
| 48 h | 18 | 14 | |||
| 72 h | 30 | 14 | 13 | 18 | 8 |
FIG. 1.
DKO mice have reduced inflammation following SL1344 infection. Streptomycin-treated WT or DKO mice were either left uninfected or infected with 5 × 107 CFU of SL1344 for 24 to 72 h prior to sacrifice, and then their ceca were examined for histological changes. A pathological score was assigned to each sample, and the average score for each analyzed feature (edema, neutrophil recruitment, goblet cell depletion, and epithelial erosion) for all mice from each group was calculated (A) as well as the average total sum of the pathological scores (B). The line graphs show the average numbers of neutrophils (C) and goblet cells (D) observed per microscopic field from infected samples over the period of infection. Error bars represent 1 standard error of the mean. Significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Nod1 and Nod2 regulate the levels of key inflammatory cytokines.
In mice, Nod1 and Nod2 lead to NF-κB-dependent expression of chemokines such as the keratinocyte-derived chemokine (KC) (similar to human IL-8) and play a critical role in neutrophil recruitment in different models of inflammation (14). Activation of NF-κB also leads to expression of the proform of IL-1β, another cytokine that plays a central role in mediating inflammation. However, release of mature IL-1β requires cleavage of pro-IL-1β by caspase 1, an enzyme whose activity is regulated via a complex termed the “inflammasome.” Caspase 1 activation and IL-1β release have been shown to be regulated by another NLR family member, ice protease activating factor (IPAF), in the context of Salmonella infection (9, 27, 34). Thus, although Nod1 and Nod2 activation lead to increased expression of pro-IL-1β, release of IL-1β is likely independent of Nod1 and Nod2 during Salmonella colitis. Hence, it is not clear what influence disruption of Nod1 and Nod2 would have on IL-1β production. Therefore, we assessed the impact of Nod1 and Nod2 on the expression of the cytokines KC and IL-1β during infection.
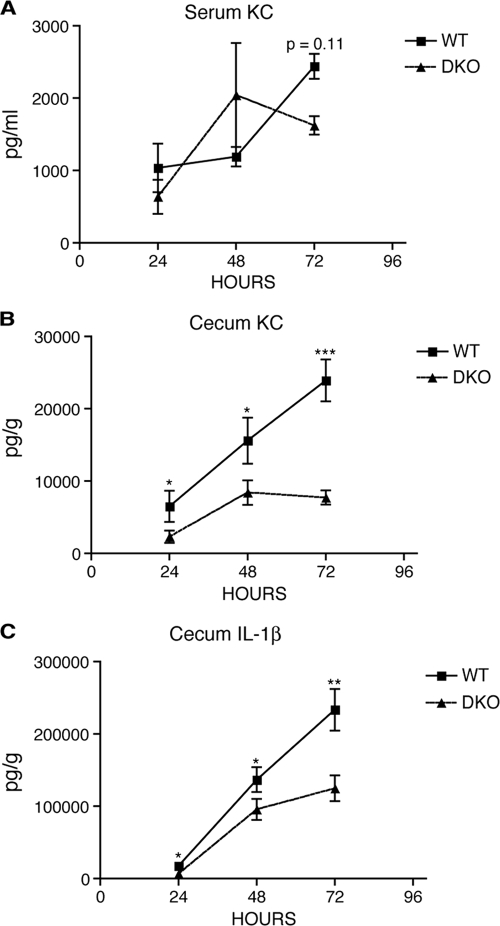
To this end, portions of the same cecum samples that were used for histological analysis as well as serum samples from these same mice were used to measure cytokine levels by ELISA. Infected Nod1- and Nod2-deficient mice had no change in cecal KC compared to that of infected WT mice (see Fig. S1 in the supplemental material). However, at 72 h postinfection there was a significant decrease in cecal IL-1β in Nod2-deficient mice, but this did not match the pathology observed in the same mice. In infected DKO mice, there were significantly reduced levels of both KC and IL-1β in the cecal tissue at all time points compared to those of WT mice; however, there was no difference in the levels of KC in the serum (Fig. 2). The greatest differences were detected at 72 h postinfection, where the levels of KC were quite consistent between samples and closely matched the pathology. In contrast, the levels of IL-1β were generally more variable and did not necessarily correlate with the pathology, perhaps reflecting an indirect influence of Nod1/2 on IL-1β production. Overall, these findings indicate that Nod1 and Nod2 regulate local tissue KC and IL-1β levels and that the levels of KC closely parallel the pathology.
FIG. 2.
Reduced cytokine production in cecal tissue from DKO mice. ELISAs were used to measure the level of KC and IL-1β in samples from streptomycin-treated WT and DKO mice infected with 5 × 107 CFU of SL1344 for 24 to 72 h. Line graphs depict the average serum levels of KC (A) and the average cecal tissue levels of KC (B) and IL-1β (C) in infected samples over time. Error bars represent 1 standard error of the mean. Significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DKO mice have increased bacterial load in cecal tissue at 48 and 72 h postinfection.
WT, Nod1-deficient, Nod2-deficient, and DKO mice were also examined for differences in colonization by SL1344. Pellets, spleens, and cecal tissue samples were collected from the same infected mice as those used for histological analysis, and the CFU in the samples were determined. No differences in CFU were observed in any samples in either Nod1- or Nod2-deficient mice (see Fig. S1 in the supplemental material). DKO mice had levels of colonization similar to those of WT mice in both spleen and pellet samples; however, there were significantly fewer bacteria isolated from cecal tissue at 24 h postinfection and significantly more bacteria in cecal tissues at 48 and 72 h (approximately 4-fold increase at both time points) (Fig. 3A to D). Thus, time-dependent differences in colonization were observed between WT and DKO mice.
FIG. 3.
Increased colonization of cecal tissue from DKO mice at 72 h postinfection. Streptomycin-treated WT and DKO mice were infected with 5 × 107 CFU of SL1344 for 24 to 72 h, and then CFU counts were performed on spleen (A), pellet (B), and cecal tissue (C) samples. In addition, the colonization of the cecum of streptomycin-treated Rip2-deficient mice infected with SL1344 for 72 h was determined (D). Hematoxylin- and eosin-stained cecum samples from 72-h-infected WT (top panels) and DKO (bottom panels) mice were also stained with Alcian Blue to visualize mucus (E). Arrowheads indicate mucinous material in the cecal lumen that is stained with Alcian Blue. The left panels show 2.5× magnification, while the right panels show 20× magnifications of the inset black rectangles. All graphs show the median CFU levels, and error bars depict the interquartile ranges. Significance: *, P < 0.05; **, P < 0.01.
It has previously been reported that Salmonella utilizes caspase 1-dependent inflammatory pathways to enhance host colonization (35). Although these results are somewhat controversial (27), it is possible that the increased cecal colonization of DKO mice at 24 h reflects a requirement for Nod1/2-driven inflammation to efficiently colonize the host. Most of the analysis was performed following infection with a relatively high infectious dose of 5 × 107 CFU because this dose produces the most consistent results for pathology. We repeated some of the analyses in a smaller group of mice by using a lower dose of 103 CFU. Using this low dose, we found that the pathology was similar to that seen using a high dose with similar trends for differences in KC and IL-1β production (see Fig. S2 in the supplemental material). At this lower dose, the reduced colonization of DKO mice persisted from 24 to 72 h postinfection in the cecal tissue as well as systemic sites (the spleen, liver, and mesenteric lymph nodes), and this translated to decreased shedding of bacteria in fecal pellets (see Fig. S2; note that statistical analyses could not be performed on most 24-h samples because most DKO samples had colonization or cytokine levels below the detection threshold). By 120 h postinfection, the levels of colonization in cecal tissue and systemic sites were similar in WT and DKO mice and appeared to be trending toward increased colonization of DKO ceca, similar to what was seen using the higher infectious dose. However, experiments at low doses were not carried out beyond 120 h because the mice began to die after this time point. Notably, there were no differences in mortality observed between WT and DKO mice at either low or high doses (data not shown). Overall, we observed that differences in colonization of WT and DKO mice were dependent on timing and the size of the inoculum.
It appears that the reduced inflammation in the DKO mice initially resulted in less-efficient colonization; however, the colonization of cecal tissue increased at later time points. This may be due to inefficient clearance of bacteria at the mucosal surface as a direct consequence of reduced fluid and mucus secretion. This is supported by the observation that in some infected DKO samples there was intense staining by Alcian Blue, a dye that stains mucus, that persisted in the cecal lumen at 72 h postinfection but was never observed in WT samples (Fig. 3E). Thus, we postulate that Nod1- and Nod2-dependent inflammation is important for physically removing Salmonella from the mucosal surface.
Both hematopoietic and nonhematopoietic Nod1 and Nod2 are involved in Salmonella colitis.
Chimeras were generated to determine the contribution of Nod1 and Nod2 signaling from different cellular compartments. Lethally irradiated WT and DKO mice were reconstituted with either WT or DKO bone marrow, generating mice with WT radioresistant (or nonhematopoietic) cells with DKO radiosensitive (or hematopoietic) cells (DKO→WT), mice with DKO radioresistant cells with WT radiosensitive cells (WT→DKO), and control mice with both compartments derived from WT (WT→WT) or DKO (DKO→DKO) mice. These chimeric mice were treated with streptomycin and infected with SL1344, and then the mice were sacrificed after 72 h of infection and the ceca were analyzed for histology and cytokine levels. DKO→DKO mice had significantly reduced overall inflammatory pathology compared to that of WT→WT mice while DKO→WT and WT→DKO mice had intermediate phenotypes (Fig. 4A and B). Similar results were seen for KC levels measured in cecal tissue, with both DKO→WT and WT→DKO mice having intermediate levels of KC (Fig. 4C). Again, the levels of KC closely matched the observed pathology. Interestingly, the levels of IL-1β were uncoupled from Nod1 and Nod2 in the chimeric mice, perhaps reflecting an influence of irradiation on the IL-1β responses (Fig. 4D). All groups of chimeras had similar levels of IL-1β in the cecal tissue, and the IL-1β levels did not correlate with observed differences in pathology. From these experiments, we conclude that both hematopoietic and nonhematopoietic Nod1/2 are involved in modulating Salmonella colitis.
FIG. 4.
Nod1/2 from both hematopoietic and nonhematopoietic compartments contribute to Salmonella colitis. Lethally irradiated WT and DKO mice were reconstituted with WT or DKO bone marrow, thus generating chimeric mice with DKO nonhematopoietic cells with WT hematopoietic cells (WT→DKO), mice with WT nonhematopoietic cells with DKO hematopoietic cells (DKO→WT), or control mice with both compartments derived from either WT (WT→WT) or DKO (DKO→DKO) mice. These chimeric mice were streptomycin treated, infected with 5 × 107 CFU of SL1344 for 72 h, and then sacrificed, and their ceca were analyzed for pathological changes and cytokine production. A pathological score was assigned to each sample, and the average score for each analyzed feature (edema, neutrophil recruitment, goblet cell depletion, and epithelial erosion) for all mice from each group was calculated (A) as well as the average total sum of the pathological scores (B). ELISAs were also used to measure and calculate the average levels of KC (C) and IL-1β (D) in the cecum samples. Error bars represent 1 standard error of the mean. Significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The role of Rip2 in Salmonella colitis is dependent on SPI-1 or SPI-2 growth conditions for infection.
While the studies on Nod1/2-deficient mice were in progress, Bruno et al. published a report indirectly suggesting that Nod1 and Nod2 do not play a role in Salmonella colitis (3). In this report, Rip2, the adaptor protein for both Nod1 and Nod2, was found to be dispensable for inflammation in the Salmonella colitis model. These findings were surprising since reports had already shown that Nod1 and Nod2 can influence responses to Salmonella (21, 28). Moreover, we found that all the phenotypes observed in DKO mice during Salmonella colitis were recapitulated in Rip2-deficient mice (data not shown and Fig. 3D and 5). We investigated the possibility that different pregrowth conditions could affect the influence of Rip2 in Salmonella colitis, since we used growth conditions that favor SPI-2 TTSS expression in our experiments while Bruno et al. used growth conditions that favored SPI-1 TTSS expression in their study. Therefore, streptomycin-treated Rip2-deficient mice or WT littermates, generated from breeding mice heterozygous for the Rip2 null allele, were infected using SL1344 grown under SPI-1- or SPI-2-favoring conditions. As expected, when we infected mice with SPI-2-expressing strain SL1344, Rip2-deficient mice had reduced inflammation and cytokine production compared to those of their WT littermates (Fig. 5). Surprisingly, when mice were infected with SPI-1-expressing SL1344 cultures, Rip2-deficient mice actually had a trend toward an increase in overall inflammation and cytokine production. Of note, SPI-1 and SPI-2 conditions did not significantly change colonization levels in the spleen or cecum (see Fig. S3 in the supplemental material). Overall, these results reproduce the previous conclusion by Bruno et al. that Rip2 is dispensable for inflammation when mice are infected with bacteria expressing the SPI-1 TTSS. However, we found that this is strictly dependent on growth conditions, since Rip2 plays a significant role in inflammation when mice are infected with bacteria expressing the SPI-2 TTSS.
FIG. 5.
Infection using SL1344 grown under SPI-1- or SPI-2-inducing growth conditions influences the role of Rip2 in Salmonella colitis. Streptomycin-treated Rip2-deficient mice or their WT littermates were infected with 5 × 107 CFU of SL1344 grown under either SPI-1- or SPI-2-inducing conditions. After 72 h of infection, the mice were sacrificed and their ceca were analyzed for pathological changes and cytokine production. A pathological score was assigned to each sample, and the average score for each analyzed feature (edema, neutrophil recruitment, goblet cell depletion, and epithelial erosion) for all mice from each group was calculated (A) as well as the average total sum of the pathological scores (B) (error bars represent 1 standard error of the mean). ELISAs were used to measure KC (C) and IL-1β (D) in the cecum samples; the circles in the scatter plots show the data from individual mice, and the horizontal bars represent the means. Significance: *, P < 0.05; **, P < 0.01.
DISCUSSION
Although Nod1 and Nod2 are key regulators of intestinal homeostasis, this is the first in-depth analysis of their role in an animal model of infectious colitis. We found that in the Salmonella colitis model, mice lacking both proteins have reduced overall pathology and cytokine production, coinciding with an increased bacterial burden in the mucosal tissue. Furthermore, Nod1/2 signaling from both hematopoietic and nonhematopoietic cells contributes to the pathology. Finally, the role of Rip2, the adaptor protein for both Nod1 and Nod2 signaling, was dependent on bacterial growth conditions that favored expression of SPI-2 TTSS.
An important paradox in the field of Nod2 research is how mutations that disrupt its function and impair its ability to induce inflammatory responses could lead to the increased inflammation observed in Crohn's disease (4, 10, 11, 14). It has been postulated that defects in Nod2 result in a constitutively weak inflammatory response that could lead to increases in intestinal bacterial load and over time lead to chronic states of inflammation (33). We found that the weaker inflammatory response of Nod1/2-deficient mice allowed for increased association of bacteria with the mucosal tissue. It is possible that in the long term this could lead to chronic states of inflammation. Unfortunately, this could not be investigated in our mice since they are deficient for the nramp1 resistance gene and die within 5 to 7 days postinfection, making it impossible to perform long-term infection studies. In addition to increased bacterial colonization, other work has shown that Nod1/2 deficiency can affect T-cell polarization, skewing responses from a Th2 to a Th1 bias (12, 32). Therefore, it is also possible that such influences on adaptive responses could further contribute toward the establishment of chronic colitis in the Salmonella colitis model.
It has previously been shown that host responses to Salmonella infection are influenced by Nod1 and Nod2 (21, 28); however, none of these previous reports provided in-depth analysis of the role of Nod1/2 in mediating inflammation during Salmonella colitis. Bruno et al. were the only other group to investigate the role of Nod1/2 in the Salmonella colitis model and, contradictory to our results, came to the conclusion that Nod1/2 signaling is not involved in the inflammatory response in this model (3). In the 1990s, there was a similar controversy regarding the mechanism of cell death that various groups observed in cultured macrophages during Salmonella infection (7, 30, 31, 36). This controversy was resolved when it was reported that infection using pregrowth conditions that favor SPI-1 expression (log-phase cultures) resulted in rapid macrophage death whereas infection using pregrowth conditions that favor SPI-2 expression (stationary-phase cultures) results in delayed cell death (40). We therefore considered the possibility that different pregrowth conditions could influence the role of Nod1/2 in the Salmonella colitis model. This was an attractive hypothesis since it had already been reported that the pathology in this model is differentially influenced by Salmonella mutants that are deficient for either SPI-1 or SPI-2 (8, 20). This is precisely what we observed; indeed, the influence of Nod1 and Nod2 on inflammation in the Salmonella colitis model was entirely dependent on growth conditions.
The mechanism leading to differential roles of Nod proteins during infection with SPI-1- and SPI-2-expressing Salmonella remains unknown. One possible explanation stems from the fact that SPI-1-deficient S. enterica serovar Typhimurium enters the host exclusively through a monocyte- and dendritic cell-dependent uptake mechanism that differs from the M-cell-dependent entry mechanism employed by WT S. enterica serovar Typhimurium (41, 43). This difference in uptake mechanisms could lead to differential means of NF-κB activation in different cell types. Thus, SPI-1- or SPI-2-dependent infections could affect the interactions with different cell types at very early stages of infection and lead to distinct mechanisms of activating the inflammatory response. In support of this idea, it has been shown that when streptomycin-treated mice are infected with Salmonella by using SPI-1 conditions or using SPI-1-deficient bacteria, the bacteria reside within unique subsets of monocytic cells (20). Interestingly, SPI-1-deficient Salmonella specifically associates with CX3CR1+ dendritic cells (19) and these cells have been implicated in mediating inflammation in the CD45RB transfer model of colitis (37). Another possibility is that effectors delivered into host cells via the SPI-1 TTSS subvert the need for host PRM recognition pathways. Indeed, SPI-1 effectors have been implicated in PRM-independent NF-κB activation (3). Therefore, using SPI-1 expression conditions for infection could be masking the influence of Nod-driven inflammatory pathways.
NOD receptors are key regulators of inflammatory responses that serve a critical function in controlling acute stages of bacterial infections by coordinating events such as the recruitment of phagocytic cells, mucus release, and programmed cell death. These aggressive measures, designed to physically remove or contain the pathogen, often come at the expense of causing localized tissue damage. Thus, it is essential to carefully regulate inflammation in order to prevent excessive damage to the host organism. This is evidenced by the fact that improper activation of these inflammatory pathways leads to various autoimmune and inflammatory disorders and is therefore an intense area of research. This report expands our understanding of how NOD receptors contribute to infectious colitis; however, much work remains to fully understand how the pleiotropic effects of Nod1/2 deficiency can result in chronic inflammation.
Supplementary Material
Acknowledgments
We thank Lily Morikawa of The Centre for Phenogenomics for advice on preparation of histological samples.
Kaoru Geddes is supported by a Canadian Association for Gastroenterology (CAG)/Canadian Institutes of Health Research (CIHR) postdoctoral fellowship. This work is supported by CIHR and The Howard Hughes Medical Institute (International Scholars Program) (to D.J.P.) and by CIHR and the Crohn's and Colitis Foundation of Canada (to S.E.G.).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 4 October 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonen, D. K., Y. Ogura, D. L. Nicolae, N. Inohara, L. Saab, T. Tanabe, F. F. Chen, S. J. Foster, R. H. Duerr, S. R. Brant, J. H. Cho, and G. Nunez. 2003. Crohn's disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology 124:140-146. [DOI] [PubMed] [Google Scholar]
- 3.Bruno, V. M., S. Hannemann, M. Lara-Tejero, R. A. Flavell, S. H. Kleinstein, and J. E. Galan. 2009. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 5:e1000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carneiro, L. A., J. G. Magalhaes, I. Tattoli, D. J. Philpott, and L. H. Travassos. 2008. Nod-like proteins in inflammation and disease. J. Pathol. 214:136-148. [DOI] [PubMed] [Google Scholar]
- 5.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. [DOI] [PubMed] [Google Scholar]
- 6.Chamaillard, M., D. Philpott, S. E. Girardin, H. Zouali, S. Lesage, F. Chareyre, T. H. Bui, M. Giovannini, U. Zaehringer, V. Penard-Lacronique, P. J. Sansonetti, J. P. Hugot, and G. Thomas. 2003. Gene-environment interaction modulated by allelic heterogeneity in inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 100:3455-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L. M., K. Kaniga, and J. E. Galan. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21:1101-1115. [DOI] [PubMed] [Google Scholar]
- 8.Coburn, B., Y. Li, D. Owen, B. A. Vallance, and B. B. Finlay. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 73:3219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchi, L., A. Amer, M. Body-Malapel, T. D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 7:576-582. [DOI] [PubMed] [Google Scholar]
- 10.Franchi, L., J. H. Park, M. H. Shaw, N. Marina-Garcia, G. Chen, Y. G. Kim, and G. Nunez. 2008. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell. Microbiol. 10:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Fritz, J. H., R. L. Ferrero, D. J. Philpott, and S. E. Girardin. 2006. Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 7:1250-1257. [DOI] [PubMed] [Google Scholar]
- 12.Fritz, J. H., L. Le Bourhis, G. Sellge, J. G. Magalhaes, H. Fsihi, T. A. Kufer, C. Collins, J. Viala, R. L. Ferrero, S. E. Girardin, and D. J. Philpott. 2007. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity 26:445-459. [DOI] [PubMed] [Google Scholar]
- 13.Geddes, K., F. Cruz, and F. Heffron. 2007. Analysis of cells targeted by Salmonella type III secretion in vivo. PLoS Pathog. 3:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geddes, K., J. G. Magalhaes, and S. E. Girardin. 2009. Unleashing the therapeutic potential of NOD-like receptors. Nat. Rev. Drug Discov. 8:465-479. [DOI] [PubMed] [Google Scholar]
- 15.Geddes, K., M. Worley, G. Niemann, and F. Heffron. 2005. Identification of new secreted effectors in Salmonella enterica serovar Typhimurium. Infect. Immun. 73:6260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 17.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 18.Hapfelmeier, S., and W. D. Hardt. 2005. A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 13:497-503. [DOI] [PubMed] [Google Scholar]
- 19.Hapfelmeier, S., A. J. Muller, B. Stecher, P. Kaiser, M. Barthel, K. Endt, M. Eberhard, R. Robbiani, C. A. Jacobi, M. Heikenwalder, C. Kirschning, S. Jung, T. Stallmach, M. Kremer, and W. D. Hardt. 2008. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J. Exp. Med. 205:437-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hapfelmeier, S., B. Stecher, M. Barthel, M. Kremer, A. J. Muller, M. Heikenwalder, T. Stallmach, M. Hensel, K. Pfeffer, S. Akira, and W. D. Hardt. 2005. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174:1675-1685. [DOI] [PubMed] [Google Scholar]
- 21.Hisamatsu, T., M. Suzuki, H. C. Reinecker, W. J. Nadeau, B. A. McCormick, and D. K. Podolsky. 2003. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124:993-1000. [DOI] [PubMed] [Google Scholar]
- 22.Imler, J. L., and J. A. Hoffmann. 2003. Toll signaling: the TIReless quest for specificity. Nat. Immunol. 4:105-106. [DOI] [PubMed] [Google Scholar]
- 23.Inohara, N., T. Koseki, L. del Peso, Y. Hu, C. Yee, S. Chen, R. Carrio, J. Merino, D. Liu, J. Ni, and G. Nunez. 1999. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J. Biol. Chem. 274:14560-14567. [DOI] [PubMed] [Google Scholar]
- 24.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 25.Kawai, T., and S. Akira. 2005. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 17:338-344. [DOI] [PubMed] [Google Scholar]
- 26.Kimbrell, D. A., and B. Beutler. 2001. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2:256-267. [DOI] [PubMed] [Google Scholar]
- 27.Lara-Tejero, M., F. S. Sutterwala, Y. Ogura, E. P. Grant, J. Bertin, A. J. Coyle, R. A. Flavell, and J. E. Galan. 2006. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203:1407-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Bourhis, L., J. G. Magalhaes, T. Selvanantham, L. H. Travassos, K. Geddes, J. H. Fritz, J. Viala, K. Tedin, S. E. Girardin, and D. J. Philpott. 2009. Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection. Infect. Immun. 77:4480-4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, J., T. Moran, E. Swanson, C. Julian, J. Harris, D. K. Bonen, M. Hedl, D. L. Nicolae, C. Abraham, and J. H. Cho. 2004. Regulation of IL-8 and IL-1beta expression in Crohn's disease associated NOD2/CARD15 mutations. Hum. Mol. Genet. 13:1715-1725. [DOI] [PubMed] [Google Scholar]
- 30.Lindgren, S. W., I. Stojiljkovic, and F. Heffron. 1996. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 93:4197-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundberg, U., U. Vinatzer, D. Berdnik, A. von Gabain, and M. Baccarini. 1999. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J. Bacteriol. 181:3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magalhaes, J. G., J. H. Fritz, L. Le Bourhis, G. Sellge, L. H. Travassos, T. Selvanantham, S. E. Girardin, J. L. Gommerman, and D. J. Philpott. 2008. Nod2-dependent Th2 polarization of antigen-specific immunity. J. Immunol. 181:7925-7935. [DOI] [PubMed] [Google Scholar]
- 33.Marks, D. J., M. W. Harbord, R. MacAllister, F. Z. Rahman, J. Young, B. Al-Lazikani, W. Lees, M. Novelli, S. Bloom, and A. W. Segal. 2006. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet 367:668-678. [DOI] [PubMed] [Google Scholar]
- 34.Miao, E. A., C. M. Alpuche-Aranda, M. Dors, A. E. Clark, M. W. Bader, S. I. Miller, and A. Aderem. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 7:569-575. [DOI] [PubMed] [Google Scholar]
- 35.Monack, D. M., D. Hersh, N. Ghori, D. Bouley, A. Zychlinsky, and S. Falkow. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. U. S. A. 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niess, J. H., and G. Adler. 2010. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J. Immunol. 184:2026-2037. [DOI] [PubMed] [Google Scholar]
- 38.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276:4812-4818. [DOI] [PubMed] [Google Scholar]
- 39.Philpott, D. J., and S. E. Girardin. 2010. Nod-like receptors: sentinels at host membranes. Curr. Opin. Immunol. 22:428-434. [DOI] [PubMed] [Google Scholar]
- 40.van der Velden, A. W., S. W. Lindgren, M. J. Worley, and F. Heffron. 2000. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype typhimurium. Infect. Immun. 68:5702-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 42.Werts, C., S. E. Girardin, and D. J. Philpott. 2006. TIR, CARD and PYRIN: three domains for an antimicrobial triad. Cell Death Differ. 13:798-815. [DOI] [PubMed] [Google Scholar]
- 43.Worley, M. J., G. S. Nieman, K. Geddes, and F. Heffron. 2006. Salmonella typhimurium disseminates within its host by manipulating the motility of infected cells. Proc. Natl. Acad. Sci. U. S. A. 103:17915-17920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.