Abstract
The first step of Plasmodium development in vertebrates is the transformation of the sporozoite, the parasite stage injected by the mosquito in the skin, into merozoites, the stage that invades erythrocytes and initiates the disease. The current view is that, in mammals, this stage conversion occurs only inside hepatocytes. Here, we document the transformation of sporozoites of rodent-infecting Plasmodium into merozoites in the skin of mice. After mosquito bite, ∼50% of the parasites remain in the skin, and at 24 h ∼10% are developing in the epidermis and the dermis, as well as in the immunoprivileged hair follicles where they can survive for weeks. The parasite developmental pathway in skin cells, although frequently abortive, leads to the generation of merozoites that are infective to erythrocytes and are released via merosomes, as typically observed in the liver. Therefore, during malaria in rodents, the skin is not just the route to the liver but is also the final destination for many inoculated parasites, where they can differentiate into merozoites and possibly persist.
Keywords: intravital imaging, Plasmodium, schizogony
Malarial infection starts with the inoculation of Plasmodium sporozoites by mosquitoes probing the vertebrate skin for blood. The highly motile sporozoites eventually invade host target cells where they differentiate and divide into numerous merozoites, the parasite form that invades erythrocytes and initiates the pathogenic phase of malarial infection. The host cell type in which sporozoites transform into merozoites, however, differs between Plasmodium species. In species that infect birds, sporozoites differentiate inside macrophages primarily in the skin but also in the spleen, liver, and bone marrow (1). In species that infect mammals, sporozoites are known to differentiate only inside hepatocytes in the liver (2–4).
The first demonstration that sporozoites of mammal-infecting Plasmodium species develop inside hepatocytes was made in 1948 after i.v. inoculation of sporozoites of P. cynomologi into rhesus monkeys (2). In addition to reporting fully mature parasites inside hepatocytes, the authors also documented the persistence of immature and dormant forms of the parasite in the liver several months after the initial inoculation, which they proposed to be the cause of relapses (5), and were later called hypnozoites (6). Subsequent work indicated that sporozoites of species that infect humans (7) also undergo complete development inside hepatocytes.
Since these early studies, P. berghei and the related P. yoelii species, which infect rodents, have been used as practical and safe models for studying the pre-erythrocytic phase of malaria. These parasites were shown to differentiate in the liver of laboratory rodents (8), and the P. berghei/rodent system was used to demonstrate that the majority of sporozoites were inoculated by mosquitoes in the skin rather than directly into the blood circulation (9), as traditionally assumed. More recently, the generation of fluorescent P. berghei parasites, along with the development of intravital imaging approaches applicable to rodents, have allowed rapid progress in our understanding of the fate of Plasmodium sporozoites in the mammalian host.
Intravital imaging of P. berghei sporozoites confirmed that sporozoites were injected in the skin of the mouse, where they display vigorous motility (10). Quantitative analysis revealed that more than half of the P. berghei sporozoites inoculated by mosquitoes in the mouse ear skin were still present at the bite site after 1 h (11), and ∼40% stayed as long as 6 h (12), when sporozoites are no longer actively motile. Of the sporozoites that left the mosquito bite site, ∼70% were found to take the blood route and ∼30% the lymphatic route (11). The latter terminate their journey in the first draining lymph node, where most die in a few hours, though a few develop, at least partially, in association with podoplanin-expressing cells (11). A similar tripartite fate of sporozoites staying in the skin, leaving the skin via the blood or via the lymph, was later also described for P. yoelii using quantitative PCR analysis (13).
In this work, we analyze the fate of sporozoites of rodent-infecting Plasmodium species in the skin of mice.
Results and Discussion
P. berghei Develops Inside Skin Cells in the Mouse.
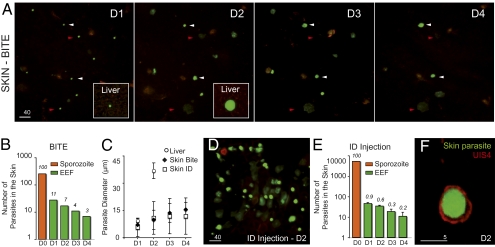
We first analyzed the fate of the P. berghei sporozoites that remain in the skin of the host. Anopheles stephensi mosquitoes were allowed to transmit WT green fluorescent sporozoites (14) into the ear of SKH1 hairless mice (15), which display little autofluorescence in the skin, and parasites were imaged daily using spinning-disk confocal microscopy (16). Typically, inside hepatocytes, parasites round up and increase their size to become spherical exoerythrocytic forms (EEF) of ∼40-μm average diameter, undergo schizogony, and produce thousands of uninucleate merozoites in ∼50–70 h (4). Approximately 11% of the elongated sporozoites detected in the skin soon after the bite were still observed after 24 h (day 1, D1) as brightly fluorescent, round parasites (Fig. 1 A and B). The average size and fluorescence intensity of the EEF steadily increased with time (Fig. 1 A and C). However, the maximal diameter of skin EEF, typically reached at D3–D4, remained 2–3 times smaller than the maximal diameter of liver EEF, reached at D2 (Fig. 1 A and C). When parasite survival was assessed after intradermal injection of ∼5,000 sporozoites into the ear skin, only ∼1% and 0.2% were found to brightly fluoresce at D1 and D4, respectively (Fig. 1 D and C). Despite the ∼10-fold decrease in the percentage of parasites present at D1 after needle injection compared with mosquito delivery, the fate of surviving parasites was similar in the two cases, as judged by the proportion (Fig. 1 B and E) and the average size (Fig. 1C) of fluorescent EEF.
Fig. 1.
P. berghei differentiation in the ear skin of a hairless mouse. (A) Parasites (in green) imaged for 4 d (autofluorescence in red) after sporozoite inoculation by the bite of a single mosquito. Images are maximal Z-projections of 13–21 contiguous pictures separated by 5 μm. Red arrowheads, fluorescent parasites fading over time; white arrowheads, brightly fluorescent parasites until day 4 (D4). The lower-right inset shows a liver stage at the same scale at D1 and D2. (Scale bar, 40 μm.) (B) Cumulative numbers of developing parasites in six different bite sites from two independent experiments. Orange bar (D0), number of sporozoites detected after the bite (n = 258); green bars, number of brightly fluorescent EEF; numbers above the bars, percentages of developing parasites versus sporozoites imaged at D0. (C) Parasite diameter (average ± SD), estimated by the EEF maximum projection area, in the liver (circles) and in the skin (diamond, after bite; square, after injection). (D) Parasites (in green) at D2 after microinjection of 5,200 sporozoites. The image is a maximal Z-projection of 35 pictures covering 70 μm in depth. (Scale bar, 40 μm.) (E) Numbers of developing parasites after intradermal injection. Orange bar (D0), no. of injected sporozoites (5,200); green bars, numbers of brightly fluorescent EEF (average ± SD), in four injection sites; numbers above the bars, percentages of developing parasites vs. sporozoites injected at D0. Similar results were obtained after injection of larger number of sporozoites (75,000–300,000 parasites). (F) Green fluorescent EEF surrounded by a parasitophorous vacuole stained with anti-UIS4 polyclonal antibody (in red) at D2. (Scale bar, 5 μm.)
We then asked whether parasite maturation in the skin occurred within host cells. In hepatocytes, parasites develop inside a parasitophorous vacuole (PV) formed upon sporozoite entry into the host cell (17). The sporozoite transmembrane protein UIS4 inserts into the PV membrane and is essential for liver-stage development (18). Staining of skin cryosections with anti-UIS4 antibodies (Fig. 1F) showed that 53% and 65% of the green fluorescent EEF were delineated by a clear red UIS4 signal at D1 and D2, respectively. We also analyzed the development in the mouse skin of sporozoites lacking the P36p protein. P36p is important for the formation and/or maintenance of the PV membrane, and a P. berghei P36p knockout clone generates ∼5–10% of the EEF produced by WT sporozoites inside hepatocytes (19, 20). We constructed the P. berghei clone P36p-G bearing both the P36p- null mutation and a GFP-expression cassette (Fig. S1), and sporozoites of the clone were coinjected with red-fluorescent WT sporozoites of the L733 clone (21) into the skin of mice. Approximately 10-fold fewer green P36p-G than red WT EEF were observed at D1 at the injection site (Fig. S1). Together, these data suggested that most WT P. berghei parasites surviving in the mouse skin were developing intracellularly inside a PV.
P. berghei Develops in the Epidermis and Dermis and in Association with Hair Follicles.
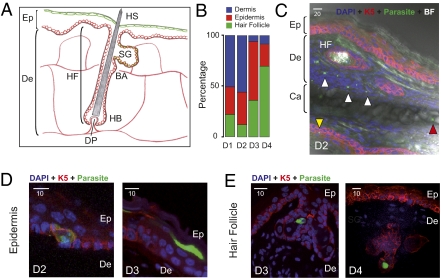
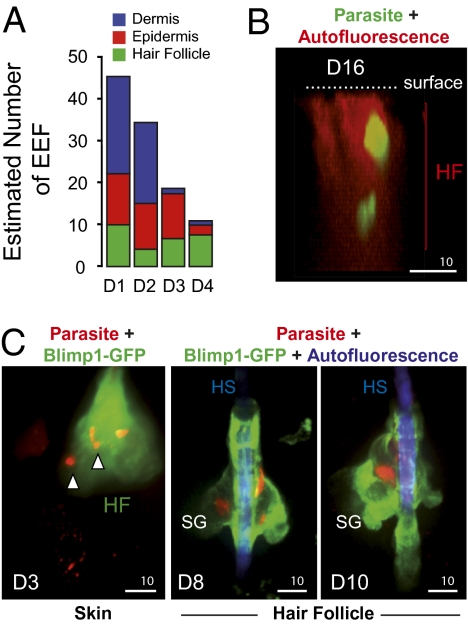
We next localized the EEF in the skin using an immunohistological approach (see Fig. 2A for a schematic representation of the mammalian skin). P. berghei sporozoites (2 × 105) were injected into the ear skin of hairless mice, and at various days postinoculation ∼10-μm cryosections of fixed ear tissues were labeled with DAPI and immunostained using antibody K5, which recognizes keratin5 in keratinocytes of the basal layer of the epidermis (22). EEF were present in multiple sites in the skin. (i) At D1 and D2, ∼50% of the EEF were located in the dermis (Fig. 2 B and C and Fig. S2A). Dermal EEF behaved similarly to liver EEF, with a sharp decrease between D2 and D3, and only represented ∼7% of the skin EEF at D3 and D4 (Fig. 2B). (ii) EEF were also found in the epidermis. At D1 and D2, epidermal EEF were associated mostly with keratin5-positive keratinocytes lying on the basement membrane, whereas at D3 and D4 they were mostly in keratin5-negative cells in the superficial layers of the epidermis (Fig. 2D and Fig. S2B). The number of epidermal EEF did not significantly change up to D3, and only slightly decreased at D4 (Fig. 3A). (iii) More surprisingly, EEF were found in close association with hair follicles, appearing as keratin5-positive keratinocyte-bound tubular invaginations of the epidermis (Fig. 2E and Fig. S2C). In hairless mice, EEF associated with the autofluorescent (and rudimentary) hair follicles were frequently located close to the sebaceous glands (Fig. 2E and Fig. S2C). Their numbers remained stable up to D4 (Fig. 3A), representing 70% of the skin EEF at D4 (Fig. 2B), and parasites were still detected in hair follicles after more than 2 wk postinoculation (Fig. 3B).
Fig. 2.
Localization of P. berghei skin EEF. (A) Schematic view of the epidermis, dermis, and hair follicle of the mammalian skin. Drawn are the keratin5-positive keratinocytes (in red) that rest on the basement membrane separating the dermis from the epidermis and line the invagination of the HF; the Blimp1-positive cells (in green) associated with the superficial layer of the epidermis and the HF; and the vascularization in the dermis (red lines). Ep, epidermis; De, dermis; HF, hair follicle; HS, hair shaft; SG, sebaceous gland; BA, bulge area; HB, hair bulb; DP, dermal papilla. (B) Percentage of dermal (blue), epidermal (red), and hair follicle-associated (green) parasites in the mouse ear estimated by immunofluorescence microscopy at various days after intradermal injection of sporozoites. Number of analyzed EEF for each time point: 33–63. (C) Confocal image showing EEF (in green) in the deep dermis (white arrowheads), the epidermis (yellow arrowhead), and the cartilage (red arrowhead). Abbreviations are as in A; Ca, cartilage. (Scale bar, 20 μm.) (D) Confocal images of epidermal EEF (in green), associated with keratin5-positive keratinocytes of the basal layer of the epidermis (Left) or with keratin5-negative keratinocytes of the superficial layers of the epidermis (Right). Abbreviations are as in A. (Scale bar, 10 μm.) (E) Confocal images of hair follicle-associated EEF. EEF (in green) are located in the upper portion of the HF, in keratin5-positive or keratin5-negative cells, often near the sebaceous glands. Abbreviations are as in A. (Scale bar, 10 μm.)
Fig. 3.
P. berghei association with hair follicles. (A) Numbers of dermal, epidermal, and hair follicle-associated EEF after intradermal injection of sporozoites, obtained by multiplying the numbers of skin EEF counted by intravital microscopy (Fig. 1E) by the percentages of dermal, epidermal, and hair follicle-associated parasites counted by histology (Fig. 2B). (B) Intravital confocal image showing EEF surviving inside a hair follicle in the ear of a hairless mouse at D16 after microinjection of sporozoites. The images are a lateral view of a 3D reconstruction of the skin. Parasites are in green and the hair follicle autofluoresces in red. (Scale bar, 10 μm.) (C) Intravital confocal images showing red fluorescent EEF in the ear skin of a Blimp1-GFP mouse. (Left) D3: Z-projection of 70 slices covering 35 μm showing red fluorescent EEF inside a hair follicle (white arrowheads). (Center) D8 and (Right) D10: red EEF in a hair follicle in close association with Blimp1-GFP-positive cells. (Scale bars, 10 μm.)
Parasite association with hair follicles was also imaged in a Blimp1-GFP mouse (23). In this mouse, a group of cells expressing the transcriptional repressor B lymphocyte-induced maturation protein 1 (Blimp1) and residing near the bud site of the sebaceous glands, which act as sebocyte progenitor cells, are green fluorescent (24). After injection of red fluorescent WT sporozoites into the ear of a Blimp1-GFP mouse, red fluorescent EEF were found associated with the green fluorescent area of hair follicles (Fig. 3C, skin). As in the hairless mice, hair follicle-associated EEF were observed persisting for several days, in the vicinity of the GFP-fluorescent zone of the sebaceous glands (Fig. 3C, hair follicle). It is still unclear, however, whether these parasites were simply growing slowly in the skin or might include true dormant (growth-arrested) forms.
P. berghei Developing in the Mouse Skin Generates Merozoites.
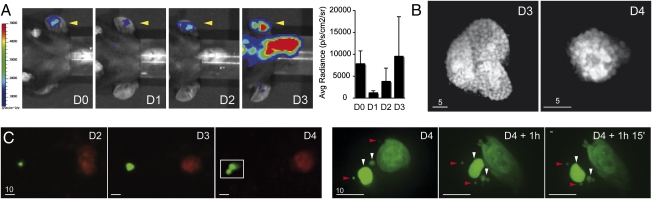
We next imaged parasite growth in the skin using bioluminescence. For this, we used sporozoites of the transgenic P. berghei clone 676cl1 (PbGFP-LUCSCH) expressing a GFP-luciferase fusion gene via the EEF1α promoter (25), which constitutively produces the fusion protein throughout the parasite life cycle. Sporozoites (2 × 104) were inoculated in the ear skin of mice and luciferin injected immediately before real-time whole-body imaging of mice using the IVIS system (26). Bioluminescent signals were detected only in the ear and the liver at D2 (Fig. S3). The main signal was detected in the liver, peaking at D2, and the signal in the ear skin peaked at D3. Quantification of the intensity of bioluminescence signals using the Living Image software showed that the signal in the ear skin increased ∼threefold between D1 and D2, and ∼2.5-fold between D2 and D3 (Fig. 4A). This suggested that, like liver parasites, skin parasites could actively grow.
Fig. 4.
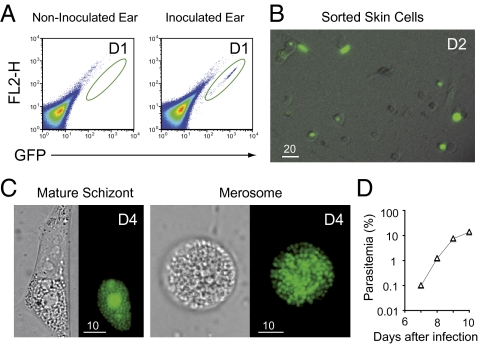
P. berghei complete development inside skin cells. (A) Parasite development in the skin measured by bioluminescence. The 2 × 104 GFP::LUC sporozoites were microinjected in the ear of C57BL6 mice (yellow arrowhead) and recorded from D0 to D3 following injection of luciferin. The graph represents the difference between the average radiance of the inoculated ear and the contralateral ear at D0, 1, 2, and 3 (mean ± SD; n = 3). (B) Mature schizonts in the skin. Intravital imaging of merozoite-filled EEF in the ears of mice at D3 and D4. (Scale bars, 5 μm.) (C) Skin EEF release merosomes. (Left) Skin EEF growing and budding between D3 and D4 after mosquito bite. (Right) Time-lapse recording of the squared area depicted at D4 and shows the release and movement of fluorescent structures of various sizes. White arrowheads, merosomes; red arrowheads, merozoites. (Scale bars, 10 μm.)
To investigate schizogony in skin parasites, we examined individual skin EEF at D3 and D4, when most had reached their maximal size, by confocal microscopy. In hairless mice, fluorescent parasites were seen undergoing nuclear divisions (Fig. S4) and generating individual merozoite-like progeny (Fig. 4B). Further, infected cells in the skin were frequently observed giving rise to cell extensions reminiscent of the merosomes that extrude from infected hepatocytes (Fig. S5A). Hepatocyte-derived merosomes contain tens to thousands of merozoites wrapped in the host cell membrane, bud off, and detach from the infected cell to reach the blood in the liver sinusoids (27). Brightly fluorescent merosome-like extensions were also observed detaching from infected cells (Fig. 4C) and moving in the skin (Fig. 4C and Fig. S5B).
To test whether the fluorescent progeny seen inside skin cells were indeed merozoites, we tested their capacity to invade and multiply in mouse erythrocytes. One day after injection of green fluorescent sporozoites into the skin of mice (1.0–2.5 × 105 sporozoites per ear in 2–4 animals), i.e., before the first merozoites are formed in the liver, the skin tissue at the injection site was dissected and treated with collagenase and trypsin to obtain a single cell suspension. Infected fluorescent cells were sorted by FACS (Fig. 5A) and incubated for several days at 37 °C in vitro in DMEM 10% FCS. Examination of the sorted cells confirmed the diversity of skin cell types that were infected in situ (Fig. 5B), and after 4 d (1 d in the skin and 3 d in vitro), merozoites were detected inside sorted cells (Fig. 5C). At D4, cells were scratched and the host cell/parasite mixture injected i.v. into mice. In three independent experiments (Fig. 5D), mice became infected and parasitemia increased at a normal rate (∼10-fold increase/24 h). This demonstrates that P. berghei development in skin cells can generate infective merozoites, and thus that the parasite developmental pathway in the skin indeed reproduced that in the liver.
Fig. 5.
Merozoite production by and in vivo infectivity of P. berghei skin EEF. (A) Sorting of infected skin cells. The 1.0–2.5 × 105 sporozoites were microinjected in the ear of C57BL6 mice. The pseudocolor plot shows the distribution of skin cells obtained from the ears of noninfected (Left) and infected mice (Right) 1 d postinfection. The green oval represents the gate used for sorting the infected skin cells, which were collected in 96-wells cell culture plate and kept at 37 °C, 5% CO2 in DMEM 10% FCS. No events were detected using the noninfected ear. (B) Wide-field microscopy of sorted cells showing the variety of infected skin cell types (bright field and green) at D2—1 d in the skin and 1 d in vitro. (Scale bar, 20 μm.) (C) Generation of merozoites within skin cells. Presence of merozoites inside an adherent skin cell (Left) and in a floating merosome (Right). (Scale bar, 10 μm.) (D) A representative parasitemia curve following injection of merozoite-filled skin cells at D4—1 d in the skin and 3 d cultured in vitro. The number of events sorted at D1 in this experiment was 1,200 (∼600 GFP+ cells), resulting in approximately four mature schizonts after 3 d in culture. The parasitemia was accessed by FACS and blood smear.
In Situ Infectivity of P. berghei Merozoites Generated in Skin Cells.
To test whether merozoites generated in skin cells (skin-derived merozoites) were capable of invading erythrocytes in situ and sufficient for generating a blood stage infection, transplantation experiments of infected skins onto naive mice were complicated by the different timings of maximal release of skin-derived merozoites (D2–D3 post-sporozoite inoculation) and of efficient vascularization of the skin graft. Strikingly, we observed that primaquine, a drug known to inhibit liver EEF development, had a much weaker effect on skin EEF development (Fig. S6A). After injection of luciferase-producing sporozoites in the ear of mice, treatment with 25 mg/kg primaquine at D0 and D1 abolished the bioluminescence signal in the liver without affecting that in the ear. However, this differential primaquine effect could not be exploited to show the in vivo infectivity of skin-derived merozoites. Indeed, the 25-mg/kg primaquine treatment did not completely prevent liver EEF maturation in all animals (5 of 52 primaquine-treated mice and surgically deprived of the infected ear at D1 became patent; see Fig. S7) and impaired skin EEF development between D2 and D3 (Fig. S6A), whereas 30 mg/kg primaquine significantly affected skin EEF development at earlier time points (Fig. S6A).
In any case, the skin-derived merozoites are clearly outnumbered by their liver-derived counterparts, and their contribution to the onset of blood stage infection would be minimal at best. After injection of luciferase-producing sporozoites in the skin of mice, the maximal intensity signal at the injection site, reached at D3, was found to be ∼2% of the maximal intensity signal in the liver, reached at D2 (Fig. S6B). Not surprisingly, when sporozoites were injected in the ear of mice and the infected or controlateral ear was surgically removed at D1, no statistical difference was detected in the parasitemia of animals lacking or having skin EEF (Fig. S6C).
P. yoelii Generates Merozoites in the Mouse Skin.
Last, we studied the development of sporozoites of Plasmodium yoelii, another species that infects rodents, in the mouse skin. Like sporozoites of P. falciparum, the species most lethal to humans, P. yoelii sporozoites are known to invade only certain hepatocytic lines in vitro, in a CD81-dependent manner (28). Additionally, quantitative PCR analysis of mouse ear inoculated with P. yoelii sporozoites showed that the parasite DNA was still detected 42 h postinjection in the mouse skin (13). We analyzed the fate of P. yoelii sporozoites expressing GFP (29) or RedStar (Fig. S8) after intradermal injection in mice. At D1, ∼0.2% and ∼0.15% of the inoculated sporozoites transformed in the ear skin of hairless and Swiss mice, respectively (Fig. S9A). Despite the ∼fivefold smaller number of P. yoelii parasites observed in the skin compared with P. berghei at D1, P. yoelii EEF, like P. berghei EEF, grew both in the epidermis and the dermis of mice (Fig. S9B). P. yoelii merozoites were detected from D2 onward (Fig. S9C) and were seen moving across the skin (Fig. S9D), indicating that P. yoelii EEF completed their development in the skin of mice.
Conclusions
This study shows that the P. berghei and P. yoelii rodent-infecting species can undergo complete exoerythrocytic schizogony not just inside hepatocytes but also inside skin cells. However, although the P. berghei infective merozoites released and moving in the skin in merosome-like extensions might occasionally invade erythrocytes in the mouse, and thus constitute a potential secondary reservoir of infective merozoites, skin-derived merozoites do not significantly contribute to erythrocyte infection in normal conditions. Whether other mammal-infecting Plasmodium species, particularly the human-infecting species, can also develop in the skin of the host remains to be addressed.
The data also suggest that parasites might persist in association with hair follicles, which constitute an immunoprivileged site of the mammalian body characterized by the virtual absence of major histocompatibility complex (MHC) class I expression and a strongly immunosuppressive environment (30). This raises the hypothesis that parasites might become quiescent when associated with hair follicles and act as a source of infection relapses, as proposed for the hypnozoites of P. vivax in the liver (31).
Perhaps the most important implication of this work is immunological. Whereas previous data (11, 12) showed that in the mouse ear model about half of the P. berghei sporozoites inoculated by mosquitoes stayed at the bite site, the present data indicate that ∼10% of the injected sporozoites are developing at D1 in the skin. This implies that the lymph node that drains the injection site will receive parasite antigens not just from sporozoites (sporozoites actively reaching the lymph node or dead sporozoites left in the skin) but also from differentiating parasites (skin EEF aborting at various stages of their development). Overall, in the P. berghei/ear skin model system, the draining lymph node receives up to 70% of the parasite antigens inoculated by mosquito bite. The tolerogenic or protective properties of the various kinds of skin-derived parasite antigens will have to be assessed, in natural as well as immunizing conditions. This would be particularly important in the case of live attenuated (irradiated or genetically modified) sporozoites, which have been known for many years to act as powerful vaccines in animal models (32) and will soon undergo human trials (33–35), because in humans they can only be inoculated in the skin.
Materials and Methods
Parasites, Mice, and Mosquitoes.
We used the P. berghei ANKA clone expressing GFP under the control of the hsp70 promoter (14). The P. yoelii GFP-expressing parasite clone was obtained from the MR4 repository (ATCC no. MRA-817; 17XNL PYGFP). The red fluorescent P. berghei line (line 733) contains the RedStar-expressing cassette integrated at the 230p genomic locus. The red fluorescent P. yoelii clone contains the RedStar cassette integrated at the d-ssu-rrna locus. We used the P. berghei clone 676cl1 (PbGFP-LUCSCH) expressing a GFP-luciferase fusion gene via the EEF1α promoter (25). C57BL/6, Swiss and hairless SKH1 mice were purchased from Charles River Laboratories. All experiments were approved by the committee of Institut Pasteur and were performed in accordance with the applicable guidelines and regulations. A. stephensi (Sda500 strain) mosquitoes were reared using standard procedures (16). For intradermal injection of sporozoites into rodents, salivary gland sporozoites were dissected out and a small volume (0.2–10 μL) containing 5 × 103 to 3 × 105sporozoites was deposited in the dermis of the ear by using a 35- to 36-gauge needle with a NanoFil syringe (World Precision Instruments).
Intravital Imaging and Immunolabelings.
Intravital imaging was performed as described (16). For immunolabelings, ears were excised, fixed with 4% paraformaldehyde/PBS for 2 h, and dehydrated in 10% and 30% sucrose/PBS before embedding in OCT. Ten-micrometer sections were cut on a CM3050S cryostat (Leica) and adhered to Superfrost Plus Slides (VWR). Sections were permeabilized and blocked in PBS containing 0.1% Triton X-100 (Sigma) and 5% FCS, followed by staining with anti-keratin5 polyclonal primary antibody (Covance) or anti-UIS4 polyclonal primary antibody, AlexaFluor 546 conjugate (Molecular Probes), and DAPI (Molecular Probes). Stained slides were mounted with Prolong Gold (Invitrogen), and 3D image stacks were acquired on a SP5 confocal microscope (Leica). Images are displayed as 2D maximum-intensity projections.
Supplementary Material
Acknowledgments
We thank Stéphane Vincent (Institut Pasteur) and Mitinori Saitou (Riken Center for Developmental Biology) for the kind gift of Blimp1-GFP mice. We thank Spencer Shorte, Marie Nguyen-de Bernon, and Marie-Anne Nicola and the Imagopole team (Institut Pasteur) for help with microscopy, cytometry, and bioluminescence; Catherine Bourgouin, Isabelle Thiéry and the other members of the CEPIA platform (Institut Pasteur) for rearing mosquitoes; Masao Yuda (Mie University) for the gift of anti-HSP70 antibodies; Stephan Kappe (Seattle Biomedical Research Institute) for the gift of anti-UIS4 antibodies; and Jean-Jacques Panthier and Geneviève Aubin-Houzelstein (Institut Pasteur) for helpful discussions. We acknowledge funding from Fundação para a Ciência e Tecnologia Grant SFRH/BPD/48340/2008 (to J.T.) and Institut Pasteur, Natixis, the BioMalPar European Network of Excellence, the Agence Nationale pour la Recherche, and the Howard Hughes Medical Institute (to R.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009346107/-/DCSupplemental.
References
- 1.Huff CG. Life cycle of malarial parasites. Annu Rev Microbiol. 1947;1:43–60. [Google Scholar]
- 2.Shortt HE, Garnham PC. Pre-erythrocytic stage in mammalian malaria parasites. Nature. 1948;161:126. doi: 10.1038/161126a0. [DOI] [PubMed] [Google Scholar]
- 3.Vanderberg JP. Plasmodium berghei exoerythrocytic forms develop only in the liver. Trans R Soc Trop Med Hyg. 1981;75:904–905. doi: 10.1016/0035-9203(81)90445-4. [DOI] [PubMed] [Google Scholar]
- 4.Meis JF, Verhave JP. Exoerythrocytic development of malarial parasites. Adv Parasitol. 1988;27:1–61. doi: 10.1016/s0065-308x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 5.Shortt HE, Garnham PC. Demonstration of a persisting exo-erythrocytic cycle in Plasmodium cynomolgi and its bearing on the production of relapses. BMJ. 1948;1:1225–1228. doi: 10.1136/bmj.1.4564.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krotoski WA, et al. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am J Trop Med Hyg. 1982;31:1291–1293. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- 7.Shortt HE, Fairley NH, Covell G, Shute PG, Garnham PC. The pre-erythrocytic stage of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1951;44:405–419. doi: 10.1016/s0035-9203(51)80019-1. [DOI] [PubMed] [Google Scholar]
- 8.Yoeli M, Vanderberg JP, Upmanis RS, Most H. Primary tissue phase of Plasmodium berghei in different experimental hosts. Nature. 1965;208:903. doi: 10.1038/208903a0. [DOI] [PubMed] [Google Scholar]
- 9.Sidjanski S, Vanderberg JP. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am J Trop Med Hyg. 1997;57:426–429. doi: 10.4269/ajtmh.1997.57.426. [DOI] [PubMed] [Google Scholar]
- 10.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Amino R, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- 12.Kebaier C, Voza T, Vanderberg JP. Kinetics of mosquito-injected Plasmodium sporozoites in mice: Fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 2009;5:e1000399. doi: 10.1371/journal.ppat.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9:1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol. 2006;59:1175–1184. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- 15.Panteleyev AA, et al. Towards defining the pathogenesis of the hairless phenotype. J Invest Dermatol. 1998;110:902–907. doi: 10.1046/j.1523-1747.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 16.Amino R, et al. Imaging malaria sporozoites in the dermis of the mammalian host. Nat Protoc. 2007;2:1705–1712. doi: 10.1038/nprot.2007.120. [DOI] [PubMed] [Google Scholar]
- 17.Baldacci P, Ménard R. The elusive malaria sporozoite in the mammalian host. Mol Microbiol. 2004;54:298–306. doi: 10.1111/j.1365-2958.2004.04275.x. [DOI] [PubMed] [Google Scholar]
- 18.Mueller AK, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005;58:1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk MR, et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci USA. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturm A, et al. Alteration of the parasite plasma membrane and the parasitophorous vacuole membrane during exo-erythrocytic development of malaria parasites. Protist. 2009;160:51–63. doi: 10.1016/j.protis.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Dai X, Segre JA. Transcriptional control of epidermal specification and differentiation. Curr Opin Genet Dev. 2004;14:485–491. doi: 10.1016/j.gde.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohinata Y, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- 24.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke-Fayard B, et al. Simple and sensitive antimalarial drug screening in vitro and in vivo using transgenic luciferase expressing Plasmodium berghei parasites. Int J Parasitol. 2008;38:1651–1662. doi: 10.1016/j.ijpara.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Franke-Fayard B, Waters AP, Janse CJ. Real-time in vivo imaging of transgenic bioluminescent blood stages of rodent malaria parasites in mice. Nat Protoc. 2006;1:476–485. doi: 10.1038/nprot.2006.69. [DOI] [PubMed] [Google Scholar]
- 27.Sturm A, et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 28.Silvie O, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 29.Ono T, Tadakuma T, Rodriguez A. Plasmodium yoelii yoelii 17XNL constitutively expressing GFP throughout the life cycle. Exp Parasitol. 2007;115:310–313. doi: 10.1016/j.exppara.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellor AL, Munn DH. Immune privilege: A recurrent theme in immunoregulation? Immunol Rev. 2006;213:5–11. [Google Scholar]
- 31.Cogswell FB. The hypnozoite and relapse in primate malaria. Clin Microbiol Rev. 1992;5:26–35. doi: 10.1128/cmr.5.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulligan HW, Russell PF, Mohan BN. Active immunization of fowls against Plasmodium gallinaceum by injections of killed homologous sporozoites. J Malaria Inst India. 1941;4:25–34. [Google Scholar]
- 33.Luke TC, Hoffman SL. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol. 2003;206:3803–3808. doi: 10.1242/jeb.00644. [DOI] [PubMed] [Google Scholar]
- 34.Matuschewski K. Vaccine development against malaria. Curr Opin Immunol. 2006;18:449–457. doi: 10.1016/j.coi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Mikolajczak SA, Aly AS, Kappe SH. Preerythrocytic malaria vaccine development. Curr Opin Infect Dis. 2007;20:461–466. doi: 10.1097/QCO.0b013e3282ef6172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.