Abstract
Tigecycline is a new-generation of tetracycline (glycylcyclines) and is active in vitro against bacteria that possess any of the classical genes that confer tetracycline resistance through ribosomal protection or efflux pumps. Herein, tigecycline disposition in patients with community- or hospital-acquired pneumonia was described using a population pharmacokinetic model. Additionally, the influence of covariates, such as body surface area, severity of illness, and clinical laboratory measures, on tigecycline disposition was evaluated. An intravenous loading dose of 100 mg was followed by 50 mg of tigecycline every 12 h. The final population pharmacokinetic model was a two-compartment model with linear elimination and with a relationship between tigecycline clearance and body surface area and creatinine clearance. The model was parameterized using total clearance (CL), the volume of the central compartment, distributional clearance from the central to the peripheral compartment, and volumes of distribution at steady state. Relationships between body surface area and creatinine clearance were identified as significant predictors of interindividual variability on CL. This model will serve as the basis for estimating tigecycline exposure for pharmacokinetic-pharmacodynamic analyses for efficacy and safety among patients with community- or hospital-acquired pneumonia.
Tetracyclines were originally derived from the soil bacterium Streptomyces. Trace amounts of tetracycline have been found in Nubian bones dating back over 2 millennia (3). While ancient peoples did not realize they were consuming tetracycline, they may have been doing so for medicinal purposes. American botanist Benjamin Duggar discovered the first tetracycline, chlortetracycline, in 1945 while studying soil collected around graveyards (8). During the last 60 years, other natural (oxytetracycline, dimethylchlortetracycline, and tetracycline) and semisynthetic (doxycycline, minocycline, and lymecycline) tetracyclines have been developed. However, widespread bacterial antimicrobial resistance has greatly diminished the utility of tetracyclines (15).
Tigecycline represents the first of a new generation of tetracyclines, the glycylcyclines. Tigecycline is active against bacteria that possess any of the classical genes that confer tetracycline resistance through ribosomal protection or efflux pumps (13). Tigecycline has proven to be safe and effective for the treatment of community-acquired pneumonia, complicated intra-abdominal infections, and complicated skin and skin structure infections (17). Additionally, tigecycline's safety and efficacy have been studied in patients with hospital-acquired pneumonia, including those with ventilator-associated pneumonia.
The pharmacokinetics of tigecycline has been studied in intensively sampled volunteers and in sparsely sampled patients with complicated intra-abdominal or complicated skin and skin structure infections (9, 16). The development and application of a population pharmacokinetic (PK) model for predicting tigecycline exposure have proven to be very valuable when investigating tigecycline exposure-response relationships for safety and efficacy in patients with complicated abdominal or complicated skin and skin structure infections (10, 12).
Herein, we describe the development of a population PK model to describe the disposition of tigecycline in a pooled data set of patients with community- or hospital-acquired pneumonia. The objectives of this analysis were (i) to develop a tigecycline population PK model using a pooled population of patients from three phase 3 trials, one with patients with hospital-acquired pneumonia and two with patients with community-acquired pneumonia, and (ii) to examine which patient demographic and disease characteristics (covariates) explain significant portions of the interindividual variability in tigecycline PK parameters.
MATERIALS AND METHODS
Analysis population and PK sampling strategy.
The population for this analysis consisted of tigecycline-treated patients from three different studies. One study in 2007 involved patients suffering from hospital-acquired pneumonia (8a), while two studies in 2006 included patients suffering from community-acquired pneumonia (studies 3074a1-308-WW and 3074a1-313-WW [CSR-67076], protocol 3047A-3088-WW [CSR-63128], and protocol 3047A1-313-WW [CSR-61261], data on file, Wyeth Research, Philadelphia, PA). All patients meeting the eligibility criteria, as defined in the protocols, and who also had at least one evaluable serum tigecycline concentration were included in the analysis.
In brief, the study involving patients with hospital-acquired pneumonia was a phase 3, multicenter, randomized, double-blind comparison of the efficacy and safety of tigecycline (with or without ceftazidime with or without an aminoglycoside) with that of imipenem-cilastatin (with or without vancomycin with or without an aminoglycoside). Those patients randomized to tigecycline received an intravenous (i.v.) loading dose of 100 mg of tigecycline, followed by 50 mg i.v. of tigecycline every 12 h. The tigecycline infusion time was variable in order to maintain the blind, given that prolonged infusions were used in some patients receiving larger doses of imipenem-cilastatin.
The two studies involving hospitalized patients with community-acquired pneumonia were phase 3, multicenter, randomized, double-blind comparisons of the efficacy and safety of tigecycline with those of levofloxacin. For both studies, those patients randomized to tigecycline received an i.v. loading dose of 100 mg of tigecycline, followed by 50 mg i.v. of tigecycline every 12 h. In one study, tigecycline was infused over 30 min, while in the other study, tigecycline was infused over 60 min.
In all three studies, blood samples for analysis of tigecycline concentrations were drawn from patients at selected sites at the following time points. (i) On day 1, the first blood sample (0 h) was collected prior to the first tigecycline dose. (ii) On any day on or after day 3 (after a minimum of 6 tigecycline doses), while patients were still receiving the i.v. study drug, samples were taken before the infusion (0 h, within 30 min before infusion) and within the interval 10 min before the end of the infusion and within 1 min after the end of the infusion. (iii) On any day on or after day 3 (after a minimum of 6 tigecycline doses), while patients were still receiving the i.v. study drug, samples were taken at 3 and 6 h from the start of the infusion. A 30-min window on either side of each of these time points was allowed.
It is important to note that the above sampling scheme was designed to provide as much information as possible regarding drug clearance, while sacrificing the ability to estimate other parameters, within the logistical constraints of the trials.
Concentrations of tigecycline in serum were determined by sensitive and specific liquid chromatography methods with tandem mass spectrometry detection (14). The assay had a lower limit of quantitation (LLOQ) of 10.0 ng/ml.
Missing or outlier drug concentration data.
For the tigecycline concentrations, suspected outlier observations were tested and, if justified, excluded based on the following procedure. Data for each patient were fit with and without the individual suspected outlier concentrations. If the difference between the value of the fitted concentration and the observed concentration was at least 3 error standard deviations, the trajectory of the PK profile was altered significantly, and an improvement was seen in the fit of the remaining samples for that patient, the observation was declared a significant outlier and was excluded from the analysis. If all of the samples for a given patient were excluded, that patient was removed from the analysis. This process was carried out during structural model development.
Concentrations below the limit of quantitation (BLQ) were identified and dealt with using the Beal M3 method (5), an algorithm that considers the BLQ value as normally distributed somewhere between negative infinity and the limit of quantification.
Structural model development.
Structural model development was initiated using the data from the two community-acquired pneumonia studies, as these data became available first. Subsequently, when the data from the hospital-acquired pneumonia study became available, the structural PK model derived from the community-acquired pneumonia studies was fit to these data using Monte Carlo parametric expectation maximization (MCPEM) as described below. If similar mean population parameter estimates were obtained when the model was fitted to the populations separately, the data were to be pooled in order to potentially improve the precision of the mean population PK parameter estimates.
Candidate PK models were fit to serum data using MCPEM as implemented in the open-source software S-ADAPT (4). S-ADAPT (also called scriptable ADAPT) is a version of ADAPT II that contains an augmented interface, as well as additional simulation and optimization abilities. The augmented portions of the S-ADAPT software were written by Robert J. Bauer. It utilizes the computational engine of ADAPT II release 4, by David D'Argenio and Alan Schumitsky at the University of Southern California Department of Biomedical Simulations Resource (6, 7). Model discrimination was accomplished according to the rule of parsimony based on Akaike's information criterion (1). Given past population analyses of tigecycline (16), a two-compartment model with short-term i.v. infusion input was attempted first with plans to investigate other models only if an adequate fit of the data could not be obtained. The two-compartment model was initially parameterized using the total clearance (CLt), the volume of the central compartment (Vc), the distributional clearance (CLd), and the steady-state volume of distribution (Vss).
Weighting of each output (serum tigecycline concentrations) was based on the reciprocal of the estimated observation variance (the “error” standard deviation [SD] squared for that observation), which was predicted as a function of the fitted concentration. Relationships between concentration and variance (using “error” variance models) were estimated based on the performance (as assessed by the interday percent coefficient of variation [%CV] and sensitivity) of the assay for the drug in serum. A combined additive and proportional model was used; the additive component (termed SDin) was fixed across the population, while the proportional component (termed SDsl) was fit but still constant across all patients (often termed a “fixed effect”). The initial value of SDin was set at 0.005 (one-half of the LLOQ in μg/ml) and altered as necessary, depending on the fit of the model to the data. S-ADAPT method 8 was chosen for determination of the dispersion of mean PK parameters. This method calculates the standard error (SE) of the mean using the full second derivative matrix and using third and fourth central moments (4).
Covariate analyses.
After an adequate structural model was identified and fit to the data derived, individual post-hoc parameter estimates were generated for each patient. The PK parameters were then merged with patient demographics and disease characteristics for use in the statistical analysis. A combined data set (community- and hospital-acquired pneumonia) was then used to evaluate the apparent impact of demographic and disease characteristics on the primary PK parameters (CLt and Vss). The characteristics evaluated are summarized in Table 1.
TABLE 1.
Demographic and disease characteristics evaluated for impact on pharmacokinetics of patients with community- or hospital-acquired pneumoniag
| Continuous variables |
Categorical variables |
||
|---|---|---|---|
| Demographic | Disease | Demographic | Disease(s) |
| Age | Albumina | Ethnicity | Alcohol abuse |
| Hta | CrCLa | Nursing home residence | Cerebrovascular disease |
| Wta | APACHE II score (HAPh only)a | Sex | CHFd |
| BMIb | COPDe | ||
| BSA | Current or previous smoking | ||
| IBWc | Diabetes, Fine score (CAPf only),a liver disease, neoplastic disease, renal disease, ventilator-associated pneumonia | ||
Baseline values were used for these variables.
BMI, body mass index.
IBW, ideal body weight.
CHF, congestive heart failure.
COPD, chronic obstructive pulmonary disease.
CAP, community-acquired pneumonia.
The equations used to generate BSA, BMI, IBW, and CrCL are provided in the supplemental material.
HAP, hospital-acquired pneumonia.
Covariate screening was conducted using SYSTAT 11 (Systat Software, Inc., Richmond, CA). The first step in covariate screening involved the univariate graphical examination of plots of PK parameters versus covariates. In the second step, multivariate general linear modeling (GLM) was used to determine the significance of the association of all covariates. Using forward stepping, all covariates which were at least of “borderline” significance (P < 0.1) were included in the model for the next step. The third step of covariate screening involved centering those continuous covariates that were significant, assigning numerical (e.g., 0 for no or 1 for yes) values to the significant categorical covariates and then creating a multiple linear regression model with only those covariates which passed the initial screen (as described in step 2). When the graphical analyses suggested a nonlinear function might be needed, similar procedures, using nonlinear regression, were employed.
The results of the multiple linear regression analysis were used as initial estimates in the final step of covariate analysis conducted using S-ADAPT as described below. The entire process was conducted for CLt first. The resultant model (with covariates for CLt included) then served as the base model during construction of the covariate model for Vss.
A forward-stepwise approach was used to construct the covariate models in S-ADAPT. All of the covariates deemed significant using multiple linear regression were added, one at a time, to the model. That covariate for which the addition resulted in the largest significant improvement in the objective function (α = 0.05) was retained, and the resulting model was used for the next step. The process was then repeated until none of the remaining covariates provided an improvement in the objective function when added to the model. This full multivariate model was then subjected to a backward elimination procedure in which the criteria for retention in the model were made more stringent (α = 0.001 or an increase in the value of the objective function of >10.83 units after removal of that covariate).
Evaluation of the final model.
In order to evaluate the robustness of the model, a bootstrap evaluation was performed using the following process. (i) Fifty sets of bootstrapped data were created, each of which contained the same number of patients as the actual population. Patients were randomly sampled with replacement. Each data set contained the same dosing and sampling scheme as found in an actual patient with the tigecycline concentrations simulated using the final population PK model (structural model, covariate relationships, error model). Covariate values for each simulated patient were not altered from those observed in the actual patients. (ii) Each of the 50 data sets was analyzed separately using the final covariate model. (ii) The distribution of the mean parameter estimates from the bootstrap analysis was compared (both visually and statistically) to the mean estimates from the actual analysis.
RESULTS
Data.
The complete pooled data set (i.e., before data cleansing and removal of outliers) contained a total of 2,090 serum drug concentrations from 485 patients. Using the process described above, 54 samples from the community-acquired pneumonia studies were deemed significant outliers and removed from the data set; 36 of these concentrations constituted the full complement of samples for 10 patients, and thus these patients were removed from the analysis. The cleansing process for the hospital-acquired pneumonia study resulted in the removal of 385 samples; 243 of these concentrations constituted the full complement of samples for 50 patients, and thus these patients were removed from the analysis. The main reason for data removal was lack of an adequate dosing history, while the other reasons for data removal included pretreatment (BLQ) samples, missing sample collection times, pretreatment samples recorded at an incorrect time, and patients with all concentrations documented as missing or BLQ. The outlier analysis for the HAP study resulted in the removal of 70 samples that were deemed significant outliers; 51 of these concentrations constituted the full complement of samples for 13 patients and these patients were removed from the analysis. Thus, the final data set for structural model development contained 1,581 serum drug concentrations from 412 patients (1,119 concentrations from 289 community-acquired pneumonia patients and 462 concentrations from 123 hospital-acquired pneumonia patients). The majority of the patients had the protocol-specified number of samples (four), and the distribution of time since the previous dose was as expected, given the sampling scheme and the fact that these were phase 3 trials. Summary statistics for the demographic variables are provided in Tables 2 and 3. Note that two patients from the hospital-acquired pneumonia study, who were included in structural model development, had inadequate covariate information and were excluded from the covariate analysis.
TABLE 2.
Demographics characteristics of the analysis population of patients with community- or hospital-acquired pneumonia (continuous variables)
| Variable | n | Mean (SD) | Median (range) |
|---|---|---|---|
| Age (yr) | 410 | 55.2 (17.5) | 56.0 (18.0-92.0) |
| Wt (kg) | 410 | 74.7 (17.8) | 74.0 (33.8-140) |
| Ht (cm) | 410 | 168 (10.5) | 168 (136-198) |
| IBWb (kg) | 410 | 62.5 (10.5) | 63.2 (45.0-91.3) |
| BMIc (kg/m2) | 410 | 26.5 (5.98) | 25.7 (14.6-55.1) |
| BSA (m2) | 410 | 1.83 (0.232) | 1.83 (1.23-2.42) |
| CrCL (ml/min/1.73 m2) | 410 | 79.7 (35.1) | 75.7 (18.4-247) |
| Albumin (g/dl) | 410 | 3.02 (0.675) | 3.02 (1.20-5.30) |
| APACHE II score (HAPd only) | 121 | —a | 12.0 (3.00-30.0) |
—, the mean and standard deviation were not calculated for the APACHE II score, as this variable is ordinal in nature.
IBW, ideal body weight.
BMI, body mass index.
HAP, hospital-acquired pneumonia.
TABLE 3.
Demographic and disease characteristics of the analysis population of patients with community- or hospital-acquired pneumonia (categorical variables)
| Variable | n | % |
|---|---|---|
| Sex | ||
| Male | 250 | 61.0 |
| Female | 160 | 39.0 |
| Race | ||
| White | 299 | 72.9 |
| Black | 30 | 7.32 |
| Hispanic | 67 | 16.3 |
| Other | 14 | 3.48 |
| Fine score (CAPa only) | ||
| I | 50 | 17.3 |
| II | 94 | 32.5 |
| III | 83 | 28.7 |
| IV | 60 | 20.8 |
| V | 2 | 0.69 |
| Alcohol abuse (present) | 41 | 10.0 |
| Cerebrovascular disease (present) | 41 | 10.0 |
| CHFb (present) | 34 | 8.29 |
| COPDc (present) | 52 | 12.7 |
| Current smoking (yes) | 143 | 34.9 |
| Diabetes (present) | 69 | 16.8 |
| Liver disease (present) | 27 | 6.59 |
| Neoplastic disease (present) | 11 | 2.68 |
| Nursing home residence (present) | 19 | 4.63 |
| Previous smoking (yes) | 225 | 54.9 |
| Renal disease (present) | 32 | 7.80 |
| Ventilator-associated pneumonia (HAPd only) | 44 | 10.7 |
CAP, community-acquired pneumonia.
CHF, congestive heart failure.
COPD, chronic obstructive pulmonary disease.
HAP, hospital-acquired pneumonia.
Population PK model.
The most robust fit to the community-acquired pneumonia data was obtained using a two-compartment model (one central, one peripheral) with zero-order i.v. infusion and first-order linear elimination. The model was parameterized using CLt, Vc, CLd, and Vss. Due to the excellent fits obtained overall, no other structural models were attempted. The residual error model employed included an additive term (fixed at 0.025) and a proportional term (fitted as an intraindividual variability parameter). Covariate analyses using the community-acquired pneumonia data alone indicated that, of all of the covariates tested, only body surface area (BSA) and age explained a significant amount of the interindividual variability in CLt when evaluated using S-ADAPT. None of the covariates explained a significant portion of the interindividual variability in Vss.
After pooling of the community- and hospital-acquired pneumonia data sets and exclusion of the two patients from the hospital-acquired pneumonia study who had inadequate covariate information, the previously described covariate model was subjected to a backward stepping analysis, which confirmed the significance of the effects of BSA and age on clearance. Subsequently, univariate explorations of the data showed that there were several additional apparent relationships between CLt and certain demographic and disease characteristics. Thus, it was decided to include all of the covariates in the initial screen using GLM. The only significant covariates from the GLM model were albumin, baseline weight, creatinine clearance (CrCL), diabetes, and sex. Of these, only CrCL explained a significant portion of the interindividual variability in clearance in addition to BSA and age when evaluated using S-ADAPT. This expanded model (relationships between age, BSA, and CrCL and drug clearance) was subjected to backward stepping, which showed that the effect of CrCL was slightly more significant than that of age, which was then removed from the model. As was seen in the analysis of the community-acquired pneumonia data alone, none of the covariates explained a significant portion of the interindividual variability in Vss.
The final population PK model for tigecycline was a two-compartment model with linear elimination and with a relationship between tigecycline clearance and BSA and CrCL. The final parameter estimates and associated SEs are provided in Table 4. The precision of the mean fitted PK parameters was high, as evidenced by low %SE values. Despite the inclusion of covariates to explain some of the interindividual variability in clearance, there was a high degree of correlation between the PK parameters (correlation coefficients ranged from 0.544 for CLt-Vc to 0.908 for Vc-Vss; the full covariance matrix is provided in the supplemental material).
TABLE 4.
Parameter estimates and %SEs from the final population PK model for tigecycline-treated patients with community- or hospital-acquired pneumoniaa
| Parameter | Population mean |
Magnitude of interindividual variability (%CV) |
||
|---|---|---|---|---|
| Final estimate | %SE | Final estimate | %SE | |
| CL (liters/h) | 19.2 | NAb | 40.4 | 8.53 |
| Vc (liters) | 65.2 | 7.86 | 82.1 | 11.6 |
| CLd (liters/h) | 85.1 | 9.35 | 110 | 11.8 |
| Vss (liters) | 398 | 7.04 | 40.2 | 23.0 |
| CLt intercept (liters/h) | 19.6 | 2.64 | NA | NA |
| CLt BSA slope (liters/h·m2) | 10.2 | 13.7 | NA | NA |
| CLt CrCL slope [(liters/h)·(ml/min/1.73 m2)] | 0.0638 | 16.0 | NA | NA |
| SDin | 0.0250 | NA | NA | NA |
| SDsl | 0.146 | 4.77 | NA | NA |
Minimum value of the objective function, −3,126.
NA, not applicable due to the parameterization of the model.
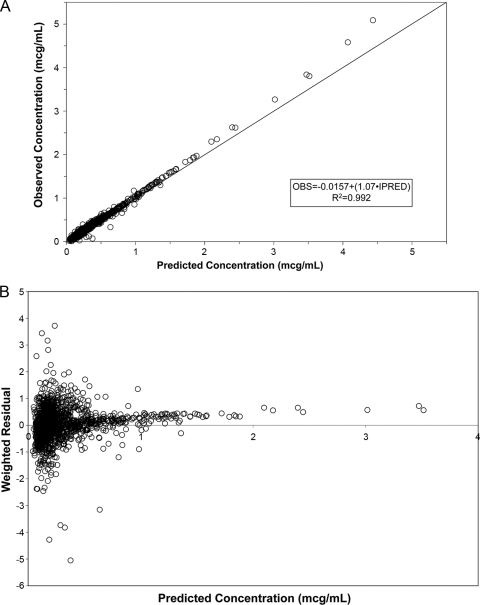
Overall, excellent fits to the data were obtained. Figure 1A shows the scatter plot of observed versus individual post-hoc predicted data; an overall r2 value of 0.992 was obtained. There was, however, a trend for some of the peak concentrations to be slightly underpredicted. This can also be seen in the plot of individual weighted residuals versus individual predicted concentrations (Fig. 1B).
FIG. 1.
Scatter plot of observed versus individual predicted tigecycline concentrations for the final population model for patients with either community- or hospital-acquired pneumonia (A). Scatter plot of individual weighted residuals versus individual predicted tigecycline concentrations for the final population model (B). Note that the solid line represents the line of identity. OBS, observed; PRED, predicted.
The equation describing the relationships between CLt and BSA and CrCL for the final model is CLt = 19.6 + [10.2·(BSA − 1.73)] + [0.0638·(CrCL − 100)].
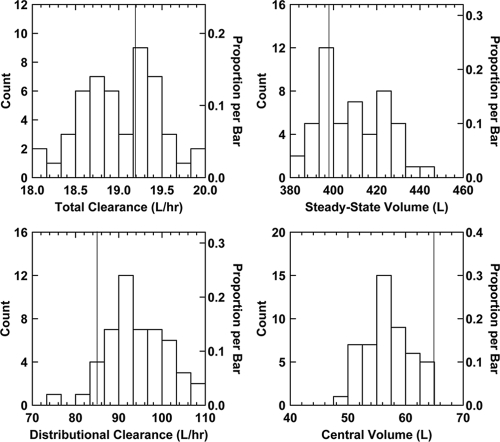
Using a bootstrap procedure, it was determined that, overall, the PK parameters were estimated with relatively little bias and an acceptable degree of precision (Fig. 2). For CLt, the mean estimate from the original population (19.2 liters/h) fell very close to the center of the distribution of CLt estimates from the bootstrap (median of the mean estimates from the bootstrap = 19.0 liters/h). The dispersion of the mean CLt estimates from the bootstrap was very tight (%CV = 2.4%), which indicates that the estimates of clearance were also very precise. The apparent bias in the estimations of Vc and, to a lesser extent, Vss and CLd is likely due to the sparse sampling scheme. The sampling scheme was designed to provide robust estimates of CLt (in order to obtain strong area estimates of the under the concentration-time curve [AUC]) while sacrificing the ability to obtain robust estimates of Vc, Vss, and CLd. Summary statistics for all of the bootstrap estimates are provided in the supplemental material.
FIG. 2.
Frequency distribution histograms of mean parameter estimates from analysis of the 50 bootstrap data sets. Note that the vertical line represents the mean estimate from the analysis of the original data set.
DISCUSSION
The main objective of this analysis was to develop a population PK model to describe the pharmacokinetics of tigecycline in a pooled phase 3 population of patients with community- or hospital-acquired pneumonia. The goal was to characterize the exposure in these patients (which would be of use for subsequent PK-pharmacodynamic [PD] analyses based on data for these same patients) as opposed to developing a model that would be considered predictive of tigecycline pharmacokinetics in other populations. To this end, excellent fits to the data were obtained overall with a two-compartment model with linear elimination. The finding that a two-compartment model was most appropriate in describing the pharmacokinetics of tigecycline was expected, given the results of a previous analysis of data from patients with complicated skin and skin structure or intra-abdominal infections (16).
Although the utilization of different population methods (MCPEM in this analysis, NONMEM in the previous analysis [16]), differences in the patients and infections studied, and the differences in study design make exact comparisons difficult, there is value in comparing the mean parameter estimates between the two models. When comparing the basic structural models, the tigecycline clearance values were similar: 19.2 liters/h in the present analysis versus ∼17 liters/h in the NONMEM analysis. While distributive clearance values are also comparable (85.1 liters/h in the present analysis versus 70.9 liters/h in the NONMEM analysis), the volume parameters differed substantially (Vc/Vss of 65.2/398 liters for this analysis versus 115/644 liters in the NONMEM analysis). There are several possible explanations for this difference, but the most likely cause may be the difficulty in obtaining accurate estimates of Vss for multicompartmental drugs when using a sparse-sampling strategy, or simply differences in the patients included in the studies. Using the population PK parameter estimates, the terminal elimination half-life in HAP/CAP patients would be expected to be approximately 17 h. This is significantly shorter than other estimates in the literature, which estimate a terminal elimination half-life of approximately 40 h (11, 16). This discrepancy is likely due to the fact that the sampling scheme for these studies was optimized for the determination of clearance (and thus AUC) and not for the determination of the terminal elimination half-life.
Our second objective was to identify those patient factors that help explain the interindividual variability in tigecycline PK parameters. The initial analysis, involving patients with community-acquired pneumonia only, identified a relationship between total clearance and both BSA and age. In the final analysis, where patients with community- and hospital-acquired pneumonia were pooled, a relationship between total clearance and both BSA and CrCL was identified. The replacement of the age effect with CrCL when analyzing the pooled data set was not unexpected, given that (i) the pooled data set contained a broader range of CrCL values and (ii) there was a high degree of correlation between age and CrCL. In fact, CrCL was only minimally more predictive of CLt than age. This change in the covariate model brings our results somewhat more in line with the previous analysis of patients with complicated skin and skin structure or intra-abdominal infections (16). The only difference in covariate models for CLt between our current model and the previous model is the lack of a sex effect. The lack of an impact of sex in the present analysis may simply be due to the more appropriate “explanation” of certain body size factors using BSA versus weight, which obviated the differences between males and females seen in the NONMEM analysis. It is important to note that although the covariate relationships were statistically significant in both analyses, they were not necessarily clinically significant, as evidenced by the small amount of the interindividual variability in clearance explained. Thus, none of these covariate relationships would be expected to translate into the need to adjust doses based on patient demographics (e.g., body size, CrCL, or sex).
Analysis of the pooled data from patients with community- or hospital-acquired pneumonia confirmed the previous results from patients with community-acquired pneumonia alone regarding the lack of any covariate effects on Vss. The lack of covariate effects on Vss was somewhat unexpected but may be explained by the difficulty in obtaining accurate estimates of Vss. This can result in the high degree of correlation observed between CLt and Vss and perhaps precluded the identification of significant predictors of the interindividual variability in Vss once those for CLt were included in the model.
The PK-PD measure most closely associated with tigecycline efficacy is the ratio of the AUC to the MIC of tigecycline for the microorganism (AUC/MIC ratio) (2). Based on the concordance with the findings of the previous population PK analysis (16) and the results of the model evaluation in the current analysis, our confidence in the model-derived individual PK estimates of tigecycline clearance is high. As clearance simply transforms to AUC (dose/clearance), individual estimates of patient exposures (AUC) should be accurate and precise. Therefore, this population PK model will allow the future exploration of relationships between tigecycline exposure and response to therapy for safety and efficacy endpoints among patients with community- or hospital-acquired pneumonia.
In conclusion, the final population PK model for tigecycline based on pooled data from phase 3 studies of patients with community- or hospital-acquired pneumonia was a two-compartment model with linear elimination and distribution and zero-order i.v. infusion. Covariate analyses identified significant relationships between tigecycline total clearance and both BSA and CrCL. Results of the bootstrap evaluation suggest that the mean estimates of total clearance (and thus AUC) and of Vss from the final model are unbiased and precise. This model will serve as the basis for estimating tigecycline exposure for future PK-PD analyses for efficacy and safety among patients with community- or hospital-acquired pneumonia.
Supplementary Material
Footnotes
Published ahead of print on 4 October 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Akaike, H. 1979. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 66:237-242. [Google Scholar]
- 2.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 3.Bassett, E. J., M. S. Keith, G. J. Armelagos, D. L. Martin, and A. R. Villnueva. 1980. Tetracycline labeled human bone from ancient Sudanese Nubia. Science 209:1532-1534. [DOI] [PubMed] [Google Scholar]
- 4.Bauer, R. J. 2006. S-ADAPT/MCPEM user's guide: software for pharmacokinetic, pharmacodynamic and population data analysis. Biomedical Simulations Resource, Los Angeles, CA. http://bmsr.usc.edu/Software/ADAPT/SADAPTsoftware.html.
- 5.Beal, S. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 2:481-504. [DOI] [PubMed] [Google Scholar]
- 6.D'Argenio, D. Z., and A. Schumitzky. 2003. ADAPT II user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA.
- 7.D'Argenio, D. Z., and A. Schumitzky. 1979. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput. Programs Biomed. 9:115-134. [DOI] [PubMed] [Google Scholar]
- 8.Duggar, B. M. 1948. Aureomycin: a product of the continuing search for new antibiotics. Ann. N. Y. Acad. Sci. 51:177-181. [DOI] [PubMed] [Google Scholar]
- 8a.Freire, A. T., V. Melnyk, M. J. Kim, O. Datsenko, O. Dzyublik, F. Glumcher, Y.-C. Chuang, R. T. Maroko, G. Dukart, C. A. Cooper, J. M. Korth-Bradley, N. Dartois, and H. Gandjini for the 311 Study Group. 2010. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 68:140-151. [DOI] [PubMed] [Google Scholar]
- 9.Meagher, A. K., P. G. Ambrose, T. H. Grasela, and E. J. Ellis-Grosse. 2005. Pharmacokinetic/pharmacodynamic profile for tigecycline—a new glycylcycline antimicrobial agent. Diagn. Microbiol. Infect. Dis. 52:165-171. [DOI] [PubMed] [Google Scholar]
- 10.Meagher, A. K., J. A. Passarell, B. B. Cirincione, S. A. Van Wart, K. Liolios, T. Babinchak, E. J. Ellis-Grosse, and P. G. Ambrose. 2007. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob. Agents Chemother. 51:1939-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muralidharan, G., M. Micalizzi, J. Speth, D. Raible, and S. Troy. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passarell, J. A., A. K. Meagher, B. B. Cirincione, S. A. Van Wart, T. Babinchak, A. J. Ellis-Grosse, and P. G. Ambrose. 2008. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob. Agents Chemother. 52:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen, P. J., N. V. Jacobus, W. J. Weiss, P. E. Sum, and R. T. Testa. 1999. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t butylglycylamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodvold, K. A., M. H. Gotfried, M. Cwik, J. M. Korth-Bradley, G. Dukart, and E. J. Ellis-Grosse. 2006. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 58:1221-1229. [DOI] [PubMed] [Google Scholar]
- 15.Schnappinger, D., and W. Hillen. 1996. Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 165:359-369. [DOI] [PubMed] [Google Scholar]
- 16.Van Wart, S. A., J. S. Owen, E. A. Ludig, A. K. Meagher, J. M. Korth-Bradley, and B. B. Cirincione. 2006. Population pharmacokinetics of tigecycline in patients with complicated intra-abdominal or skin and skin structure infections. Antimicrob. Agents Chemother. 50:3701-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyeth Pharmaceuticals, Inc. 2009. Tygacil (tigecycline) for injection. Wyeth Pharmaceuticals, Inc., Philadelphia, PA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.