Abstract
Autoimmunity to heat shock protein 60 (HSP60) has been related to atherosclerosis. Chlamydia pneumoniae (CP), the most studied infectious agent implicated in promoting atherosclerosis, produces a form of HSP60, which can induce an autoimmune response, due to high antigenic homology with human HSP60 (hHSP60). In this study, we evaluated the correlations among anti-hHSP60 antibodies, CP infection, and cardiovascular disease (CVD) in a high-risk population, such as patients undergoing hemodialysis (HD). Thirty-two patients (67.9 ± 13.9 years; male/female, 23:9) on regular HD were enrolled. Global absolute cardiovascular risk (GCR) was assessed using the Italian CUORE Project’s risk charts, which evaluate age, gender, smoking habits, diabetes, systolic blood pressure, and serum cholesterol. The occurrence of cardiovascular events during a 24-month follow-up was recorded. Seropositivity to CP and the presence of anti-hHSP60 antibodies were tested by specific enzyme-linked immunosorbent assays. Inflammation was assessed by measurement of C-reactive protein (CRP) serum levels. Fifteen healthy sex and age-matched (61.9 ± 9.5 years; male/female, 11:4) subjects were the control group. Fifteen of 32 patients resulted seropositive for CP. CP + patients were older than CP−, while they did not differ for GCR, CRP, and dialytic parameters. CVD incidence was significantly higher in CP+ (9 CP+ vs 2 CP−, p < 0.05). Cox analysis recognized that the incidence of CVD was independently correlated with seropositivity to CP (HR, 7.59; p = 0.01; 95% CI = 1.63–35.4). On the other hand, there were no significant differences in anti-hHSP60 levels among CP+, CP− and healthy subjects: 18.11 μg/mL (14.8–47.8), 31.4 μg/mL (23.2–75.3), and 24.72 μg/mL (17.7–41.1), respectively. Anti-hHSP60 did not correlate to GCR, CRP, and incidence of CVD. In conclusion, our data suggest that anti-hHSP60 autoimmune response is not related to CP infection and CP-related CVD risk in HD patients.
Keywords: HSP60, Anti-human HSP60, Chlamydia pneumoniae, Cardiovascular risk, Hemodialysis
Introduction
Heat shock protein 60 (HSP60) belongs to the HSPs family or chaperonins, a group of highly conserved proteins involved in protein folding and degrading of denaturated proteins. HSP60 is mainly located in mitochondria, but under stress conditions, changes in the intracellular location and cell surface expression have been reported (Soltys and Gupta 1997)
Autoimmunity against HSP60 has been related to inflammation and cardiovascular diseases (CVD). In vitro experiments showed that anti-HSP60 antibodies induce complement and antibody-dependent cellular cytotoxicity on heat stressed endothelial cells and peripheral blood mononuclear cells (PBMC; Schett et al. 1997). Moreover, it has been recently described that anti-HSP60 antibodies mediate endothelial damage and exert prothrombotic activity in a murine experimental model (Dieudé et al. 2009). In addition, clinical studies reported significantly higher serum levels of anti-hHSP60 immunoglobulin G (IgG) antibodies in acute coronary syndrome patients when compared to patients with stable ischemic heart disease or controls (Hoshida et al. 2005).
Interestingly, HSP60 autoimmunity has been also related to chronic infections, in particular to Chlamydia pneumoniae (CP) infection.
CP, the most studied infectious agent implicated in promoting atherosclerosis (Watson and Alp 2008), is a Gram-negative obligate intracellular bacterium, which accesses the organism via the respiratory tract, invades circulation, where it may persist asymptomatically, and then localizes in arteries and atherosclerotic tissues (Borel et al. 2008).
CP chronic infection, evaluated both by the presence of IgG and immunoglobulin A (IgA) antibodies and, more recently, by the measurement of CP DNA in PBMC, has been related to increased risk of CVD (Gattone et al. 2001; Mitusch et al. 2005). CP could have a direct and/or indirect effect on the infected vessel wall, by the induction of cytokines and adhesion molecules (Högdahl et al. 2008). It has been also suggested that CP components or products, such as lipopolysaccharide (LPS), LPS-like products and chlamydial HSP60 (cHSP60), stimulate inflammation, leading to atherosclerosis (Netea et al. 2000; Pesonen et al. 2007). CP may establish a persistent infection, which could be a source of cHSP60. The persistent expression of this antigen in CP-infected patients can induce not only an immune reaction against cHSP60 but also an autoimmune response against hHSP60, through the mechanism of molecular mimicry; in fact, chlamydial and human HSP60 share 85% homology (Xu 2003). It has been demonstrated that anti-hHSP60 IgA, but not anti-hHSP60 IgG or anti-cHSP60 antibodies, are related to a significant risk for coronary events, especially when associated to CP seropositivity and high c-reactive protein (CRP) serum levels (Huittinen et al. 2002). For these reasons, a pathogenetic role of anti-hHSP60 antibodies in course of CP infection has been hypothesized but, so far, poorly investigated. In this study, we evaluated whether autoimmunity to hHSP60 is associated to CP infection and CVD risk in a high-risk population, such as patients undergoing hemodialysis (HD), who present an elevated risk of cardiovascular morbidity and mortality.
Patients and methods
Patients
We enrolled 32 clinically stable uremic patients in regular dialytic treatment for at least 1 year prior to the study [male/female = 23:9, mean age of 67.9 (13.9) years, mean dialytic age of 62.8 (42.5) months]. Written informed consent was obtained. All patients were dialysed three times a week using low-flux synthetic AM-BIO-1000Wet membrane (Asahi Kasei Medical Europe GmbH, Frankfurt, Germany). Dialysis session time was of 3.5–4.0 h.
Patients with conditions influencing immune response, such as acute infections, active immunological diseases, immunosuppressive therapy, previous transplantation, or history of malignancies, were excluded from the study. Dialysis adequacy was assessed by the evaluation of delivered dose of dialysis, using a single-pool urea kinetic model (spKt/V urea). At baseline, cardiovascular risk was assessed by global absolute cardiovascular risk (GCR), using the validated risk charts of the Italian CUORE Project.
The CUORE study is a large prospective cohort followed up study, whose aim was to develop a 10-year coronary risk predictive equation, specific to the Italian population (Ferrario et al. 2005). It evaluates well-known cardiovascular risk factors, such as age, gender, smoking habits, diabetes, systolic blood pressure, and serum cholesterol. On the basis of these factors, a GCR score was generated (from 1 to 6), which corresponds to well-defined 10-year cardiovascular risk classes (from 1 to 6: <5%, 5–9%, 10–14%, 15–19%, 20–29%, and ≥30%, respectively).
After the initial assessment, patients were followed up for 24 months. During the follow-up, the occurrence of cardiovascular events (myocardial infarction, heart failure, stroke, mesenteric infarction, and peripheral vascular disease) was recorded. Fifteen healthy subjects matched by sex and age [male/female = 11:4, mean age of 61.9 (9.5) years], were considered the control group.
Laboratory measurements
Blood samples were collected before the dialytic treatment. Serum lipids and nitrogen measurements were performed using standard methods in routine clinical laboratory. CRP was measured by nephelometry. Anti-CP IgG and IgA antibodies were determined using a commercially available semi-quantitative kit (Labsystems OY, Helsinki, Finland), which is 100% specific and 97% sensitive. IgG and IgA seropositivity for CP was defined by the presence of detectable antibodies at dilution of ≥1:64 and ≥1:32, respectively. Anti-hHSP60 serum levels were measured by an enzyme-linked immunosorbent assay (EIA), which detected IgG, IgA, and IgM isotypes, with a sensitivity of 2.88 ng/mL and a range of 7.81–250 ng/mL (Stressgen Biotechnologies Corporation, Victoria, BC, Canada). Briefly, samples from healthy subjects and HD patients were added in duplicate to a pre-coated hHsp60 immunoassay plate and then incubated. Captured anti-human HSP60 antibodies were detected with a goat polyclonal antibody conjugated with peroxidase (HRP), specific for human IgG, IgA, and IgM isotypes. Color intensity was measured in a microplate reader at 450 nm and then plotted to a standard curve to determine sample concentrations.
Data analysis
To summarize quantitative variables, we used means and SD, or medians [interquartile range (IQR)] if they were not normally distributed (Shapiro Test), and count and percentage for qualitative variables. Differences between infected and non infected patients were assessed by the Student T test or the Mann-Whitney’s U test for quantitative variables, and by the chi-square (χ2) test or Fisher exact test for qualitative ones. Kaplan–Meier method was used to illustrate the incidence of CVD events. Univariate and multivariate Cox’s proportional hazard models were fitted to identify the most important predictors of CVD (GCR and CRP serum levels). The proportional hazard assumption was verified by means of Schoenfeld residuals. All tests were two sides, and p < 0.05 was considered statistically significant. Data analysis was performed with the STATA statistical package (ver. 10.0, 209, Stata Corporation, College Station, TX, USA).
Results
Patient characteristics and CP infection
Table 1 shows characteristics of the patients enrolled. Fifteen of 32 patients (47%) were seropositive for CP (CP+). In particular, 11 patients were exclusively positive to IgG, one patient to IgA, while three patients presented both IgG and IgA class of antibodies.
Table 1.
Patients characteristics
| CP+ | CP− | |
|---|---|---|
| N (%) | 15 (47) | 17 (53) |
| Age (years) | 74.2 [8.2]* | 61.7 [15.5] |
| Gender (male/female) | 12:3 | 11:6 |
| Time on HD treatment (months) | 65.6 [33.3] | 60.4[50.1] |
| Mean HD session duration (hours) | 3.83 [0.24] | 3.76 [0.25] |
| spKT/V urea | 1.3 [0.1] | 1.3 [0.1] |
| Systolic pre-dialysis BP (mmHg) | 129 [19.7] | 136.2 [21.7] |
| Diastolic pre-dialysis BP (mmHg) | 68.7 [13.1] | 73.2 [11.2] |
| Current smokers (N) | 4 | 3 |
| Diabetes (N) | 5 | 3 |
| Total cholesterol serum level (mg/dl) | 161.5 [43.1] | 158.5 [29.7] |
| GCR | 3.3 [1.2] | 2.4 [1.4] |
| CRP (mg/dl)a | 0.53 (0.22–1.21) | 0.34 (0.15–0.90) |
| Cardiovascular events, N (%) | 9 (60)* | 2 (11) |
| Myocardial infarction, N | 3 | 1 |
| Heart failure, N | 3 | 0 |
| Stroke, N | 1 | 0 |
| Mesenteric infarction, N | 1 | 0 |
| Peripheral vascular disease, N | 1 | 1 |
| Anti-hHSP 60 serum levels (μg/mL)a | 18.11 (14.8–47.8) | 31.4 (23.2–75.3) |
Data are expressed as mean [SD]
CP+ seropositive to Chlamydia pneumoniae (IgA and/or IgG), CP− seronegative to Chlamydia pneumoniae (IgA and/or IgG), HD hemodialysis, spKT/V urea delivered HD dose, evaluated according to single-pool urea kinetic model, BP blood pressure, GCR global cardiovascular risk score, evaluated according to the CUORE Project (see text), CRP C-reactive protein, anti-hHSP 60 anti-human heat shock protein 60
*p < 0.05 vs CP−
aMedian (25–75%)
CP + patients were older than seronegative (CP−) patients [mean (SD), 74.2 (8.2) vs 61.7 (15.5) years, p = 0.009), whereas they did not present any difference in dialytic age, duration of HD sessions, and dialytic adequacy, as well as in cardiovascular risk factors. GCR, evaluated as reported above, was also not different between CP+ and CP− [3.0 (1.3) vs 2.4 (1.4), p = 0.115]. Furthermore, also CRP serum median (IQR) also was not significantly different between the two groups: CP+, 0.53 (0.22–1.21) vs CP−, 0.34 (0.15–0.90)
CP infection and CVD events
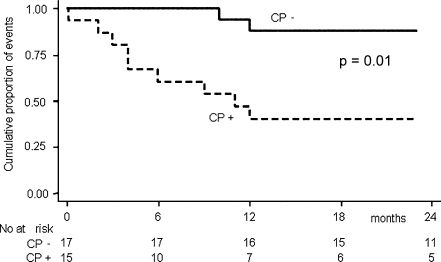
The mean follow-up time was 23 months. During the follow-up, 11 patients died (seven for cardiovascular events, mean follow-up of 16 months; four for non-cardiovascular diseases, mean follow-up of 15 months). Eleven cardiovascular events occurred, and the prevalence of CVD was significantly higher in CP+ (9/15 CP+ vs 2/17 CP−, p = 0.008). The number of patients suffering from separate cardiovascular events is reported in Table 1. On univariate Cox regression analysis, the incidence of CVD was significantly associated to GCR [hazard ration (HR), 2.03; p = 0.01; 95% CI, 1.38–2.99, for every point of the score]. CVD incidence was also strongly associated to CP seropositivity (HR, 7.59, p = 0.01; 95% CI, 1.63–35.4; Fig. 1). Moreover, on multivariate Cox analysis, the strength of the association between CP seropositivity and CVD incidence further increased after data adjustment for GCR and CRP (HR, 16.77, p = 0.05; 95% CI, 2.20/127.63). We also evaluated the relationship between CVD incidence and anti-CP antibodies titer, but we did not find any association.
Fig. 1.
Kaplan–Meier estimate survival for cardiovascular events occurred during the follow-up (myocardial infarction, heart failure, stroke, mesenteric infarction, and peripheral vascular disease) in Chlamydia pneumoniae seropositive (CP+) and seronegative (CP−) HD patients
Anti-hHSP60 autoimmune response
Anti-hHSP60 antibodies were present in all analyzed subjects; there were no significant differences in antibody serum medians comparing CP seropositive (CP+) and CP seronegative (CP−) HD patients [18.11 μg/mL (14.8–47.8) vs 31.4 μg/mL (23.2–75.3), respectively], as well as to healthy control subjects [−24.72 μg/mL (17.7–41.1)]. Moreover, anti-hHSP60 titer was not different between patients affected and not by CVD events: 21.3 μg/mL (IQR, 14.8–77.8) vs 31.3 μg/mL (IQR, 18.2–52.7), respectively. Correlations among anti-hHSP60 serum levels, dialytic parameters, CRP levels, GCR, as well as incidence of CVD during the follow-up were not found.
Discussion
HD patients present an increased risk of cardiovascular diseases (Foley et al. 1998). Traditional cardiovascular risk factors cannot fully justify such an elevated risk; therefore, new and non-traditional risk factors, such as inflammation and chronic infections, have been evaluated (Kendrick and Chonchol 2008). CP infection has been related to the development and progression of atherosclerosis in HD patients and, more recently, also in patients undergoing peritoneal dialysis (Kim et al. 2008). In particular, both anti-CP IgG and IgA antibodies titers have been associated to progression of carotid atherosclerosis and ischemic heart disease (Kato et al. 2004; Wszola et al. 2006). However, in the clinical setting, together with a great deal of studies reporting the close association between CP infection and CVD, both in general and HD population (Lentine et al. 2006), conflicting evidence by observational and interventional studies also exists (Ieven and Hoymans 2005; O’Connor et al. 2003). Some authors have proposed CP infection only having a subsidiary role in atherosclerosis development in HD patients (Kato et al. 2006). In particular, it has been suggested that the link between CP seropositivity and CVD risk is confounded by the presence of other risk factors (Zoccali et al. 2003). Our data agree with former studies, showing a significant association between CP seropositivity and incidence of CVD, which was also relevant after adjustment for the so-called traditional risk factors, such as diabetes, hypertension, hypercholestolemia, etc., as summarized in the GCR score. The reasons for this discrepancy among the reported studies could be due, first of all, to the different methods and definitions of CP infection (seropositivity or DNA detection) and second, to individual differences of the evaluated patients (Paldanius et al. 2006). For instance, regarding HD patients, it is well known that different dialytic modalities and membranes may affect immune response and inflammation. In fact, there is evidence that the use of bioincompatible dialytic devices results both in increased levels of pro-inflammatory cytokines (such as IL-1, IL-6, and IL-12) and impaired ability of lymphocytes to respond to antigenic stimuli (Libetta et al. 2004). In order to minimize these potential confounding factors, we evaluated a homogeneous sample of patients undergoing a regular HD treatment, with similar dialytic prescriptions. On the basis of our findings, we confirm the hypothesis that CP infection could be considered an additional non-traditional risk factor in HD patients, even if we admit that the real impact of CP infection on clinical outcomes in HD should be better elucidated in specific-designed studies. It has been supposed that in HD patients, as well as in general population, there are several potential pathogenetic mechanisms to explain the role of CP in atherosclerosis, but the exact pathways are still unknown. In vitro experiments have shown that the growth of CP in human monocytes induces the production of pro-inflammatory cytokines (TNF-α, IL-1, and IL-6) and chemokines (IL-8, monocyte chemotactic protein-1) (Kern et al. 2009; Tsirpanlis et al. 2003; Stenvinkel et al. 2002). In addition, CP invasion of endothelial cells determines the expression of adhesion molecules, such as intercellular adhesion molecule, and stimulates transendothelial migration of neutrophils and monocytes (Summersgill et al. 2000; Molestina et al. 1999). In the present study, we tested the hypothesis that, in HD patients, CP infection could promote an autoimmune response to hHSP60, mediated by the mechanism of molecular mimicry, due to the high antigenic homology between human and chlamydial HSP60. We found that anti-hHSP60 antibodies levels were not different between healthy subjects and HD patients and, in turn, between CP seropositive and seronegative HD patients. Moreover, anti-hHSP60 antibodies were not related to CP infection, cardiovascular risk factors, or incidence of CVD. These data agree with a recent paper, which reports, in an ex vivo model on coronary artery, the ability of CP to induce arterial thickening without the presence of a host immune response to HSP60 (Deniset et al. 2010). These findings let us suppose that anti-hHSP60 autoimmunity does not play a role in the pathogenesis of CP infection and CP-associated cardiovascular risk. As above reported, previous studies showed pro-inflammatory and pro-atherotrombotic effects of anti-hHSP60 antibodies, but actually inconclusive results have been also reported. In fact, while a strong correlation between anti-hHSP60 and atherosclerosis has been found in general population and in patients suffering from acute myocardial infarction (Heltai et al. 2004), it has also been demonstrated that anti-hHSP60 antibodies are not associated to CVD events in diabetic and cardiopathic patients (Gruden et al. 2009; Hoymans et al. 2008). Regarding HD patients, high serum levels of anti-hHSP60 antibodies were reported in children and young HD patients, whereas adult subjects did not show different serum levels of anti-hHSP60 when compared to healthy control group (Musial et al. 2009; Esposito et al. 2010). Similar data resulted from the present study confirms our previous report. However, it is noteworthy that, in the specific setting of HD, regulation of immune system is quite complex, and several factors could influence antibody levels. It is well established that HD patients present a state of immune system dysfunction that makes them liable to infections and malignancies and unresponsiveness to vaccinations (Eleftheriadis et al. 2007). This immunodeficiency depends mainly on the altered function of various types of immune cells, including polymorphonuclear leukocytes, monocytes, and B and T lymphocytes (Sardenberg et al. 2006; Lim et al. 2007). The leading causes of this immune response impairment still are not clear. It is believed that iron overload, HD treatment per se, and metabolic disturbances linked to uremic status, accompanied by the accumulation of numerous toxic substances, could play a role in this immunity impairment (Cohen et al. 1997). This immunodeficiency could explain, at least in part, the low anti-hHSP60 titer we found in our HD patients. At the same time, it is important to underline that HD treatment by standard HD techniques and devices, such as what we used in this study, is not able to remove serum antibodies from the circulation. In fact, HD membranes are optimized for the removal of low molecular weight solutes (<100 Da), while antibodies are too large (~150 kDa) to cross dialysis membrane. Taken together, these considerations remark that the evaluation of immune response and antibodies production in the setting of HD is difficult. Nevertheless, in our opinion, our data add a new interesting contribution to the debate on the role of anti-hHSP60 in atherosclerosis, bringing into question the real impact of HSP60 autoimmunity in the clinical setting. However, our study presents some limitations, mainly due to the limited number of studied subjects and the short-term follow-up, which make us unable to perform in-depth statistical and clinical analysis. Furthermore, we did not completely evaluate factors potentially influencing anti-hHSP60 response, such as immune and nutritional status and dialysis-specific factors, testing, for example, the effects of different dialytic techniques and membranes. In conclusion, our data suggest that in HD patients, while CP seropositivity is associated to higher incidence of CVD, even after adjustment for traditional risk factors, the autoimmune response to hHSP60 is not related to CP infection. However, because of the highly potential clinical impact of the relationship among infections, autoimmunity, and cardiovascular disease, we think that larger prospective studies are required to better evaluate the nature of this association, mainly in high-risk populations, such as HD patients.
References
- Borel N, Summersgill JT, Mukhopadhyay S, Miller RD, Ramirez JA, Pospischil A. Evidence for persistent Chlamydia pneumoniae infection of human coronary atheromas. Atherosclerosis. 2008;199(1):154–161. doi: 10.1016/j.atherosclerosis.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Cohen G, Haag-Weber M, Hörl WH. Immune dysfunction in uremia. Kidney Int Suppl. 1997;62:S79–S82. [PubMed] [Google Scholar]
- Deniset JF, Cheung PK, Dibrov E, Lee K, Steigerwald S, Pierce GN. Chlamydophila pneumoniae infection leads to smooth muscle cell proliferation and thickening in the coronary artery without contributions from a host immune response. Am J Pathol. 2010;176(2):1028–1037. doi: 10.2353/ajpath.2010.090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudé M, Gillis MA, Théorêt JF, Thorin E, Lajoie G, Levine JS, Merhi Y, Rauch J. Autoantibodies to heat shock protein 60 promote thrombus formation in a murine model of arterial thrombosis. J Thromb Haemost. 2009;7(4):710–719. doi: 10.1111/j.1538-7836.2009.03305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20:440–451. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Esposito P, Libetta C, Rampino T, Gregorini M, Gabanti E, Portalupi V, Dal Canton A. Autoimmune response to heat shock protein 60 in haemodialysis patients. J Intern Med. 2010;267(4):440. doi: 10.1111/j.1365-2796.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- Ferrario M, Chiodini P, Chambless LE, et al. Prediction of coronary events in a low incidence population. Assessing accuracy of the CUORE cohort study prediction equation. Int J Epidemiol. 2005;34:413–421. doi: 10.1093/ije/dyh405. [DOI] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- Gattone M, Iacoviello L, Colombo M, et al. Chlamydia pneumoniae and cytomegalovirus seropositivity, inflammatory markers, and the risk of myocardial infarction at a young age. Am Heart J. 2001;142(4):633–640. doi: 10.1067/mhj.2001.118118. [DOI] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, et al. Anti-HSP60 and anti-HSP70 antibody levels and micro⁄macrovascular complications in type 1 diabetes: the EURODIAB. J Intern Med. 2009;266(6):527–536. doi: 10.1111/j.1365-2796.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- Heltai K, Kis Z, Burian K, Endresz V, Veres A, Ludwig E, Gönczöl E, Valyi-Nagy I. Elevated antibody levels against Chlamydia pneumoniae, human HSP60 and mycobacterial HSP65 are independent risk factors in myocardial infarction and ischaemic heart disease. Atherosclerosis. 2004;173(2):339–346. doi: 10.1016/j.atherosclerosis.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Högdahl M, Söderlund G, Kihlström E. Expression of chemokines and adhesion molecules in human coronary artery endothelial cells infected with Chlamydia (Chlamydophila) pneumoniae. APMIS. 2008;116(12):1082–1088. doi: 10.1111/j.1600-0463.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- Hoshida S, Nishino M, Tanouchi J, Kishimoto T, Yamada Y (2005) Acute Chlamydia pneumoniae infection with heat-shock-protein-60-related response in patients with acute coronary syndrome. Atherosclerosis 183(1):109–12 [DOI] [PubMed]
- Hoymans VY, Bosmans JM, Herck PL, Ieven MM, Vrints CJ. Implications of antibodies to heat-shock proteins in ischemic heart disease. Int J Cardiol. 2008;123(3):277–282. doi: 10.1016/j.ijcard.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Huittinen T, Leinonen M, Tenkanen L, Mänttäri M, Virkkunen H, Pitkänen T, Wahlström E, Palosuo T, Manninen V, Saikku P. Autoimmunity to human heat shock protein 60, Chlamydia pneumoniae infection, and inflammation in predicting coronary risk. Arterioscler Thromb Vasc Biol. 2002;22(3):431–437. doi: 10.1161/hq0302.104512. [DOI] [PubMed] [Google Scholar]
- Ieven MM, Hoymans VY. Involvement of Chlamydia pneumoniae in atherosclerosis: more evidence for lack of evidence. J Clin Microbiol. 2005;43(1):19–24. doi: 10.1128/JCM.43.1.19-24.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Takita T, Maruyama Y, Hishida A. Chlamydial infection and progression of carotid atherosclerosis in patients on regular haemodialysis. Nephrol Dial Transplant. 2004;19(10):2539–2546. doi: 10.1093/ndt/gfh416. [DOI] [PubMed] [Google Scholar]
- Kato A, Takita T, Furuhashi M, Maruyama Y, Hishida A. Association between seroprevalence of anti-chlamydial antibodies and long-term cardiovascular mortality in chronic hemodialysis patients. Atherosclerosis. 2006;188(1):120–125. doi: 10.1016/j.atherosclerosis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kendrick J, Chonchol MB. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2008;4(12):672–681. doi: 10.1038/ncpneph0954. [DOI] [PubMed] [Google Scholar]
- Kern JM, Maass V, Maass M. Chlamydia pneumoniae adversely modulates vascular cell properties by direct interaction with signalling cascades. Thromb Haemost. 2009;102(6):1064–1070. doi: 10.1160/TH09-06-0348. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kim HJ, Han SH, Lee JE, Moon SJ, Kim BS, Kang SW, Choi KH, Lee HY, Han DS. Chlamydia pneumoniae accompanied by inflammation is associated with the progression of atherosclerosis in CAPD patients: a prospective study for 3 years. Nephrol Dial Transplant. 2008;23(3):1011–1018. doi: 10.1093/ndt/gfm696. [DOI] [PubMed] [Google Scholar]
- Lentine KL, Parsonnet J, Taylor I, Wrone EM, Lafayette RA. Associations of serologic markers of infection and inflammation with vascular disease events and mortality in American dialysis patients. Clin Exp Nephrol. 2006;10(1):55–62. doi: 10.1007/s10157-005-0392-5. [DOI] [PubMed] [Google Scholar]
- Libetta C, Zucchi M, Gori E, Sepe V, Galli F, Meloni F, Milanesi F, Canton D. Vitamin E-loaded dialyzer resets PBMC-operated cytokine network in dialisi patients. Kidney Int. 2004;65(4):1473–1481. doi: 10.1111/j.1523-1755.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- Lim WH, Kireta S, Leedham E, Russ GR, Coates PT. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int. 2007;72:1138–1148. doi: 10.1038/sj.ki.5002425. [DOI] [PubMed] [Google Scholar]
- Mitusch R, Luedemann J, Wood WG, Berger K, Schminke U, Suter M, Kessler C, John U, Rupp J, Kentsch M, Maass M. Asymptomatic carotid atherosclerosis is associated with circulating Chlamydia pneumoniae DNA in younger normotensive subjects in a general population survey. Arterioscler Thromb Vasc Biol. 2005;25(2):386–391. doi: 10.1161/01.ATV.0000151284.49967.a7. [DOI] [PubMed] [Google Scholar]
- Molestina RE, Miller RD, Ramirez JA, Summersgill JT. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67(3):1323–1330. doi: 10.1128/iai.67.3.1323-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musial K, Szprynger K, Szczepańska M, Zwolińska D. Heat shock proteins in children and young adults on chronic hemodialysis. Pediatr Nephrol. 2009;24(10):2029–2034. doi: 10.1007/s00467-009-1197-7. [DOI] [PubMed] [Google Scholar]
- Netea MG, Selzman CH, Kullberg BJ, Galama JM, Weinberg A, Stalenhoef AF, Meer JW, Dinarello CA. Acellular components of Chlamydia pneumoniae stimulate cytokine production in human blood mononuclear cells. Eur J Immunol. 2000;30(2):541–549. doi: 10.1002/1521-4141(200002)30:2<541::AID-IMMU541>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- O’Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, Benner RJ, Fisher MR, Cook TD; Investigators in the WIZARD Study (2003) Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA 17; 290(11):1459–1466 [DOI] [PubMed]
- Paldanius M, Leinonen M, Virkkunen H, Tenkanen L, Sävykoski T, Mänttäri M, Saikku P. Chlamydia pneumoniae antibody levels before coronary events in the Helsinki heart study as measured by different methods. Diagn Microbiol Infect Dis. 2006;56(3):233–239. doi: 10.1016/j.diagmicrobio.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Pesonen E, Andsberg E, Ohlin H, Puolakkainen M, Rautelin H, Sarna S, Persson K. Dual role of infections as risk factors for coronary heart disease. Atherosclerosis. 2007;192(2):370–375. doi: 10.1016/j.atherosclerosis.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Sardenberg C, Suassuna P, Andreoli MC, Watanabe R, Dalboni MA, Manfredi SR, Santos OP, Kallas EG, Draibe SA, Cendoroglo M. Effects of uraemia and dialysis modality on polymorphonuclear cell apoptosis and function. Nephrol Dial Transplant. 2006;21:160–165. doi: 10.1093/ndt/gfi095. [DOI] [PubMed] [Google Scholar]
- Schett G, Metzler B, Mayr M, Amberger A, Niederwieser D, Gupta RS, Mizzen L, Xu Q, Wick G. Macrophage-lysis mediated by autoantibodies to heat shock protein 65/60. Atherosclerosis. 1997;128(1):27–38. doi: 10.1016/S0021-9150(96)05975-8. [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Cell surface localization of the 60 kDa heat shock caperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;21(5):315–320. doi: 10.1006/cbir.1997.0144. [DOI] [PubMed] [Google Scholar]
- Stenvinkel P, Heimburger O, Jogestrand T. Elevated interleukin-6 predicts progressive carotid artery atherosclerosis in dialysis patients: association with Chlamydia pneumoniae seropositivity. Am J Kidney Dis. 2002;39(2):274–278. doi: 10.1053/ajkd.2002.30546. [DOI] [PubMed] [Google Scholar]
- Summersgill JT, Molestina RE, Miller RD, Ramirez JA. Interactions of Chlamydia pneumoniae with human endothelial cells. J Infect Dis. 2000;181(Suppl 3):S479–S482. doi: 10.1086/315620. [DOI] [PubMed] [Google Scholar]
- Tsirpanlis G, Chatzipanagiotou S, Ioannidis A, Ifanti K, Bagos P, Lagouranis A, Poulopoulou C, Nicolaou C. The effect of viable Chlamydia pneumoniae on serum cytokines and adhesion molecules in hemodialysis patients. Kidney Int Suppl. 2003;84:S72–S75. doi: 10.1046/j.1523-1755.63.s84.42.x. [DOI] [PubMed] [Google Scholar]
- Watson C, Alp NJ. Role of Chlamydia pneumoniae in atherosclerosis. Clin Sci (Lond) 2008;114(8):509–531. doi: 10.1042/CS20070298. [DOI] [PubMed] [Google Scholar]
- Wszola M, Kwiatkowski A, Nosek R, et al. Chlamydia pneumoniae infection and ischemic heart disease in hemodialysis patients. Transplant Proc. 2006;38(1):31–34. doi: 10.1016/j.transproceed.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Xu Q. Infections, heat shock proteins, and atherosclerosis. Curr Opin Cardiol. 2003;18(4):245–252. doi: 10.1097/00001573-200307000-00001. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Benedetto FA, Bonanno G, Seminara G, Fatuzzo P, Rapisarda F, Malatino LS. Chlamydia pneumoniae, overall and cardiovascular mortality in end-stage renal disease (ESRD) Kidney Int. 2003;64(2):579–584. doi: 10.1046/j.1523-1755.2003.00095.x. [DOI] [PubMed] [Google Scholar]