Abstract
Longstanding results with calorie and growth factor restriction plus recent results with the first interventional drug suggest that retarding the pace of aging to improve the quality of life of older people is at hand. The biological system targeted by these approaches is the target of rapamycin (TOR), which is central for cellular responses to a variety of stimuli including stressors, growth factors, and nutrients and energy states. That the life-extending response to reducing its activity is highly conserved from yeast to mammals is consistent with the evolution of aging as a strategy to preserve reproductive potential of young cells and animals.
Keywords: mTOR, Rapamycin, Healthy lifespan, Growth factor restriction, Caloric restriction
Aging: the deepest and hardest problem in biology?
Because everyone ages, and every biologist has his/her perspectives from which they view life, almost all who become interested in aging (and many scientists do because it is a common problem) initially have a unique angle on the answer to the problem. If you are a geneticist, the nucleus is where it’s at. If you are an immunologist, the immune system is the ticket. Here is the dilemma: everyone is probably correct in his or her view. The reason for this, I believe, is because aging is so interwoven into the whole fabric of life, that is it is fairly easy to pick some aspect and say—ah HA! that’s it! It is also why, I believe, that many of our late adult-onset diseases, such as cancer, and cardiovascular and neurodegeneration, are all age-related. If this is correct, the base problem in all of these issues is aging, which means, to paraphrase Dobzhansky, nothing about them makes any sense except in the light of aging.
Is aging so central to life that one might say ‘life is aging,’ or ‘aging is life’? Another way I have thought about this is that life, as we know it, may not be possible without aging. But, is there any evidence of such a position? Quite a lot, I submit.
First, let us start with very small organisms (notice I did not say simple) beginning with work on asymmetrical partitioning of damaged proteins in symmetrically dividing bacteria. In Escherichia coli, there is a systematic accumulation of protein aggregates in the old pole of new cells, which leads to a “progressively greater aggregate load in older cells” [1]. The same authors then showed that the presence of aggregates leads to decreased growth potential. Maisonneuve et al. [2] proposed that increased aggregate accumulation constituted an aging factor involved in cell death. This type of subcellular polarization is a common strategy that prokaryotes exploit to regulate essential functions [3, 4]. Another example of the importance of polarity and aging in the prokaryotic world is cell senescence observed in the asymmetrically dividing microorganism, Caulobacter crescentus [5]. Thus, very early in its evolution, life appears to have taken advantage of asymmetrical partitioning of damaged goods into older cells as a way of maintaining reproductive capacity in younger cells.
In single cell eukaryotes, there is also evidence of segregation of damaged macromolecules. In symmetrically dividing Schizosaccharomyces pombe, damage-enriched daughters show evidence of prolonged generation times and aging, which is dependent upon sir2 [6]. In budding yeast, there is also evidence of asymmetric partitioning of damaged macromolecules to mother cells as a strategy to rejuvenate the replicative potential of daughters. Egilmez and Jazwinski [7] initially observed this in Saccharomyces cerevisiae, and described it as an unknown “diffusible cytoplasmic factor(s)”. Asymmetric sir2-dependent partitioning of oxidatively damaged proteins to mothers occurs during cytokinesis [8, 9], on which calorie restriction (CR) has a beneficial effect [10]. Recently, Liu et al. [11] identified the polarisome as a required component in the segregation of damaged protein aggregates to yeast mother cells. Extrachromosomal rDNA circles also partition to the mothers [12] by a process involving a septin-dependent diffusion barrier in the nuclear envelope [13], but this was recently questioned [14]. Thus, ample evidence indicates that asymmetric partitioning of damaged goods resulting in aged cells has a vital role in maintaining the rejuvenation of offspring in unicellular prokaryotes and eukaryotes. What about metazoans?
Rujano et al. [15] examined aggresomes and their localization in several models and provided evidence that (a) they do not hinder mitosis in tissue culture cells and they are asymmetrically partitioned in daughters; (b) they are asymmetrically inherited in the de novo-generated neuroblast cells in Drosophila melanogaster that express the Huntington’s Htt-Q128 protein; (c) they are also differentially localized in the committed crypt cells, but not stem cells, in intestine obtained from patients with a protein folding disease (SCA3).
Macara and Mili [4] proposed that “polarity evolved very early and is a universal and essential attribute of cellular organisms.” They also posit that the initial driver of this evolutionary strategy was a mechanism to deal with accumulated damage materials, i.e. aging.
Finally, evidence of this evolutionary principle is found in insect superorganisms. In social insects, there is an age-dependent division of labor (temporal polyethism) wherein younger workers tend queens and the brood, while older workers perform the more dangerous out-of-the-nest tasks such as foraging. Thus, it appears that older members (comparable to cells carrying more damaged goods) are expended to insure that the reproductive members (comparable to the germ line) provide for reproduction and survival of the colony as a whole. Hölldobler and Wilson [16] provide an elegant discussion of the programming of these stages, which are guided by natural selection. Another example of this evolutionary principle in the insect world was recently reported. In the gall-forming aphids Quadrartus yoshinomiyai, older postreproductive females defend the colony by sticking themselves to predators with a waxy secretion that accumulates with age [17], thus preserving reproductive capacity of younger members.
TOR and its signaling
So, if aging is necessary for life, should we be fooling around trying to diminish its effect on the quality and quantify of life? While eliminating the outcome of aging is highly unlikely, retarding its pace and its detrimental effects on the quality of life is now an achievable goal in mammals. Years of work showing the effectiveness of calorie and growth factor restriction is the basis of this statement and implies that aging can be regulated, or at least modulated. And, if it can be regulated, what is/are the regulator(s)/modulator(s)? In recent years, a strong candidate has emerged: it is the target of rapamycin (TOR) signaling pathway, which functions cell-autonomously in the regulation of cellular responses to a variety of stimuli including cell stressors, nutrients and growth factors. Evidence continues to mount that TOR is a prime causal factor in the forces that lead to a phenotype that everyone recognizes as an aged animal. Additionally, these studies strongly suggest that TOR function is, at least partially, dispensable in, and perhaps detrimental to, aging eukaryotes, a process referred to as antagonistic pleiotropy [18]. These finding suggest that limiting TOR activity in adults by a compound such as rapamycin would have beneficial effects on the pace of aging.
The intracellular target of the macrocyclic lactone, rapamycin (reviewed in reference [19]), in eukaryotes belongs to a conserved family of stress response kinases (phosphatidylinositol kinase-related kinases) including ataxia telangiectasia mutated, ataxia telangiectasia and Rad3 related, and DNA-dependent protein kinase, catalytic subunit. The defining feature of TOR kinases is an FKBP12/rapamycin-binding domain through which rapamycin allosterically inhibits TOR kinase activity (reviewed in reference [19]).
In mammals, TOR participates in two complexes, mTORC1 and mTORC2. One other protein, mLST8, is common to both C1 and C2. Raptor is the defining member of C1, while Rictor specifies C2. C1 is sensitive to rapamycin, C2 is not except in a subset of cell types [20]. Little is known regarding the sensitivity of these complexes to acute or sustained rapamycin treatments in the organs and cells of differing types in mammals.
Figure 1 illustrates the aging-related aspects of mammalian TOR complex 1 signaling. Note that mTORC1 integrates inputs from cell stresses, growth factor stimuli, and energy and nutrient states. There are excellent reviews and recent original reports on the role of mTOR in translation regulation [21, 22], autophagy [23], lipid synthesis [24], cell growth/division [25], and cell senescence [25, 26]. Of special interest for aging is a recent leap in our understanding regarding nutrient signaling. It arrived with the discovery that Rag GTPases and a companion regulator (Ragulator) play a vital role in relaying amino acid levels to the mTORC1 system. Since, CR is the gold standard for an antiaging intervention, it is important to discuss this aspect in more detail.
Fig. 1.
Mammalian target of rapamycin complex 1 signaling pertinent to aging. a In a replete or wild-type setting, mTORC1 receives inputs from growth factor receptors such as IGF-I via Akt and the tuberous sclerosis complex (TSC2). Energy status is monitored by AMPK, which also signals through TSC2. Recently it has been found that amino acids signal to mTORC1 via Rag GTPases (RagA-RagD) in conjunction with a regulator (Ragulator) complex on endosomal membranes (reviewed in reference [122]. Two major outputs of mTORC1 signaling are S6 kinase 1 (S6K1) and 4E-BP1 translation (cap dependent) repressor. Various types of cell stresses limit mTORC1 activity through p53 or Redd1/2 [123]. In this setting, mTORC1 promotes anabolic activities and is nonpermissive for autophagy. b mTORC1-directed interventions that reduce its activities and increase lifespan include CR (hypothetical at this point), genetic models with restriction of growth factor signaling [57, 58], and an mTOR inhibitor, rapamycin [63]. Biguanides, such as metformin, have been recently shown to inhibit mTORC1 indirectly via the nutrient (amino acid)-responsive Rag GTPases [95], and may promote longevity. This is an oversimplification of a much more complex system with increasingly varied cell autonomous and systemic functions [19]
Rag GTPases were identified as critical effectors linking amino acid signaling with mTORC1 activation [27, 28]. Heterodimeric Rags containing RagA/BGTP or RagC/DGDP (e.g. RagAGTP·RagCGDP, RagBGTP·RagCGDP or RagBGTP·RagDGDP) promote the activation of the mTORC1 complex [27, 28]. The presumptive site of activation occurs at the endosomal membrane compartment through a sequence of protein–protein interactions that drive the activation of mTORC1 by amino acids [27, 28]. At this compartment, RagBGTP·RagDGDP heterodimers serve as a bridge linking a membrane-bound heterotrimeric scaffolding complex (called Ragulator) to mTORC1 [29]. Another GTPase at this location, Rheb, is a well-established activator of mTORC1 that acts in response to amino acids and other anabolic signaling such as growth factors. Sancak et al. [29] also showed in cell-based assays that insulin signaling to mTORC1 is dependent upon amino acids and the integrity of the Ragulator, suggesting that the well-established responses of mTORC1 to mitogens and cytokines may also signal via these endosomal complexes. These important discoveries place Rag GTPases and their Ragulator at the center of cellular responses to nutrients and other anabolic stimuli that are transmitted via mTORC1.
TOR in age modulation
Because decreasing TOR activity (by a variety of mechanisms) in a wide range of eukaryotes (see below) results in extended longevity, one general conclusion can be reached at the outset, which is in tune with my initial comments: these systems and their responses to interventions must be highly conserved [30, 31].
Yeast
This single-cell eukaryote has been used extensively for aging studies. It is popular for genetic screens, which have identified candidate mechanisms (evolutionarily conserved) mediating lifespan extension by CR. More recently this organism has been used to screen for compounds that enhance or suppress cellular responses to rapamycin [32]. In yeast, decreasing TOR signaling extends chronological lifespan [33]. Considering this and other findings, Huber et al. [34] proposed a model in which yeast TOR, Sch9 (S6K1 ortholog, see also reference [35] for longevity study), and PKA are targets for extension of both chronological and replicative lifespan by CR [36]. Additional evidence supports models in which altered translation, stress responses [37, 38], and/or mitochondrial function [39, 40] in response to reduced TOR activity are also associated with lifespan increases in yeast.
Rapamycin as a prolongevity treatment has also been investigated yeast. Powers et al. [33] showed that decreasing TOR activity by treatment with rapamycin resulted in extended chronological lifespan. Medvedik et al. [41] showed that it extended replicative lifespan, which also requires a transcriptional response.
Nematode worms
These small metazoans are also very useful for genetic studies, which have yielded numerous genes and their products important in aging and lifespan extension [42]. For an excellent review of reduced activity of insulin/insulin-like growth factor I (IGF-I) signaling (IIS) in lifespan extension in Caenorhabditis elegans see reference [31]. Importantly, IIS pathways also interact closely with the TOR system and its downstream effector, RSK-1 (S6K) in C. elegans. Extended longevity is also strongly correlated with decreased activity of TOR in adult C. elegans [43–45], including a decrease associated with a reduction in CeRaptor (daf15, [46]). Longevity resulting from a reduction of either CeTOR or rsks-1 (S6K ortholog) requires a transcriptional response mediated by pha-4, a gene encoding a FoxA transcription factor [47].
Syntichaki et al. [48] showed that loss of a specific eIF4E isoform (IFE-2), which functions as a Cap-binding translation regulatory factor specifically in somatic tissues, reduces global protein synthesis, and extends lifespan in C. elegans. IFE-2 deficiency also further extends the already long-lived worms carrying mutations in the Age, Daf, Clk or Eat genes. A reduction of CeTOR results in an additional increase the longevity of ife-2 mutants. Pan et al. [49] observed that reductions in components of the translation initiation complex, including ifg-1, the worm homologue of the gene encoding mammalian eIF4G (scaffolding protein for eIF4F initiation complex [21]) and rsk-1 extend lifespan. These studies and a report by Hamilton et al. [50] are strong arguments for the involvement of altered translation as one mechanism for lifespan extension. Mair and Dillin [51] have cautioned that RNA inhibition of let-363 (CeTOR) in C. elegans needs further tests to conclude that its reduction is causal in lifespan extension. To my knowledge, rapamycin treatment of adult animals has not been tested for lifespan extension in C. elegans. Rapamycin treatment did result in developmental defects, which reverted upon removal of the drug [52].
Fruit fly
The positive effect of reduced IIS and diet restriction, both of which are linked to the TOR system in Drosophila melanogaster, has been discussed extensively by Fontana et al. [31]. In D. melanogaster, Kapahi et al. [53] showed that reduced Drosophila TOR (dTOR) activity is also associated with extended longevity. Later, Zid et al. [54] also showed that d4E-BP (another downstream effector of TOR) is necessary for a normal lifespan, and its overexpression increases longevity. As in C. elegans, lifespan extension by CR in flies does not depend upon the dFOXO transcription factor, which is required for longevity (when the ortholog of daf-16 is altered), although dFOXO possibly modulates the response to CR [55]. These findings are consistent with the above model in Fig. 1, in which mTORC1 integrates insulin/growth factor and nutrient signaling, and its reduction alone is sufficient to extend lifespan. If there is a role for nuclear signaling in longevity in this setting, it appears to be minimal.
Recent work by Bjedov et al. [56] showed that treatment with rapamycin extends the lifespan of D. melanogaster. In this study, the authors also provided evidence that dTORC1, but not dTORC2, mediates the lifespan extension by affecting autophagy and translation. Interestingly, rapamycin treatment of flies undergoing CR also extends lifespan, concomitant with a reduction in protein synthesis in concert with the findings in worms and yeast. In flies carrying weak mutations in the insulin receptor, rapamycin treatment also extends lifespan. The authors concluded that (a) based on results in mice and flies, the mechanisms involved in lifespan extension are evolutionarily conserved; (b) dTORC1 is responsible for the lifespan extension following rapamycin treatment; (c) the extension of lifespan following rapamycin treatment includes aspects of CR and reduced IIS. The last point is consistent with the above model in which TOR integrates these inputs for appropriate responses to external and internal stimuli, and, when reduced, leads to extended longevity.
Small mammals
Long-lived dwarf mice, a model of growth factor restriction, show downregulation in mTOR effectors in liver and skeletal muscle [57, 58]. Selman et al. [59] showed that gene expression profiles of S6K1 −/− (a downstream effector of mTOR, Fig. 1) mice are similar to those of calorie-restricted mice. In females, abrogation of S6K1 resulted in lifespan extension. These studies support the view that the mTORC1 pathway is integral to the lifespan extension by CR, although to date little direct evidence for mTORC1 downregulation in the major organs over the lifespan of calorie-restricted mice has been reported.
Lifespan is extended in mice and numerous other organisms by limiting nutrients (CR) [60] or limiting growth factor signaling in genetic models [61] or both [62]. It has been postulated [19] that rapamycin, an allosteric inhibitor of mTOR, would pharmacologically mimic one or both or these conditions to effect an extension of lifespan in mice. Harrison et al. [63] in the NIA Intervention Testing Program (ITP) rigorously tested a novel form of enterically delivered rapamycin for extension of lifespan in four-way cross, genetically heterogeneous mice (reviewed in reference [19]). Remarkably, a significant extension of lifespan in older male and female mice (20 months of age, 60 in human years) was observed at all three of the ITP test sites [63]. Since CR in most studies works best when started early [60], this was a surprising proof of principle that an aging intervention does not necessarily have to be started early in life. The results in a second cohort of mice started on rapamycin chow at 9 months of age by the ITP were also reported as interim results by Harrison et al. [63]. This second study has now been completed, and again the results show that chronic treatment with microencapsulated rapamycin extends the lifespan of ITP mice [64]. Additionally, the question of whether the mice are healthier was also addressed by obtaining activity assessments of the 9-month cohort at each of the ITP test sites. Chronic rapamycin reduced the rate of loss of movement, suggesting that long-term mTORC1 inhibition may extend the healthy lifespan. What is unclear at this point is how chronic enteric rapamycin, a purported immunosuppressant, extends longevity (see below).
Humans
In a study of Ashkenazi Jewish centenarians, Suh et al. [65] found an over-representation of heterozygous mutations in the IGF-I receptor gene of females that was associated with higher levels of endocrine IGF-I. Importantly, in transformed lymphocytes, an association between receptors carrying an Arg-407→His mutation with lower IGF-I response was documented by immunoassays of Akt phosphorylation. Thus, longevity in humans is associated with downregulation of signaling systems that intersect with mTORC1. In this vein, it would be interesting to determine if these altered IGF-IRs mediate a modulated responsiveness at the level of mTORC1. In a related study, Kuningas et al. [66] showed that variants of the human gene encoding FOXO transcription factor (ortholog of the C. elegans and D. melanogaster factors discussed above) are associated with human longevity.
CR in humans has been shown to be associated with traits consistent with those observed in other animal models [31, 67–69]. In an impressively designed and performed study of CR in rhesus monkeys, Colman et al. [70] reported results indicating an expected benefit in longevity and youthful appearance. However, the significance of the longevity in this study has been questioned [71]. Because of their relatively short life history, other primates ideally suited to the study of the mTOR system in general (more specifically rapamycin) in an aging context would be marmosets and tamarins [72].
Mechanism of rapamycin effect
CR mimic?
As discussed by Harrison et al. [63], CR reduces body weight, but rapamycin in the four-way cross mouse studies did not, except for a slight weight loss in cohort 3 mice that started rapamycin at 9 months [64]. In most studies reported, CR resulted in little if any benefit when started late (550 days or later). Since it is predicted that rapamycin is allosterically inhibiting mTOR kinase in most, if not all, tissues, perhaps it is mimicking both calorie and growth factor restriction. At this point, much study is needed to determine if the rapamycin effect(s) is (are) cell-autonomous, organs/system-specific or both. While visceral adipose tissue is sensitive to chronic rapamycin [63], studies are under way to determine if other organs show similar levels of sensitivity. Another question is: are there temporal (acute vs. chronic), and/or sex-specific responses to enteric rapamycin? We have much to learn concerning rapamycin’s ability to extend lifespan, and in the process gain additional knowledge about aging itself.
Translation?
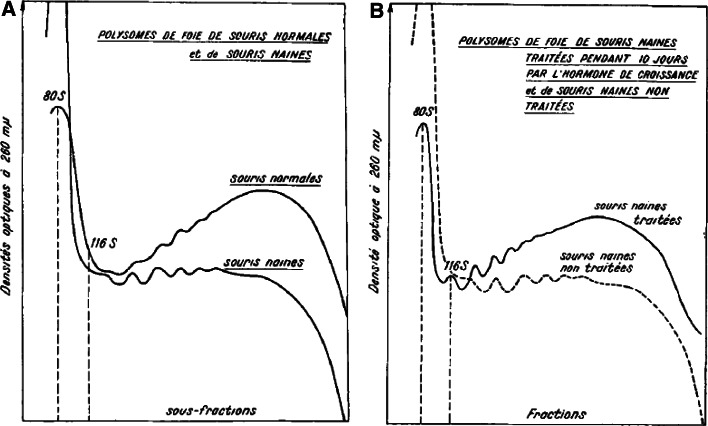
A modulation of cap-dependent translation was discussed above in the sections on yeast, worm and fly studies. Interestingly, it was, in a way, also addressed many years ago in early studies of protein synthesis in long-lived (not known at the time) pituitary dwarf mice. Endocrine growth hormone (GH) and IGF-I signaling is greatly reduced in dwarf mice, and administration of GH or IGF-I restores somatic growth. In 1973, Korner [73] argued that, irrespective of the types of proteins, the increase in the rate of protein synthesis in the liver stimulated by GH is regulated at the cytoplasmic level. GH treatment of Snell dwarfs (also deficient in GH/IGF-I signaling) is associated with an increase in liver growth [74] and rate of protein synthesis [75]. Similar to the reduction of polysomes in long-lived yeast [76] and flies [55], comparisons of polysome profiles in dwarf and normal-size mice also showed a lower proportion of polysomes to monosomes, which are restored to normal with GH, but not with T3, treatment [77]. Figure 2 shows the reduced polysome profile of Snell dwarfs compared to normal-size siblings (Fig. 2a), and that treatment of dwarfs with GH restores that profile (Fig. 2b) [77]. It would be interesting to determine the effect that CR and/or rapamycin has on GH- and IGF-I-stimulated increases in polysome profiles in these dwarf mice.
Fig. 2.
Snell dwarf mouse liver polysome profiles. a Plots show the reduced polysome profile of dwarf mice (souris naines) compared to normal size siblings (souris normales). b GH treatment of dwarf mice (souris naines traitées) restores the polysome profile. These are Figs. 2 and 1, respectively, from reference [77]
Autophagy?
An increase in autophagy is another predicted mechanism that could be involved in longevity promoted by chronic rapamycin treatment. A role for autophagy in C. elegans lifespan extension is discussed above. Food restriction increases macroautophagy and has been suggested to provide a “cleansing” effect that promotes longevity [78]. Importantly, chronic rapamycin treatment has been shown to result in an increase in autophagy in the brains of mice [79], consistent with this expectation.
Gcn4 and general amino acid control?
Steffen et al. [76], reported that lifespan extension in response to depletion of 60S subunits in yeast is dependent upon functional Gcn4, a transcription factor regulated by nutrition. Furthermore, TOR inhibition increases Gcn4 [76]. This is supported by the work of Alvers et al. [80], which demonstrated a requirement for Gcn4 in lifespan extension in yeast in response to amino acid supplementation of cells deficient in ATB1 or ATG7 (autophagy factors). In response to nutrient stress, Gcn4, which is a transcription factor, activates expression of genes encoding proteins functional in the amino acid biosynthetic pathway [81]. Upon inhibition of TOR signaling, Gcn4 activity increases [82–84]. It has been speculated that, as cells age, endoplasmic reticulum stress may increase to a level such that ribosomal subunit gene transcription is no longer supported [76]. Reduction in 60S, but not 40S, subunits blocks this response [85, 86], and in the process may prolong viability.
Cell senescence?
A hallmark of cell senescence is an increase in size [87]. TOR plays a central role in the regulation of cell size [88, 89]. Based on this, treatment of cells with rapamycin might be expected to delay or inhibit cell senescence [90], thereby promoting longevity. These predicted effects on cell senescence were dramatically confirmed by Castilho et al. [91] who showed that rapamycin blocks hair follicle alterations and stem cell senescence in a Wnt-overexpression model. A question raised by this work is: are stem cells in other tissues of aging animals responsive to rapamycin?
Metformin as an antiaging intervention?
One potential pharmacological approach toward the development of new aging interventions that might work when started in older organisms (similar to rapamycin) is metformin and its related biguanide, phenformin. For 50 years, metformin has been used to treat diabetes. It is currently the front-line drug for treatment of type 2 diabetes. Metformin treatment results in an increase in AMPK activity, which is associated with an increase in fatty acid oxidation, an inhibition of lipogenic genes, a decrease in liver glucose production, an increase in intestinal glycolytic lactate production, and a stimulation of peripheral glucose uptake (reviewed in reference [92]). AMPK activation by metformin is not direct, with the mechanism of its activation being the subject of debate [92]. Regardless of the mechanism, metformin treatment of cultured cells results in a decrease in mTORC1 activation, as assessed by 4E-BP1 and S6K1 phosphorylation status [93]. Based on the work of Dowling et al. [94], AMPK activation by biguanides and repression of mTORC1 was thought to involve AMPK phosphorylation of TSC2 (Fig. 1). AMPK phosphorylation of TSC2 enhances its GAP activity leading to an increase in the levels of GDP-bound Rheb and mTORC1 inhibition [95]. Recently, however, it was found that biguanides repress mTORC1 in cells lacking TSC1/2 and AMPK [96]. Remarkably, the same authors found that instead biguanide repression of mTORC1 is dependent upon Rag GTPases discussed above.
That metformin inhibits mTORC1 via the Rag GTPases at the nexus of cellular responsiveness to amino acids provides a strong rationale for testing chronic metformin for its ability to extend lifespan in older mice in a way similar to that shown for rapamycin. However, the results thus far are mixed in studies using metformin as an antiaging and age-related disease intervention. The earliest go back 30 years with a report that phenformin suppressed chemically induced tumors in rats, and reduced spontaneous tumors in mice [97], resulting in a 23% increase in lifespan [98]. Since then, a number of studies have been performed in mice, rats and hamsters on the effects of biguanides (and other antidiabetic drugs) on the inhibition of spontaneous and carcinogen-induced tumors (summarized in reference [99]). A tumor-prone mouse model (HER-2/neu) responds to metformin treatment (subcutaneous injections) by a reduction in the number of tumors per mouse and a delay in their onset, and little if any increase in lifespan, except for the last 10% of survivors [100, 101].
Two studies examined the ability of metformin to extend lifespan in mice not prone to tumor. Anisimov et al. [100] found evidence of increased lifespan in SHR mice (an outbred strain [102]) treated with metformin in their drinking water (100 mg/kg). The strongest effect in this study was in the last 10% of surviving mice. In a study of Fisher 344 rats, Smith et al. [103] found that metformin treatment using food supplementation (300 mg/kg) had no effect on lifespan. Caveats associated with both of these studies are: (a) no evidence was provided of expected metabolic effects (glucose and insulin) of metformin in either of these delivery systems; (b) no measurements of blood levels or tissue effects (including mTORC1 inhibition) were done; (c) although there were effects of metformin on food consumption, Anisimov et al. [100] had no pair fed controls. While metformin/phenformin looks promising as a pharmacological approach to slow down aging, further studies are needed.
mTORC1 inhibition and age-related diseases
One of the tenets of aging research is that by reducing the rate of aging, one will delay the onset of age-related diseases [30, 104]. This is based on the outcomes of many years research on diet- and growth factor-restricted models. As examples, food-restricted rats have an overall reduced incidence of cancers [105], Ames dwarf mice (a model of growth factor restriction) have a delayed occurrence and reduced severity of cancers [106], and female mice deficient in S6K1 have extended longevity, while both sexes show evidence of resistance to age-related pathologies including bone, immune, motor dysfunction and loss of insulin sensitivity [59].
In the treatment of neurological disorders including Huntington’s, Alzheimer’s disease (AD) and Parkinson’s disease, mTORC1 inhibition is likely to be useful via attenuation of protein aggregate accumulation, increased autophagy and translation of the tau protein in AD [107–109]. The effectiveness of rapamycin analogs (rapalogs) in autoimmune disorders including rheumatoid arthritis, psoriasis, multiple sclerosis, and Parkinson’s disease [110] is under investigation. There is a suggestion that rapalogs may be effective in the treatment of familial cardiomyopathy and Wolff-Parkinson-White syndrome [111, 112]. This raises the question: would preemptive and prolonged rapamycin treatment of predisposed individuals prevent or delay the declaration of symptomatic diseases in these classes?
In the ITP studies, treated and control cohort 2 mice (rapamycin at 20 months) showed the same pattern of diseases [63]. In cohort 3 ITP mice (rapamycin at 9 months), the apparent causes of death of control and treated mice were also the same with modest changes in hemangiosarcoma, lymphoma, and lung carcinoma [64]. One interpretation of these findings is that chronic rapamycin is somehow delaying the onset or reducing the severity of diseases of age that are the cause of mortality. Cross sectional data are needed to distinguish between these or other potential reasons underlying the ability of rapamycin to extend lifespan.
Recently two studies on model mice consuming rapamycin chow underscore its potential to positively affect one of the worst age-related afflictions, AD. Spilman et al. [79] showed that long-term treatment with enteric rapamycin prevents cognitive impairments associated with AD, and lowers the levels of a major toxic species in AD (Aβ42) in the PDAPP transgenic mouse model. These investigators also found that autophagy is upregulated in response to high levels of Aβ in this model [79]. In another study, Caccamo et al. [113], showed in the 3xTg-AD mouse model that a build up of Aβ is associated with an increase in mTOR activation, while chronic treatment with enteric rapamycin reduces Aβ levels. Importantly, chronic rapamycin treatment again rescues cognitive impairment and ameliorates Aβ and Tau pathologies, which is also associated with an increase in autophagy. Thus, in these two separate studies, chronic rapamycin restored impairments in learning and memory associated with AD, suggesting that similar approaches using FDA-approved rapamycin (or rapalogs) in human patients could have similar positive effects.
What’s on the horizon?
The mTOR pathways are, in my opinion, a gold mine for the discovery of novel targets and compounds that will have antiaging benefits. Approval of rapamycin, metformin and other newly discovered drugs that modulate aging for use in humans, will require that the FDA modifies its current policy of only approving drugs for specific indications (diseases). Likely clinical trials involving antiaging intervention in humans, when they are permitted, will initially involve patients who are either predisposed to a specific age-related disease or those in early disease stages where an intervention might ameliorate its progression.
For rapamycin (and rapalogs), the issue of immunosuppression needs addressing before long-term use is warranted in humans. In recent preclinical studies, treatment of mice with rapamycin had immunostimulatory effects on the generation of memory CD8 T cells [114]. Rapamycin-induced autophagy also improves vaccination efficacy [115]. Do these unexpected effects have antiaging implications for an increase in healthy lifespan? And, are there other biological systems upon which rapamycin will have effects contrary to current expectations and would they promote a healthy lifespan? In an indirect way, this issue has also been addressed in cancer patients. In long-term stable disease settings, rapalogs as a monotherapy are well tolerated, and, importantly, show little if any immunosuppressive side effects [116–120]. Thus, the supposed immunosuppressive effects of chronic rapamycin treatment may be less of an issue than envisioned.
As mentioned previously, rapamycin in complex with FKBP12 is an allosteric inhibitor of mTOR kinase activity. Will kinase inhibitors that inhibit both mTORC1 and C2 (reviewed in reference [121]) be better or worse as antiaging agents? The issue for these agents is toxicity, which is still in the process of being determined [122]. My current view is that allosteric inhibition may be a key for the ability of rapamycin to extend a healthy lifespan, which would likely allow a wider dose range for efficacy and a lower level of unwanted side effects. A smaller dose range for kinase inhibitors may also be just as effective. Another question is: how will TOR modulation interact with the other signaling/metabolic pathways also involved in age regulation/modulation?
Conclusion
Slowly, aging, one of the deepest and most difficult of biological problems is yielding some of her secrets, one of which is the TOR system, whose downmodulation has significant benefits for aging. While we will probably not be able to reverse the effects of aging, there is now real hope that we can, by further dissecting these pathways for the discovery of new interventions, minimize its effects until near the very end.
Acknowledgments
Numerous discussions with the experts in aging research in the Institute of Biotechnology and Barshop Institute for Longevity and Aging Studies are gratefully acknowledged. I would also like to thank ITP investigators, Randy Strong, Rich Miller, David Harrison and Nancy Nadon for their knowledge and expertise in conducting aging studies, and for being wonderful colleagues in this ongoing adventure. Work in the author’s laboratory is supported by a Grand Opportunity grant from the NIH and by the Glenn Foundation. Finally, I would like to thank the anonymous reviewers for very helpful and insightful suggestions for improving the paper.
References
- 1.Lindner AB, Madden R, Demarez A, Stewart EJ, Taddei F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc Natl Acad Sci U S A. 2008;105:3076–3081. doi: 10.1073/pnas.0708931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisonneuve E, Ezraty B, Dukan S. Protein aggregates: an aging factor involved in cell death. J Bacteriol. 2008;190:6070–6075. doi: 10.1128/JB.00736-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro L, McAdams HH, Losick R. Generating and exploiting polarity in bacteria. Science. 2002;298:1942–1946. doi: 10.1126/science.1072163. [DOI] [PubMed] [Google Scholar]
- 4.Macara IG, Mili S. Polarity and differential inheritance – universal attributes of life? Cell. 2008;135:801–812. doi: 10.1016/j.cell.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Stearns SC, Jenal U. Senescence in a bacterium with asymmetric division. Science. 2003;300:1920. doi: 10.1126/science.1083532. [DOI] [PubMed] [Google Scholar]
- 6.Erjavec N, Cvijovic M, Klipp E, Nyström T. Selective benefits of damage partitioning in unicellular systems and its effects on aging. Proc Natl Acad Sci U S A. 2008;105:18764–18769. doi: 10.1073/pnas.0804550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egilmez NK, Jazwinski SM. Evidence for the involvement of a cytoplasmic factor in the aging of the yeast Saccharomyces cerevisiae. J Bacteriol. 1989;171:37–42. doi: 10.1128/jb.171.1.37-42.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erjavec N, Nyström T. Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2007;104:10877–10881. doi: 10.1073/pnas.0701634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 10.Reverter-Branchat G, Cabiscol E, Tamarit J, Ros J. Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae. J Biol Chem. 2004;279:31983–31989. doi: 10.1074/jbc.M404849200. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Larsson L, Caballero A, Hao X, Öling D, Grantham J, Nyström T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair DA, Guarente L. Extrachromosomal rDNA circles – a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 13.Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y. A mechanism for asymmetric segregation of age during yeast budding. Nature. 2008;454:728–734. doi: 10.1038/nature07212. [DOI] [PubMed] [Google Scholar]
- 14.Khmelinskii A, Keller PJ, Lorenz H, Schiebel E, Knop M. Segregation of yeast nuclear pores. Nature. 2010;466:E1. doi: 10.1038/nature09255. [DOI] [PubMed] [Google Scholar]
- 15.Rujano MA, Bosveld F, Salomons FA, Dijk F, van Waarde MAWH, van der Want JJL, de Vos RAI, Brunt ER, Sibon OCM, Kampinga HH. Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 2006;4:2325–2335. doi: 10.1371/journal.pbio.0040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hölldobler B, Wilson EO. The superorganism: the beauty, elegance and strangeness of insect societies. New York: W. W. Norton; 2009. [Google Scholar]
- 17.Uematsu K, Kutsukake M, Fukatsu T, Shimada M, Shibao H. Altruistic colony defense by menopausal female insects. Curr Biol. 2010;20:1182–1186. doi: 10.1016/j.cub.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 18.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 19.Sharp ZD, Strong R. The role of mTOR signaling in controlling mammalian life span: what a fungicide teaches us about longevity. J Gerontol A Biol Sci Med Sci. 2010;65:580–589. doi: 10.1093/gerona/glp212. [DOI] [PubMed] [Google Scholar]
- 20.Sarbassov DD, Ali SM, Sengupta S, Sheen J-H, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 23.Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowling RJO, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blagosklonny MV. Rapamycin and quasi-programmed aging: four years later. Cell Cycle. 2010;9:1859–1862. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- 27.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasty P. Rapamycin: the cure for all that ails. J Mol Cell Biol. 2009;2:17–19. doi: 10.1093/jmcb/mjp033. [DOI] [PubMed] [Google Scholar]
- 31.Fontana L, Partridge L, Longo VD. Extending healthy life span – from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghajan M, Jonai N, Flick K, Fu F, Luo M, Cai X, Ouni I, Pierce N, Tang X, Lomenick B, Damoiseaux R, Hao R, del Moral PM, Verma R, Li Y, Li C, Houk KN, Jung ME, Zheng N, Huang L, Deshaies RJ, Kaiser P, Huang J. Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nat Biotech. 2010;28:738–742. doi: 10.1038/nbt.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber A, Bodenmiller B, Uotila A, Stahl M, Wanka S, Gerrits B, Aebersold R, Loewith R. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 2009;23:1929–1943. doi: 10.1101/gad.532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy BK, Smith ED, Kaeberlein M. The enigmatic role of Sir2 in aging. Cell. 2005;123:548–550. doi: 10.1016/j.cell.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Kaeberlein M, Burtner CR, Kennedy BK. Recent developments in yeast aging. PLoS Genet. 2007;3:e84. doi: 10.1371/journal.pgen.0030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaeberlein M, Kennedy BK. Protein translation. Aging Cell. 2007;6:731–734. doi: 10.1111/j.1474-9726.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 39.Schieke SM, Finkel T. TOR and aging: less is more. Cell Metab. 2007;5:233–235. doi: 10.1016/j.cmet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Bonawitz ND, Shadel GS. Rethinking the mitochondrial theory of aging: the role of mitochondrial gene expression in lifespan determination. Cell Cycle. 2007;6:1574–1578. doi: 10.4161/cc.6.13.4457. [DOI] [PubMed] [Google Scholar]
- 41.Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson TE. Caenorhabditis elegans 2007: the premier model for the study of aging. Exp Gerontol. 2008;43:1–4. doi: 10.1016/j.exger.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 44.Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 46.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 47.Sheaffer KL, Updike DL, Mango SE. The target of rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr Biol. 2008;18:1355–1364. doi: 10.1016/j.cub.2008.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445:922–926. doi: 10.1038/nature05603. [DOI] [PubMed] [Google Scholar]
- 49.Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 52.Yu H, Larsen PL. DAF-16-dependent and independent expression targets of DAF-2 insulin receptor-like pathway in Caenorhabditis elegans include FKBPs. J Mol Biol. 2001;314:1017–1028. doi: 10.1006/jmbi.2000.5210. [DOI] [PubMed] [Google Scholar]
- 53.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell. 2008;7:187–198. doi: 10.1111/j.1474-9726.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 56.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2005;60:293–300. doi: 10.1093/gerona/60.3.293. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh CC, Papaconstantinou J. Akt/PKB and p38 MAPK signaling, translational initiation and longevity in Snell dwarf mouse livers. Mech Ageing Dev. 2004;125:785–798. doi: 10.1016/j.mad.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Selman C, Tullet JMA, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson ICA, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp Gerontol. 2003;38:1353–1364. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 63.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller RA, Strong R, Sharp ZD, Harrison DE, Nadon NL et al (in press) Rapamycin, but not resveratrol or simvastatin, extends lifespan of genetically heterogeneous mice. J Gerontol [DOI] [PMC free article] [PubMed]
- 65.Suh Y, Atzmon G, Cho M-O, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuningas M, Magi R, Westendorp RG, Slagboom PE, Remm M, van Heemst D. Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. Eur J Hum Genet. 2007;15:294–301. doi: 10.1038/sj.ejhg.5201766. [DOI] [PubMed] [Google Scholar]
- 67.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E, Pennington CALERIE Team Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Austad S. Recent advances in vertebrate aging research 2009. Aging Cell. 2010;9:297–303. doi: 10.1111/j.1474-9726.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 72.Tardif SD, Araujo A, Arruda MF, French JA, Sousa MB, Yamamoto ME. Reproduction and aging in marmosets and tamarins. Interdiscip Top Gerontol. 2008;36:29–48. doi: 10.1159/000137678. [DOI] [PubMed] [Google Scholar]
- 73.Korner A. Growth hormone effects on RNA and protein synthesis in liver. J Cell Physiol. 1965;66(Suppl 1):153–162. doi: 10.1002/(SICI)1097-4652(200001)182:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 74.van Buul-Offers S, Van den Brande JL. Cellular growth in organs of dwarf mice during treatment with growth hormone, thyroxine and plasma fractions containing somatomedin activity. Acta Endocrinol (Copenh) 1982;99:150–160. doi: 10.1530/acta.0.0990150. [DOI] [PubMed] [Google Scholar]
- 75.Bates PC, Holder AT. The anabolic actions of growth hormone and thyroxine on protein metabolism in Snell dwarf and normal mice. J Endocrinol. 1988;119:31–41. doi: 10.1677/joe.0.1190031. [DOI] [PubMed] [Google Scholar]
- 76.Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, Pak DN, Fields S, Kennedy BK, Kaeberlein M. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mellet J. Etude de l’effectif ribosomique du foie chez la souris normale et chez la souris naine. Biochimie. 1973;55:189–194. doi: 10.1016/s0300-9084(73)80391-8. [DOI] [PubMed] [Google Scholar]
- 78.Cuervo AM. Calorie restriction and aging: the ultimate “Cleansing Diet”. J Gerontol A Biol Sci Med Sci. 2008;63:547–549. doi: 10.1093/gerona/63.6.547. [DOI] [PubMed] [Google Scholar]
- 79.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alvers AL, Fishwick LK, Wood MS, Hu D, Chung HS, Dunn WA Jr, Aris JP. Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell. 2009;8:353–369. doi: 10.1111/j.1474-9726.2009.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 82.Cherkasova VA, Hinnebusch AG. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev. 2003;17:859–872. doi: 10.1101/gad.1069003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kubota H, Obata T, Ota K, Sasaki T, Ito T. Rapamycin-induced translational derepression of GCN4 mRNA involves a novel mechanism for activation of the eIF2 alpha kinase GCN2. J Biol Chem. 2003;278:20457–20460. doi: 10.1074/jbc.C300133200. [DOI] [PubMed] [Google Scholar]
- 84.Valenzuela L, Aranda C, Gonzalez A. TOR modulates GCN4-dependent expression of genes turned on by nitrogen limitation. J Bacteriol. 2001;183:2331–2334. doi: 10.1128/JB.183.7.2331-2334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y, Sohn JH, Warner JR. Autoregulation in the biosynthesis of ribosomes. Mol Cell Biol. 2003;23:699–707. doi: 10.1128/MCB.23.2.699-707.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyoshi K, Tsujii R, Yoshida H, Maki Y, Wada A, Matsui Y, Toh EA, Mizuta K. Normal assembly of 60S ribosomal subunits is required for the signaling in response to a secretory defect in Saccharomyces cerevisiae. J Biol Chem. 2002;277:18334–18339. doi: 10.1074/jbc.M201667200. [DOI] [PubMed] [Google Scholar]
- 87.Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol Rev. 1993;73:617–638. doi: 10.1152/physrev.1993.73.3.617. [DOI] [PubMed] [Google Scholar]
- 88.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 90.Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–2102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- 91.Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804:581–591. doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 93.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dowling RJO, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 95.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 96.Kalender A, Selvaraj A, Kim SY, Gulati P, Br˚lÈ S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a Rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dilman VM, Berstein LM, Zabezhinski MA, Alexandrov VA, Bobrov JF, Pliss GB. Inhibition of DMBA-induced carcinogenesis by phenformin in the mammary gland of rats. Arch Geschwulstforsch. 1978;48:1–8. [PubMed] [Google Scholar]
- 98.Dilman VM, Anisimov VN. Effect of treatment with phenformin, diphenylhydantoin or l-dopa on life span and tumour incidence in C3H/Sn mice. Gerontology. 1980;26:241–246. doi: 10.1159/000212423. [DOI] [PubMed] [Google Scholar]
- 99.Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- 100.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 101.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blagosklonny MV, Campisi J. Cancer and aging: more puzzles, more promises? Cell Cycle. 2008;7:2615–2618. doi: 10.4161/cc.7.17.6626. [DOI] [PubMed] [Google Scholar]
- 103.Smith DL, Elam CF, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB. Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci. 2010;65A:468–474. doi: 10.1093/gerona/glq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Katewa SD, Kapahi P. Dietary restriction and aging, 2009. Aging Cell. 2010;9:105–112. doi: 10.1111/j.1474-9726.2010.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Higami Y, Yu BP, Shimokawa I, Bertrand H, Hubbard GB, Masoro EJ. Anti-tumor action of dietary restriction is lesion-dependent in male Fischer 344 rats. J Gerontol A Biol Sci Med Sci. 1995;50:B72–B77. doi: 10.1093/gerona/50a.2.b72. [DOI] [PubMed] [Google Scholar]
- 106.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:B291–B296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 107.Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O’Kane CJ, Rubinsztein DC. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 108.Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. FEBS J. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- 109.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 110.Young DA, Nickerson-Nutter CL. mTOR – beyond transplantation. Curr Opin Pharmacol. 2005;5:418–423. doi: 10.1016/j.coph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 111.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 112.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 113.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mTOR, amyloid β and tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 116.Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther. 2003;2:S169–S177. [PubMed] [Google Scholar]
- 117.Mita MM, Mita A, Rowinsky EK. Mammalian target of rapamycin: a new molecular target for breast cancer. Clin Breast Cancer. 2003;4:126–137. doi: 10.3816/cbc.2003.n.018. [DOI] [PubMed] [Google Scholar]
- 118.Mahalingam D, Sankhala K, Mita A, Giles FJ, Mita MM. Targeting the mTOR pathway using deforolimus in cancer therapy. Future Oncol. 2009;5:291–303. doi: 10.2217/fon.09.9. [DOI] [PubMed] [Google Scholar]
- 119.Sankhala K, Mita A, Kelly K, Mahalingam D, Giles F, Mita M. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target Oncol. 2009;4:135–142. doi: 10.1007/s11523-009-0107-z. [DOI] [PubMed] [Google Scholar]
- 120.Mita M, Sankhala K, Abdel-Karim I, Mita A, Giles F. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin Investig Drugs. 2008;17:1947–1954. doi: 10.1517/13543780802556485. [DOI] [PubMed] [Google Scholar]
- 121.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 122.Garber K. Targeting mTOR: something old, something new. J Natl Cancer Inst. 2009;101:288–290. doi: 10.1093/jnci/djp034. [DOI] [PubMed] [Google Scholar]
- 123.Abraham RT. Lysosomal Rag-ulation of mTOR complex 1 activity. Cell Metab. 2010;11:341–342. doi: 10.1016/j.cmet.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 124.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]