Abstract
Background
Studies have reported young ages at cancer diagnosis in HIV-infected people, suggesting that HIV accelerates carcinogenesis. However, these comparisons did not account for differences in population age structures.
Objective
To compare ages at diagnosis for non-AIDS-defining cancers arising in the AIDS and general populations, after adjusting for differences between these populations in age and other demographic characteristics.
Design
Registry linkage study
Setting
15 U.S. HIV/AIDS and cancer registry databases.
Participants
People with AIDS in the HIV/AIDS Cancer Match Study (N=212,055, 1996–2007).
Measurements
We compared the age-at-diagnosis distributions of cancers in the AIDS and general populations, after adjusting for age and other demographics.
Results
The proportion of person-time contributed by older persons (65+ years) was far smaller in the AIDS (1.5%) than the general population (12.5%). Reflecting this difference, the ages at diagnosis for most cancers were ~20 years younger among people with AIDS. However, after adjustment for differences in the populations at risk, the ages at diagnosis in the AIDS and general populations did not differ for most cancers (e.g., no difference in the median ages of colon, prostate or breast cancers; all p>0.1). In contrast, ages at lung (median 50 vs. 54 years) and anal cancer (42 vs. 45 years) were significantly younger in people with AIDS than expected in the general population (p<0.001), and the age at Hodgkin lymphoma was significantly older (42 vs. 40 years, p<0.001).
Limitations
We lacked information on other cancer risk factors, including cigarette smoking. Further, our analysis was restricted to non-Hispanic whites and non-Hispanic blacks who had AIDS, perhaps limiting the generalizability of our findings to other racial and ethnic groups and to people with HIV, but without AIDS.
Conclusions
For most cancers, the age of diagnosis is similar in the AIDS and general populations, after accounting for the ages of the populations at risk. Modest age differences remained for a few cancers, perhaps reflecting acceleration of carcinogenesis by HIV or earlier exposure to cancer risk factors.
Introduction
Human immunodeficiency virus (HIV) infection increases the risk of certain cancers. The risk of Kaposi sarcoma, non-Hodgkin lymphoma, and cervical cancer is so high among HIV-infected individuals that these cancers are included in the Centers for Disease Control and Prevention’s definition of acquired immune deficiency syndrome (AIDS) (advanced HIV infection) (1). Additionally, HIV-infected people are at an elevated risk of certain non-AIDS-defining cancers (2–6), largely attributable to HIV-related immune suppression, resulting in loss of control of oncogenic infections (7;8), and a high prevalence of exposure to other carcinogens (e.g., tobacco, alcohol) (2;9). With use of highly active antiretroviral therapy (HAART), survival among HIV-infected people has dramatically improved and AIDS incidence has decreased (10–12). However, the burden of cancer, particularly non-AIDS-defining cancers, will likely increase as HIV-infected people live longer.
Elevated cancer incidence, coupled with increased risk of other conditions with typical onset at older ages (e.g., cardiovascular and bone disease, cognitive impairment, and general frailty) have suggested that HIV-infected people are vulnerable to a syndrome of premature aging (13;14). For cancer, premature aging would manifest not only as overall elevated cancer risk, but also as a downward shift in the age distribution at cancer diagnosis. In support of this possibility, studies have noted that the ages at diagnosis of certain non-AIDS-defining cancers are 10–20 years younger among people with HIV compared to the general population, e.g., in studies of lung (15–17), liver (18;19), anal (20) and colorectal cancers (21).
Importantly, before concluding that HIV-infected people generally develop cancer at young ages, it is important to consider the differences in age distributions of the underlying HIV and general populations. Notably, due to the young age at HIV acquisition in the U.S. and other western countries, and the shorter life expectancy of people with HIV, the proportion of persons with HIV who are older-aged is far smaller than in the general population. For example, in the U.S., only 3% of HIV-infected individuals compared to 13% of the general population were 65+ years old in 2007 (22;23). As overall cancer incidence is ten times higher in persons age 65+ years than in persons <65 years (24), the truncated age distribution among persons with HIV prohibits observation of most cancers that would occur at older ages, which might plausibly explain the dramatic age differences reported by prior studies.
Using data from the U.S. HIV/AIDS Cancer Match Study, we evaluated the ages at diagnosis for 26 non-AIDS-defining cancers in the AIDS and general populations, after accounting for differences in population age structure. These analyses help clarify the potential effects of HIV infection on cancer development.
Methods
Study design
The HIV/AIDS Cancer Match Study links 15 U.S. population-based HIV/AIDS and cancer registries in Colorado; Connecticut; Florida; Illinois; Georgia; Massachusetts; Michigan; New Jersey; Texas; Los Angeles, San Diego, and San Francisco, California; New York City, New York; Seattle, Washington; and Washington, D.C. (25). Registry areas were selected to include a large HIV-infected population. This analysis focused on people who contributed follow-up information during the HAART era (1996–2007) for the period 4–60 months after AIDS diagnosis. We restricted the study to non-Hispanic whites and blacks as data on Hispanic ethnicity were not consistently available for all years across cancer registries.
Reporting of invasive malignancies to each cancer registry is mandated by law. Malignancies were categorized according to a modified version of the Surveillance, Epidemiology, and End Results (SEER) program’s “Site recode with KS and mesothelioma” (26). Stage was coded by cancer registries using SEER summary stage algorithms. We restricted analysis to non-AIDS-defining cancers that occurred in 10 or more individuals with AIDS.
The study was approved by institutional review boards at each participating registry and funded by the Intramural Research Program of the National Cancer Institute.
Statistical analysis
For each cancer, we depict the age-at-diagnosis distributions for people with AIDS and those in the general population, based on cancer registry data. However, these age distributions are strongly dependent on the age structure of the underlying populations that give rise to these cancers, and differences in these age structures can lead to bias. Therefore, we additionally considered the ages at cancer diagnosis for cases that would be expected to arise in the general population if the general population had the same demographic structure as the AIDS population. Specifically, we estimated expected counts for cancers in the general population by applying the general population’s observed cancer incidence rates to the accumulated person-time among people with AIDS, stratified by single years of attained age, sex, race, calendar year and registry (i.e., indirect standardization). Thus, expected cases are general population cases after adjusting for population characteristics, and address the question: what would be the age-at-diagnosis distribution of cancers in the general population, if the general population was demographically similar to the AIDS population?
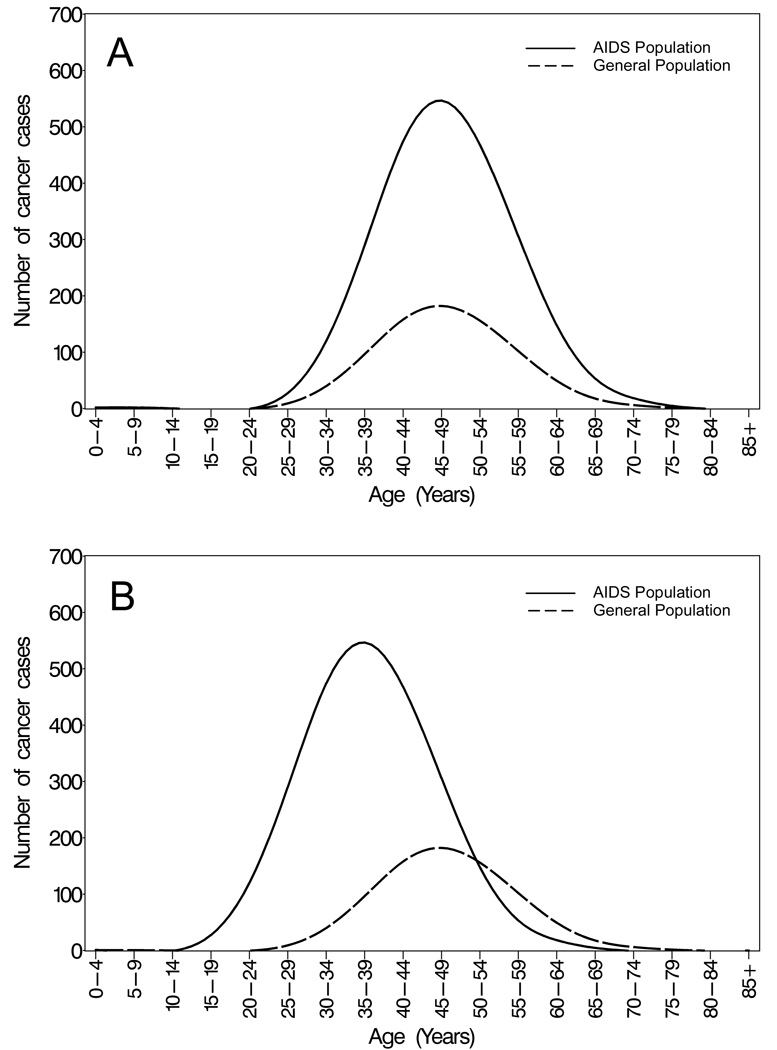
If the risk of a specific cancer is higher among people with AIDS than in the general population, we envision two possibilities (Figure 1). First, if HIV merely increases the risk of that cancer, we would expect that the age-at-diagnosis curve for the AIDS population would have the same shape as the curve for the expected cases in the general population (including the same median age), but that the curve for the AIDS cases would enclose a larger area (Figure 1, panel A). In contrast, if HIV accelerates the development of that cancer, then the age-at-diagnosis curve for the AIDS population would be shifted to the left and the median age would be lower than for the expected cases in the general population (Figure 1, panel B).
Figure 1. Hypothetical examples of age distributions of cancers in the AIDS and general populations.
The panels show hypothetical age-at-diagnosis distributions of cancers in the AIDS and general population if A) the risk of cancer is increased in the AIDS population, but the age distribution is the same; and B) the risk of cancer is increased in the AIDS population, and the age distribution is younger. The solid line represents the cases observed in the AIDS population. The dashed line represents cases expected in the general population, which are derived using indirect standardization, i.e., by applying general population rates to the accumulated person-time among people with AIDS, stratified by single years of attained age, sex, race, calendar year and registry. These expected cancers represent cases that would be seen in the general population if the general population had the same underlying demographic structure as the AIDS population. In addition to illustrating differences in age at onset, the curves provide information on relative risk for cancer in people with AIDS, because the ratio of the areas that they enclose is the standardized incidence ratio (observed cancers / expected cancers).
To formally test whether the ages at cancer diagnosis observed in people with AIDS differed from those expected in the general population, we assumed that rates applied to estimate expected cancer cases in the general population were known without error. We therefore multiplied expected case counts by 10,000, and then created a dataset with one record per observed case in persons with AIDS and expected case in the general population. We tested for differences in median age using the Brown-Mood test (27) for each cancer. A Bonferroni correction was applied to account for multiple comparisons; a p-value<0.0019 (i.e., 0.05/26 cancers) was considered statistically significant.
To further evaluate the age-at-diagnosis distributions of non-AIDS-defining cancers in the AIDS and general populations, we estimated standardized incidence ratios [SIRs], which measure risk in people with AIDS relative to the general population. SIRs were calculated as the number of observed cases in the AIDS population divided by the number of expected cases, if the general population was demographically similar to the AIDS population. For select cancers, we estimated SIRs overall and in age groups: 0–29, 30–39, 40–49, 50–59, 60–69, and 70+ years. Exact two-sided confidence intervals (CIs) of the SIRs were calculated, and trends in SIRs were evaluated with Poisson regression. If age acceleration is present, relatively more cancers would occur at young ages, which would manifest as higher SIRs at younger than older ages (Figure 1). Finally, for select carcinomas, we calculated SIRs by cancer stage.
Results
Description of study subjects
This analysis included 212,055 individuals with AIDS who were followed for cancer during the HAART era in the U.S. The study sample was predominately male (76.1%) and included a larger proportion of blacks (57.7%) than whites (42.3%). The median age at AIDS diagnosis was 38 years. During 1996–2007, 2,540 non-AIDS-defining cancers occurred during 591,378 person-years of follow-up after AIDS onset. The most common cancers were lung cancer (n=605, 24%), anal cancer (n=282; 11%), and Hodgkin lymphoma (n=226; 9%).
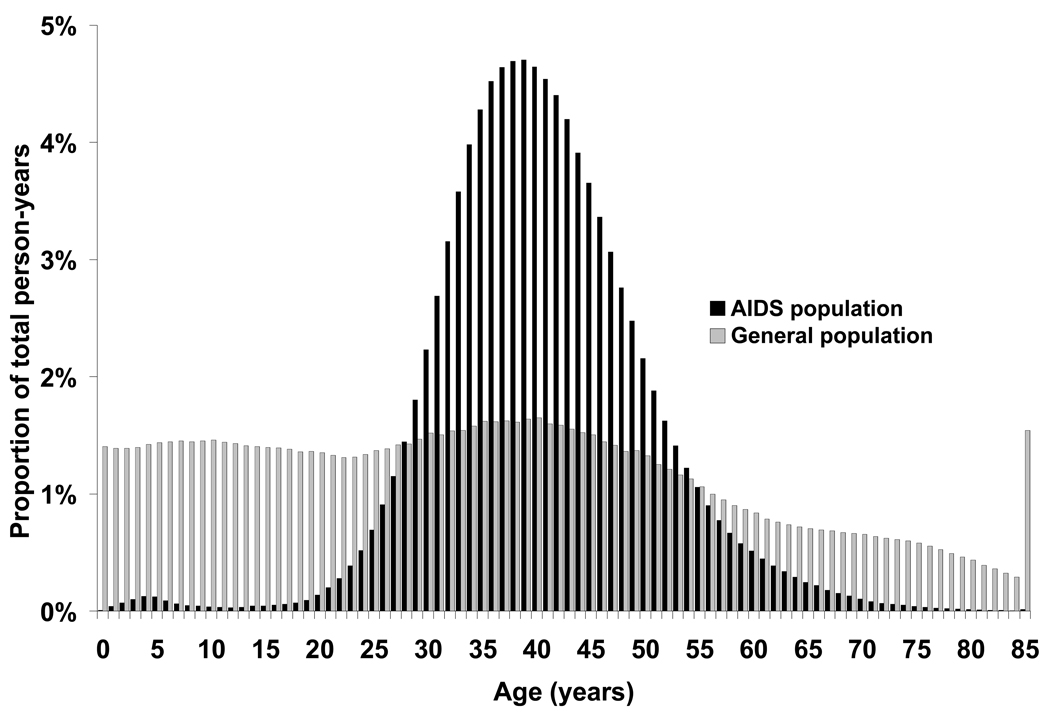
The age distributions of the AIDS and general populations for the registry areas included in this study are presented in Figure 2. Although the median age of people at risk of cancer was slightly higher in the AIDS population (40 years in the AIDS population vs. 35 years in the general population), the age distribution was much wider in the general population. In particular, the proportion of total person-years contributed by older persons (65+ years), who are at the greatest risk of developing cancer, was much smaller among people with AIDS than in the general population (1.5% vs. 12.5%, respectively).
Figure 2. Age distribution of the AIDS and general populations (HIV/AIDS Cancer Match Study, 1996–2007).
The figure shows follow-up time at risk of cancer by age in the AIDS and general populations, for cancer regions covered by the HIV/AIDS Cancer Match Study (1996–2007). Black bars indicate the distribution for people with AIDS, and light bars indicate the distribution for the general population.
Age at cancer diagnosis
Reflecting the difference in age structures of the AIDS and general populations, for most cancers the median observed ages at diagnosis were far younger (~20 years) among people with AIDS than in the general population (Table 1). However, after adjusting for the underlying population structures, we found either no difference or very small differences in the ages at cancer diagnosis observed in the AIDS population and expected in the general population. For example, the median observed age at diagnosis for colon cancer in people with AIDS was 52 years compared to 72 years in the general population. However, the median age observed in the AIDS population and expected in the general population were identical (52 years, p=0.53).
Table 1.
Age at cancer diagnosis among people with AIDS and in the general population, U.S. (1996–2007).
| Age in years at cancer diagnosis, by category of cancer | |||||||
|---|---|---|---|---|---|---|---|
| Observed in the general population |
Observed in the AIDS population |
Expected in the general population |
|||||
| Cancer type | Median | N | Median |
25th, 75th percentile |
Median |
25th, 75th percentile |
p-value* |
| Oral cavity and pharynx | 63 | 137 | 49 | (44, 57) | 50 | (44, 56) | 0.40 |
| Esophagus | 69 | 36 | 50.5 | (45, 58.5) | 54 | (48, 60) | 0.50 |
| Stomach | 72 | 36 | 49.5 | (44, 58) | 51 | (44, 59) | 0.50 |
| Colon | 73 | 61 | 52 | (44, 62) | 52 | (46, 61) | 0.53 |
| Rectum | 69 | 52 | 46 | (39, 52.5) | 51 | (45, 58) | 0.007 |
| Anus | 62 | 282 | 42 | (37, 48) | 45 | (40, 51) | <0.001† |
| Liver | 66 | 98 | 49 | (45, 55) | 50 | (45, 55) | 0.53 |
| Pancreas | 71 | 21 | 51 | (49, 57) | 53 | (46, 60) | 0.59 |
| Larynx | 65 | 72 | 48 | (44, 55.5) | 52 | (47, 59) | 0.003 |
| Lung | 70 | 605 | 50 | (44, 56) | 54 | (47, 61) | <0.001† |
| Soft tissue including heart | 58 | 26 | 40.5 | (34, 52) | 43 | (37, 50) | 0.42 |
| Melanoma | 60 | 74 | 46 | (39, 54) | 45 | (39, 53) | 0.45 |
| Breast | 62 | 110 | 44.5 | (38, 53) | 46 | (41, 52) | 0.177 |
| Ovary | 63 | 13 | 42 | (37, 44) | 46 | (40, 53) | 0.025 |
| Vulva | 70 | 12 | 41.5 | (38, 56) | 44 | (39, 50) | 0.39 |
| Prostate | 68 | 176 | 58.5 | (53.5, 63.5) | 58 | (52, 64) | 0.40 |
| Testis | 34 | 33 | 35 | (33, 39) | 38 | (34, 42) | 0.018 |
| Penis | 68 | 16 | 49.5 | (38.5, 54.5) | 48 | (43, 56) | 0.58 |
| Urinary bladder | 73 | 23 | 48 | (41, 58) | 54 | (47, 62) | 0.069 |
| Kidney and renal pelvis | 66 | 47 | 49 | (43, 54) | 50 | (44, 57) | 0.171 |
| Brain | 56 | 16 | 43 | (38, 52) | 45 | (38, 52) | 0.61 |
| Thyroid | 47 | 22 | 43.5 | (36, 52) | 43 | (37, 49) | 0.72 |
| Hodgkin lymphoma | 37 | 226 | 42 | (36, 47) | 40 | (34, 46) | <0.001† |
| Myeloma | 70 | 45 | 47 | (40, 52) | 52 | (46, 59) | 0.004 |
| Lymphocytic leukemia | 25 | 11 | 43 | (26, 52) | 43 | (37, 51) | 0.88 |
| Myeloid/monocytic leukemia | 68 | 46 | 48 | (39, 55) | 45 | (38, 53) | 0.178 |
P-value compares median ages among the observed cancer cases in people with AIDS and expected cancer cases in the general population. Expected cases are adjusted for age, sex, race, calendar year and registry.
Comparison was statistically significant after Bonferroni correction for multiple comparisons (p<0.0019).
Nonetheless, ages at cancer diagnosis among persons with AIDS were younger than expected in the general population for anal cancer (median 42 vs. 45 years, p<0.001, respectively) and lung cancer (50 vs. 54 years, p<0.001) (Table 1). In contrast, ages at diagnosis for Hodgkin lymphoma were significantly older in people with AIDS than expected in the general population (median 42 vs. 40 years, p<0.001).
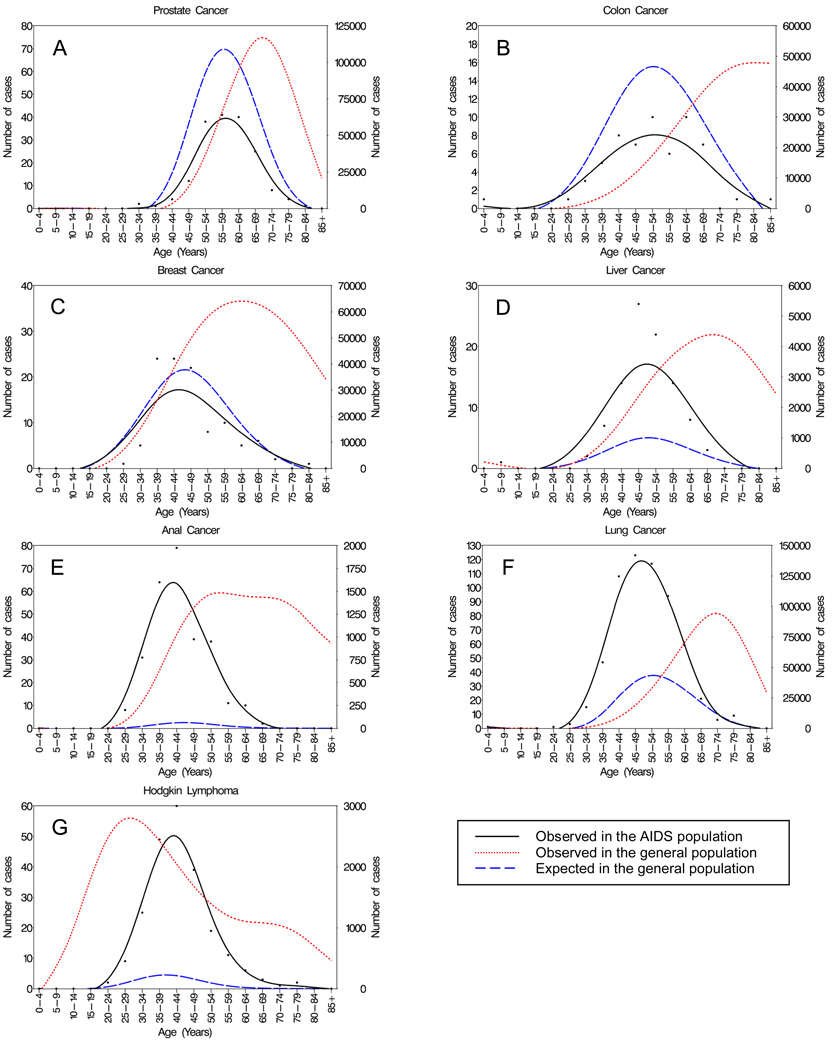
Figure 3 illustrates age-at-diagnosis distributions for selected cancers: anal cancer, lung cancer, Hodgkin lymphoma and liver cancer, which are common among people with AIDS, and prostate, breast and colon cancers, which are common in the general population. For prostate (A), colon (B) and breast (C) cancers, the age-at-diagnosis curves for observed cases in the general population are much older than the AIDS population, but after adjustment, the age-at-diagnosis distributions for expected cases in the general population are similar to the AIDS population distributions. Of note, the areas under the curve for these cancers are smaller for people with AIDS than expected in the general population, reflecting a reduced risk of prostate, breast, and colon cancers in the AIDS population. Though the reason for this reduced risk is unclear, it has been seen previously, and may reflect a protective effect of HIV infection or differences in cancer screening (5;6;28). For liver cancer (D), the age-at-diagnosis curve is similar for the observed cases in people with AIDS and expected cases from the general population, but the larger area under the curve for people with AIDS reflects their elevated overall risk for liver cancer.
Figure 3. Age at cancer diagnosis among people with AIDS and the general population, U.S. (1996–2007).
Panels depict the age-at-diagnosis distributions for select non-AIDS-defining cancers. Points represent cancers observed among persons with AIDS. Smoothed curves show cancers observed in persons with AIDS (left axis), expected cancers in the general population (left axis) and observed cancers in the general population (right axis).
For anal cancer (E), lung cancer (F), and Hodgkin lymphoma (G), people with AIDS have a higher risk than the general population, corresponding to greater areas under the age-at-diagnosis curves for the AIDS population than for expected cases in the general population. After adjustment, the expected age-at-diagnosis curves for anal and lung cancers in the general population are shifted downward from the observed curves in the general population, but they remain slightly older than the AIDS population. In contrast, for Hodgkin lymphoma, the age-at-diagnosis curves show that observed cases in the AIDS population occur at older ages than expected in the general population. Notably, in the general population, Hodgkin lymphoma has a bimodal age-at-diagnosis distribution; however, the age-at-diagnosis distributions for observed cases in the AIDS population and expected cases in the general population are unimodal.
Standardized incidence ratios for cancer
SIRs are shown in Table 2 for the same malignancies as in Figure 3. Overall SIRs for anal cancer, lung cancer, Hodgkin lymphoma and liver cancer were elevated. SIRs for anal cancer and lung cancer were highest in the youngest ages, and declined significantly across age groups (all p-trend<0.001). In contrast, SIRs for Hodgkin lymphoma increased significantly across age groups (p-trend<0.001) with highest SIRs at the oldest ages. No significant trends in SIRs were observed across age groups for liver, prostate, breast or colon cancers.
Table 2.
Standardized incidence ratios for selected cancers across age groups, comparing persons with AIDS to the general population.
| Standardized incidence ratio (95% confidence interval) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Overall | 0–29 years | 30–39 years | 40–49 years | 50–59 years | 60–69 years | 70+ years | p-trend* | |
| Cancer | ||||||||
| Anus | 25.1 | 97.4 | 39.0 | 22.1 | 19.7 | 17.4 | 0 | <0.001 |
| (22.3, 28.3) | (42.0, 191.9) | (31.6, 47.7) | (18.3, 26.5) | (14.6, 26.1) | (9.0, 30.3) | (0, 19.6) | ||
| Lung | 3.0 | 21.6 | 7.1 | 3.9 | 2.8 | 1.8 | 1.2 | <0.001 |
| (2.8, 3.2) | (7.0, 50.4) | (5.4, 9.0) | (3.4, 4.4) | (2.5, 3.2) | (1.4, 2.2) | (0.66, 1.9) | ||
| Hodgkin lymphoma | 10.8 | 5.9 | 8.7 | 13.1 | 12.6 | 14.9 | 24.2 | <0.001 |
| (9.4, 12.3) | (3.0, 10.6) | (6.9, 11.0) | (10.6, 15.9) | (8.5, 17.9) | (6.8, 28.2) | (5.0, 70.8) | ||
| Liver | 3.4 | 6.1 | 5.0 | 3.4 | 3.3 | 3.3 | 0 | 0.117 |
| (2.7, 4.1) | (0.16, 34.2) | (2.3, 9.5) | (2.5, 4.6) | (2.3, 4.6) | (1.6, 5.9) | (0, 4.2) | ||
| Prostate | 0.54 | 0 | 3.3 | 0.36 | 0.57 | 0.58 | 0.46 | 0.80 |
| (0.47, 0.63) | (0, 231) | (0.69, 9.8) | (0.21, 0.59) | (0.45, 0.70) | (0.45, 0.74) | (0.25, 0.79) | ||
| Breast | 0.83 | 0.70 | 1.1 | 0.75 | 0.62 | 1.1 | 0.94 | 0.48 |
| (0.68, 1.0) | (0.02, 3.9) | (0.76, 1.6) | (0.55, 1.0) | (0.37, 0.97) | (0.55, 2.0) | (0.19, 2.8) | ||
| Colon | 0.59 | 4.0 | 0.87 | 0.49 | 0.46 | 0.80 | 0.39 | 0.49 |
| (0.45, 0.75) | (0.49, 14.5) | (0.38, 1.7) | (0.27, 0.81) | (0.26, 0.75) | (0.47, 1.29) | (0.08, 1.13) | ||
P-trend is calculated across age categories.
Finally, Supplemental Table 1 presents SIRs by stage of anal cancer and lung cancer (carcinomas where observed age in persons with AIDS was younger than expected in the general population). The SIR for local stage anal cancer was higher than the SIR for distant stage, while SIRs for local and distant stage lung cancers were similar.
Discussion
When we did not account for the underlying population age structures, we found that many cancers occurred at much younger ages in people with AIDS than in the general population, as suggested in previous studies (15–21). However, these differences were almost completely driven by differences in the underlying age structures of the populations at risk of cancer. The prior studies that have reported on younger ages at cancer diagnosis in persons with HIV/AIDS have not taken into account that very few HIV-infected people who are at risk of cancer had attained older age, when most cancers develop. For example, in the present study only 1.5% of person-time among people with AIDS was contributed by those aged 65+ years. This underlying age difference creates a bias when the ages of the cancer cases arising in these populations are compared.
Cancer has recently been considered as a component of a potential syndrome of premature aging caused by HIV infection, motivated by clinical observations that the average age of onset of age-related cancers is younger in HIV-infected individuals than in the general population (15–21). However, as shown here, these dramatic age differences were influenced by age differences in the populations at risk. Although we are not aware of previous mention of this type of confounding in the literature on HIV/AIDS and cancer, a similar mechanism has been discussed in studies of inherited diseases, such as Crohn disease. Studies of parent-child pairs with Crohn disease have observed that children are younger than their parents when diagnosed (29;30). However, these generational age differences arose because children were younger than their parents when assessed for disease status, and were thus not followed across the same age range as their parents (31;32). After properly accounting for differences in the periods at risk for Crohn disease, these age differences were eliminated (32). This analysis highlights that statistical adjustment for differences in time at risk is essential when comparing ages at diagnosis (32). In our study, indirect standardization allowed a comparison of the ages at cancer diagnosis in the AIDS and general populations after controlling for differences in the distributions of age and other demographics.
Small, but statistically significant, differences in the ages at diagnosis for anal cancer, lung cancer, and Hodgkin lymphoma were observed after adjusting for differences in population structure. These malignancies are among the select group where cancer risk is elevated among HIV-infected people (2–5;8). We propose two potential explanations for the earlier age at diagnosis among people with AIDS for anal and lung cancers. First, the earlier age at diagnosis may represent an effect of HIV on the development of these cancers. For example, HIV increases risk for cancer by inducing loss of immune control of oncogenic infections (e.g., human papillomavirus [HPV] for anal cancer) (7;8). By increasing the transition rate through intermediate stages of infection on the pathway to cancer, this biological mechanism dramatically increases the number of people with cancer, and may also lead to slightly earlier ages at cancer diagnosis. Second, an early onset of cancer in people with AIDS could reflect differences in the timing or intensity of exposure to other key risk factors for these cancers, e.g., earlier age at initiation of tobacco smoking or sexual debut (leading to HPV infection) or a greater number of cigarettes smoked per day. These explanations are not mutually exclusive, and both could explain the younger age at diagnosis for cancers known to be linked to HIV infection. Nonetheless, if AIDS directly accelerates anal and lung cancer development, one would likely observe more rapidly growing, distant stage cancers. However, we did not observe higher SIRs for distant stage anal and lung cancers, compared to local and regional stage cancers.
One additional explanation for these age differences is increased medical surveillance of persons with AIDS, resulting in lead time bias. This explanation is partly supported by our data for anal cancer. The SIR for anal cancer was highest for local stage disease, which would be compatible with a stage shift due to screening with anal Pap tests (targeted toward HIV-infected men who have sex with men). However, because many HIV-infected people do not receive regular medical care or cancer screening (33;34), the overall magnitude and direction of this effect on age at cancer diagnosis are uncertain.
Among persons with AIDS, Hodgkin lymphoma was diagnosed at an older age than in the general population, but interpretation of this observation is hindered by the complexities of Hodgkin lymphoma epidemiology. In the general population, Hodgkin lymphoma exhibits a bimodal pattern in its age at onset. Nodular sclerosis often affects teenagers and young adults and is less strongly associated with Epstein Barr virus (EBV) than other subtypes, while mixed cellularity (often EBV positive) is the most common subtype among older adults (35). Among persons with HIV, Hodgkin lymphomas mainly resemble this second peak, i.e., the mixed cellularity subtype predominates (36;37), and EBV is detectable in 80–100% of cases (38). Of note, the age distribution of Hodgkin lymphoma cases in people with AIDS did not show the bimodal pattern seen in the general population (Figure 3), reflecting the relative lack of young and old people with AIDS (Figure 2). Thus, the single peak in people with AIDS actually represents a mixture of both EBV-negative and EBV-positive Hodgkin lymphomas. We speculate that the apparent shift to older ages in the observed cases among people with AIDS represents a strong increase in the risk of the EBV-positive cases that occur at older ages, rather than a shift of EBV-negative cases to older ages. Indeed, HIV may accelerate the development of the EBV-positive Hodgkin lymphomas that occur at older ages, e.g., by leading to loss of immune control of EBV infection.
Our study has several strengths. Most importantly, our comparisons of age at cancer diagnosis were corrected for bias due to the differing underlying age structures of the AIDS and general populations. Additionally, the HIV/AIDS Cancer Match Study includes data on a large and representative sample of persons with AIDS in the U.S. (e.g., these analyses included approximately 20% of the 1.05 million cumulative AIDS cases in the U.S.). The main limitation of our study was the lack of risk factor information, including information on cigarette smoking, which prohibited us from directly assessing how exposure to known cancer risk factors influenced the age at cancer diagnosis. Additionally, our study was restricted to non-Hispanic whites and non-Hispanic blacks with AIDS, perhaps limiting the generalizability of our findings. However, any biologic effect of HIV in accelerating the development of cancer should be similar across racial and ethnic groups, and the lack of acceleration in cancer development among people with AIDS, who are most immune compromised, argues against an important effect in people with earlier HIV disease. Finally, we assumed that cancer rates were known without error, because the cancer registries included in our study cover a very large population with over 875 million person-years of follow-up. If this assumption is incorrect, then the variance of our estimates would be underestimated, increasing the probability of observing a statistically significant age difference when one does not exist. However, as we observed no age differences for most cancers, we do not believe that making this assumption biased our results.
To conclude, our results do not support inclusion of cancer as part of a general syndrome of premature aging in HIV-infected people. Apparent earlier ages at cancer diagnosis arise largely because there have been very few people with AIDS followed during older age when most cancers arise. As the AIDS population continues to age, we would expect more non-AIDS-defining cancers to occur at older ages, attenuating or eliminating the apparent age differences at cancer diagnosis. Our results do not support an accelerated screening schedule in HIV-infected individuals for most cancers (e.g., prostate, colon and breast cancers). However, HIV-infected individuals should still receive regular cancer screening based on recommendations made for the general population and established guidelines made specifically for HIV-infected people for those cancers where risk is particularly high (e.g. for cervical and anal cancers) (39).
Supplementary Material
Acknowledgements
The authors thank the HIV/AIDS and cancer registry staff in Colorado; Connecticut; Florida; Illinois; Georgia; Massachusetts; Michigan; New Jersey; Texas; New York City, New York; San Diego, Los Angeles and San Francisco, California; Seattle, Washington; and Washington, D.C., and Tim McNeel (Information Management Systems) for database management.
Grant Support: Intramural research program of the National Cancer Institute
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record
Reproducible Research Statement:
Protocol: not available
Statistical code: Available to interested readers by contacting Dr. Shiels at shielsms@mail.nih.gov
Data: not available
Reference List
- 1.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 3.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 4.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS. 2008;22(4):489–496. doi: 10.1097/QAD.0b013e3282f47082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of Types of Cancer among HIV-Infected Persons Compared with the General Population in the United States, 1992 . Annals of internal medicine. 2008;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 6.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52(5):611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23(17):2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97(6):425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 10.Detels R, Munoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280(17):1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 11.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg MJ, Wegner SA, Milazzo MJ, McKaig RG, Williams CF, Agan BK, et al. Effectiveness of highly-active antiretroviral therapy by race/ethnicity. AIDS. 2006;20(11):1531–1538. doi: 10.1097/01.aids.0000237369.41617.0f. [DOI] [PubMed] [Google Scholar]
- 13.Bhavan KP, Kampalath VN, Overton ET. The aging of the HIV epidemic. Curr HIV /AIDS Rep. 2008;5(3):150–158. doi: 10.1007/s11904-008-0023-3. [DOI] [PubMed] [Google Scholar]
- 14.Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alshafie MT, Donaldson B, Oluwole SF. Human immunodeficiency virus and lung cancer. Br J Surg. 1997;84(8):1068–1071. [PubMed] [Google Scholar]
- 16.Brock MV, Hooker CM, Engels EA, Moore RD, Gillison ML, Alberg AJ, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: implications for patient care. J Acquir Immune Defic Syndr. 2006;43(1):47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 17.Demopoulos BP, Vamvakas E, Ehrlich JE, Demopoulos R. Non-acquired immunodeficiency syndrome-defining malignancies in patients infected with human immunodeficiency virus. Arch Pathol Lab Med. 2003;127(5):589–592. doi: 10.5858/2003-127-0589-NISMIP. [DOI] [PubMed] [Google Scholar]
- 18.Brau N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47(4):527–537. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Puoti M, Bruno R, Soriano V, Donato F, Gaeta GB, Quinzan GP, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS. 2004;18(17):2285–2293. doi: 10.1097/00002030-200411190-00009. [DOI] [PubMed] [Google Scholar]
- 20.Crum-Cianflone NF, Hullsiek KH, Marconi VC, Ganesan A, Weintrob A, Barthel RV, et al. Anal cancers among HIV-infected persons: HAART is not slowing rising incidence. AIDS. 2010;24(4):535–543. doi: 10.1097/QAD.0b013e328331f6e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman C, Aboulafia DM, Dezube BJ, Pantanowitz L. Human immunodeficiency virus-associated adenocarcinoma of the colon: clinicopathologic findings and outcome. Clin Colorectal Cancer. 2009;8(4):215–219. doi: 10.3816/CCC.2009.n.036. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009 Dec 17;
- 23.U.S.Census Bureau. Annual Estimates of the Resident Population by Sex and Five-Year Age Groups for the United States: April 1, 2000 to July 1, 2008. 2009
- 24.Horner M, Ries L, Krapcho M, Neyman N, Aminou R, Howlader N, et al., editors. Bethesda, MD: National Cancer Institute; 2009. SEER Cancer Statistics Review, 1975–2006. [Google Scholar]
- 25.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285(13):1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. International Classification of Diseases for Oncology. Geneva: World Health Organization; 2000
- 27.Brown GW, Mood AM. On median tests for linear hypotheses. Proc Second Berkeley Symp on Math Statist and Prob. 1951:159–166. [Google Scholar]
- 28.Goedert JJ, Schairer C, McNeel TS, Hessol NA, Rabkin CS, Engels EA. Risk of breast, ovary, and uterine corpus cancers among 85,268 women with AIDS. Br J Cancer. 2006;95(5) doi: 10.1038/sj.bjc.6603282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandbastien B, Peeters M, Franchimont D, Gower-Rousseau C, Speckel D, Rutgeerts P, et al. Anticipation in familial Crohn's disease. Gut. 1998;42(2):170–174. doi: 10.1136/gut.42.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polito JM, Rees RC, Childs B, Mendeloff AI, Harris ML, Bayless TM. Preliminary evidence for genetic anticipation in Crohn's disease. Lancet. 1996;347(9004):798–800. doi: 10.1016/s0140-6736(96)90870-3. [DOI] [PubMed] [Google Scholar]
- 31.Frisch M, Olsen J, Andersen PK. Follow-up time bias and Crohn's disease. Lancet. 1996;347(9014):1551–1552. [PubMed] [Google Scholar]
- 32.Picco MF, Goodman S, Reed J, Bayless TM. Methodologic pitfalls in the determination of genetic anticipation: the case of Crohn disease. Ann Intern Med. 2001;134(12):1124–1129. doi: 10.7326/0003-4819-134-12-200106190-00013. [DOI] [PubMed] [Google Scholar]
- 33.D'Souza G, Cook RL, Ostrow D, Johnson-Hill LM, Wiley D, Silvestre T. Anal cancer screening behaviors and intentions in men who have sex with men. J Gen Intern Med. 2008;23(9):1452–1457. doi: 10.1007/s11606-008-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobias C, Cunningham WE, Cunningham CO, Pounds MB. Making the connection: the importance of engagement and retention in HIV medical care. AIDS Patient Care STDS. 2007;21 Suppl 1:S3–S8. doi: 10.1089/apc.2007.9992. [DOI] [PubMed] [Google Scholar]
- 35.Mani H, Jaffe ES. Hodgkin lymphoma: an update on its biology with new insights into classification. Clin Lymphoma Myeloma. 2009;9(3):206–216. doi: 10.3816/CLM.2009.n.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108(12):3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clifford GM, Rickenbach M, Lise M, Dal ML, Battegay M, Bohlius J, et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood. 2009;113(23):5737–5742. doi: 10.1182/blood-2009-02-204172. [DOI] [PubMed] [Google Scholar]
- 38.Carbone A, Gloghini A, Serraino D, Spina M. HIV-associated Hodgkin lymphoma. Curr Opin HIV AIDS. 2009;4(1):3–10. doi: 10.1097/COH.0b013e32831a722b. [DOI] [PubMed] [Google Scholar]
- 39.Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58(RR-11):1–166. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.