Abstract
Purpose
It is not yet possible to estimate the number of cases required for a beginner to become expert in laparoscopic radical prostatectomy. We estimated the learning curve of laparoscopic radical prostatectomy for positive surgical margins compared to a published learning curve for open radical prostatectomy.
Materials and Methods
We reviewed records from 8,544 consecutive patients with prostate cancer treated laparoscopically by 51 surgeons at 14 academic institutions in Europe and the United States. The probability of a positive surgical margin was calculated as a function of surgeon experience with adjustment for pathological stage, Gleason score and prostate specific antigen. A second model incorporated prior experience with open radical prostatectomy and surgeon generation.
Results
Positive surgical margins occurred in 1,862 patients (22%). There was an apparent improvement in surgical margin rates up to a plateau at 200 to 250 surgeries. Changes in margin rates once this plateau was reached were relatively minimal relative to the CIs. The absolute risk difference for 10 vs 250 prior surgeries was 4.8% (95% CI 1.5, 8.5). Neither surgeon generation nor prior open radical prostatectomy experience was statistically significant when added to the model. The rate of decrease in positive surgical margins was more rapid in the open vs laparoscopic learning curve.
Conclusions
The learning curve for surgical margins after laparoscopic radical prostatectomy plateaus at approximately 200 to 250 cases. Prior open experience and surgeon generation do not improve the margin rate, suggesting that the rate is primarily a function of specifically laparoscopic training and experience.
Keywords: prostatic neoplasms, laparoscopy, prostatectomy, learning
We previously reported on the learning curve of LRP for BCR, and we found that recurrence rates were nearly halved as surgeons developed surgical experience.1 That said, we also reported that the learning curve for LRP appears to accrue more slowly than for open surgery.2 Whereas the learning curve for ORP rises steeply up to 250 to 350 prior procedures and then plateaus, the curve for LRP increases linearly up to approximately 750 operations.
BCR is a problematic end point for studies of surgery for prostate cancer because events take many years to accrue. As such, many studies use PSM rates to determine the effects of changes in technique or training. In this study we estimated the overall PSM rate among patients treated with LRP without robotic assistance at North American and European institutions, and estimated the learning curve using PSMs as an end point. In addition, we evaluated the effects of prior open experience and whether first generation surgeons had results different from those of the second generation who benefitted from experience. We also compared the laparoscopic RP learning curve for PSMs to that of open RP, and examined the margins learning curve in light of the learning curve for BCR.
MATERIALS AND METHODS
Study Cohort and Data Sources
Our cohort consisted of 9,336 patients with clinically localized prostate cancer treated with LRP between January 1998 and July 2007 at 1 of 14 participating institutions (see Appendix). We excluded from study those patients who received adjuvant (7) or neoadjuvant therapy (588), or those missing data for the treating surgeon (2), for SMs (26) or for clinical covariates (PSA, Gleason grade or stage, 169), leaving 8,544 eligible for analysis. All information was obtained with appropriate institutional review board waivers and data were de-identified before analysis.
Eligible patients were treated by 1 of 51 surgeons, all of whom saw patients only at a study institution while on staff there. Surgeons who had previously conducted open or laparoscopic RP at a nonstudy institution were asked to provide the number of prior cases. Only 1 surgeon reported previous laparoscopic experience (285 procedures). A total of 31 surgeons reported experience with ORP before conducting their first laparoscopic procedure and 13 (25%) performed at least 100 prior ORPs. Therefore, for all but 1 of the study surgeons we were able to obtain the complete surgical history of all laparoscopically treated patients to date and have a good estimate of any previous open experience. A PSM was defined as the presence of tumor cells at the inked margin in the prostatectomy specimen according to the respective institutional processing protocol.
Statistical Methods
For each patient the surgeon experience was coded as the number of LRPs performed by the surgeon before the patient's operation, including operations that the surgeon performed at nonstudy institutions or on patients who were ineligible for this analysis. Only a single leading surgeon was recorded for each operation. Operations at which a surgeon assisted such as during fellowship training were not counted toward surgeon prior experience. Thus, surgeon experience differed for each patient treated by a particular surgeon. Surgeons were categorized as first or second generation. First generation was defined as those surgeons who began LRP before January 2001 (3 years after the first reported LRP), had performed at least 100 procedures before the end of this study (July 2007) and were the first or second surgeon at their institution to perform this operation. All other surgeons were classified as second generation.
For our initial descriptive analysis we categorized patients into 4 groups according to the surgeon experience at the time of the patient's procedure (less than 50, 50 to 99, 100 to 249, or 250 or more prior lifetime LRPs, table 1). These cut points were chosen to reflect clinical judgments about different levels of surgeon experience. They are for illustrative purposes only and were not entered into any statistical model. Thus, our findings are unaffected by our choice of cut points.
Table 1.
Distribution of surgeons by number of prostatectomies performed
| No. Surgeons (%) | |
|---|---|
| No. LRP:* | |
| Less than 50 | 22 (43) |
| 50–99 | 4 (8) |
| 100–249 | 13 (25) |
| 250 or More | 12 (24) |
| Total | 51 (100) |
| No. ORP:† | |
| 0 | 20 (39) |
| 1–10 | 5 (10) |
| 11–99 | 13 (25) |
| 100 or More | 13 (25) |
| Total | 51 (100) |
The most experienced surgeon completed 1,066 cases excluding prior open surgeries and 1,434 cases including prior open surgeries.
Lifetime total.
Before first laparoscopic procedure.
To adjust for differences in case mix among surgeons we created a logistic regression model to predict SM status, and included as predictors preoperative PSA, pathological stage (presence or absence of 3 separate variables of extracapsular extension, seminal vesicle invasion and lymph node involvement) and pathological Gleason score of the surgical specimen. Although we included year of surgery as a predictor in our previous analysis of the learning curve for ORP,3 we did not include it in these analyses for several reasons. We did not expect stage migration in this contemporary cohort (1998 to 2007), whereas our former cohort (1987 to 2004) clearly includes the period in which stage migration had an important impact. In addition, we evaluated the association between year of surgery and tumor characteristics. Earlier year of surgery was not associated with Gleason score or organ confined disease, although it was significantly associated with a small increase in PSA (p = 0.008). Year of surgery was not a predictor of SM status (univariate OR 1.01, p = 0.7; multivariable OR 1.02, p = 0.6). The degree to which our model corrected for case mix was assessed by the area under the receiver operating characteristic curve.
As the relationship between surgeon experience and outcome is likely nonlinear, surgeon experience was modeled using restricted cubic splines with knots at the ter-tiles. Data from different patients treated by the same surgeon are not independent. Therefore, we incorporated within-surgeon clustering in our analyses by specifying the cluster option in Stata® statistical software (v9.2). It is possible that institution could affect the PSM rate if pathologists rate SMs differently. However, this is a longitudinal study that models the change in PSM rates over time, and all but 1 surgeon practiced only at 1 center. Therefore, it is unlikely that institution would affect the rate of SMs over time independent of surgeon. When the cases of the surgeon who did switch institutions were excluded from analysis our results did not change. To produce a learning curve we used the mean value of covariates to calculate the probability of a PSM predicted by the model for each level of surgical experience. CIs for the difference between selected points on the curve were obtained by bootstrapping.
To compare the SM learning curve of LRP to our previously published learning curve for ORP we fit 2 separate prespecified multivariable models, 1 using data from the laparoscopic cohort and 1 using data from the open cohort. Both models were adjusted for case mix using the mean values of predictors from the laparoscopic cohort. To account for stage migration we restricted the open cohort to patients treated after 1995 and included a term for year of treatment. A permutation test was used to compare the open and laparoscopic LCs, the details of which were reported previously.1 The difference in the LC slope between approaches was calculated by comparing the LC slopes over various levels of experience. To test the hypotheses that prior open experience and/or surgeon generation affect the learning curve for SMs we entered prior open experience (yes/no) and surgeon generation (first/ second) in the multivariable model. All p values are 2-sided.
RESULTS
The distribution of the number of previous ORPs together with the total lifetime number of LRPs is shown in table 1. Although many of the surgeons (43%) had performed fewer than 50 procedures during their careers to date, a substantial percentage (49%) had performed at least 100 LRPs in total. Clinical and pathological characteristics of the study cohort are shown in table 2. Approximately half of the patients (4,809, 56%) were treated by a surgeon who had performed fewer than 250 prior LRPs and the other half (3,735, 44%) were treated by a surgeon who had performed 250 or more prior surgeries. PSA and age at operation did not differ significantly with increasing surgeon experience. Differences in pathological Gleason grade were statistically significant (p = 0.002), with a higher Gleason score associated with greater surgeon experience. This finding possibly reflects the increased reliance on active surveillance in recent years. However, most importantly it argues against case selection as an explanation for the LC.
Table 2.
Patient and tumor characteristics by surgeon experience
| Surgeon Operations Before Incident Case |
|||||
|---|---|---|---|---|---|
| Less Than 50 | 50–99 | 100–249 | 250–1,100 | p Value | |
| No. pts (%) | 1,456 (17) | 1,134 (13) | 2,219 (26) | 3,735 (44) | |
| Median ng/ml preop PSA (IQR) | 6.9 (5.0–9.6) | 6.9 (5.0–10.0) | 6.6 (4.9–9.6) | 6.0 (4.5–8.7) | 0.3 |
| Median pt age at surgery (IQR) | 63 (59–67) | 63 (58–67) | 62 (57–67) | 61 (56–66) | 0.069 |
| No. RPs (%):* | <0.001 | ||||
| 1998–2002 | 664 (46) | 426 (38) | 649 (29) | 431 (12) | |
| 2003 | 237 (16) | 257 (23) | 435 (20) | 606 (16) | |
| 2004 | 221 (15) | 122 (11) | 531 (24) | 867 (23) | |
| 2005 | 276 (19) | 221 (19) | 322 (15) | 1,044 (28) | |
| 2006–2007 | 58 (4) | 108 (10) | 282 (13) | 787 (21) | |
| No. Gleason score (%): | 0.002 | ||||
| 6 or Less | 776 (53) | 575 (51) | 1,172 (53) | 1,628 (44) | |
| 7 | 588 (40) | 476 (42) | 881 (40) | 1,848 (49) | |
| 8 or Greater | 92 (6) | 83 (7) | 166 (7) | 259 (7) | |
| No. extracapsular extension (%) | 436 (30) | 349 (31) | 655 (30) | 952 (25) | 0.4 |
| No. seminal vesicle invasion (%) | 114 (8) | 102 (9) | 175 (8) | 282 (8) | 0.9 |
| No. lymph node involvement (%) | 14 (1) | 23 (2) | 35 (2) | 67 (2) | 0.2 |
| No. nonorgan confined Ca (%)† | 441 (30) | 357 (32) | 664 (30) | 979 (26) | 0.4 |
| No. PSMs (%)‡ | 373 (26) | 257 (23) | 456 (21) | 776 (21) | 0.005 |
Linear regression to test the association between number of RPs performed and year of surgery.
Presence of extracapsular invasion, seminal vesicle invasion or lymph node involvement.
Unadjusted outcome.
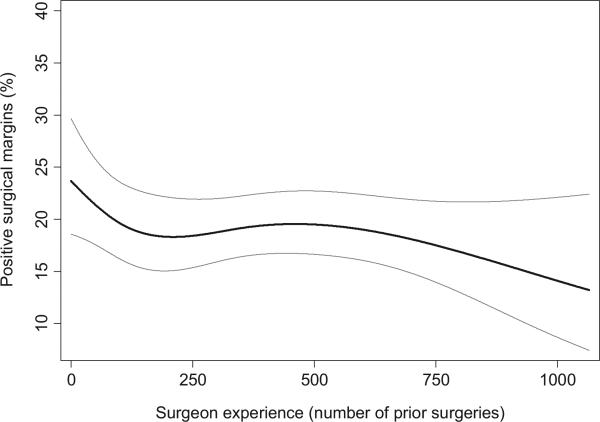
PSMs were reported in 1,862 patients (22%), 838 (14%) with organ confined disease and 1,024 (42%) with nonorgan confined disease. Our initial descriptive analysis revealed that the rate of PSMs was lower among patients who were treated by surgeons with more prior experience than among those treated by surgeons with less prior experience (table 2). Because these differences in outcomes may have resulted from differences in case mix, we fit a prespecified multivariable model that controls for case mix by adjusting for clinical and pathological variables. The predictive accuracy of the covariates was moderate (area under the curve of 0.72), suggesting that our multivariable model provided good control for case mix. In the adjusted model greater surgeon experience was associated with a lower PSM rate, although this did not reach statistical significance (p = 0.14). Figure 1 shows the predicted probability of a PSM plotted against surgeon experience and, thus, provides the learning curve for SMs after LRP. There was an apparent improvement in SM rates up to a plateau at 200 to 250 surgeries. Changes in margin rates once this plateau was reached were relatively minimal relative to the CIs. The absolute risk difference for 10 vs 250 prior surgeries was 4.8% (95% CI 1.5, 8.5) and for 250 vs 750 prior surgeries was 0.9% (95% CI 0.2, 4.6). As a post hoc analysis we modeled the LC for the first 250 surgeries separately, and found a significant association between greater surgeon experience and lower margin rate (p = 0.007). We did not conduct an analysis restricted to surgeons with at least 250 surgeries because it would be highly confounded by prior open experience as all but 1 of the surgeons who reached 250 or more surgeries had prior ORP experience.
Figure 1.
Surgical learning curve for SMs with LRP controlling for preoperative PSA, pathological Gleason score and pathological stage. X-axis represents number of cases before incident case, excluding prior open cases. Gray lines represent 95% CI.
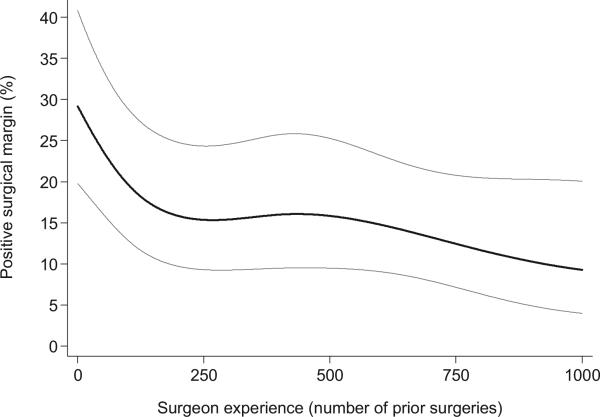
Figure 2 shows the predicted probability of PSMs for ORP, where the learning curve for ORP was estimated using a previously described multi-insti tutional data set with patients treated after 1995.3 The curve for ORP appears to start higher and end lower than that for LRP. However, the vertical distance between the 2 curves depends on appropriate case mix adjustment between cohorts and may be confounded by institutional differences. Nevertheless, the shape of each curve will remain constant regardless of case mix adjustment and we can conclude that although fairly similar, the rate of decrease in PSMs was higher in the open cohort (p = 0.025). This is similar to our previously reported finding that the LC for recurrence was more rapid for open vs laparoscopic RP.1
Figure 2.
Surgical learning curve for SMs with ORP controlling for preoperative PSA, pathological Gleason score and pathological stage. To make open and laparoscopy learning curves more comparable, open cohort was restricted to patients seen after 1995 and model covariates were set to mean of patients in laparoscopy learning curve. Gray lines represent 95% CI.
Table 3 shows the results when surgeon generation and prior ORP experience were added to the multivariable model for laparoscopic surgery. Although neither variable was statistically significant, there was some evidence of higher margin rates for surgeons who had started their careers performing ORP. As the estimate was negative, prior open experience did not appear to provide an advantage.
Table 3.
Odds ratios for positive surgical margins
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Cumulative experience* | — | — | 0.14 | — | — | 0.2 |
| PSA (ng/ml) | 1.02 | 1.01–1.03 | <0.0005 | 1.02 | 1.01–1.03 | <0.0005 |
| Gleason 7 vs 6 | 1.55 | 1.27–1.89 | <0.0005 | 1.57 | 1.29–1.92 | <0.0005 |
| Gleason 8 vs 6 | 1.79 | 1.36–2.35 | <0.0005 | 1.80 | 1.39–2.34 | <0.0005 |
| Extracapsular extension | 3.07 | 2.39–3.93 | <0.0005 | 3.09 | 2.41–3.96 | <0.0005 |
| Seminal vesicle invasion | 1.92 | 1.52–2.42 | <0.0005 | 1.89 | 1.50–2.38 | <0.0005 |
| Lymph node involvement | 0.90 | 0.57–1.42 | 0.6 | 0.90 | 0.58–1.40 | 0.6 |
| Surgeon generation (1st vs 2nd) | — | — | — | 0.90 | 0.56–1.46 | 0.7 |
| Prior ORP (yes vs no) | — | — | — | 1.38 | 0.92—2.07 | 0.12 |
Odds ratios calculated from multivariable models including all variables except surgeon generation and prior ORP (model 1) or including all variables shown (model 2).
No odds ratio given due to use of nonlinear terms.
DISCUSSION
The number of cases needed to become proficient in LRP has been the subject of continuous debate. There has been no consistent definition of the minimum number of procedures per surgeon for good outcomes. This is largely because previous authors have focused on outcomes of only indirect medical importance (blood loss, operating time) and have used analytic methods with predetermined cut points. For example, if the investigator divides surgeon experience into categories of 0 to 99, 100 to 199 and 200 to 299,4,5 then clearly the minimum number of procedures required per surgeon will be a number divisible by 100. Moreover investigators have typically reported the results from single surgeon series.4,6,7 Conversely in this study we focused on an end point of oncologic relevance, that is SMs, we used nonlinear modeling to present the LC graphically and we included data from a large number of surgeons at multiple institutions.
It is reasonable to believe that not all surgeons reach the same level of proficiency regardless of the number of procedures performed. Bianco et al demonstrated substantial variation in margin rates between high volume surgeons, even at the same institution. 8 It may be that an experienced surgeon merely repeats the same intraoperative technical error in a larger number of cases than the less experienced surgeon.9 Thus, reaching proficiency depends on learning from experience, not merely from accruing experience. Exactly how surgeons learn and what specific aspects of technique improve with experience are areas ripe for empirical research.
Although the PSM rate can be reduced by resecting some or all of the periprostatic tissue, wide dissection jeopardizes postoperative erectile function while the close dissection optimal for nerve sparing can create a PSM depending on tumor location. Therein lies one of the greatest challenges of this procedure, that is ensuring negative SMs while preserving urinary and erectile function.10 Therefore, the learning curve for radical prostatectomy is not solely or adequately represented by margin status. Instead multiple LCs exist for the different outcome variables. It is incumbent on the urological community to create prospective databases that include validated, patient reported functional outcomes so that the LC for functional outcomes may be better understood. In particular it is critical to evaluate further how LCs may differ by surgical modality. Given that we have published data on the learning curve for open and laparoscopic RP, learning curves for robotically assisted surgery constitute a research priority. Preliminary data do suggest that robotic assistance may speed the LC.11,12 Naturally our findings cannot be extrapolated to robotic techniques.
Because the learning curve for LRP is long, minimally invasive urology programs need to find ways of improving laparoscopy teaching. A modulated method of teaching LRP technique to fellows and residents has proven to be effective in achieving reasonable perioperative outcomes.13 Other strategies to improve LRP outcomes are the correlation of intraoperative video recordings with pathology findings to understand how PSMs occurred, plus an institutional multi-departmental process of continued evaluation of surgical technique and outcomes.14,15
Comparing the results of this study with our previous report on the learning curve for BCR allows us to reflect on the value of margins as a surrogate.2,16 Two results, the slower LC being for laparoscopic vs open RP and the small effect of surgical generation, were consistent for margins and recurrence end points. Our key finding was the existence of the learning curve, which was similar for both end points, although the findings were far less clear for margins. Despite a smaller number of patients (4,702 vs 8,544), the results for recurrence were highly significant (p = 0.005) whereas the findings for margins were only significant when examining the early part of the LC. A final finding reported in the recurrence study (that previous open experience is harmful) was not replicated here. We found a smaller increase in risk that was not statistically significant. This suggests that although analysis of SMs can indeed provide clues as to eventual onco-logic outcomes, it is far from a perfect surrogate and may be insufficiently sensitive to identify important effects of surgical technique.
CONCLUSIONS
PSMs were reported in 1,862 patients (22%) of this multicenter series. The learning curve for PSMs after LRP, although fairly similar to that of the open technique, appears to be slower. SM rates improve with increasing surgeon experience until a plateau at around 200 to 250 cases. Prior open experience and surgeon generation do not improve margin rates, suggesting that these are primarily a function of specifically laparoscopic experience.
ACKNOWLEDGMENTS
Dr. Janet Novak and Melissa Bogen provided editorial assistance supported by Memorial Sloan-Kettering Cancer Center.
Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers.
Abbreviations and Acronyms
- BCR
biochemical recurrence
- LC
learning curve
- LRP
laparoscopic radical prostatectomy
- ORP
open radical prostatectomy
- PSA
prostate specific antigen
- PSM
positive surgical margin
- RP
radical prostatectomy
- SM
surgical margin
APPENDIX
APPENDIX.
| Hospital | Country |
|---|---|
| 1. Centre Hospitalier Universitaire Henri Mondor | France |
| 2. Cleveland Clinic Foundation | USA |
| 3. Hospital Universitario La Paz | Spain |
| 4. Institut Mutualiste Montsouris | France |
| 5. Johns Hopkins Medical Institutions | USA |
| 6. Jules Bordet Institute | Belgium |
| 7. Klinikum Heilbronn | Germany |
| 8. Krankenhaus der Elisabethinen | Austria |
| 9. Lahey Clinic | USA |
| 10. Malmö University Hospital Lund University | Sweden |
| 11. Memorial Sloan-Kettering Cancer Center | USA |
| 12. North Hampshire Hospital | UK |
| 13. University of Leipzig | Germany |
| 14. Vincenzo Pansadoro Foundation | Italy |
REFERENCES
- 1.Vickers AJ, Savage CJ, Hruza M, et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol. 2009;10:475. doi: 10.1016/S1470-2045(09)70079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 3.Vickers A, Bianco F, Cronin A, et al. The learning curve for surgical margins after open radical prostatectomy: implications for margin status as an oncological end point. J Urol. 2010;183:1360. doi: 10.1016/j.juro.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viney R, Gommersall L, Zeif J, et al. Ultrasensitive prostate specific antigen assay following laparoscopic radical prostatectomy–an outcome measure for defining the learning curve. Ann R Coll Surg Engl. 2009;91:399. doi: 10.1308/003588409X428289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atug F, Castle EP, Srivastav SK, et al. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol. 2006;49:866. doi: 10.1016/j.eururo.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson GG, Ames CD, Weld KJ, et al. Prospective evaluation of learning curve for laparoscopic radical prostatectomy: identification of factors improving operative times. Urology. 2005;66:840. doi: 10.1016/j.urology.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Eden CG, Neill MG, Louie-Johnsun MW. The first 1000 cases of laparoscopic radical prostatectomy in the UK: evidence of multiple ‘learning curves’. BJU Int. 2009;103:1224. doi: 10.1111/j.1464-410X.2008.08169.x. [DOI] [PubMed] [Google Scholar]
- 8.Bianco FJ, Jr, Riedel ER, Begg CB, et al. Variations among high volume surgeons in the rate of complications after radical prostatectomy: further evidence that technique matters. J Urol. 2005;173:2099. doi: 10.1097/01.ju.0000158163.21079.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastham JA, Kattan MW, Riedel E, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170:2292. doi: 10.1097/01.ju.0000091100.83725.51. [DOI] [PubMed] [Google Scholar]
- 10.Saranchuk JW, Kattan MW, Elkin E, et al. Achieving optimal outcomes after radical prostatectomy. J Clin Oncol. 2005;23:4146. doi: 10.1200/JCO.2005.12.922. [DOI] [PubMed] [Google Scholar]
- 11.Prasad SM, Maniar HS, Soper NJ, et al. The effect of robotic assistance on learning curves for basic laparoscopic skills. Am J Surg. 2002;183:702. doi: 10.1016/s0002-9610(02)00871-1. [DOI] [PubMed] [Google Scholar]
- 12.Caballero Romeu JP, Palacios Ramos J, Pereira Arias JG, et al. Radical prostatectomy: evaluation of learning curve outcomes laparoscopic and robotic-assisted laparoscopic techniques with radical retropubic prostatectomy. Actas Urol Esp. 2008;32:968. doi: 10.1016/s0210-4806(08)73974-3. [DOI] [PubMed] [Google Scholar]
- 13.Stolzenburg JU, Rabenalt R, Do M, et al. Modular training for residents with no prior experience with open pelvic surgery in endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2006;49:491. doi: 10.1016/j.eururo.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Touijer K, Kuroiwa K, Saranchuk JW, et al. Quality improvement in laparoscopic radical prostatectomy for pT2 prostate cancer: impact of video documentation review on positive surgical margin. J Urol. 2005;173:765. doi: 10.1097/01.ju.0000146574.52402.d5. [DOI] [PubMed] [Google Scholar]
- 15.Touijer K, Kuroiwa K, Vickers A, et al. Impact of a multidisciplinary continuous quality improvement program on the positive surgical margin rate after laparoscopic radical prostatectomy. Eur Urol. 2006;49:853. doi: 10.1016/j.eururo.2005.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickers AJ, Bianco FJ, Gonen M, et al. Effects of pathologic stage on the learning curve for radical prostatectomy: evidence that recurrence in organ-confined cancer is largely related to inadequate surgical technique. Eur Urol. 2008;53:960. doi: 10.1016/j.eururo.2008.01. [DOI] [PMC free article] [PubMed] [Google Scholar]